Abstract
Background
Electroencephalogram (EEG) discontinuity can occur at high concentrations of anesthetic drugs, reflecting suppression of electrocortical activity. This EEG pattern has been reported in children and reflects a deep state of anesthesia. Isoelectric events on the EEG, a more extreme degree of voltage suppression, have been shown to be associated with worse long-term neurologic outcomes in neonates undergoing cardiac surgery. However, the clinical significance of EEG discontinuities during pediatric anesthesia for noncardiac surgery is not yet known and merits further research. In this study, we assessed the incidence of EEG discontinuity during anesthesia induction in neurologically normal infants and the clinical factors associated with its development. We hypothesized that EEG discontinuity would be associated with sevoflurane-induced alpha (8-12 Hz) power during the period of anesthesia induction in infants.Methods
We prospectively recorded 26 channels of EEG during anesthesia induction in an observational cohort of 54 infants (median age, 7.6 months; interquartile range [IQR] [4.9-9.8 months]). We identified EEG discontinuity, defined as voltage amplitude <25 microvolts for >2 seconds, and assessed its association with sevoflurane-induced alpha power using spectral analysis and multivariable logistic regression adjusting for clinically important variables.Results
EEG discontinuity was observed in 20 of 54 subjects (37%), with a total of 25 discrete events. Sevoflurane-induced alpha power in the posterior regions of the head (eg, parietal or occipital regions) was significantly lower in the EEG discontinuity group (midline parietal channel on the electroencephalogram, International 10-20 System [Pz]; 8.3 vs 11.2 decibels [dBs]; P = .004), and this association remained after multivariable adjustment (adjusted odds ratio [aOR] = 0.51 per dB increase in alpha power [95% CI, 0.30-0.89]; P = .02). There were no differences in the baseline (unanesthetized) EEG between groups in alpha power or power in any other frequency band.Conclusions
We demonstrate that EEG discontinuity is common during anesthesia induction and is related to the level of sevoflurane-induced posterior alpha power, a putative marker of cortical-thalamic circuit development in the first year of life. This association persisted even after adjusting for age and propofol coadministration. The fact that this difference was only observed during anesthesia and not in the baseline EEG suggests that otherwise hidden brain circuit properties are unmasked by general anesthesia. These neurophysiologic markers observed during anesthesia may be useful in identifying patients who may have a greater chance of developing discontinuity.Free full text

Decreased electroencephalographic (EEG) alpha power during anesthesia induction is associated with EEG discontinuity in human infants
Abstract
Background:
Electroencephalogram (EEG) discontinuity can occur at high concentrations of anesthetic drugs, reflecting suppression of electrocortical activity. This EEG pattern has been reported in children and reflects a deep state of anesthesia. Isoelectric events on the EEG, a more extreme degree of voltage suppression, have been shown to be associated with worse long-term neurologic outcomes in neonates undergoing cardiac surgery. However, the clinical significance of EEG discontinuities during pediatric anesthesia for non-cardiac surgery is not yet known and merits further research. In this study, we assessed the incidence of EEG discontinuity during anesthesia induction in neurologically normal infants and the clinical factors associated with its development. We hypothesized that EEG discontinuity would be associated with sevoflurane-induced alpha (8 to 12 Hz) power during the period of anesthesia induction in infants.
Methods:
We prospectively recorded 26 channels of EEG during anesthesia induction in an observational cohort of 54 infants (median age 7.6 IQR [4.9, 9.8] months). We identified EEG discontinuity, defined as voltage amplitude <25 microvolts for >2 seconds, and assessed its association with sevoflurane-induced alpha power using spectral analysis and multivariable logistic regression adjusting for clinically important variables.
Results:
EEG discontinuity was observed in 20 of 54 subjects (37%), with a total of 25 discrete events. Sevoflurane-induced alpha power in the posterior regions of the head (e.g., parietal or occipital regions) was significantly lower in the EEG discontinuity group (midline parietal channel [Pz], 8.3 vs. 11.2 dBs, p = 0.004), and this association remained after multivariable adjustment (adjusted odds ratio [aOR] = 0.51 per dB increase in alpha power [95% CI: 0.30 – 0.89], p = 0.02). There were no differences in the baseline (unanesthetized) EEG between groups in alpha power, or power in any other frequency band.
Conclusions:
We demonstrate that EEG discontinuity is common during anesthesia induction and is related to the level of sevoflurane-induced posterior alpha power, a putative marker of cortical-thalamic circuit development in the first year of life. This association persisted even after adjusting for age and propofol co-administration. The fact that this difference was only observed during anesthesia and not in the baseline EEG suggests that otherwise hidden brain circuit properties are unmasked by general anesthesia. These neurophysiologic markers observed during anesthesia may be useful in identifying patients who may have a greater chance of developing discontinuity.
Introduction
Electroencephalogram (EEG) discontinuity can occur at high concentrations of anesthetic drugs reflecting a pattern of suppression or transient attenuation of electrocortical activity. This EEG pattern occurs frequently during general anesthesia in adults as well as in children1,2, and is defined by the presence of EEG amplitudes less than 25 microvolts that persist for longer than 2 seconds3. Discontinuous EEG patterns during general anesthesia have been reported to occur with a prevalence as high as 52–63%1,2 and may be an indicator of unnecessarily deep anesthesia. While EEG discontinuities can be present in normal preterm infants during sleep-wake cycling, a pattern labeled tracé alternant3, in full-term infants suppression to less than 15 microvolts for longer than 2 seconds (tracé discontinu) is non-physiologic and abnormal3. A recent study found that increased duration of isoelectric events on the EEG, a more extreme degree of suppression to less than 2 microvolts, is associated with worse long-term neurologic outcomes in neonates undergoing cardiac surgery4, but the clinical significance of EEG discontinuities during pediatric anesthesia for non-cardiac surgery is not yet known.
