Abstract
Background
We aimed to investigate antioxidant and neuroprotective properties of chlorogenic acid in spinal cord injury (SCI).Methods
Twenty-one rats were divided into three groups. Laminectomy was performed in group L (n=7), spinal cord trauma was induced in group T (n=7), and spinal cord trauma was induced and chlorogenic acid treatment was started in group C (n=7). Blood samples were collected to analyze baseline values and the 12th h, 1st day, 3rd day, and 5th day catalase, native thiol (NT), total thiol (TT), disulfide (SS), SS/TT, SS/NT, and NT/TT levels. Functional analysis with Basso-Beattie and Bresnahan scores was performed at the same time points. Total antioxidant status (TAS), total oxidative stress, oxidative stress index, and cyclooxygenase-2 (Cox-2) were examined in the spinal cord of rats euthanized on day 7; results were statistically analyzed.Results
On day 7, catalase levels in Group C were significantly higher than baseline levels, whereas those in Group T were significantly lower than baseline levels; Group L showed no significant difference (p=0.008). SS values on day 7 were lower in Group T than in Groups C and L. Group C showed the lowest decrease in NT/TT level after trauma. On day 7, SS/TT level was high in Group T but stable in Groups C and L (p=0.04). Histopathological examination revealed significantly lower Cox-2 and TAS levels in Group C than in Group T (p=0.003, p=0.017, respectively).Conclusion
In this study, SCI was primarily examined through thiol-SS balance, and it was demonstrated by experimental models that chlorogenic acid has antioxidant and neuroprotective effects in SCI.Free full text

Can chlorogenic acid reduce oxidative stress and in an experimental spinal cord injury?
ABSTRACT
BACKGROUND:
We aimed to investigate antioxidant and neuroprotective properties of chlorogenic acid in spinal cord injury (SCI).
METHODS:
Twenty-one rats were divided into three groups. Laminectomy was performed in group L (n=7), spinal cord trauma was induced in group T (n=7), and spinal cord trauma was induced and chlorogenic acid treatment was started in group C (n=7). Blood samples were collected to analyze baseline values and the 12th h, 1st day, 3rd day, and 5th day catalase, native thiol (NT), total thiol (TT), disulfide (SS), SS/TT, SS/NT, and NT/TT levels. Functional analysis with Basso-Beattie and Bresnahan scores was performed at the same time points. Total antioxidant status (TAS), total oxidative stress, oxidative stress index, and cyclooxygenase-2 (Cox-2) were examined in the spinal cord of rats euthanized on day 7; results were statistically analyzed.
RESULTS:
On day 7, catalase levels in Group C were significantly higher than baseline levels, whereas those in Group T were significantly lower than baseline levels; Group L showed no significant difference (p=0.008). SS values on day 7 were lower in Group T than in Groups C and L. Group C showed the lowest decrease in NT/TT level after trauma. On day 7, SS/TT level was high in Group T but stable in Groups C and L (p=0.04). Histopathological examination revealed significantly lower Cox-2 and TAS levels in Group C than in Group T (p=0.003, p=0.017, respectively).
CONCLUSION:
In this study, SCI was primarily examined through thiol-SS balance, and it was demonstrated by experimental models that chlorogenic acid has antioxidant and neuroprotective effects in SCI.
INTRODUCTION
Spinal cord injury (SCI) can cause primary injury which initially occurs due to the acute effect of trauma, followed by development of the secondary injury. In primary damage caused by mechanical damage, oxidative stress occurs as a result of cellular events in the cytoplasm and mitochondria and persists during secondary damage due to ongoing neuroinflammation.[1–3] One of the last measurable parameters of oxidative stress is thiol-disulfide (SS) levels. Thiols are in equilibrium with their SS forms in plasma. Many studies have shown a correlation between thiol-SS levels and the severity, type, and outcome of body damage.[4–8]
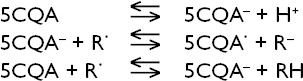
Chlorogenic acid (5-0-caffeoylquinic acid, [5CQA]) is a phytochemical substance found in coffee and coffee beans. It has antidiabetic, antioxidant, anti-inflammatory, antimicrobial, and antihyperlipidemic bioactivities.[3,9–13] Experimental studies have shown that it stops cell growth in tumors and does not cause toxicity even at high doses.[14] Furthermore, brain studies have shown that it has protective effects against ischemia.[9,15,16] The main factors that increase complications in post-traumatic SCI are increased oxidant capacity, ischemia, infection, diabetes as a comorbidity, and obesity. Another possible theory is that 5CQA reduces damage in SCI due to its antidiabetic, anti-inflammatory, and antimicrobial activities. 5CQA can also protect dopaminergic neurons by suppressing neuroinflammation.[17]
Based on the above-mentioned information, we functionally, biochemically, and histopathologically investigated the effects of chlorogenic acid in an experimental SCI model.
