Abstract
Free full text

Disturbance of the Circadian System in Shift Work and Its Health Impact
Abstract
The various non-standard schedules required of shift workers force abrupt changes in the timing of sleep and light-dark exposure. These changes result in disturbances of the endogenous circadian system and its misalignment with the environment. Simulated night-shift experiments and field-based studies with shift workers both indicate that the circadian system is resistant to adaptation from a day- to a night-oriented schedule, as determined by a lack of substantial phase shifts over multiple days in centrally controlled rhythms, such as those of melatonin and cortisol. There is evidence that disruption of the circadian system caused by night-shift work results not only in a misalignment between the circadian system and the external light-dark cycle, but also in a state of internal desynchronization between various levels of the circadian system. This is the case between rhythms controlled by the central circadian pacemaker and clock genes expression in tissues such as peripheral blood mononuclear cells, hair follicle cells, and oral mucosa cells. The disruptive effects of atypical work schedules extend beyond the expression profile of canonical circadian clock genes and affects other transcripts of the human genome. In general, after several days of living at night, most rhythmic transcripts in the human genome remain adjusted to a day-oriented schedule, with dampened group amplitudes. In contrast to circadian clock genes and rhythmic transcripts, metabolomics studies revealed that most metabolites shift by several hours when working nights, thus leading to their misalignment with the circadian system. Altogether, these circadian and sleep-wake disturbances emphasize the all-encompassing impact of night-shift work, and can contribute to the increased risk of various medical conditions. Here, we review the latest scientific evidence regarding the effects of atypical work schedules on the circadian system, sleep and alertness of shift-working populations, and discuss their potential clinical impacts.
Shift Work in Modern Society
Shift work is essential in today’s 24/7 society, including in health care and emergency services, hospitality, transport, and manufacturing. In an effort to monitor global trends in working conditions, the International Labour Organization and the European Foundation for the Improvement of Living and Working Conditions compared exposure to shift work across 187 countries covering approximately 1.2 billion workers (Eurofound and International Labour Organization, 2019). Between 10% and 30% of workers are working night shifts at least once a month whereas working on a rotating or regular night-shift schedule was reported by 12% to 13% of the workforce in North America (Yong et al., 2017; Rydz et al., 2020), although other types of atypical shifts (e.g., split shifts, irregular shifts, on call) also occur and are more difficult to define and quantify. When only regular night shifts are considered, a 3.6% to 4.4% prevalence was reported (Yong et al., 2017; Bureau of Labor Statistics, 2019). Women represent roughly half of the shift workers, and are more likely to work part time and experience work-life conflicts (Eurofound and International Labour Organization, 2019).
The aim of this narrative review is to summarize the latest scientific evidence on disturbances of the circadian system associated with working atypical schedules and to discuss their impacts on the physical and mental health of shift-working populations. We focused on circadian misalignment and internal desynchrony rather than intervention studies, with an emphasis on work done in real shift workers.
The Human Circadian System
From gene expression to behavior, nearly every function is influenced by the endogenous circadian timing system. In humans, physiological parameters (e.g., body temperature, heart rate variability, brain waves, resting energy expenditure), biological processes (e.g., hormone, metabolites, clock gene, and protein expression), and behavior (sleep propensity and organization, cognitive abilities and performance) demonstrate circadian variations.
The Central Clock and Molecular Clockwork
In 2017, the Nobel Prize in Physiology and Medicine was awarded to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for their discoveries which clarified the molecular mechanisms underlying self-sustained intracellular circadian oscillations (Young, 2018). The core of the molecular clockwork is composed of transcriptional autoregulated feedback loops and a set of clock genes whose transcription can generate and maintain circadian rhythms in the absence of environmental time cues (Honma, 2018; Cox and Takahashi, 2019). At the cellular level, the clock genes CLOCK and ARNTL (alias BMAL1) encode activators of the main feedback loop, whereas period circadian regulators 1 to 3 (PER1, PER2, PER3) and cryptochrome circadian regulators 1 to 2 (CRY1, CRY2) encode repressors. The processes of translation/transcription and accumulation/degradation of these core components form the basic circadian clock and cycles with a period of approximately 24 h. Besides these main core clock genes and associated feedback loops, additional components have been described, adding to the complexity and precision of the clockwork mechanisms. Clock-controlled genes are considered the molecular output of the circadian clock as they are the links between the core clockwork and observable rhythms in cells, tissues, functions, and behaviors.
Peripheral Clocks
Today, we know that the molecular clockwork underlying circadian rhythms is intrinsic to most cells and tissues in mammals (Yamazaki et al., 2000), including in humans (Bjarnason et al., 2001; Archer et al., 2008; Akashi et al., 2010; Cuesta et al., 2017; Kervezee et al., 2019b; Du and Brown, 2021). The suprachiasmatic nucleus (SCN) is considered the central clock, while other tissues generating self-sustained circadian rhythms are named peripheral clocks (Brown et al., 2019). Contrary to the SCN which can self-sustain circadian rhythms for weeks without environmental cues, the oscillations observed in peripheral tissues usually dampen after a few days in vitro at the tissue level (Yamazaki et al., 2000), but not necessarily at the individual cell level (Welsh et al., 2004).
The existence of peripheral clocks in humans was demonstrated in oral mucosa cells (Bjarnason et al., 2001), then in peripheral blood mononuclear cells (PBMCs) and other blood cells (Boivin et al., 2003; Kusanagi et al., 2004; James et al., 2007a), in hair follicles (Akashi et al., 2010), skin cells (Brown et al., 2005; Wu et al., 2018), and in adipose fat tissues (Gomez-Abellan et al., 2008; Garaulet et al., 2011). We and others have shown the existence of non-SCN clocks in human post-mortem brain tissues, with desynchronized or dampened rhythmicity in Alzheimer’s disease or major depressive disorders (Cermakian et al., 2011; Li et al., 2013; Lim et al., 2013). A comprehensive study of the transcriptome carried out in 64 tissues collected in 12 baboons (one animal per time point) revealed that more than 80% of the detected protein-coding genes exhibited a 24-h rhythm in at least one tissue, with only limited overlap between tissues (Mure et al., 2018). Interestingly, Ruben et al. (2018) used an open access database of post-mortem RNA-sequenced human donor samples, combined with an automatic ordering algorithm, to create a population-level atlas of gene expression in 13 human tissues. They showed that 44% of the protein-coding genes cycled in at least one tissue studied.
Resetting of Circadian Clocks
Light is the most powerful synchronizer of the central circadian pacemaker in humans. The light-dark information is captured by the retina via specialized intrinsically photosensitive retinal ganglion cells expressing the photopigment melanopsin (Provencio et al., 2000; Yamazaki et al., 2000; Berson et al., 2002; Panda et al., 2002; Ruby et al., 2002). Based on animal studies, rods and cones are not necessary to photoentrainment (Freedman et al., 1999), but probably modulate the response to light (Foster et al., 2020). The light information is then directly transmitted to the SCN via monosynaptic connections of the retinohypothalamic tract. In humans, light exposure in the morning causes a phase advance of the circadian system, whereas exposure in the evening/early night causes a phase delay (Czeisler et al., 1989; Minors et al., 1991; Czeisler and Buxton, 2011; Vetter et al., 2021). Light administered in the middle of the day exerts only a small or non-discernible effect. The resetting effect of a light stimulus is also influenced by its intensity (Boivin et al., 1996; Zeitzer et al., 2000), duration (Dewan et al., 2011) and spectral composition (Ruger et al., 2013). Exposure to light can displace not only rhythms regulated by the central circadian clock such as those of cortisol and melatonin secretion, but also those of peripheral clocks (Yamazaki et al., 2000; James et al., 2007b; Ackermann et al., 2009; Cuesta et al., 2017; Kervezee et al., 2019b). Initial studies have suggested that the central circadian clock can be shifted faster than peripheral clocks in humans (James et al., 2007b) and rats (Yamazaki et al., 2000). However, it was later shown that bright light exposure in humans can synchronize peripheral clock gene expression more rapidly than previously suspected (Cuesta et al., 2017).
In humans, only a few nonphotic stimuli have been reported to modify the rhythmicity of the central circadian system under very dim light conditions or in free-running blind individuals, including exogenous melatonin (Lockley et al., 2000; Burgess et al., 2010) and exercise (Buxton et al., 2003; Barger et al., 2004). Social contacts have also been proposed to entrain the circadian system in mammals and humans (Mistlberger and Skene, 2004), but their resetting effects remain controversial as social interactions may rather modulate the daily pattern of light-dark exposure than directly shift the circadian system.