In this paper, we performed an analysis of prospectively collected EEG data to identify periods of EEG discontinuity and estimate its incidence during the period of anesthesia induction. Inhalational induction is a dynamic period consisting typically of rapid wash-in of volatile anesthesia with possible later co-administration of intravenous agents. This period may be uniquely susceptible to the development of EEG discontinuity due to the important clinical need to rapidly obtain a deep level of sedation/hypnosis to avoid unwanted airway hyperreflexia (e.g., laryngospasm)5. We hypothesized that EEG discontinuity would occur during anesthesia induction at a rate as high as 50–60%1–2. Sevoflurane, propofol, and other gamma-aminobutyric acidergic (GABAergic) anesthetics produce stereotyped slow (0.1 to 1 Hz) activity and frontal alpha (8 to 12 Hz) oscillations in the EEG during unconsciousness which can be quantified using power spectral analysis6–11. The amount of energy or power in the EEG signal within these frequency bands may be calculated from the power spectrum (e.g., alpha band power)6–11. Frontal alpha band activity appears to be generated by prefrontal cortical and thalamic circuits12. It is absent in neonates and begins developing at approximately 6 months of age, suggesting it may be a neurophysiologic biomarker of brain development13–19. In adults, the size of the anesthesia-induced frontal alpha activity decreases with age, is correlated with cognitive function18,19, and is inversely associated with the likelihood of anesthesia-induced discontinuities20. Therefore, our secondary hypothesis was that there would be an association between early sevoflurane-induced alpha power during anesthetic induction and development of EEG discontinuity.
Methods
This is a prospective observational study approved by the Institutional Review Board (IRB) of Albert Einstein College of Medicine in which we collected EEG data and coded a priori for a variety of EEG features during anesthesia. In a prior report, we described epileptiform changes21. In this present manuscript, we describe our analyses of EEG discontinuity. Written informed consent was obtained from a legal surrogate, the parents or legal guardians for all subjects. This manuscript adheres to the applicable STROBE statement guidelines. We described details of cohort recruitment and characteristics in our previous report21.
Anesthesia induction protocol
All subjects received sevoflurane as the sole inhalational agent in oxygen alone without nitrous oxide or air; no subjects were premedicated. The sevoflurane concentration was started at or increased to 8% in all subjects. Anesthetic management was otherwise at the discretion of the responsible anesthesiologist.
Definitions of anesthesia induction, emergence and emergence excitation/delirium
We estimated the incidence of EEG discontinuity during the period of anesthesia induction, defined as the probability of EEG discontinuity to occur any time between (ta, tb), among subjects who did not have EEG discontinuity at the time ta, where:
ta = the time of initiation of anesthesia induction when EEG recording started, and,
tb = end time of EEG analysis, 10 minutes after ta, and
tb – ta = the first 10 minutes of anesthesia
We explored potential associations of EEG discontinuity with increased emergence time and emergence excitation/delirium. We defined emergence time as td – tc, where:
tc = (1) time of last stable end tidal sevoflurane measurement in the electronic medical record immediately prior to a decrease in the inspired sevoflurane concentration or (2) time of first increased oxygen gas flow signaling the provider’s intent to begin washing out sevoflurane to begin anesthesia emergence; and
td = the time the patient left the operating as indicated by the anesthesia provider
We further coded for an agitation/crying variable by assessing PACU nursing flowsheets and noting whether any agitation or crying was recorded, which could be a proxy of emergency excitation/delirium.
EEG recordings
26-channels of EEG with synchronized video were recorded at scalp locations designated by the International 10–20 System with the reference at the midline occipital channel (Oz) (microEEG System, Biosignal, Acton, MA)21,22. We recorded at least 5 minutes of baseline EEG data in the preoperative area prior to anesthesia induction. Patient physiologic data including vital signs, end-tidal anesthetic concentrations, and intravenous anesthetic administration were reliably obtained in real time from the video-EEG, written annotations, and/or from the electronic medical record (EPIC, Verona, WI). EEG discontinuity was the primary outcome of interest, defined as an amplitude less than 25 microvolts lasting greater than 2 seconds3. Two neurologists (AL, EY) analyzed the recordings using EEG review software (Persyst, Solana Beach, CA). Agreement on EEG discontinuity was measured by Cohen’s Kappa statistic. For discordant assessments, a third neurologist (SS) was introduced and we facilitated a process of discussion until consensus was achieved. The number of discrete EEG discontinuity events and their durations were recorded for each subject. If periods of EEG discontinuity (including a burst-suppression pattern) were followed by a clear return to a continuous EEG and then followed by another discontinuity, these were counted as separate events.
Quantitative EEG analyses
Custom EEG analysis scripts were written using MATLAB (version R2017a, MathWorks, Natick, MA), employing functions in the Chronux and Fieldtrip toolboxes23. We compared the EEG spectral features of patients who developed discontinuity (Figure 1) to those who did not at baseline prior to anesthesia administration (Supplemental Figure 1) as well as during the period of anesthesia induction. We excluded the first three minutes of anesthetic induction from EEG analysis to avoid potential artifacts due to patient movement or clinical interactions such as airway management or positioning. Thereafter, power spectral features were analysed on a minute-by-minute basis, requiring for each minute-long epoch at least 30 seconds of data that were free of both artifacts and suppression events. EEG data were band-pass filtered from 0.1 to 40 Hz. We also examined power spectral features in the period of transition before, during, and after EEG discontinuity (Supplemental Figure 2).