MATERIALS AND METHODS
Wistar-Hannover albino adult female rats (weighing 250–300 g, n=21) were divided into three groups. During the experiment, all groups were held in a standard postoperative care room (at 20–25°C, 50–60% humidity, in polycarbonate cages) with standard feeding conditions and 12-h light and dark cycles.
A commercial kit was used for measurement of total antioxidant status (TAS) and total oxidative status in tissue.[18,19] The protein level of tissue was determined using the Lowry method.[20] Oxidative Stress Index (OSI) was obtained by dividing total oxidant capacity (TOS) values by TAS values (OSI [Arbitrary Unit] = [TOS, μmol H2O2 eq/L]/[TAS, μmol Trolox eq/L]).
Serum thiol SS was measured by an automatic analyzer (Roche, cobas 501, Mannheim, Germany) using the method created by Erel and Neşelioğlu. In this method, SS bonds in the sample were converted to functional thiol groups by NaBH4. The total thiol (TT) content in the sample was calculated using Ellman reagent. The serum SS level was determined with the formula (serum TT − serum native thiol [NT])/2.[5]
Study Design
Wistar-Hannover albino adult female rats (weighing 250–300 g, n=21) were divided into three groups with seven randomly selected rats in each group. During the experiment, all groups were held in a standard post-operative care room (at 20–25°C, 50–60% humidity, in polycarbonate cages) with standard feeding conditions and 12-h light and dark cycles. T 7-8-9 laminectomy was performed on the rats in the control group (Group L, laminectomy group). After laminectomy was performed on the rats in the trauma group (Group T; trauma group), trauma was induced with the modified Allen weight dropping model (using a 10-cm long glass tube with a diameter of 0.5 cm, 10 g of weight was dropped on solid dura from a height of 10 cm). Following laminectomy and trauma induction, rats in the treatment group (Group C, chlorogenic acid group) received 60 mg/kg/day chlorogenic acid (Sigma-Aldrich, St. Louis, MO, USA) by gastric lavage, with the initial dose given immediately after trauma at the 15th min.
Surgical Procedure
After 6 h of fasting, 1 mg/kg ketamine (Ketalar, Pfizer, New York, USA) and 1 mg/kg xylazine (Alfazyne, Alfasan, Woerden, Netherland) were intraperitoneally administered, and the rats were left for spontaneous breathing at a suitable room temperature. Rats were fixed face down, their T7-8-9 levels were determined, and they were cleaned before surgery. Antisepsis was provided with Povidone-iodine. A midline incision was made, skin and subcutaneous tissue were crossed, and paravertebral muscles were subperiosteally dissected. After T7-8-9 laminectomy, the procedures described above were performed for each group. All rats were closed in line with anatomical integrity after the procedure. Rats were euthanized on the 7th day.
Functional Analysis
Basso-Beattie and Bresnahan scores were evaluated in all rats on postoperative 12 h, day 1, day 3, day 5, and day 7. The results were evaluated statistically.
Biochemical Analysis
Tail blood samples were taken before anesthesia induction, and at 12th h, 1st day, 5th day, and 7th day after the surgical procedure. Albumin, catalase, ischemia-modified albumin, and thiol-SS balance were examined by the spectrophotometric method. After the rats were euthanized, spinal cord tissue in the T7-8-9 region was removed, and TOS, TAS, and OSI were studied and statistically evaluated.
Histopathological Analysis
On gross examination perpendicular cut sections of spinal cords were processed and embedded in paraffin blocks. Then, 4-μm sections were deparaffinized in xylene, and rehydrated through a graded series of ethanol in order to perform hematoxylin-eosin staining and immunohistochemistry. Immunohistochemistry was performed using cyclooxygenase-2 (Cox-2) ab (D-12: sc-166475, Santa Cruz Biotechnology, Inc.) with a 1:100 dilution by Leica bond auto-stain system according to manufacturer’s guideline. In this system slides are visualized with 3,3-diaminobenzidine (Fig. 1).