Sex Differences in Circadian Physiology
Women have, on average, a shorter intrinsic circadian period than men and are more likely than men to have a circadian period shorter than 24 h (Duffy et al., 2011). Probably as a consequence, the circadian phases of many biological processes (e.g., melatonin and core body temperature) occur earlier in women than men for a similar habitual sleep timing (Baehr et al., 2000; Mongrain et al., 2004; Cain et al., 2010; Duffy et al., 2011; Boivin et al., 2016). It is presumed these sex differences in circadian physiology can affect the timing of sleep and waking, although differences tend to disappear with aging (Roenneberg et al., 2007).
The circadian variations of sleep parameters (e.g., sleep efficiency, sleep onset latency, REM sleep, and non-REM sleep propensity) have been shown to be advanced in women compared to men when rhythms are aligned by their habitual wake time (Boivin et al., 2016), although these results are not consistent across studies (Santhi et al., 2016). For similar sleep times, women also presented an advanced alertness rhythm compared to men (Boivin et al., 2016), with lower nocturnal alertness levels and/or performances (Boivin et al., 2016; Santhi et al., 2016). Moreover, menstrual phase or hormonal contraceptives can affect the circadian variation of sleep (Shechter et al., 2010; Boivin et al., 2016) and alertness (Wright and Badia, 1999; Boivin et al., 2016). These observations underline the role of sex and gonadotropic steroids on circadian physiology, although contradictory results persist and more studies are needed, especially field studies of shift-working populations. These are important to better understand the reported reduced tolerance to shift work (Saksvik et al., 2011) and greater risk for work injury (Wong et al., 2011) observed in women compared to men.
Circadian Disturbances In Shift Work
General Observations
For the impact of shift work on rhythms controlled by the central circadian clock, such as cortisol, melatonin, and core body temperature, a search of the PubMed database was conducted using the following strategy: (“shift work” OR “night shift”) AND ([circadian rhythms] OR (circadian misalignment)) AND (“cortisol” OR “melatonin” OR “body temperature”). Only studies published since 2000 with real shift workers were considered. A total of 482 references were obtained and 30 original studies were kept after screening titles and abstracts. Three relevant studies known to the authors were added to the list. Search results are summarized in Table 1. In general, markers of the central circadian pacemaker such as melatonin, cortisol, and body temperature are reduced in amplitude or distorted when working atypical shifts, especially night shifts (e.g., Goh et al., 2000; Harris et al., 2010; Bostock and Steptoe, 2013; Bracci et al., 2016). With some exceptions (e.g., Gibbs et al., 2007; Hansen et al., 2010), rhythms when working night shifts remain misaligned to a night-oriented schedule, showing few signs of circadian adaptation (e.g., Ferguson et al., 2012; Gomez-Acebo et al., 2015; Bracci et al., 2016; Daugaard et al., 2017; Molzof et al., 2019; Razavi et al., 2019).
Table 1.
Central clock markers in shift workers.
| References | Population | Tissue and Circadian Markers | Observations |
|---|---|---|---|
| Barnes et al. (1998) | Offshore oil-rig workers | Urine: 6-sulfatoxymelatonin | Phase: ~1.5 h/day partial phase shift |
| Goh et al. (2000) | Navy personnel | Saliva: Melatonin | Mesor: 17% ↓ Profile: 19% distorted peaks/troughs Phase: 52% profiles misaligned, 12% partial delay |
| Saliva: Cortisol | Profile: Disrupted peaks and troughs in night work | ||
| Zuzewicz et al. (2000) | Air traffic controllers | Urine: Cortisol | Mesor: ↓ during night vs. day shifts |
| Yamauchi et al. (2001) | Shift-working nurses | Urine: 6-sulfatoxymelatonin | Phase: ↓ at night for night vs. day shifts |
| Gibbs et al. (2002) | Offshore oil-rig workers | Urine: 6-sulfatoxymelatonin | Phase: 5.4 h phase delay after 7 night shifts |
| Lac and Chamoux (2004) | Process control worker | Saliva: Cortisol | Mesor: ↓ peak for night vs. day shifts Phase: Later peak on 3rd night vs. day shift (1100 h vs. 0700 h) |
| Hansen et al. (2006) | Nurses | Urine: 6-sulfatoxymelatonin | Mesor: ↓ for night vs. day shifts |
| Gibbs et al. (2007) | Offshore oil-rig workers | Urine: 6-sulfatoxymelatonin | Phase: 83% > 3 h phase delay after 7 night shifts |
| Kudielka et al. (2007) | Manufacturing workers | Saliva: Cortisol | Profile: ↓ awakening response after night shifts Phase: n.s. between fixed and rotating night workers |
| Grundy et al. (2009) | Nurses | Saliva: Melatonin | Phase: Peak between 2300 h and 0700 h for night and day shifts |
| Urine: 6-sulfatoxymelatonin | Profile: ↓ upon awakening after night vs. day shifts | ||
| Hansen et al. (2010) | Offshore oil-rig workers | Urine: 6-sulfatoxymelatonin | Phase: 4 h delay after 7 nights |
| Harris et al. (2010) | Offshore oil-rig workers | Saliva: Cortisol | Profile: ↓ awakening response when working nights vs. days. n.s. timing of peak relative to awakening |
| Grundy et al. (2011) | Nurses | Saliva: Melatonin | Phase: Peak between 2300 h and 0700 h for both night and day shifts |
| Urine: 6-sulfatoxymelatonin | Profile: Similar morning excretion after night vs. day shifts | ||
| Leichtfried et al. (2011) | Doctors and medical students | Urine: 6-sulfatoxymelatonin | Profile: ↓ peak between 1900 h and 2300 h of 24 h shifts Phase: Peak in same 4 h time bin before and after 24 h shifts |
| Dumont et al. (2012) | Telecommunication workers | Urine: 6-sulfatoxymelatonin | Mesor: Similar 24 h excretion in night and day shifts Profile: ↓ during day vs. night sleep periods |
| Ferguson et al. (2012) | Remote mining operators | Saliva: Melatonin | Phase: ~30 min delay after 7 night vs. day shifts |
| Peplonska et al. (2012) | Nurses and midwives | Urine: 6-sulfatoxymelatonin | Profile: Similar between 0600 h-0800 h after night or day shifts |
| Bostock and Steptoe (2013) | Short-haul airline pilots | Saliva: Cortisol | Profile: ↓ awakening response for late vs. early shifts |
| Mirick et al. (2013) | Health care workers | Urine: 6-sulfatoxymelatonin | Profile: ↓ during day vs. night sleep periods |
| Urine: Cortisol | Profile: ↑ during day vs. night sleep periods | ||
| Serum: Cortisol | Profile: ↓ morning levels after night shifts morning levels after night shifts | ||
| Papantoniou et al. (2014) | Assorted occupations/industries | Urine: 6-sulfatoxymelatonin | Mesor: ↓ for night workers Amp.: ↓ for night workers Phase: Later for night vs. day workers (0842 h vs. 0536 h) |
| Gomez-Acebo et al. (2015) | Nurses and teachers | Urine: 6-sulfatoxymelatonin | Mesor: ↓ for rotating night workers Amp.: ↓  for rotating night workers for rotating night workersPhase: Later for night vs. day shifts (0831 h vs. 0713 h) |
| Serum: Cortisol | Profile: n.s. morning levels for night and day workers | ||
| Niu et al. (2015) | Nurses | Saliva: Cortisol | Profile: ↓ awakening response for 4 night shifts |
| Bracci et al. (2016) | Nurses | Wrist skin temperature | Mesor: ↑ for shift workers Amp.: ↓ for shift workers Phase: n.s. |
| Saliva: Melatonin | Profile: n.s. Phase: Peak in same 3 h bin for shift and day workers | ||
| Saliva: Cortisol | Profile: ↓ peak for shift workers Phase: Peak in same 3 h bin for shift and day workers | ||
| Hung et al. (2016) | Hospital employees | Urine: Cortisol | Mesor: 16.7% ↓ for night vs. day workers Profile: Flatter rhythm for night vs. day shifts |
| Jensen et al. (2016) | Police officers | Saliva: Melatonin | Profile: ↓ peak after 4 nights Phase: n.s. |
| Saliva: Cortisol | Profile: n.s Phase: 8.83 h (2nd night) to 11.52 h (7th night) delay | ||
| Leung et al. (2016) | Hospital employees | Urine: 6-sulfatoxymelatonin | Mesor: ↓ after 1 night shift Phase: Earlier in night vs. day shifts (0343 h vs. 0423 h) |
| Morris et al. (2016) | Current self-identified shift workers | Blood: Melatonin | Phase: n.s. |
| Daugaard et al. (2017) | Assorted occupations/industries | Saliva: Melatonin | Mesor: 15% ↓ for night vs. day workers Phase: n.s. |
| Jang et al. (2017) | Manufacturing company employees | Wrist skin temperature | Mesor: n.s. Amp.: ↓ for shift vs. day workers Phase: Later for shift vs. day workers (0803 h vs. 0411 h) |
| Stone et al. (2018) | Physicians and nurses | Urine: 6-sulfatoxymelatonin | Phase: 1.1 h delay for 3rd/4th shifts (0421 vs. 0518 h) |
| Koshy et al. (2019) | Police officers | Saliva: Cortisol | Profile: ↓ difference between wake and bedtimes after 7 night shifts |
| Urine: 6-sulfatoxymelatonin | Mesor: ↓ after night shifts Amp.: ↓ after night shifts Phase: 7.6 h phase delay after 7 nights (0425 h vs. 1159 h) | ||
| Molzof et al. (2019) | Nurses | Core body temperature | Phase: Delayed for night vs. day shifts (0816 h vs. 0252 h) |
| Razavi et al. (2019) | Nurses | Urine: 6-sulfatoxymelatonin | Mesor: ↓ for night vs. day workers Profile: ↓ peak for night vs. day workers Phase: Delayed for night vs. day workers (0543 h vs. 0406 h) |
Abbreviation: n.s. = non-significant. Studies were screened for results on mesor, amplitude (amp.), and acrophase (phase). When the amplitude was not available, profile information, such as peak modifications and rhythm distortions, were reported. All studies were conducted in the field, with one exception (i.e., Morris et al., 2016). Clock times expressed in 24-h time format hhmm.