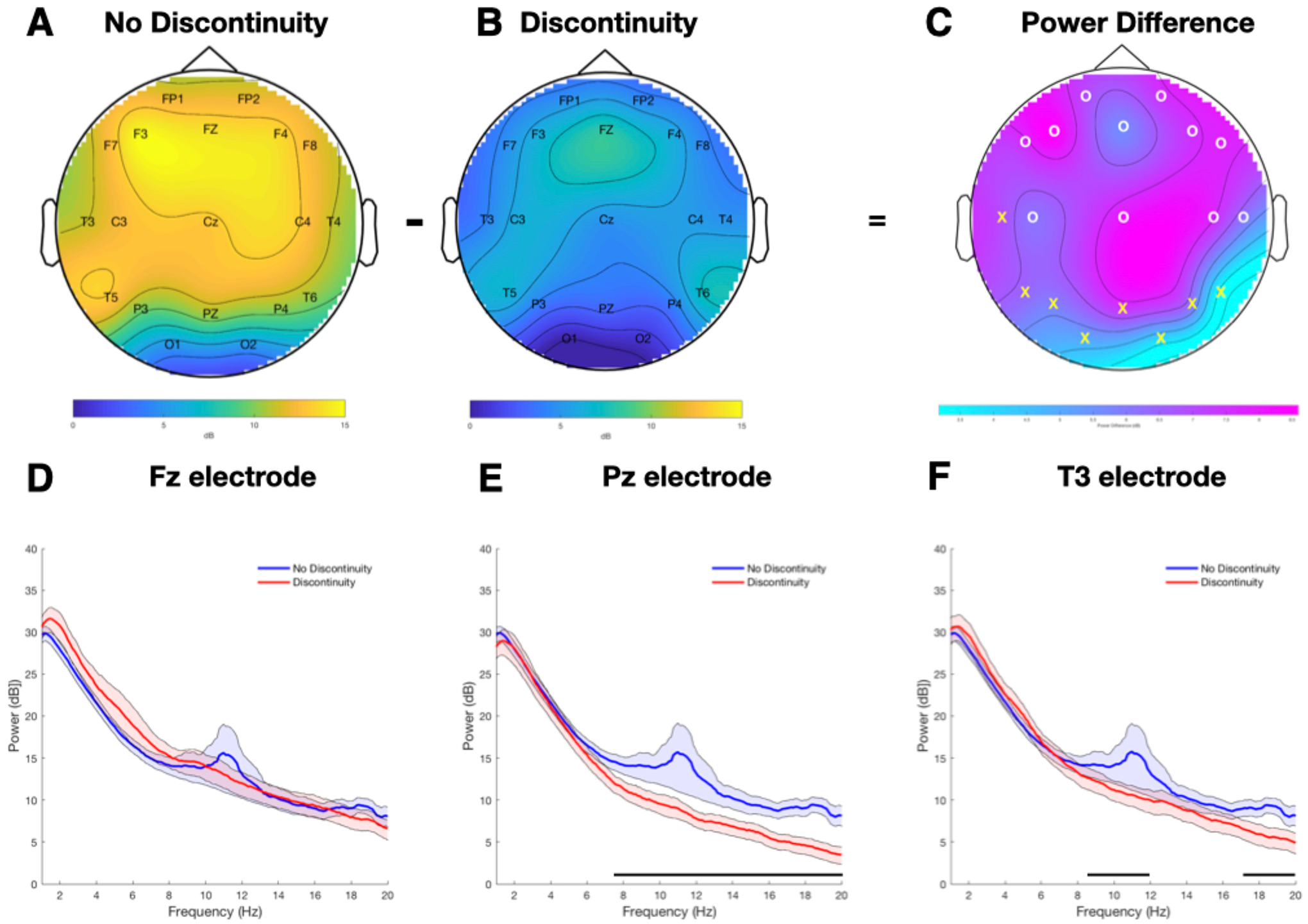
Comparison of alpha power in infants with electroencephalogram (EEG) discontinuity (n=18) and without EEG discontinuity (n=34) during anesthesia induction. (A-C) Topographic distribution over the head of the group-mean power spectrum in the alpha band (8 to 12 Hz) between subjects who did not develop discontinuity in their EEG (A) compared to those who did (B). The power difference between the two groups is presented in (C) with statistically significant differences represented by “X” observed in temporal, parietal, and occipital leads. Non-significant differences are represented by “O”. (D-F) Comparison of average power spectra in infants with EEG discontinuity (red, n=18) and without EEG discontinuity (blue, n= 34) during anesthesia induction with their respective 95% CIs estimated using the bootstrap method in the (D) Fz electrode, (E) Pz electrode and (F) T3 electrode. The horizontal black line parallel to the X axis in (E) and (F) represents the frequency segment in which the 95% CIs do not cross zero and therefore the difference between the groups is considered to be statistically significant.
Power spectra were computed for each patient using the multitaper method implemented in the Chronux toolbox23. The parameters were as follows: window length T = 5 s with no overlap, time-bandwidth product TW = 3, number of tapers K = 5, and spectral resolution of W = 1.2 Hz. Group-averaged spectra were computed by taking the mean power across subjects at each frequency for discontinuity compared to no discontinuity groups across the entire epoch at every electrode separately. We calculated 95% confidence intervals (CIs) for each spectral estimate and differences between power spectra using a bootstrap procedure23. To account for the spectral resolution of the power spectral estimates, for frequencies f > 2W, we deemed differences in spectra to be significant only if their CIs did not include zero within at least one spectral resolution (2W = 1.2 Hz).
For each electrode, alpha power was estimated by computing the average power from 8 to 12 Hz. Delta power was estimated by computing the average power from 1 to 4 Hz. We then averaged across subjects to obtain the group-level average power with 95% CIs. To characterize the topographic distribution of the power spectrum in the alpha band, we used the topoplot function implemented in the Fieldtrip toolbox24.
Statistical Analysis
Data were reported as means and standard deviations or medians and interquartile ranges for continuous variables and frequencies and percentages for categorical variables. Differences in clinical characteristics between those with and without EEG discontinuity were compared using Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. A two-sided p value of less than 0.05 was considered statistically significant (Table 1). We counted the number of EEG discontinuity events and described their characteristics after sevoflurane and propofol (Table 2).