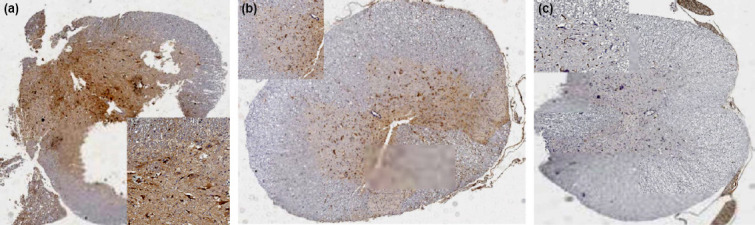
Inlets demonstrate the strong cytoplasmic immunohistochemical expression. Although background stain is prominent, the cells with strong expression are counted (a) Group L - Control group, (b) Group T - Trauma group, (c) Group C - Chlorogenic acid group.
A pathologist, who is blind to the groups, examined hematoxylin-eosin stained and immunohistochemically Cox-2 applied sections. Cox-2 positive cells were counted per 3 HPF for each spinal cord.
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) 15 (SPSS Inc; Chicago; IL, USA) software. The suitability of numerical variables to normal distribution was examined by Kolmogorov–Smirnov tests. The difference between independent groups was analyzed with the Kruskal–Wallis test followed by the Mann–Whitney U test, and the difference between dependent groups was analyzed with the Friedman test. The results were expressed as mean±SD, and p<0.05 was considered statistically significant.
RESULTS
Investigation of Plasma Levels
There was no statistically significant difference between the groups in terms of baseline plasma values of the markers examined (p>0.005) (Table 1). Although catalase levels did not show a statistically significant difference between the groups at the 12th h, there was an overall decrease in all groups (p=0.489). On day 1, there was a statistically significant difference between the catalase levels of Group L and Groups C and T; catalase levels decreased in Groups C and T, whereas they increased in group L (p=0.003). In Group L, there was no significant change in catalase levels between baseline levels and day 7 (from 77.07±8.63 kU/L to 74.64±9.838 kU/L). There was a decrease in catalase levels in Group T (from 75.71±7.42 kU/L to 48.33±22.25 kU/L) and an increase in Group C (from 77.69±16.95 kU/L to 85.20±9.36 kU/L). In addition, a statistically significant difference was found between Group T and Group C on day 7 (p=0.008). This pattern was the same when evaluated according to the 12th h. When the change in catalase levels was examined within the groups over time, there was no statistically significant difference in Group L (p=0.296), but there was a statistically significant difference in Group T and Group C (p=0.041, p<0.001, respectively) (Table 1 and Fig. 2).
Table 1
Oxidative and antioxidative state of the rats
| Parameters | Group | Baseline | Time | p-value | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 12th h | 1st day | 3rd day | 5th day | 7th day | ||||
| Catalase | L | 77.07±8.63 | 55.50±32.70 | 76.67±13.67 | 70.04±13.07 | 66.00±12.85 | 74.64±9.38 | 0.296 |
| T | 75.709±7.42 | 75.59±12.25 | 63.01±18.74 | 58.08±11.03 | 59.77±13.5 | 48.33±22.25 | 0.041 | |
| C | 77.69±16.95 | 74.83±8.99 | 37.39±9.91 | 86.96±7.29 | 61.00±12.61 | 85.20±9.36 | <0.001 | |