For the impact of shift work on peripheral clocks, a search of the PubMed database was conducted using the following string “shift work” AND “clock genes” with no time restrictions. Only studies including >12-h sampling in at least one peripheral tissue, in real or simulated shift work, without interventions were considered. A total of 236 references were obtained, and five original studies were kept after screening titles and abstracts. One pertinent study found in the reference lists of these papers was added. Search results are summarized in Table 2, upper panel.
Table 2.
Peripheral clocks and omic studies in shift work.
| References | Population | Tissue and Circadian Markers | Observations |
|---|---|---|---|
| Clock gene expression in peripheral tissues | |||
 Akashi et al. (2010) Akashi et al. (2010) | Rotating shift workers | Scalp hair follicle cells: PER2, PER3, NR1D1, NR1D2 | Phase: ~2 h delay |
 Cuesta et al. (2017) Cuesta et al. (2017) | Healthy participants | PBMCs: PER1 | Amp.: ↓ during night shifts (trend) Phase: 3.17 h delay |
| PBMCs: PER2, PER3, NR1D1 | Amp.: ↓ during night shifts Phase: n.s. | ||
| PBMCs: ARNTL | Phase: 2.45 h delay | ||
 Besco et al. (2018) Besco et al. (2018) | Healthy participants | Scalp hair follicle cells: PER1, PER3, NR1D1, NR1D2 | Similar profile between simulated night and day shifts |
 Skene et al. (2018) Skene et al. (2018) | Healthy participants | Blood cells: PER3 | Phase: n.s. |
 Hattammaru et al. (2019) Hattammaru et al. (2019) | Daytime workers, Nurses and doctors, Factory workers | Facial hair follicle cells: PER3 | Mesor: ↓ after consecutive night shifts Amp.: Fewer workers with sig. 24 h rhythms after night shifts Phase: n.s. |
| Facial hair follicle cells: NR1D1 | Mesor: n.s. Amp.: n.s. Phase: n.s | ||
| Facial hair follicle cells: NR1D2 | Mesor: ↓ after one night shift Amp.: Fewer workers with sig. 24 h rhythms after 1 vs. ≥3 night shifts Phase: n.s. | ||
 Koshy et al. (2019) Koshy et al. (2019) | Police officers | Oral mucosa cells: PER1 | Phase: 11 h delay |
| Oral mucosa cells: PER2, PER3, ARTNL, NR1D1, NR1D2 | Amp.: Loss of group rhythm after night shifts | ||
 Resuehr et al. (2019) Resuehr et al. (2019) | Nurses | PBMCs: PER1, PER3, ARNTL | Amp.: Only rhythmic in night-shift workers |
| PBMCs: PER2, NR1D1 | Amp.: Not rhythmic in day- or night-shift workers | ||
| Omic studies in peripheral tissues | |||
 Kervezee et al. (2018) Kervezee et al. (2018) | Healthy participants | PBMCs: Transcriptome | Mesor: Heterogenous Amp.: ↓  in rhythmic transcripts with simulated night shifts. in rhythmic transcripts with simulated night shifts.Phase: Misalignment of most (~73%) rhythmic transcripts with simulated night shifts. |
 Resuehr et al. (2019) Resuehr et al. (2019) | Nurses | PBMCs: Transcriptome | Only 20 rhythmic transcripts in both day- and night-shift groups (out of 446 and 341, respectively). |
 Skene et al. (2018) Skene et al. (2018) | Healthy participants | Plasma: Metabolome | Mesor: Heterogenous Amp.: Heterogenous Phase: Phase shift of most (~95%) rhythmic metabolites with simulated night shifts |
 Kervezee et al. (2019a) Kervezee et al. (2019a) | Healthy participants | Plasma: Metabolome | Mesor: Heterogenous Amp.: Heterogenous Phase: Phase shift of most (~75%) rhythmic metabolites with simulated night shifts |
Abbreviations: PBMC = peripheral blood mononuclear cell; n.s. = non-significant; sig. = significant. Studies were screened for results on mesor, amplitude (amp.) and acrophase (phase). Results on amplitudes also includes changes in the number of participants with significant rhythms.
For the impact of shift work on the transcriptome and metabolome, a search of the PubMed database was conducted using the following string, “shift work” AND (“transcriptome” OR “metabolome”) with no time restrictions. Only studies including >12-h sampling in at least one peripheral tissue, in real or simulated shift work, without interventions were considered. A total of 22 references were obtained, from which four original studies were kept after screening titles and abstracts, including one which was already identified in the previous search on clock genes. Search results are summarized in Table 2, lower panel.
In general, peripheral clocks remain adjusted to a day-oriented schedule, with dampened rhythms at the group level. This is the case for clock gene expression in PBMCs (Cuesta et al., 2017; Resuehr et al., 2019), blood cells (Skene et al., 2018), hair follicle cells (Akashi et al., 2010; Bescos et al., 2018; Hattammaru et al., 2019), and oral mucosa cells (Koshy et al., 2019), as well as transcriptomic rhythms in PBMCs (Kervezee et al., 2018; Resuehr et al., 2019). In contrast, the majority of metabolite rhythms rapidly adjust to the night-oriented schedule. Overall, a misalignment between circadian rhythms (either central or peripheral) and the environment is observed when working nights. An internal desynchrony also occurs between transcriptomic and metabolomic rhythms.
Circadian Misalignment
The various non-standard hours and rosters required of shift workers inevitably force abrupt changes in the sleep-wake and light-darkness schedules to which the central and peripheral clocks are usually entrained. These changes result in circadian misalignment, which describes a state of desynchronization between circadian clocks and the environment (Boivin and James, 2002a; Boudreau et al., 2013a; Skene et al., 2018; Kervezee et al., 2019a). Another immediate effect of night-shift work is the reduction in amplitude or distortion of circadian rhythms such as those of melatonin and cortisol secretion (Touitou et al., 1990; Dijk et al., 2012; Mirick et al., 2013). As schematically presented in Figure 1, simulated night-shift experiments and field-based studies with shift workers both indicate that the central clock is resistant to adaptation from a day-oriented to a night-oriented schedule, as determined by the magnitude of phase shifts in rhythms of melatonin, cortisol, and body temperature over multiple days (Crowley et al., 2004; Boudreau et al., 2013a; Jensen et al., 2016; Molzof et al., 2019; Resuehr et al., 2019; Jensen et al., 2020). Thus, the nadir of cortisol, peak of melatonin, and trough of body temperature, which normally occur in the first, middle, and last thirds of the nocturnal sleep period, coincide with wake periods during night shifts (Boivin and James, 2002a; Benloucif et al., 2005; Resuehr et al., 2019). This misalignment of endogenous rhythms with the shifted sleep-wake cycle means that shift workers must perform their tasks and sleep at incongruous biological times.