Table 1.
Clinical and Electroencephalogram (EEG) Spectral Characteristics of Cohort
| Overall | No EEG Discontinuity (n = 34) | EEG Discontinuity (n = 20) | P value* | |
|---|---|---|---|---|
| Age (months) | 7.6 (4.9, 9.8) | 7.3 (4.9, 9.8) | 7.6 (5.0, 9.9) | 0.94 |
| Weight (kg) | 8.9 (6.7, 10.4) | 8.7 (6.8, 10.2) | 9.4 (6.0, 10.5) | 0.61 |
| Male sex | 41 (75.9) | 28 (82.4) | 13 (65.0) | 0.15 |
| Full term | 52 (96.3) | 33 (97.1) | 19 (95) | 0.99 |
| Alpha Power at Pz (dB) | 10.6 (8.0, 11.8) | 11.2 (8.7, 12.9) | 8.3 (7.8, 10.1)# | 0.004 |
| Epileptiform EEG | 4 (7.4) | 1 (2.9) | 3 (15.0) | 0.14 |
| Maximum etSEV (%) | 6.1 (5.5, 6.6) | 6.1 (5.5, 6.5) | 6.1 (5.5, 6.6) | 0.14 |
| Minimum etCO2 (mmHg) | 15.5 (10.0, 20.5) | 16.5 (11.0, 21.5) | 14.5 (9.5, 19.5) | 0.31 |
| Maximum etCO2 (mmHg) | 36.5 (32.0, 41.0) | 35.5 (30.0, 39.5) | 39.5 (33.0, 44.5) | 0.10 |
| Propofol administration | <0.001 | |||
 Propofol given Propofol given | 21 (38.9) | 4 (11.8) | 17 (85.0) | |
 Propofol not given Propofol not given | 33 (61.1) | 30 (88.2) | 3 (15.0) | |
| Propofol dose (mg/kg) | 0 (0, 2.6) | 0 (0, 0) | 2.7 (2.0, 3.3) | <0.001 |
| Fentanyl administration | 0.36 | |||
 Fentanyl given Fentanyl given | 28 (51.9) | 16 (47.1) | 12 (60.0) | |
 Fentanyl not given Fentanyl not given | 26 (48.1) | 18 (52.9) | 8 (40.0) | |
| Fentanyl dose (mcg/kg) | 0.5 (0, 1.1]) | 0 (0, 0.9) | 1.0 (0, 1.6) | 0.12 |
| Airway | <0.001 | |||
 Mask Mask | 16 (29.6) | 15 (44.1) | 1 (0.1) | |
 Laryngeal mask airway Laryngeal mask airway | 11 (20.4) | 9 (26.5) | 2 (0.1) | |
 Endotracheal tube Endotracheal tube | 27 (50.0) | 10 (29.4) | 17 (0.9) |
EEG: electroencephalogram
kg: kilogram
Pz: midline parietal channel on the electroencephalogram, International 10–20 System
dB: decibel
etSEV: end tidal sevoflurane
etCO2: end-tidal carbon dioxide
mmHg: millimeters of mercury
mg/kg: milligrams per kilogram
mcg/kg: micrograms per kilogram
Table 2.
Characteristics of Electroencephalogram (EEG) discontinuity events
| Overall | Sevoflurane alone | Propofol bolus | |
|---|---|---|---|
| Number of discontinuity events | 25 | 9 | 16 |
| EtSEV when first discontinuity begins (%) | 4.2 (2.9, 5.0) | 4.2 (3.8, 6.5) | 4.4 (2.9, 5) |
| Duration of EEG discontinuity (seconds) | 139 (14.5, 310) | 7 (2.5, 52) | 190 (115, 384) |
| Burst suppression pattern* | 7 | 2 (22.2) | 5 (31.3) |
| Time from start of anesthesia induction to onset of EEG discontinuity (seconds)# | - | 328.5 (200, 550) | - |
| Time from intravenous injection to appearance of EEG discontinuity | - | - | 15.5 (6, 26) |
EEG: electroencephalogram
EtSEV: end tidal sevoflurane
To assess whether differences in sevoflurane-induced EEG spectral features could be related to differences in sevoflurane dosing, we compared the minute-by-minute end-tidal sevoflurane levels, alpha power and delta power in the EEG discontinuity and no discontinuity groups, respectively, using two-way repeated measures analysis of variance (ANOVA) (Figure 2). We comprehensively explored all clinical covariates using univariable logistic regression with a threshold p value <0.1 for variable selection in a purposeful, multivariable logistic regression model building process (Supplemental Table 1). The objective of our models was to explore the independent association of early sevoflurane-induced alpha power during anesthesia induction and later development of EEG discontinuity and potential confounders of this association. As propofol is known to cause EEG discontinuity25, we adjusted for propofol and assessed other intravenous anesthetic administration as possible confounders. We defined an airway management variable (three-level categorical variable with reference = face mask only, 1 = laryngeal mask airway, and 2 = endotracheal tube). We used likelihood ratio tests to select variables for inclusion in multivariable models with a threshold level of p <0.05. We explored possible interaction effects by age17 and sex. Age was thought to be an important potential confounder because the appearance of frontal alpha power seems to occur between 4 to 8 months of age17 and was forced into the final model. We selected the model with the lowest Akaike Information Criterion (AIC) value as the final model. We limited multivariable models to a maximum of 4 covariates to avoid model instability (Table 3). All statistical analyses were performed using Stata 17.0 (StataCorp, College Station, TX).