| p-value | 0.713 | 0.489 | 0.003y,z | 0.002y,z | 0.522 | 0.008z | ||
| Native Thiol (umol/l) | L | 186.15±35.88 | 101.45±35.19 | 113.66±18.88 | 72.28±13.03 | 167.33±34.10 | 88.92±20.98 | <0.001 |
| T | 186.70±45.05 | 211.52±40.10 | 89.84±22.44 | 130.27±25.72 | 131.72±31.50 | 166.82±23.29 | <0.001 | |
| C | 186.67±21.08 | 137.08±16.27 | 130.05±41.47 | 118.84±6.09 | 107.34±36.15 | 81.62±23.38 | <0.001 | |
| p-value | 0.953 | 0.001x,z | 0.139 | 0.001x,y | 0.02y | 0.001x,z | ||
| Total Thiol (umol/l) | L | 254.81±52.54 | 177.98±46.04 | 156.18±20.37 | 149.07±19.08 | 215.65±41.68 | 161.74±24.63 | 0.04 |
| T | 244.40±59.62 | 266.94±41.36 | 167.03±38.91 | 211.91±35.67 | 218.37±48.10 | 238.07±339.81 | 0.045 | |
| C | 261.01±30.79 | 214.30±37.40 | 190.35±47.62 | 169.08±24.52 | 187.88±42.72 | 131.07±19.33 | <0.001 | |
| p-value | 0.932 | 0.005x,z | 0.387 | 0.007x,z | 0.437 | 0.001x,y,z | ||
| Disulfide (umol/l) | L | 34.32±10.88 | 38.26±9.32 | 21.26±6.05 | 38.39±9.73 | 24.26±5.09 | 36.40±8.65 | 0.005 |
| T | 28.85±11.27 | 27.70±7.26 | 38.59±12.71 | 40.82±13.21 | 43.21±17.46 | 35.62±11.07 | 0.23 | |
| C | 37.17±18.37 | 38.60±13.34 | 30.15±14.38 | 25.12±.9.32 | 40.27±7.78 | 24.72±7.41 | 0.025 | |
| p-value | 0.667 | 0.130 | 0.028x | 0.028y,z | 0.009y | 0.111 | ||
| Native Thiol/ | L | 73.44±5.05 | 56.30±6.92 | 72.74±7.29 | 48.76±8.76 | 7.48±2.83 | 54.86±8.83 | <0.001 |
| Total Thiol (%) | T | 76.67±5.26 | 78.96±5.61 | 54.02±8.99 | 61.86±9.85 | 61.14±11.88 | 70.49±5.50 | 0.001 |
| C | 72.34±11.52 | 64.82±7.25 | 67.87±12.66 | 71.15±7.07 | 55.82±7.64 | 61.63±11.90 | 0.068 | |
| p-value | 0.478 | 0.001x,y,z | 0.013x,y,z | 0.003x,y | 0.005x,y | 0.04x | ||
| Disulfide/Total Thiol (%) | L | 13.28±2.52 | 21.84±3.46 | 13.62±3.64 | 25.61±4.38 | 11.25±1.41 | 22.56±4.41 | <0.001 |
| T | 11.66±2.63 | 10.52±2.80 | 22.98±4.49 | 19.06±4.92 | 19.43±5.94 | 14.75±2.75 | 0.001 | |
| C | 13.82±5.76 | 17.58±3.62 | 16.06±6.33 | 14.42±5.53 | 22.08±3.82 | 17.41±7.44 | 0.068 | |
| p-value | 0.453 | 0.001x,y,z | 0.013x,y,z | 0.003x,y | 0.005x,y | 0.04x | ||
| Disulfide/Native Thiol (%) | L | 18.35±4.73 | 39.85±10.09 | 19.36±7.36 | 55.63±20.26 | 14.60±2.3 | 43.36±16.29 | <0.001 |
| T | 15.49±4.87 | 13.60±4.56 | 44.75±15.61 | 32.72±13.95 | 34.63±17.75 | 21.28±5.34 | 0.001 | |
| C | 20.83±12.69 | 27.98±8.93 | 25.94±14.34 | 20.85±6.86 | 41.09±13.02 | 33.70±15.62 | 0.068 | |
| p-value | 0.478 | 0.001x,y,z | 0.013x,y,z | 0.003x,y,z | 0.005x,y | 0.04x | ||
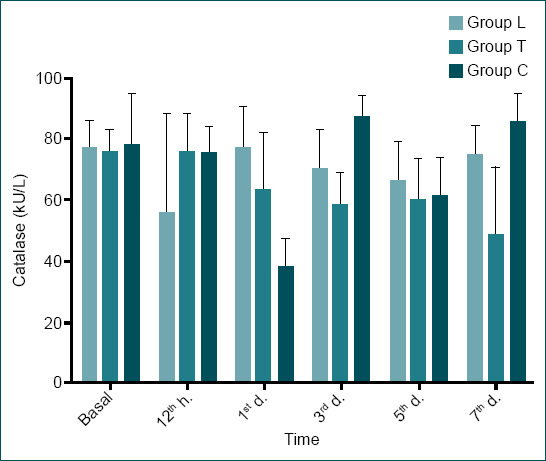
Catalase levels of all groups (mean±SD). Catalase levels decreased in all groups at the 12th h, which was the first measurement point immediately after trauma, but on day 7, catalase levels were higher in the chlorogenic acid group (Group C) and lower in the trauma group (Group T) compared to baseline levels, and no significant difference was observed in the laminectomy group (Group L).