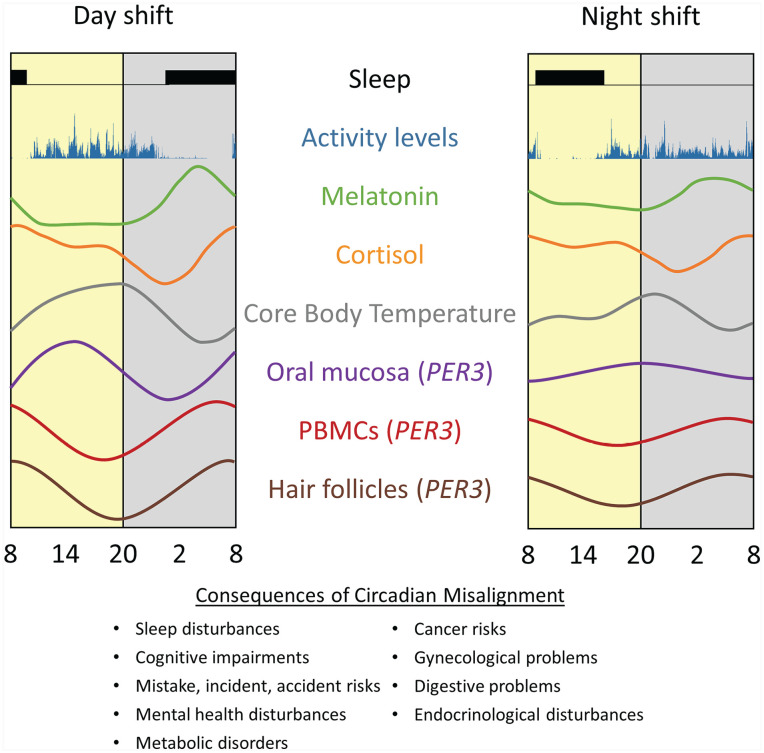
Disruption of central and peripheral rhythms by night-shift work. Under a night-oriented schedule, group rhythms are misaligned relative to the shifted rest-activity cycle and dampened in amplitude. Yellow and gray rectangles represent the environmental light and dark cycles, respectively. Rhythms are adapted from Cuesta et al. (2017) J Biol Rhythms 23; Cuesta et al. (2017); Koshy et al. (2019); Hattammaru et al. (2019). Abbreviation: PBMC = peripheral blood mononuclear cell.
Internal Desynchrony
There is evidence that disruption of the circadian system caused by night-shift work results not only in a misalignment between the circadian system and the external light-dark cycle but also in a state of internal desynchronization between several levels of the circadian system. We demonstrated this between rhythms controlled by the central circadian pacemaker (e.g., core body temperature, melatonin, cortisol) and those expressed in peripheral tissues (see Figure 1, central vs. peripheral rhythms). The discovery that rhythmic clock gene expression could be observed in PBMCs led to the exploration of the impact of a night-oriented schedule on this peripheral clock (Boivin et al., 2003; James et al., 2007a, 2007b; Cuesta et al., 2017). Under controlled laboratory conditions, rhythms of PER1, PER2, PER3, and BMAL1 clock genes expression desynchronized from the sleep-wake cycle and from each other after 3 days on a night-oriented schedule (Cuesta et al., 2017). While PER1 and BMAL1 rhythms delayed by ~2.5 to 3 h, other clock genes and rhythms of cortisol and melatonin remained adjusted to a day-oriented schedule. Three cycles of 8-h bright light exposure at night induced significant phase delays of ~7 to 9 h for central and peripheral markers, except BMAL1 (advanced by +5 h 29 minutes), thus demonstrating their endogenous circadian nature.
The disruptive effects of atypical work schedules extend beyond the expression profile of canonical circadian clock genes and affect other transcripts of the human transcriptome. In a simulated 4-night-shifts laboratory study of the transcriptome, we demonstrated that about 11.8% of transcripts in the human genome were rhythmic, and the majority of these rhythms did not adjust to a night schedule (Kervezee et al., 2018). In general, amplitudes of probe sets that were rhythmic in both conditions were significantly reduced in the night-shift condition compared with baseline. These results are consistent with those of Archer et al. (2014) who demonstrated, using a forced desynchrony protocol, that circadian disruption produced a 6-fold reduction in circadian transcripts compared with when sleeping in phase with the melatonin rhythm. In addition to circadian misalignment, sleep restriction can affect the expression of the human transcriptome and alter its circadian expression. It remains to be determined whether these rhythmic transcripts are under circadian control or are rather linked to the rest-activity cycle. The circadian nature of peripheral rhythmic transcripts is supported by their sensitivity to light-induced phase shifts (Möller-Levet et al., 2013; Archer et al., 2014; Arnardottir et al., 2014; Kervezee et al., 2019b).
Research conducted with real shift workers demonstrates a similar resistance of peripheral clocks to adapt to a night-oriented schedule (Akashi et al., 2010; Koshy et al., 2019). Akashi et al. (2010) found the phase of clock genes expression in hair follicle cells to be delayed by only ~2 h on late shifts (1500-0000 h) despite a ~7-h delay in behavioral rhythms relative to early shifts (0600-1500 h). Hattammaru et al. (2019) observed that PER3, Nr1D1 and Nr1D2 expression in facial hair follicle cells of night workers remained adjusted to a day-oriented schedule after one shift and that their phases were scattered after ≥3 consecutive nights, leading to dampened group rhythms. Koshy et al. (2019) studied 11 police officers before and after 7 days of night shifts. At baseline, central clock rhythms (urinary 6-sulfatoxymelatonin and salivary cortisol) and peripheral clock rhythms (clock genes expression in oral mucosa cells and PBMCs) were aligned to a day-oriented schedule. After seven night shifts, central group rhythms were partially adjusted and dampened, and individual rhythms were scattered (Koshy et al., 2019). In addition, rhythms of PER1-3 and REV-ERBα expression in oral mucosa cells were disrupted and the time-of-day variation in PBMCs clock genes PER1-3 was lost (Koshy et al., 2019). Recently, Resuehr et al. (2019) reported the rhythms of cortisol, melatonin, and clock gene expression in PBMCs of night nurses to be adjusted to a day-oriented schedule and more scattered, leading to a significantly dampened group rhythm. In comparison, these rhythms were clustered and well aligned to the sleep-wake cycle for day-shift nurses. Surprisingly, however, significant rhythms of the canonical circadian clock genes PER1 and PER3 were only detected in night nurses. More studies are needed on the disruption of the central and peripheral clocks of shift workers.
Altogether, these results further emphasize the all-encompassing impact of night-shift work, which not only affects rhythms controlled by the central clock but also those of peripheral clocks, and provides insight into molecular mechanisms affecting most of the entire genome.
Rate of Circadian Adaptation
Circadian adaptation to a night-oriented schedule is a gradual process requiring extended, consistent exposure to the altered work-rest cycle, and there is a high degree of variability in the capacity of night-shift workers to do so (Crowley et al., 2004; Boivin et al., 2012a, 2012b; Stone et al., 2018; Molzof et al., 2019). Without specific interventions to facilitate shifts in the central clock, it is estimated only ~25% of workers show circadian adaptation to night work (Folkard, 2008). Field-based studies indicate that most night workers are unlikely to demonstrate signs of substantial adaptation in melatonin or cortisol rhythms within three consecutive night shifts (Grundy et al., 2009; Dumont et al., 2012). However, these studies generally focused on comparing the daily patterns of hormone levels between work schedules rather than documenting changes in circadian phase (Hansen et al., 2006; Garde et al., 2009; Leung et al., 2016; Daugaard et al., 2017). While a couple of these found small reductions in melatonin levels during the night shift, none indicated phase shifts that would warrant a classification of even partial adaptation (Grundy et al., 2011; Leung et al., 2016; Daugaard et al., 2017; Stone et al., 2018). In another study, Molzof et al. (2019) monitored the core body temperature rhythm of nurses working three consecutive night or day shifts and found the temperature minimum was improperly aligned with daytime sleep. Work cycles comprising sequences of more than four or five consecutive night shifts are more likely than shorter sequences to show signs of circadian adaptation. However, even in these cases, changes in the profile of these rhythms are still highly variable between individuals and usually not large enough to represent complete adaptation of the central clock (Hansen et al., 2010; Harris et al., 2010; Ferguson et al., 2012).