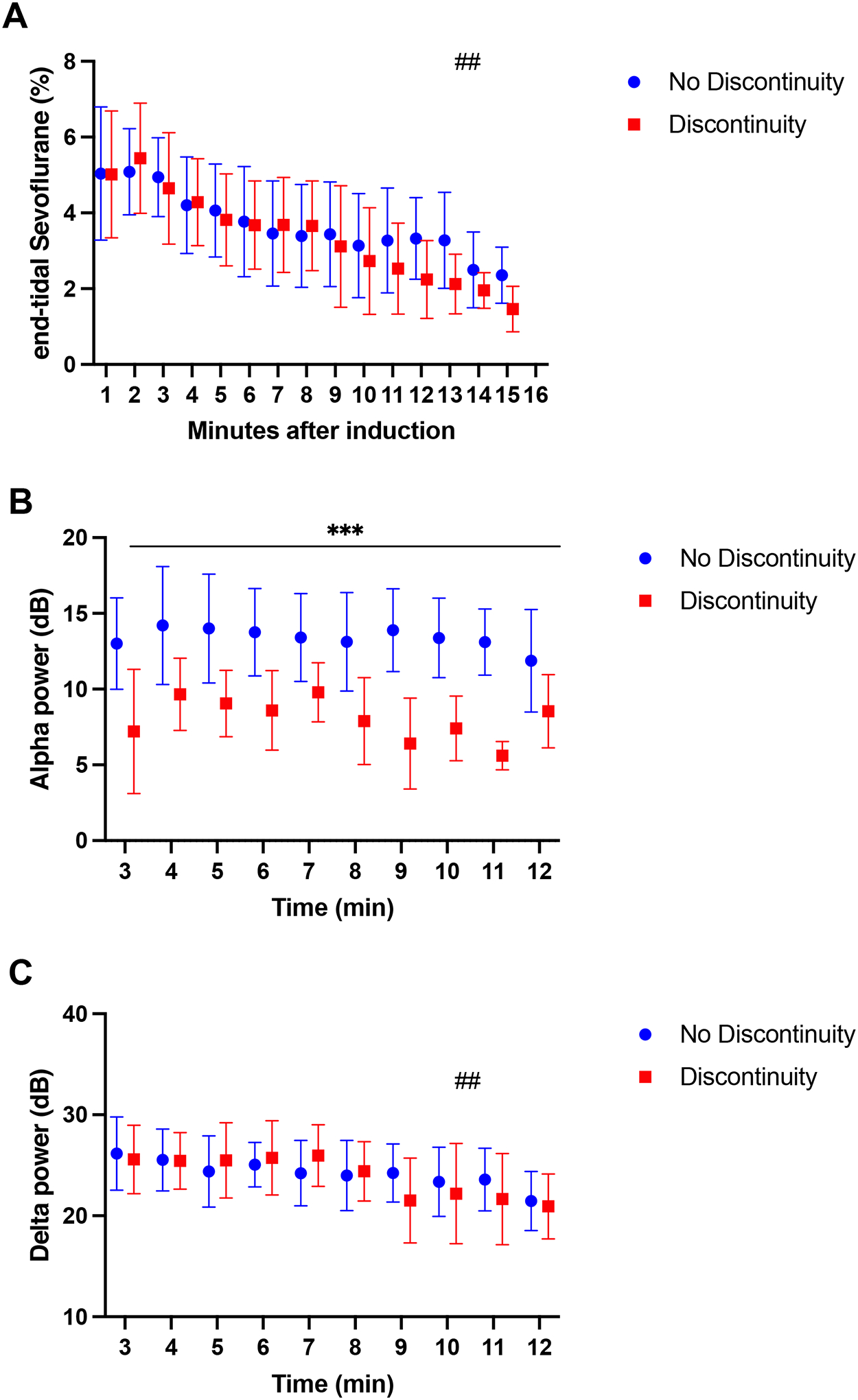
Comparison of minute-by-minute end-tidal sevoflurane, alpha and delta power between infants with and without discontinuity during anesthesia induction. (A) Average end-tidal sevoflurane concentrations. No differences were observed between the groups; however, we observed a significant reduction in end-tidal sevoflurane through time (##temporal effect, p <0.0001). (B) Average alpha power in the Pz electrode. Subjects experiencing discontinuity exhibit lower alpha power throughout anesthesia induction compared to subjects in the no discontinuity group (***group effect, p <0.0001). Alpha power remains stable in both groups through time (temporal effect, p = 0.12). (C) Average delta power in the Pz electrode. No differences in delta power were observed between groups, but average delta power decreased through time (##temporal effect, p <0.0001). All results are expressed as means and standard deviations and compared using a repeated measures two-way analysis of variance (ANOVA). Blue circles and red boxes represent the no discontinuity and discontinuity groups, respectively.
Pz: midline parietal channel on the electroencephalogram, International 10–20 System
Table 3.