There was no statistically significant difference between the groups in terms of SS levels at the 12th h (p=0.130). However, on day 5, there was a statistically significant difference in SS levels between Group L and Group C and between Group T and Group C (p=0.007). When SS levels at the 12th h and on day 7 were compared, there was a slight and insignificant decrease in Group L (from 38.26±9.32 μmol/l to 36.40±8.65 μmol/l), a significant increase was observed in Group T (from 27.70±7.26 μmol/l to 35.62±11.07 μmol/l), and a significant decrease was observed in Group C (from 38.60±13.34 μmol/l to 24.72±7.41 μmol/l). A similar pattern was observed when the baseline values were compared with the 7th day values (Table 1 and Fig. 3).
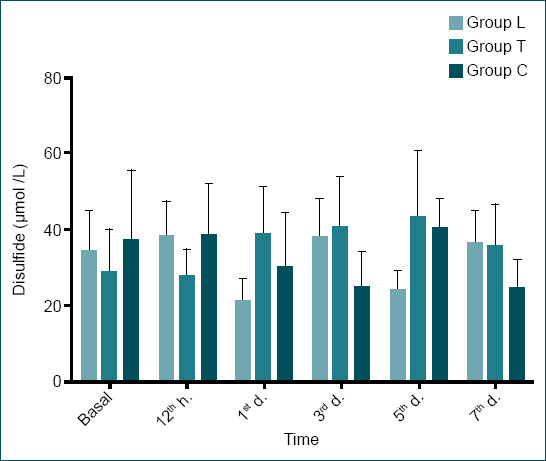
Disulfide levels of all groups (mean±SD) sulfide values increased in laminectomy and chlorogenic acid groups at the 12th h, the earliest measurement point after injury in the experiments, compared to baseline values, and showed a slight decrease in the trauma group, which was statistically insignificant. On day 7, disulfide values were higher in the trauma group compared to the 12th h values; whereas they were lower in the chlorogenic acid group compared to the 12th h values.
When SS/TT, SS/NT, and NN/TT values were compared between the 12th h and 7th day, a decrease was observed in all groups. However, this decrease was lowest in Group L (from 56.30±6.92 to 54.86±8.83), followed by Group C (from 64.82±7.25 to 61.63±11.90), and then by Group T. In terms of SS/TT values, Groups L and C followed a similar pattern while Group T followed a different pattern. When the 12th h values were compared with the 7th day values, there was no significant change in Group L (from 21.84±3.46 to 22.56±4.41) and Group C (from 17.58±3.62 to 17.41±7.44), whereas a significant increase was observed in Group T (from 10.52±2.80 to 14.74±2.75). A significant difference was observed between SS/NT, SS/TT, and NT/TT values at h 12 and day 1 between Group L and Groups T and C and between Groups T and C (p=0.001 for h 12, p=0.013 for day 1). A significant difference was found in day 3 and day 5 values only between Group L and Groups T and C (p=0.003 and p=0.005, respectively), and finally, on day 7, a statistically significant difference was found only between Group L and Group T (p=0.004). There was no longer any statistically significant difference between Group L and Group C (Table 1).
Tissue-level Investigations
Histopathological Examination
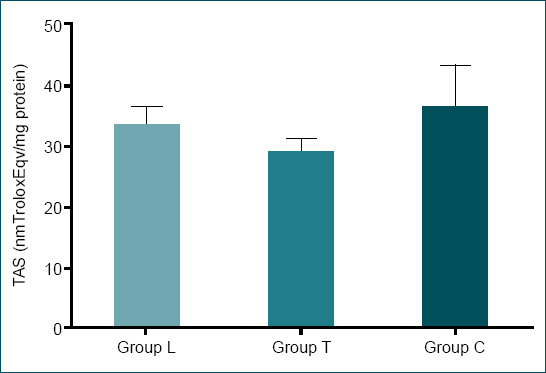
No statistically significant difference was found between the TOS and OSI levels following an examination after the rats were euthanized (p=0.107 and p=0.214, respectively). There was a statistically significant difference in TAS between Group T and Groups L and C (p=0.017). While the highest TAS values were obtained in Group C (36.27±6.96 nm Trolox Eqv/mg protein), the lowest TAS values were obtained in Group T (28.97±2.135 nm Trolox Eqv/mg protein) (Table 2 and Fig. 4).