Of the different shift-working populations that have been studied, the largest rates of adaptation to night work have consistently been reported in offshore oil-rig workers, whose isolated working environments and operating schedules are conducive to facilitating and maintaining changes to circadian alignment (Barnes et al., 1998; Gibbs et al., 2007; Hansen et al., 2010). Barnes et al. (1998) found that the acrophase of urinary sulfatoxymelatonin rhythm shifted on average ~1.3 to 1.8 h per day, with 96% of workers having their final acrophase within the second half of their daytime sleep period. Gibbs et al. (2007) and Hansen et al. (2010) found similar rates of adaptation for oil-rig workers compared with other occupations, over a week of consecutive shifts. In contrast, shift workers on similar sequences of night shifts who do not experience large phase shifts (e.g., police officers, nurses, and doctors) often have to meet work and domestic responsibilities that can interfere with their circadian adaptation (Boudreau et al., 2013a; Stone et al., 2018).
Some of the discrepancies in circadian adaptation observed in naturalistic field studies may be a consequence of the different environmental and behavioral confounders of various biomarkers of the central clock (Harris et al., 2010; Ferguson et al., 2012; Jensen et al., 2016). Indeed, the rhythms of melatonin, cortisol, and body temperature can be affected by light exposure, work-related stressors, and activity levels, respectively (Gander et al., 1986; Boivin and James, 2002b; Duffy and Dijk, 2002; van Eekelen et al., 2003). Furthermore, different metrics for assessing circadian rhythms in naturalistic environments may also influence whether adaptation can be determined (Barnes et al., 1998; Kudielka et al., 2007; Grundy et al., 2011; Jensen et al., 2020).
Interventions involving judicial exposure to light and darkness have been used to varying success for facilitating adaptation to night work. In a recent meta-analysis, Lam and Chung (2021) examined the phase-shifting effects of light therapy from the pooled-results of 13 studies comprising both real and simulated shift workers. Consistent with experimental protocols (Boivin et al., 1996; Zeitzer et al., 2000), brighter light at night was found to result in larger phase shifts and greater suppression of melatonin in a dose-responsive manner (Lam and Chung, 2021). However, the phase-shifting effects of longer or shorter treatments were more ambiguous (Lam and Chung, 2021). Light attenuation with goggles or sunglasses during the morning commute after night shifts has been shown to facilitate phase shifting (Boivin et al., 2012a), and studies focusing on the use or avoidance of blue-enriched light have demonstrated greater suppression of melatonin with more exposure (Rahman et al., 2013; Motamedzadeh et al., 2017).
Effect of Chronotype in Night-Shift Adaptation
Chronotype is a behavioral trait that describes an individual’s habitual sleep-timing preferences in relation to the 24-h light-dark cycle (Juda et al., 2013b) and is associated with the ability to adapt to specific shifts. For instance, early chronotypes generally have earlier bedtimes and wake-times than later chronotypes and typically function best in the morning than during the afternoon or evening (van de Ven et al., 2016). In contrast, late chronotypes typically have later and more flexible bedtimes, are more resilient to the consequences of night work (higher shift work tolerance), and obtain less sleep when engaging in morning shifts (Juda et al., 2013a; van de Ven et al., 2016; Kervezee et al., 2021). Moreover, early chronotypes tend to sleep for shorter durations when engaging in night work (Juda et al., 2013a), although this effect disappears when the effect of napping is considered (Kervezee et al., 2021). Increased morningness and eveningness were correlated with longer sleep duration during series of consecutive morning and evening shifts, respectively (Kervezee et al., 2021). Interestingly, Vetter et al. (2015) implemented a shift system wherein work hours were adjusted to accommodate workers’ chronotypes: morning shifts were abolished for late chronotypes, and night shifts were abolished for early chronotypes. It was found that aligning work hours and chronotype was associated with longer sleep duration across the work schedule (Vetter et al., 2015).
From an occupational health perspective, the impact of the chronotype on sleep duration and timing may mediate some of the adverse health effects associated with shift work (Kecklund and Axelsson, 2016; Kervezee et al., 2020). In a study of a large group of female hospital employees, it was shown that sleep duration is an important mediator of the relationship between shift work and metabolic syndrome (Korsiak et al., 2018). It remains to be determined whether chronotype affects this relationship. Using a cross-sectional design, Yu et al. (2015) reported that being an evening chronotype was associated with increased risk of metabolic syndromes in middle-aged adults. Similar results were reported in a case-control study, where metabolic syndrome cases were more often evening chronotypes (Assmann et al., 2020). The increased risk of metabolic syndromes in evening chronotypes would be related to modifiable lifestyle behavior rather than genetic factors (Vera et al., 2018). However, as detailed in the following section, the effect of chronotype on metabolic risks appears to differ in individuals’ working shifts.
Circadian Disruption of the Metabolome
When entrained to a day-oriented schedule, the central clock synchronizes the timing of peripheral clocks, including those related to metabolism in the liver and gut (Yamazaki et al., 2000; Brown et al., 2019). Given previous findings of internal desynchrony between central and peripheral clocks during night work, it has been proposed that circadian disruption may be one of the mechanisms behind increases in metabolic risks associated with night-shift work (Kecklund and Axelsson, 2016; Skene et al., 2018; Kervezee et al., 2019a). In a study of 100 female workers, Rotter et al. (2018) found that 70% of 44 analyzed urine metabolites after waking were altered between night and day shifts. When stratified by chronotype, working at night affected more metabolites for early chronotypes than late chronotypes. Skene et al. (2018) compared the rhythms of 132 circulating metabolites during a constant routine protocol following three simulated day versus night shifts. A shift was observed in 95% of rhythmic metabolites with 24-h rhythmicity whereas the circadian rhythms of melatonin, cortisol, and PER3 expression in PBMCs did not adapt to the shifted schedule. Kervezee et al. (2019a) also demonstrated that 75% of the metabolites that were rhythmic at both baseline and during the night-shift condition were driven by the delayed sleep-wake cycles. Thus, most rhythmic metabolites became misaligned relative to the endogenous circadian system when working at night. Further studies are needed to clarify if similar observations occurs in shift workers.
Shift Work and Sleep-Wake Disturbances
As initially proposed by Borbely (1982), sleep propensity is regulated by an interaction between homeostatic and circadian processes. The homeostatic process, known as process S, reflects the sleep pressure that builds up and dissipates exponentially over time during the wake and sleep periods, respectively. The circadian process, known as process C, varies according to a near 24-h rhythm with a circadian crest and nadir of alertness occurring in the evening and late night, respectively (Figure 2, upper panel). Sleep is initiated in the late evening because of the long period awake (Process S). The exponential decline in Process S as a function of time asleep suggests that most of the slow wave sleep needs are fulfilled within the first half of the night. The sleep period is prolonged to approximately 8 h because, despite the reduced homeostatic need for sleep, Process C promotes sleep in the second half of the night (red downward arrow, Figure 2, upper panel). After awakening in the morning, sleep propensity starts to increase. In the evening, Process C sends its strongest wake signal and promotes wakefulness until bedtime (green upward arrow, Figure 2, upper panel), even though Process S is elevated. Abrupt shifts in the timing of sleep, as frequently occurs in shift workers, disrupts the temporal harmony between processes S and C and leads to sleep-wake disturbances (Figure 2, lower panel). Typically, night-shift work gives rise to complaints of reduced sleep duration and quality, and impaired alertness, especially at night and in the early morning. Weitzer et al. (2021) reported that insomnia and daytime sleepiness can persist for years in former night-shift workers. After working at night, shift workers fall asleep rapidly in the morning as Processes S and C (red downward arrow, Figure 2, lower panel) are maximal at this moment. Compared with nocturnal sleep, workers wake up after shorter sleep duration during the day due to the exponential decline in Process S and because Process C starts to promote wakefulness. At the start of their night shift in the evening, they feel alert as Process C maximally promotes wakefulness, but feel sleepier as the night progresses.