Multivariable logistic regression models of the association between alpha power and Electroencephalogram (EEG) discontinuity
| Odds Ratio | P value | 95% confidence interval | |
|---|---|---|---|
| Model 1 | |||
 Alpha power at Pz (dB) Alpha power at Pz (dB) | 0.71 | 0.04* | (0.51 – 0.98) |
 Propofol dose (mg/kg) Propofol dose (mg/kg) | 3.77 | <0.001* | (1.81 – 7.87) |
| Model 2 | |||
 Age (months) Age (months) | 1.10 | 0.36 | (0.90 – 1.35) |
 Alpha power at Pz (dB) Alpha power at Pz (dB) | 0.70 | 0.03* | (0.51 – 0.97) |
 Propofol dose (mg/kg) Propofol dose (mg/kg) | 3.95 | <0.001* | (1.85 – 8.42) |
| Model 3 | |||
 Age (months) Age (months) | 1.17 | 0.21 | (0.92 – 1.48) |
 Alpha power at Pz (dB) Alpha power at Pz (dB) | 0.64 | 0.03* | (0.43 – 0.96) |
 Propofol dose (mg/kg) Propofol dose (mg/kg) | 4.47 | 0.001* | (1.82 – 11.00) |
 Fentanyl (mcg/kg) Fentanyl (mcg/kg) | 4.07 | 0.04* | (1.08 – 15.34) |
| Model 4 | |||
 Age (months) Age (months) | 1.04 | 0.78 | (0.81 – 1.31) |
 Alpha power at Pz (dB) Alpha power at Pz (dB) | 0.51 | 0.02* | (0.30 – 0.89) |
 Propofol dose (mg/kg) Propofol dose (mg/kg) | 4.72 | 0.002* | (1.78 – 12.56) |
 Maximum end tidal CO2 (mmHg) Maximum end tidal CO2 (mmHg) | 1.29 | 0.045* | (1.01 – 1.66) |
EEG: electroencephalogram
Pz: midline parietal channel on the electroencephalogram, International 10–20 System
dB: decibel
mg/kg: milligram per kilogram
mcg/kg: microgram per kilogram
CO2: carbon dioxide
mmHg: millimeters of mercury
Results
We obtained interpretable data in 54 of 68 (79.4%) subjects with a median (interquartile range) age of 7.6 (4.9, 9.8) months (Table 1). The neurologist readers (AL, EY) deemed 14 of 68 EEG recordings uninterpretable and these were excluded from analysis (Supplemental Figure 3). Two subjects who developed EEG discontinuity prior to the initial sampling epoch in which the spectral power was computed were excluded from the analysis. Three subjects had missing data for their end tidal gases (5.6%) because the physiological gas data were not transferred to the electronic medical record. These data values were kept as null without additional special handling. The final analytical sample was 52 subjects. EEG discontinuities were observed in 20 (37.0%) subjects with a total of 25 discrete events (concordance rate = 92.59%, Kappa = 0.84, 95% CI [0.69 – 0.99], p <0.0001) (Table 2). There were no detected differences in age, weight, sex, presence of epileptiform EEG (7.4%)21, maximum end-tidal sevoflurane, maximum and minimum end-tidal carbon dioxide (etCO2), or fentanyl administration between discontinuity groups.
Prior to anesthesia, we examined the baseline EEG power spectra of infant subjects who went on to develop EEG discontinuity during anesthesia induction (n=18) compared to those who did not (n=29). We excluded 7 subjects in this analysis because of data artifact. We found no differences in the average spectra between the groups at any frequency from 1 to 40 Hz (Supplemental Figure 1).
Under the effect of inhaled sevoflurane alone, before any intravenous agents were administered, we compared the EEG power spectra between subjects who developed discontinuity (n = 18) and those who did not (n = 34). We excluded two subjects from this analysis because they had already developed discontinuity within the first three minutes after induction, prior to the first sampling epoch. We found significant differences in sevoflurane-induced alpha power between groups at multiple electrodes in the posterior and temporal head regions (8 of 19 leads [42.1%], Figure 1C). In frontal regions, there were visible differences in the topographic plot (i.e., the group average alpha power, Figure 1A, ,1B),1B), however these differences did not reach statistical significance (Figure 1C, ,1D).1D). Alpha power at the midline parietal channel (Pz), selected as the representative lead, was statistically significantly lower in the discontinuity group, 8.3 IQR (7.8, 10.1) dBs, compared to the no discontinuity group, 11.2 IQR (8.7, 12.9) dBs (p = 0.004) (Table 1, Figure 1E). A sensitivity analysis incorporating the two subjects who were excluded found a significant alpha power difference consistent with these results (data not shown). The repeated measures ANOVA of minute-by-minute end-tidal sevoflurane concentrations showed no difference between groups (p = 0.4, Figure 2A). During this same timeframe, we observed a significantly lower Pz alpha power between groups on a minute-by-minute basis (p <0.0001; Figure 2B) whereas delta power showed no difference (P = 0.6, Figure 2C). Thus, the main difference between groups during this time period was posterior alpha power.
We characterized intravenous anesthetic administration. Overall, 21 subjects (38.9%) received propofol. A greater proportion of patients in the discontinuity group received propofol (17 of 20 subjects, 85.0%) but not all propofol administration led to discontinuity (4 of 34 subjects who received propofol did not develop EEG discontinuity, 11.8%, p <0.001) (Table 1). The majority of discontinuity events occurred after propofol administration (16 of 25 events, 64.0%) but a substantial proportion of discontinuity events occurred after sevoflurane alone (9 of 25, 36.0%) (Table 2). Median propofol dose was higher in patients exhibiting EEG discontinuity (2.7 IQR [2.0, 3.3] mg/kg) compared to those with no discontinuity (0 IQR [0, 0] mg/kg), p <0.001 (Table 1).
In logistic regression models (Table 3, Supplemental Table 1), maximum end-tidal sevoflurane was not found to be associated with EEG discontinuity. Sevoflurane-induced alpha power at Pz was significantly associated with EEG discontinuity, as was propofol administration. Biological sex was found to contribute to model instability and was not included in the model. There were no significant two-way interactions of age with propofol or alpha power, nor of sex with propofol and alpha power, suggesting that the association of propofol and alpha power on probability of developing EEG discontinuity did not vary with age and sex. The final association model consisted of age, alpha power, propofol dose, and maximum etCO2 (Model 4, Table 3). Increasing posterior alpha power (in dBs) was associated with decreasing odds of EEG discontinuity (adjusted OR [aOR] = 0.51, [95% CI: 0.30 – 0.89; p=0.02]).
In exploratory analyses, the median emergence time among subjects who did not develop EEG discontinuity was 8.5 IQR (4.5, 23) minutes compared to a median emergence time of 15 IQR (8, 32) minutes among subjects who developed EEG discontinuity. This difference approached, but did not reach, statistical significance (p = 0.07). Agitation or crying was not noted to be statistically significantly different between infants developing EEG discontinuity (2/10, 10%) compared to those who did not (9/34, 26.5%), p = 0.18.