Table 2
TAS, TOS, OSI, and Cox-2 values (mean±SD) measured from the spinal cord tissue
| Group L | Group T | Group C | p-value | |
|---|---|---|---|---|
| TAS (nmTroloxEqv/mg pro-tein) | 33.41±3.06 | 28.97±2.135 | 36.27±6.96 | 0.017 |
| TOS (nmol H2o2Eqv/mg pro-tein) | 0.75±0.17 | 0.78±0.23 | 0.97±0.23 | 0.107 |
| OSI (TOS/TAS) | 0.021±0.004 | 0.026±0.008 | 0.029±0.0.107 | 0.214 |
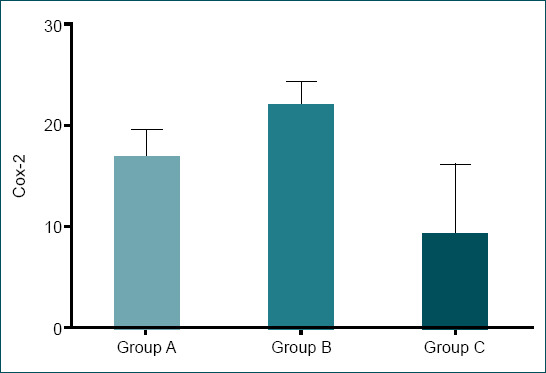
| Cox2 | 16.85±2.60 | 21.85±2.41 | 9.28±6.92 | 0.003 |
TAS: Total antioxidant status; TOS: Total oxidant status; OSI: Oxidative stress index; Cox-2: Cyclooxygenase-2; SD: Standard deviation. Group L: Control group, Group T: Trauma group, Group C: Chlorogenic acid group. xThere is a statistically significant difference between group L and group T. yThere is a statistically significant difference between group L and group C. zThere is a statistically significant difference between group T and group C.
Cox-2 levels were significantly lower in Group C compared to Groups T and L (p=0.003). The highest Cox-2 levels were observed in Group T (21.85±2.41), whereas the lowest values were observed in group C (9.28±6.92) (Table 2 and Fig. 5).
Functional Results
When all the groups were evaluated functionally, there was a statistically significant difference between Group L and Groups T and C in terms of 12th h, 1st day, 3rd day, and 5th day results (p=0.001, p=0.003, p=0.001, and p<0001, respectively). On the 7th day, a significant functional improvement was observed in group C, but there was no statistically significant difference between Group C and Group L. On the other hand, the statistically difference between Group C and Group T was still significant. The significant difference between Group T and Group L continued as observed at other time points (p<0.001) (Table 3).
Table 3
Basso-Beattie and Bresnahan scores of the rats evaluated functionally
| Time | Group L | Group T | Group C | p-value |
|---|---|---|---|---|
| 12th hour | 6.57±0.53 | 4.57±1.27 | 3±1.82 | 0.001x,y |
| 1st day | 12.71±0.48 | 10.57±1.51 | 10.71±1.60 | 0.003x,y |
| 3rd day | 13±0 | 10.57±1.51 | 11.14±1.57 | 0.001x,y |
| 5th day | 13±0 | 11±1.52 | 11.71±0.75 | <0.001x,y |
| 7th day | 21±0 | 17.85±2.11 | 20.57±0.78 | <0.001x,z |
| p-value | 0.001 | <0.001 | <0.001 |
XThere is a statistically significant difference between Group L and Group T. yThere is a statistically significant difference between Group L and Group C. zThere is a statistically significant difference between Group T and Group C.
DISCUSSION
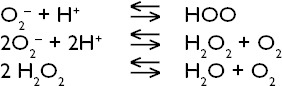
Treatment efforts for SCI have primarily focused on reducing secondary damage. Free oxygen radicals, such as O- and OH- that increase as a result of damage, are one of the main elements in reducing secondary injury. O- and OH- ions are highly active free radicals involved in cell metabolism. These are eliminated after being converted into O2 and H2O with the help of antioxidants such as ascorbic acid, glutathione, and Vitamin E, and enzymes such as superoxide dismutase (SOD), glutathione peroxidase, and especially catalase.