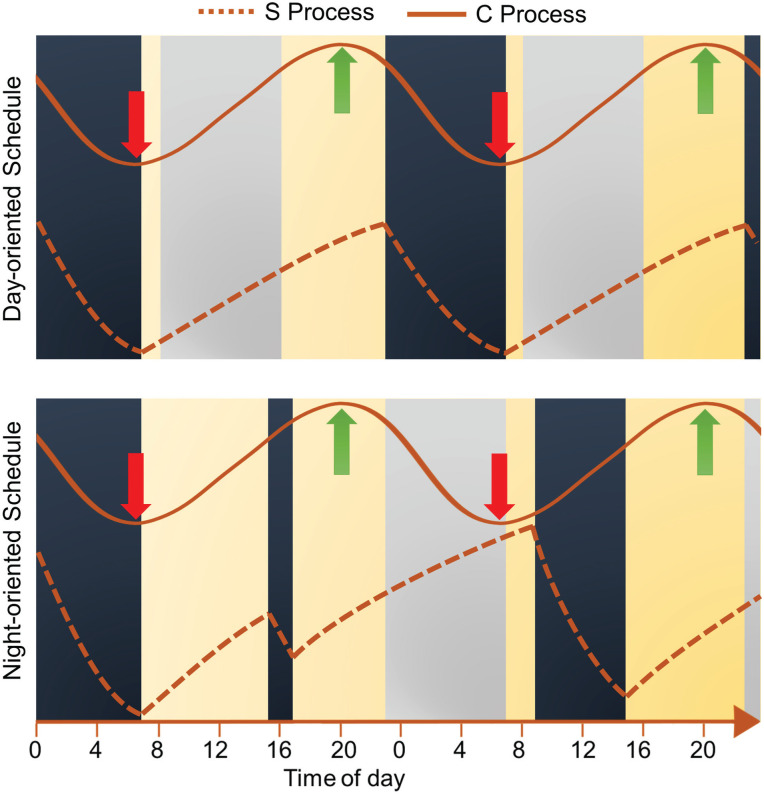
Sleep propensity as regulated by the homeostatic and circadian processes. The S process illustrates the homeostatic sleep drive, whereas the C process illustrates the wake propensity rhythm. The upper panel represents a person living on a day-oriented schedule, whereas the lower panel represents a person doing a first night shift after a nap in the afternoon. The strength of each process increases from bottom to top. Work shifts are represented by gray rectangles, sleep and nap periods by dark blue rectangles, and wake periods in yellow. Red and green arrows identify the circadian nadir and peak of wake propensity, respectively. During a typical workday, the circadian nadir of alertness occurs at the end of the nocturnal sleep period when the homeostatic drive for sleep is low. At the end of the first night shift, the circadian nadir of alertness occurs at the end of the night shift when the homeostatic drive for sleep is very high.
Sleep Disturbances
Reduced sleep quality and duration, and symptoms of insomnia are frequent in shift workers (Kecklund and Axelsson, 2016; Wyse et al., 2017; Yong et al., 2017; Moreno et al., 2019), especially those working nights, early morning, and rotating shifts (Akerstedt and Wright, 2009). Typically, the daytime sleep periods of night-shift workers end prematurely after 4 to 6 h (Akerstedt and Wright, 2009; Kecklund and Axelsson, 2016) and workers are often unable to resume sleep afterward. Early day shifts can be associated with similar levels of sleep restriction (Ganesan et al., 2019), as workers have difficulty falling asleep at earlier bedtimes coinciding with the evening wake maintenance zone, and sleep duration is curtailed to comply with the early work start. A large UK population-based study of more than 277,000 workers found that shift workers report less sleep per 24-h day and poorer sleep quality than non-shift workers (Wyse et al., 2017). A 5-year longitudinal study of 2615 hospital night-shift workers showed that the odds of reporting insomnia (Insomnia Severity Index ≥ 15) were increased by ~2-fold when working consecutive nights (vs. only one) (Lee et al., 2021). A meta-analysis summarizing 11 cross-sectional studies of police officers revealed that about 50% of workers report poor sleep quality (Pittsburgh sleep quality index > 5; Garbarino et al., 2019). In a large meta-analysis, Pilcher et al. (2000) reported an effect of the shift system on sleep duration. Compared with permanent day workers (7.0 h), permanent evening or night workers reported sleeping more (7.6 h, effect size = 0.42) or less (6.6 h, effect size = 0.35), respectively. Rotating shift workers slept more after evening shifts and less after night shifts, and this effect was more pronounced for rapid rotating schedules (evening shifts: 8.1 h, night shifts: 5.7 h, effect size ≥ 0.93). As a result of sleep loss during days of work on atypical shifts, rest days are often used for recovery and are associated with longer sleep periods (Garde et al., 2009; Paech et al., 2010; Garde et al., 2020; Kervezee et al., 2021). Recovery from one night of total sleep deprivation usually takes about one to two nights of recovery sleep (Balkin et al., 2008), whereas chronic sleep restriction takes longer. Axelsson et al. (2008) demonstrated that 7 days of recovery sleep was not enough to completely restore performances to baseline levels following 5 days of 4-h sleep per night. In a study of Norwegian nurses, Eldevik et al. (2013) found that the duration of breaks between successive work shifts was important, with quick returns (< 11 h off between shifts) associated with insomnia, excessive sleepiness, and shift work disorder. As detailed later, the negative cognitive, metabolic, and health outcomes of sleep curtailment are numerous, and probably play a role in the adverse effects associated with shift work.
Sleepiness
Sleepiness, impaired cognition and performance are common in shift workers, and have been reported in numerous studies, including in nurses (Behrens et al., 2019; Ganesan et al., 2019; Wilson et al., 2019), medical residents (Basner et al., 2017), police officers (Boivin et al., 2012a; Boudreau et al., 2013a), miners (Ferguson et al., 2010, 2011), marine pilots (Boudreau et al., 2018), professional truck drivers (Anund et al., 2018), train drivers (Jay et al., 2006), and airline pilots (Ingre et al., 2014; Sallinen et al., 2017; Aljurf et al., 2018; Sallinen et al., 2020). Extended wakefulness, lack of adequate recovery sleep between shifts, and being awake during the circadian trough of alertness, at night or in the early morning, lead to excessive sleepiness in shift workers (Mullins et al., 2014). Adjustment to consecutive night shifts is only modest under normal conditions but can improve significantly if circadian adaptation occurs (Bjorvatn et al., 2006; Boudreau et al., 2013a).
In the transportation industry, sleepiness raises important safety concerns (Akerstedt, 2019). On-the-road studies using electroencephalogram recordings in professional drivers (Kecklund and Akerstedt, 1993; Mitler et al., 1997) and inappropriate line crossing in non-professional drivers (Sagaspe et al., 2008; Hallvig et al., 2014) revealed increased incidence of severe sleepiness at night. In a group of 54 professional truck drivers, the odds of severe sleepiness (Karolinska sleepiness scale ≥ 7) were nine times higher during the first night shift compared with day and evening shifts (Pylkkonen et al., 2015). Commuting home after a night shift was also associated with higher risk of excessive sleepiness and accidents (Lee et al., 2016; Anderson et al., 2018; Liang et al., 2019). Cross-sectional studies of airline pilots revealed that about one airline pilot out of two reported to have unintentionally felt asleep while flying (Marqueze et al., 2017; Aljurf et al., 2018), which was confirmed by electroencephalogram recordings during real flights (Wright and McGown, 2001).
Performance and Cognitive Functions
Shift work, and especially night-shift work, has been associated with impairments in performance and cognitive functions (Boivin et al., 2012a; Boudreau et al., 2013a; Behrens et al., 2019; Chellappa et al., 2019; Wilson et al., 2019; Anvekar et al., 2021; Zhao et al., 2021). Circadian misalignment can impair cognitive functions (Goel et al., 2011), increase the risk of severe sleepiness, and lead to attentional errors (de Cordova et al., 2016). Performance also declines during extended work hours (Anderson et al., 2012; Rahman et al., 2021) and with shorter prior sleep duration (Ferguson et al., 2011). The first night shift usually leads to worst impairments, as both extended wakefulness and work during the circadian nadir of alertness are combined. Studies of shift workers have shown that the cognitive impairment associated with the first night shift can either gradually improve (Lamond et al., 2003; Bjorvatn et al., 2006; Santhi et al., 2007; Hansen et al., 2010), stabilize (Crowley et al., 2004; Ganesan et al., 2019), or deteriorate with consecutive night shifts (Axelsson et al., 2008; Boivin et al., 2012a; Flynn-Evans et al., 2018), probably depending on the working conditions (e.g., light exposure, work rosters, familial and social isolation), degree of circadian adaptation, and cumulative sleep debt. When circadian adaptation occurred, shift workers were found to have better sleep, have improved performance, and be more alert (Boivin et al., 2012b; Boudreau et al., 2013a; Molzof et al., 2019).
Acute (Lim and Dinges, 2010) and chronic sleep deprivation (Van Dongen et al., 2003) both contribute to impairment of performance and cognitive functions during atypical work schedules. Based on laboratory experiments carried out in a group of young adults (42 men, 6 women), it was concluded that an average human needs about 8.16 h of sleep per 24-h day to prevent cumulative neurobehavioral deficits (Van Dongen et al., 2003), although important interindividual differences exist (Van Dongen et al., 2004). As shift workers often report shorter sleep duration (Akerstedt and Wright, 2009; Kecklund and Axelsson, 2016; Wyse et al., 2017), performance and cognitive impairments are to be expected. However, the size of these effects in the field cannot be directly translated from laboratory-based studies, especially if they are carried out in a different demographic group.