Discussion
This study demonstrates a 37% incidence of EEG discontinuity during pediatric anesthesia induction, consistent with prior reports examining the period of maintenance of anesthesia1–2. Infants who develop discontinuity events exhibit significantly lower initial alpha power in temporal, parietal, and occipital regions of the brain (Figure 1C–F) under sevoflurane in the absence of any other drugs, despite equivalent end-tidal sevoflurane levels (Figure 2A). There were no significant differences between groups in alpha power, or power in any other frequency band, during the baseline period prior to any anesthesia administration (Supplemental Figure 1), suggesting that the relationship of decreased alpha power to development of EEG discontinuity reflects a different neurophysiologic process in the developing brain in response to anesthesia. This decreased posterior alpha power persisted throughout the period of EEG recording during anesthesia (Figure 2B). Sevoflurane-induced alpha power was significantly associated with EEG discontinuity even after multivariable adjustment for known confounders such as intravenous propofol administration (Table 3). This analysis shows that, all else being equal, EEG discontinuity is more likely when the patient has a low alpha power at induction, i.e., that low alpha power is a marker of susceptibility or vulnerability to suppression, whether by propofol bolus or high sevoflurane concentration. Our findings suggest that identifiable neurophysiologic markers such as alpha power are associated with later development of EEG discontinuity.
Our findings are consistent with previous work investigating the relationship between intraoperative EEG features and burst suppression, a specific pattern of EEG discontinuity1,2. In older adults, low frontal alpha power predicts burst suppression during anesthesia independent of chronological age19,26. While the most well-known alpha activity elicited by anesthesia is frontal, our study shows that posterior alpha activity may also carry important information. Previous work by Agrawal found that discontinuity episodes were preceded by EEG spectra with a significantly lower spectral edge25, consistent with our results (Supplemental Figure 2). The mechanism underlying the association between alpha power and discontinuity or burst suppression is unclear. Shao, Kahali, et al. proposed that astrocytes could mediate the link between alpha oscillations and discontinuity events, as they support both neuronal synchronization required for oscillatory dynamics as well as neuronal metabolism whose compromise leads to suppression events20. They also point out that cholinergic and noradrenergic neuromodulators can influence the activity of these astrocytes, which may explain both the age and drug dose-dependence of burst suppression under anesthesia27,28. Pertinent to the results reported here, we note that neuromodulatory systems are rapidly changing in the first year of life and may have significant between-patient variation29.
The clinical implications of anesthesia-induced discontinuity in infants are not fully understood. Isoelectric EEG, a more extreme degree of voltage suppression, has been associated with poor long-term neurological outcomes in neonates undergoing cardiac surgery4. In adults, low frontal alpha power is associated with burst suppression during anesthesia21,27, which may be associated with postoperative neurocognitive disorder20,30,31. The causal relationship between burst suppression or anesthetic depth and post-operative neurocognitive outcomes, however, is at present unclear. A recent study show that EEG-guided anesthesia that regulates or reduces anesthetic depth can reduce post-operative delirium32. On the other hand, other studies have not demonstrated a relationship between postoperative cognitive outcomes and depth of intraoperative anesthetic management employing behavioral33 or EEG guidance34. In infants and children, the clinical implications of EEG discontinuity are unknown at this time. Exploratory analyses of our dataset suggested a trend towards longer emergence times and decreased emergence excitation among infants who experienced EEG discontinuity, but additional studies are necessary. Further work is planned to investigate whether EEG discontinuity during pediatric anesthesia is a prognostic factor of clinical outcomes.
We accounted for propofol administration, which always occurred after sevoflurane administration was initiated and was typically correlated with the need for advanced airway management (e.g., endotracheal intubation) in this sample. 36% (9 of 25) of the discontinuity events occurred under the effect of sevoflurane alone without propofol (Table 2). In addition, in the subset of patients who did not develop any discontinuity, 4 of 34 (11.8%) received propofol; conversely, in the subset of patients who did develop discontinuity, 3 of 20 (15%) did not receive propofol (Table 1). Even after adjustment for the confounding effect of propofol administration, the association of alpha power with discontinuity remained significant (Table 3). Adjustment for propofol led to loss of significance of the airway variable; propofol was also thought to be a proximal event explaining airway management. Therefore, propofol was retained and airway was dropped from the model for both clinical and statistical reasons. Fentanyl blocks nociception and has a direct sedative effect through inhibitory effects on brainstem cholinergic circuits35; in combination with propofol and sevoflurane, fentanyl’s influence on arousal may promote EEG discontinuity (Model 3, Table 3). The association of maximum etCO2 might reflect the fact that significantly increased etCO2 diminishes the size of the EEG signal in premature and full-term infants36 (Model 4, Table 3). Future studies with a larger cohort will help further characterize the influence of fentanyl and other opioids, their interaction with sedative-hypnotic drugs, and the role of physiological variables such as etCO2.
This prospective observational study was not specifically powered to investigate discontinuity effects. The small sample size, which resulted in model instability when additional variables were added into the model, precluded us from accounting for all potentially relevant covariates. We also cannot rule out residual confounding. Although our sample size was moderate as was the level of statistical significance for alpha power (p = 0.02, Model 4), associations were consistently observed across multiple model formulations with no substantial change in either the effect size or the significance level of the alpha power covariate (Table 3). Inhalational induction is a dynamic period that can be difficult to observe with EEG due to its relative brevity spanning several minutes and the possibility of data artifact during airway management. However, we were able to obtain epochs of interpretable EEG data in all study subjects followed by minute-by-minute, repeated analyses. Sevoflurane in oxygen was the sole inhaled anesthetic agent used to initiate anesthesia induction in all subjects with subsequent management otherwise left to the discretion of the responsible anesthesiologist. This may have introduced some variation that could have been mitigated with a more standardized protocol. Nonetheless, the association of decreased posterior alpha power with discontinuity consistently observed during routine clinical practice suggests it may be useful in predicting which patients have a greater chance of developing discontinuity.