5CQA, which is a phytochemical substance, has been shown to have antidiabetic, antioxidant, anti-inflammatory, antimicrobial, and antihyperlipidemic activities.[3,9–13] Studies have shown that chlorogenic acid has an effect in oxidative stress through electron loss and protein transfer.[21] Furthermore, studies on the central nervous system have shown that it has protective effects against ischemia.[22] Chlorogenic acid can also protect dopaminergic neurons by suppressing neuroinflammation.[17]
The antioxidant defense system protects the cell by neutralizing harmful effects of oxidants and free radicals. Catalase, one of the important antioxidant enzymes,[23–25] is used as an indicator of damage severity and responses to therapy as well as for follow-up in therapeutic studies.[24–28] In the present study, catalase levels decreased in all groups at the 12th h, which was the first measurement point immediately after trauma, and continued to decrease on day 1 in trauma, but on day 7, catalase levels were higher in the chlorogenic acid group and lower in the trauma group compared to baseline levels, and no significant difference was observed in the laminectomy group. Although the level of antioxidant catalase decreased in response to trauma in the early period, it increased in the group treated with chlorogenic acid in the subsequent period, but decreased in the trauma group (Table 1 and Fig. 2). This result shows that chlorogenic acid increases antioxidant catalase levels after SCI. Similarly, in our tissue studies, the highest TAS values were obtained in Group C, the lowest TAS values were obtained in group T (Table 2 and Fig. 4). This result shows that chlorogenic acid also increases TAS levels after SCI in the spinal cord tissue.
The spinal cord contains high levels of antioxidants.[29–31] After SCI, a decrease in SOD and endogenous antioxidants leads to excessive accumulation of free radicals. This damages cellular lipids, proteins, and DNA. Free radicals play an important role in traumatic and ischemic spinal cord injuries.[29–32] Free radicals form on damaged cell membranes and continue to damage the membrane as the cycle continues until the endogenous antioxidants, SOD, are blocked by Vitamin E. Cellular damage occurs when a proper antioxidant response cannot be obtained and eventually leads to oxidative stress.[33] Ca++ influx to the cell leads to arachidonic acid release. Increased extracellular excitatory neurotransmitters stimulate neuronal activation. This leads to the release of Cox-2 from cortical neurons. Selective inhibition of Cox-2 has been shown to facilitate recovery after SCI in various animal experiments.[34] In the present study, the effectiveness of chlorogenic acid at tissue level was evaluated by measuring Cox-2, TAS, TOS, and OSI levels in tissue. Cox-2 levels were statistically significantly lower in the group treated with chlorogenic acid compared to the trauma group (p=0.003) (Table 2 and Fig. 5). Chlorogenic acid was seen to facilitate healing by lowering Cox-2 levels at the tissue level.
Thiol is an organic compound that contains a sulfhydryl group and is an important component of the antioxidant system. The plasma thiol pool comprises largely of albumin and protein thiols, and to a lesser extent, low molecular weight thiols such as cysteine (Cys). Thiols can form SSs through oxidation reactions. In the case of oxidative stress, reversible mix SS formation can occur between protein thiol groups and low molecular weight thiols by oxidation of Cys residues. The resulting SS can be reduced back to thiol groups, preserving dynamic thiol SS homeostasis.[4,5,7] The amount of SS formed is one of the parameters that highlight increased oxidative stress. It is used as a parameter to indicate the severity of damage in studies on thiol SS balance.[4–8,18] In the present study, thiol-SS parameters were used to evaluate the effectiveness of chlorogenic acid on oxidant-antioxidant capacity after SCI.
According to the results of this study, SS values increased in laminectomy and chlorogenic acid groups at the 12th h, the earliest measurement point after injury in the experiments, compared to baseline values, and showed a slight decrease in the trauma group, which was statistically insignificant. However, at the subsequent time points, SS values increased in the trauma group. On day 7, SS values were higher in the trauma group compared to the 12th h values; whereas they were lower in the chlorogenic acid group compared to the 12th h values (Table 1 and Fig. 3). Since SS is expected to increase when oxidative stress increases, low SS values found in the treatment group reveal that chlorogenic acid may have a positive effect on recovery. In addition, the NT/TT ratio decreased on day 7 compared to the 12th h in all groups. However, while there was a statistically significant difference between laminectomy group and trauma and chlorogenic acid groups at the 12th h (p=0.001), there was a significant difference observed on day 7 between the laminectomy and trauma groups (p=0.04), and the significant difference between the laminectomy and the chlorogenic acid groups disappeared (p>0.005). In addition, when the NT/TT ratios on day 7 were compared to the 12th h values, the lowest decrease was observed in the laminectomy group, followed by the chlorogenic acid and trauma groups, respectively. Since a lower decrease in NT/TT positively contributes to recovery of the damage, better results were observed in the chlorogenic acid group compared to the trauma group. In terms of the SS/TT ratio, higher levels were observed in the trauma group on day 7, while no significant changes were observed in the laminectomy and chlorogenic acid groups. Since an increase in SS/NT ratio is a negative parameter for a damaged tissue, its increase in the trauma group after injury compared to no change in the chlorogenic acid group suggests that chlorogenic acid had a protective effect in the treatment group by keeping the SS/NT ratio stable.