Impact of Shift Work on Physical and Mental Health
Working atypical shifts is associated with an increased risk of developing many chronic health conditions compared with day workers which may explain the high rates of absenteeism and long-term disability observed in shift workers (Violanti et al., 2011; Wong et al., 2011). Disturbed behavioral rhythms can be a contributing factor to these risks. Shift work has been associated with physical inactivity and disruption of family and social activities (Atkinson et al., 2008; Arlinghaus et al., 2019). The maintenance of regular physical activity was reported to be harder in shift workers due to several factors, including the opening hours of leisure facilities, availabilities of other team members, conflicting domestic and familial activities, and fatigue associated with shift work.
Shift work also disrupts behavioral rhythms such as the timing of meals, which a growing body of research suggests has consequences for metabolic processes and health (Banks et al., 2015; Skene et al., 2018). In a study of police officers on rotating shift schedules, Kosmadopoulos et al. (2020) found that caloric intake was significantly more dispersed across the 24-h day, with a greater proportion of caloric intake at night, on night-shift days than other types of days. This is relevant as eating later or having a greater caloric intake later in the circadian day is associated with greater body fat and reduced weight loss effectiveness, independent of total daily consumption (Garaulet et al., 2013; Reid et al., 2014; Hermenegildo et al., 2016; Ruiz-Lozano et al., 2016; McHill et al., 2017; Lopez-Minguez et al., 2018; McHill et al., 2019). The cause of this impairment of metabolism is hypothesized to be due to the circadian misalignment of peripheral clocks in the liver, pancreas, and gastrointestinal tract due to changes in the fasting/feeding cycle (Skene et al., 2018; Kervezee et al., 2019a).
Under laboratory conditions, it has been demonstrated that circadian misalignment can reduce daily energy expenditure, which could contribute to weight gain and adverse health outcomes if not accompanied by increased activity or a reduction in caloric intake (McHill et al., 2014). Thus, the displacement of typical rest-activity rhythms caused by shift work can also affect energy metabolism (McHill et al., 2014).
Physical Health
As previously described, the circadian misalignment associated with working at night has been implicated in the increased risk of cardiometabolic disorders (Brum et al., 2015; Kecklund and Axelsson, 2016), including metabolic syndrome (Khosravipour et al., 2021; Wang et al., 2021), type 2 diabetes (Vetter et al., 2018; Gao et al., 2020), and cardiovascular heart diseases (Vetter et al., 2016; Kervezee et al., 2020). Other studies have reported different forms of cancer (Schernhammer et al., 2006; Mancio et al., 2018; Ward et al., 2019), various gastrointestinal and digestive complaints (Knutsson and Bøggild, 2010; Gupta et al., 2019), menstrual irregularities, dysmenorrhea, and difficulties with pregnancy (Labyak et al., 2002; Zhu et al., 2004; Hammer et al., 2018). These are likely to be at least partially due to the circadian disruption of internal physiological processes.
Many studies identify shift work as having an adverse effect on various risk factors for metabolic and cardiovascular diseases, including elevated glucose, insulin and triacylglyceride levels, and higher white blood cell counts (Sookoian et al., 2007; van Drongelen et al., 2011; Manodpitipong et al., 2017; Wirth et al., 2017). Longitudinal studies also provide evidence for an effect of shift work on impaired glucose tolerance, being overweight, and gaining weight (Proper et al., 2016). In a cross-sectional population-based study, Sookoian et al. (2007) found that rotating shift workers had a significantly higher odds ratio (OR) for metabolic syndrome than day workers, even after controlling for age and physical activity. In another study, Manodpitipong et al. (2017) reported that night work was associated with poorer glycemic control than day work after controlling for factors such as body mass index and sleep duration. It has been hypothesized that metabolic conditions common in shift work may partly be exacerbated by disturbances of healthy gut microbiota caused by sleep loss and circadian misalignment (Reynolds et al., 2017). Working at night may also lead to altered autonomous nervous system modulation of the heart when sleep occurs at adverse circadian phases (Scheer et al., 2009; Boudreau et al., 2013b; Morris et al., 2016). Combined, these findings give credence to an effect of circadian misalignment as a risk factor of cardiometabolic disturbances, independent of behavioral changes associated with night-shift work.
A number of meta-analyses and systematic reviews have attempted to combine the various epidemiological studies that address the relationship between shift work and metabolic and cardiovascular health, providing evidence for an association between shift work and metabolic syndrome (Wang et al., 2021), diabetes mellitus (Gan et al., 2015; Gao et al., 2020), obesity (Sun et al., 2018), hypertension (Manohar et al., 2017), and cardiovascular disease (Torquati et al., 2018). Sex appears to moderate some of these relationships, as female shift workers were shown to have a higher risk of developing metabolic syndrome and diabetes mellitus compared with male shift workers (Gao et al., 2020; Wang et al., 2021), but a lower risk of hypertension (Manohar et al., 2017). However, Gan et al. (2015) reported an increased risk of diabetes mellitus in female compared with male shift workers. A recent meta-analysis of 21 studies with follow-up periods ranging from 4 to 24 years concluded that the pooled relative risk of diabetes mellitus was 1.10 (95% confidence interval [CI] [1.05, 1.14]) in shift workers compared with non-shift workers (Gao et al., 2020). A dose-response analysis comprising three cohorts of female shift workers indicated that relative risk increased by 1.05 (95% CI [1.03, 1.07]) per 5 years of exposure to shift work (Gao et al., 2020). More follow-up studies are required to assess the cumulative exposure risk of shift work and the modulating effect of sex and gender.
The type of shift schedule has also been shown to affect the risk of different health conditions. For instance, several meta-analyses indicated that a rotating shift schedule was associated with an increased risk of diabetes and hypertension compared with other types of shift schedules, even fixed night shifts (Gan et al., 2015; Manohar et al., 2017), whereas another analysis indicated permanent night-shift workers had a greater risk of developing obesity than workers on rotating shift schedules (Sun et al., 2018). In a large meta-analysis comprising 21 longitudinal and case-controls studies, Torquati et al. (2018) recently demonstrated a heightened risk of coronary heart disease and ischemic heart disease associated with shift work. Several meta-analyses have revealed dose-response effects in the relationship between health and exposure to shift work, such that more years as a shift worker is linked to poorer cardiometabolic health (Sun et al., 2018; Torquati et al., 2018; Gao et al., 2020; Wang et al., 2021). Nonetheless, there is no consensus on the definition of exposure, and more research is required to differentiate the effect of years of work and intensity of shift schedules on health outcomes (Kecklund and Axelsson, 2016). Improving understanding of the different means through which shift work affects health outcomes will facilitate the development of strategies to mitigate the burden of shift work.
A working group of the International Agency for Research on Cancer (IARC, 2020) concluded that night-shift work is probably carcinogenic to humans, based on extensive analysis of human research, experimental animal studies, and mechanistic evidence. There is evidence that night work may have a role in causing or exacerbating several specific cancers, including those of the breast (Manouchehri et al., 2021), prostate (Gan et al., 2018; Mancio et al., 2018), colon, and rectum (Wang et al., 2015). There is weak support from other meta-analyses of an increased relative risk of prostate cancer for rotating shift schedules compared with fixed daytime workers, but no increased risk for fixed night-shift work (Du et al., 2017; Gan et al., 2018; Mancio et al., 2018). A couple of recent meta-analyses found that shift work had little to no effect on breast cancer or other types of cancer risk, including prostate, pancreatic, and colorectal (Travis et al., 2016; Dun et al., 2020). These contradictory results might be explained by factors such as the lifetime duration of shift work exposure. Indeed, Wegrzyn et al. (2017) found that nurses with long-term rotating night work (≥20 years) experience had a higher risk of breast cancer, especially those who were younger when they began shift work. Support for a dose-responsive effect of night work on colorectal cancer has been demonstrated with a meta-analysis, describing an estimated 11% increase in risk for every 5 years of exposure (Wang et al., 2015). More recent epidemiological research by Papantoniou et al. (2018) also found that nurses with more than 15 years of shift work exposure had a higher risk of rectal cancer. In shift workers, it was hypothesized that photic suppression of melatonin at night may be a plausible mechanism for the increased risk of breast cancer (IARC, 2020). Some studies support a carcinogenic effect of light exposure at night (Yang et al., 2014), whereas others do not (Dun et al., 2020). Some studies in humans have also found an association between the risk of breast cancer and either the homozygous or heterozygous 5-repeat allele of PER3 (Zhu et al., 2005) or the Ala394Thr polymorphism of the NPAS2 gene (Zhu et al., 2008). However, other analyses which found marginal associations between polymorphisms of ARNTL and CRY1 and breast cancer revealed insignificant results after statistically adjusting for multiple comparisons (Grundy et al., 2013). While the data from these separate studies do not uniformly report effects of specific clock genes on cancer, they raise the possibility that desynchronized rhythms of clock gene expression in peripheral tissues provide a plausible mechanism for increased risk of cancer development.