The brain develops rapidly during the first year of life37–39 and anesthesia-induced brain rhythms vary systematically during this process of maturation13–15,17. In particular, anesthesia-induced alpha waves are absent in early infancy and develop between 4 to 10 months of age, a putative consequence of increasing GABAergic inhibitory tone within thalamocortical circuits17. At the same time, individual children may also develop at different rates during infancy40 and may have different anesthetic requirements. Our study appears to connect these two ideas by showing how individual patients’ sevoflurane-induced alpha power might be associated with their anesthetic requirements, as measured by their tendency to experience discontinuity. Future studies will expand on these findings with a larger sample size, explore potential mechanisms, and identify any associations with postoperative outcomes.
Supplementary Material
Supplemental Data File (.doc, .tif, .pdf, etc., Published Online Only)_1
Supplemental Figure 1. Comparison of alpha power at baseline prior to anesthesia induction in infants who later developed EEG discontinuity (n=18) and infants who do not develop EEG discontinuity (n=29). (A-C) Average power spectra in infants who later develop EEG discontinuity (red) and who do not develop EEG discontinuity (blue) with their respective 95% CIs estimated using the bootstrap method in the (A) Fz electrode, (B) Pz electrode and (C) O2 electrode.
Supplemental Data File (.doc, .tif, .pdf, etc., Published Online Only)_2
Supplemental Figure 2. (A) Representative raw electroencephalogram (EEG) tracing of transition into a discontinuity event with voltage attenuation with (B) average EEG spectrogram for subjects who developed discontinuity and (C) no discontinuity. The white arrow indicates the beginning of the transition into discontinuity on the spectrogram
Supplemental Data File (.doc, .tif, .pdf, etc., Published Online Only)_3
Supplemental Figure 3. Flowchart of study subject recruitment and selection.
Supplemental Data File (.doc, .tif, .pdf, etc., Published Online Only)_4
Acknowledgements
The authors would like to thank all patients and family members who engaged in conversation about participation in this clinical study.
The authors would also like to thank Samah Baki, M.D. and James Henson, Eng. of Biosignal Group (Acton, MA, USA) for their assistance with the EEG recording system.
Funding:
National Institutes of Health (NIH) National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Numbers UL1 TR002556 and KL2 TR002558 (to JC).
Glossary of Terms:
| EEG | electroencephalogram |
| EtCO2 | end-tidal carbon dioxide |
| GABAergic | gamma-aminobutyric acidergic |
| Pz | midline parietal channel on the electroencephalogram, International 10–20 System |
| Oz | midline occipital channel on the electroencephalogram, International 10–20 System |
Footnotes
Conflicts of Interest/Financial Disclosures:
Patrick L. Purdon is an inventor on patents pending on brain monitoring technologies assigned to Massachusetts General Hospital; is an inventor on a patent assigned to Massachusetts General Hospital and licensed nonexclusively to Masimo Corporation; received speaker’s honoraria from Masimo Corporation; and is a cofounder of PASCALL Systems, Inc., a startup company developing closed-loop physiological control systems. The corresponding author confirms on behalf of all the other authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
References
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/121160873
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1213/ane.0000000000005864
Article citations
Changes in the Term Neonatal Electroencephalogram with General Anesthesia: A Systematic Review with Narrative Synthesis.
Anesthesiology, 141(4):670-680, 01 Oct 2024
Cited by: 0 articles | PMID: 38775960 | PMCID: PMC11389889
Review Free full text in Europe PMC
A Window into the Developing Brain: Toward a Deeper Understanding of Pediatric Anesthesia.
Anesthesiology, 140(5):863-864, 01 May 2024
Cited by: 0 articles | PMID: 38592355
Developmental trajectories of EEG aperiodic and periodic components in children 2-44 months of age.
Nat Commun, 15(1):5788, 10 Jul 2024
Cited by: 1 article | PMID: 38987558 | PMCID: PMC11237135
Intraoperative pediatric electroencephalography monitoring: an updated review.
Korean J Anesthesiol, 77(3):289-305, 17 Jan 2024
Cited by: 0 articles | PMID: 38228393 | PMCID: PMC11150110
Review Free full text in Europe PMC
Effect of raw electroencephalogram-guided anesthesia administration on postoperative outcomes in elderly patients undergoing abdominal major surgery: a randomized controlled trial.
BMC Anesthesiol, 23(1):337, 06 Oct 2023
Cited by: 3 articles | PMID: 37803259 | PMCID: PMC10557275
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of Isoelectric Electroencephalography Events in Infants and Young Children Undergoing General Anesthesia.
Anesth Analg, 130(2):462-471, 01 Feb 2020
Cited by: 6 articles | PMID: 31107263
Electroencephalographic discontinuity during sevoflurane anesthesia in infants and children.
Paediatr Anaesth, 27(3):251-262, 08 Feb 2017
Cited by: 12 articles | PMID: 28177176
Electroencephalographic density spectral array monitoring during propofol/sevoflurane coadministration in children, an exploratory observational study.
Anaesth Crit Care Pain Med, 43(2):101342, 22 Dec 2023
Cited by: 0 articles | PMID: 38142866
Using Electroencephalography (EEG) to Guide Propofol and Sevoflurane Dosing in Pediatric Anesthesia.
Anesthesiol Clin, 38(3):709-725, 01 Sep 2020
Cited by: 9 articles | PMID: 32792193
Review
Funding
Funders who supported this work.
NCATS NIH HHS (2)
Grant ID: UL1 TR002556
Grant ID: KL2 TR002558
NICHD NIH HHS (1)
Grant ID: P50 HD105352