Up until day 5, there was a statistical difference between the laminectomy group and the trauma and chlorogenic acid groups in terms of functional results, but there was no difference between the trauma and the chlorogenic acid groups. However, on day 7, a significant improvement was observed in the chlorogenic acid group compared to the trauma group (p<0.001) (Table 3). As a result, better functional results were obtained in the group treated with chlorogenic acid compared to the trauma group.
Conclusion
The severity of oxidative stress seen during primary and secondary damage following SCI is directly proportional to the severity of the damage. Oxidative-antioxidative homeostasis has, therefore, been a primary focus for therapeutic studies. Thiol-SS balance is one of the most important parameters indicating oxidative stress. In this article, the therapeutic efficacy of chlorogenic acid in SCI was also evaluated in terms of Thiol/SS balance. The effect of chlorogenic acid on oxidative stress was evaluated at both plasma and tissue levels. According to the results, chlorogenic acid reduces oxidative stress in SCI and shows antioxidative and neuroprotective properties.
Footnotes
Ethics Committee Approval: Ethical approval for this study was obtained from the local ethics committee of Kobay AŞ (No: 279). All applicable international, national, and institutional guidelines for the care and use of animals were followed. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (Date: 23.03.2018).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: E.B., C.B., A.Ş., B.G., B.B., Ö.F.T.; Design: E.B., C.B., A.Ş., B.G., B.B., Ö.F.T.; Supervision: E.B., C.B., A.Ş., B.G., B.B., Ö.F.T.; Resource: E.B., Ş.H., A.S.A., B.G., B.B., Ö.F.T.; Materials: E.B., Ş.H., A.S.A., B.G., B.B., Ö.F.T.; Data: E.B., Ş.H., A.S.A., B.G., B.B., Ö.F.T.; Analysis: E.B., Ş.H., A.S.A., C.B., A.Ş., B.G.; Literature search: EE.B., Ş.H., A.S.A., C.B., A.Ş., B.G.; Writing: E.B., Ş.H., A.S.A., C.B., A.Ş., B.G., B.B., Ö.F.T.; Critical revision: E.B., C.B., A.Ş., B.B., Ö.F.T.
Conflict of Interest: None declared.
Financial Disclosure: This study was funded by Ankara Yildirim Beyazit University, grant number 4887. The sponsor had no role in the design or conduct of this research.
REFERENCES
Articles from Turkish Journal of Trauma & Emergency Surgery are provided here courtesy of Turkish Association of Trauma and Emergency Surgery
Full text links
Read article at publisher's site: https://doi.org/10.14744/tjtes.2020.89499
Read article for free, from open access legal sources, via Unpaywall:
https://jag.journalagent.com/z4/download_fulltext.asp?pdir=travma&plng=eng&un=UTD-89499
Citations & impact
Impact metrics
Article citations
Quality Evaluation of Lonicerae Flos Produced in Southwest China Based on HPLC Analysis and Antioxidant Activity.
Molecules, 29(11):2560, 29 May 2024
Cited by: 0 articles | PMID: 38893434 | PMCID: PMC11173438
Effects of lercanidipine on traumatic spinal cord injury: an experimental study.
Ulus Travma Acil Cerrahi Derg, 30(2):73-79, 01 Feb 2024
Cited by: 1 article | PMID: 38305651 | PMCID: PMC10977502
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Anti-inflammatory and antioxidative effects of genistein in a model of spinal cord injury in rats.
Asian Biomed (Res Rev News), 15(5):233-243, 29 Oct 2021
Cited by: 0 articles | PMID: 37551326 | PMCID: PMC10388775
Administration of chlorogenic acid alleviates spinal cord injury via TLR4/NF‑κB and p38 signaling pathway anti‑inflammatory activity.
Mol Med Rep, 17(1):1340-1346, 06 Nov 2017
Cited by: 17 articles | PMID: 29115619
[NEUROPROTECTIVE EFFECTS OF MANGIFERIN ON ACUTE SPINAL CORD INJURY IN RATS AND ITS MECHANISM].
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 30(8):1019-1025, 01 Aug 2016
Cited by: 3 articles | PMID: 29786235
The Importance of Natural Antioxidants in the Treatment of Spinal Cord Injury in Animal Models: An Overview.
Oxid Med Cell Longev, 2019:3642491, 12 Nov 2019
Cited by: 27 articles | PMID: 32676138 | PMCID: PMC7336207
Review Free full text in Europe PMC