Mental Health and Well-being
There is a large body of research demonstrating adverse effects of shift work on mental health and general well-being (Eldevik et al., 2013; James et al., 2017; Sletten et al., 2020). Atypical working time arrangements are commonly associated with deterioration of social and familial life because workers are forced to live on a pattern that diverges from that of their family and community (Arlinghaus et al., 2019). Combined with the disruption caused by circadian misalignment, this temporal isolation from family and community provides context for the mental health concerns often experienced by shift workers, including an increased prevalence of burnout (Woo et al., 2020), depression and anxiety (Nabe-Nielsen et al., 2011; Angerer et al., 2017; Brown et al., 2020), insomnia or excessive sleepiness (Eldevik et al., 2013; Wright et al., 2013; Richter et al., 2016), and suicide ideation (Violanti et al., 2008; Petrie et al., 2020).
Burnout is a psychological syndrome described by the International Classification of Diseases (ICD-11) as an occupational phenomenon characterized by emotional exhaustion, depersonalization, and reduced sense of accomplishment resulting from chronic workplace stress (World Health Organization, 2019). There is evidence that the nature of many emotionally demanding occupations largely comprised of shift workers, such as nursing and policing, can lead to burnout (Bakker and Heuven, 2006).
In terms of other mental health conditions, Angerer et al. (2017) conducted a systematic review of longitudinal studies on the relationship between depression and night work. In this systematic review, most prospective studies of health care workers conducted over 2-year periods were inconclusive (Nabe-Nielsen et al., 2011; Thun et al., 2014). For example, Thun et al. (2014) found that day-working nurses who became night workers during the study period did not have worse symptoms of depression or anxiety than at baseline. However, nurses who changed from working at night to working during the day did report improvements in depression and anxiety symptoms over time (Thun et al., 2014). In contrast, other prospective studies of night workers recruited from the general population and followed for a minimum of 2 years did show an elevated risk of depression after several years (Angerer et al., 2017). In a meta-analysis, Zhao et al. (2019) reported an association between mental health disturbances (psychological distress or depressive symptoms) and shift work. The strength of this association was stronger for irregular/unpredictable shifts than permanent night and evening shifts. In addition to the type of shift schedule, the time off between shifts (Eldevik et al., 2013) and the number of hours per week was shown to affect mental health outcomes. In a study of the work hours of 2706 randomly selected junior doctors, Petrie et al. (2020) found that those who worked more than 55 h per week were more than twice as likely to report a mental disorder or suicide ideation than doctors who worked 40 to 44 h per week.
There is evidence that individual factors such as gender and sex can modulate the effects of shift work on mental health. A meta-analysis of five studies showed a 42% increase in the risk of depression among night workers (Angerer et al., 2017), whereas another comprising seven longitudinal studies reported a 33% higher risk of depressive symptoms in shift workers (Torquati et al., 2019). In the latter study, 90% of the heterogeneity was explained by a gender difference; female shift workers were more likely to report depressive symptoms than female non-shift workers (OR = 1.73). A cross-sectional study on the payroll records of 111 police officers found that suicide ideation increased for policewomen with symptoms of depression as percentage of total work hours beginning between 0400 h and 1100 h increased (Violanti et al., 2008). Suicide ideation increased for men who had high post-traumatic stress as the percentage of work beginning between 2000 h and 0300 h (Violanti et al., 2008).
Concluding Remarks
Shift work is prevalent in modern society and affects between 10% and 30% of the adult working population. The non-standard and often irregular work times force abrupt frequent changes in the timing of sleep and waking. This situation leads to a state of misalignment between the endogenous circadian system and the sleep-wake and light-dark cycles as well as between the various oscillatory components of the circadian system (Figure 1). Shift work leads to acute and chronic disturbances of sleep and alertness and an increased risk of fatigue-related incidents and accidents (Kecklund and Axelsson, 2016; Fischer et al., 2017). An increased risk of various physical and mental health conditions is observed in shift-working populations suggesting that, over time, the stress imposed by sleep-wake disruption and living at unfavorable circadian phases represent risk factors for these conditions (Boivin and Boudreau, 2014; Kecklund and Axelsson, 2016; Moreno et al., 2019). Even though it remains difficult to establish clear causal relationships between shift work and health outcomes, numerous factors have been identified as contributors to these increased health risks, including sleep curtailment, circadian disturbances, altered behavioral rhythms, and personal characteristics such as age, sex, gender, and chronotype. As a take-home message, managing exposure to these factors in exposed individuals appears a wise and clinically relevant practice, although it might be challenging to implement in various workplaces due to operational constraints. The size effects, in real working environments, of specific factors mediating the health impacts of atypical shifts remain unclear. It is thus difficult to set clear limits as to specific parameters of shift work exposure, more specifically its intensity, duration, or type of rosters, above which reasonable health and safety risks would be exceeded. More longitudinal and dose-response studies are also needed on personal biological and behavioral factors affecting individual susceptibility to shift work, as well as mediating factors involved in the development of its health consequences. Besides scientific evidences, shift work situations must be analyzed as individual cases in point, and several other considerations including societal, economic, and legal aspects must simultaneously be considered and balanced.
Footnotes
Conflict of Interest Statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.B.B. provides consultation and medico-legal advice on shift work–related cases.
ORCID iDs: Diane B. Boivin  https://orcid.org/0000-0002-3896-2710
https://orcid.org/0000-0002-3896-2710
Philippe Boudreau  https://orcid.org/0000-0002-9367-2919
https://orcid.org/0000-0002-9367-2919
Anastasi Kosmadopoulos  https://orcid.org/0000-0002-3510-2811
https://orcid.org/0000-0002-3510-2811
References
Full text links
Read article at publisher's site: https://doi.org/10.1177/07487304211064218
Read article for free, from open access legal sources, via Unpaywall:
https://journals.sagepub.com/doi/pdf/10.1177/07487304211064218
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/120102348
Article citations
Night shift-induced circadian disruption: links to initiation of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and risk of hepatic cancer.
Hepatoma Res, 2394-5079.2024.88, 30 Oct 2024
Cited by: 0 articles | PMID: 39525867 | PMCID: PMC7616786
Integrity of the circadian clock determines regularity of high-frequency and diurnal LFP rhythms within and between brain areas.
Mol Psychiatry, 29 Oct 2024
Cited by: 0 articles | PMID: 39472662
Interactive correlations between artificial light at night, health risk behaviors, and cardiovascular health among patients with diabetes: A cross-sectional study.
J Diabetes, 16(10):e70008, 01 Oct 2024
Cited by: 1 article | PMID: 39397260 | PMCID: PMC11471435
Personalized sleep and nutritional strategies to combat adverse effects of night shift work: a controlled intervention protocol.
BMC Public Health, 24(1):2555, 19 Sep 2024
Cited by: 0 articles | PMID: 39300419 | PMCID: PMC11414285
Computational Analyses Reveal Deregulated Clock Genes Associated with Breast Cancer Development in Night Shift Workers.
Int J Mol Sci, 25(16):8659, 08 Aug 2024
Cited by: 0 articles | PMID: 39201344 | PMCID: PMC11355052
Go to all (84) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Disruption of central and peripheral circadian clocks and circadian controlled estrogen receptor rhythms in night shift nurses in working environments.
FASEB J, 38(11):e23719, 01 Jun 2024
Cited by: 0 articles | PMID: 38837828
The Impact of Shift Work on Sleep, Alertness and Performance in Healthcare Workers.
Sci Rep, 9(1):4635, 15 Mar 2019
Cited by: 118 articles | PMID: 30874565 | PMCID: PMC6420632
Circadian phase, sleepiness, and light exposure assessment in night workers with and without shift work disorder.
Chronobiol Int, 29(7):928-936, 01 Aug 2012
Cited by: 44 articles | PMID: 22823876
Impacts of shift work on sleep and circadian rhythms.
Pathol Biol (Paris), 62(5):292-301, 20 Sep 2014
Cited by: 192 articles | PMID: 25246026
Review
Funding
Funders who supported this work.
Canadian Institutes of Health Research (1)
Grant ID: MOP-102724
Fonds de Recherche du Quebec - Sante (1)
Grant ID: 273443
Institut de Recherche Robert-Sauve en Sante et en Securite du Travail (1)
Grant ID: 2013-0046




