Abstract
Objective
This study explores provider preferences regarding anal cancer screening indications, initiation age, tools, and referral threshold to high resolution anoscopy (HRA).Methods
International Anal Neoplasia Society affiliates were invited to complete an online survey. Options for initiation age and tools were delineated by sub-groups. HRA referral thresholds separately queried recommendations by patient immune status.Results
One hundred forty respondents participated. Although consensus was lacking with regard to specific screening initiation age, more respondents recommended younger initiation ages for men who have sex with men (MSM) living with HIV (LWH) compared with MSM not LWH (p < 0.01). "No age threshold" ranged 44-55% among sub-groups with lower genital tract disease. Cytology and digital anorectal exam (DARE) were the most frequently selected tools for all sub-groups (ranges 77-90% and 74-86%, respectively). HRA was recommended significantly more frequently for MSM LWH (58%) and patients with vulvar cancer (52%) compared to others (p < 0.01). "Any [test] abnormality" was more often selected as indication for HRA for immunocompromised (56%) and immunocompetent (46%) patients than a specific cytology test result (29%, 36% respectively).Conclusion
Cytology and DARE were preferred screening tools; screening initiation age and HRA referral threshold showed less consensus. Evidence-based guidelines are needed and may lead to more consistent screening practices.Free full text

Provider preferences for anal cancer prevention screening: Results of the International Anal Neoplasia Society survey
Abstract
Objective
This study explores provider preferences regarding anal cancer screening indications, initiation age, tools, and referral threshold to high resolution anoscopy (HRA).
Methods
International Anal Neoplasia Society affiliates were invited to complete an online survey. Options for initiation age and tools were delineated by sub-groups. HRA referral thresholds separately queried recommendations by patient immune status.
Results
One hundred forty respondents participated. Although consensus was lacking with regard to specific screening initiation age, more respondents recommended younger initiation ages for men who have sex with men (MSM) living with HIV (LWH) compared with MSM not LWH (p < 0.01). “No age threshold” ranged 44-55% among sub-groups with lower genital tract disease. Cytology and digital anorectal exam (DARE) were the most frequently selected tools for all sub-groups (ranges 77-90% and 74-86%, respectively). HRA was recommended significantly more frequently for MSM LWH (58%) and patients with vulvar cancer (52%) compared to others (p < 0.01). “Any [test] abnormality” was more often selected as indication for HRA for immunocompromised (56%) and immunocompetent (46%) patients than a specific cytology test result (29%, 36% respectively).
Conclusion
Cytology and DARE were preferred screening tools; screening initiation age and HRA referral threshold showed less consensus. Evidence-based guidelines are needed and may lead to more consistent screening practices.
Abbreviations and acronyms
- Abnormal squamous intraepithelial lesions of unknown significance
- (ASCUS)
- Abnormal squamous cells that cannot rule out HSIL
- (ASC-H)
- Confidence intervals
- (CI)
- Digital anorectal examination
- (DARE)
- External genital warts
- (EGWs)
- High-grade squamous intraepithelial lesions
- (HSIL)
- High-resolution anoscopy
- (HRA)
- High-risk human papillomavirus
- (hrHPV)
- International Anal Neoplasia Society
- (IANS)
- Person-years
- (PY)
- Living with HIV
- (LWH)
- Low grade squamous intraepithelial lesion
- (LSIL)
- Lower genital tract disease
- (LGTD)
- Men who have sex with men
- (MSM)
- Transgender women
- (TGW)
1. Introduction
Anal cancer is rare in the general population, with an incidence of 1.8/100,000 person-years (PY) in the United States, similar to low rates reported in other countries where data are available [1,2]. However, for several high-risk populations, the rates of anal cancer surpass incidences of other cancers for which routine screening is recommended. For persons living with HIV (LWH), Silverberg et al. reported incidences of 131, 46 and 30 per 100,000 PY respectively for men who have sex with men (MSM), non-MSM males, and females [3,4]. Colón et al. calculated a standardized incidence ratio of 19.1 in persons LWH compared to the general population. HIV uninfected MSM, transgender women (TGW), women with high-risk human papillomavirus (hrHPV)-associated lower genital tract disease (LGTD), and people with non-HIV-related immunosuppression also have anal cancer incidences several-fold higher than the general population, enough to consider the benefit of screening practices for cancer prevention [[5], [6], [7]].
Most anal cancers are squamous cell carcinomas caused by hrHPV infections arising from high-grade squamous intraepithelial lesions (HSIL) in the anal canal or perianus [8]. Anal cancer is preceded years to decades by asymptomatic HSIL, affording an opportunity for detection and treatment of these pre-cancerous lesions [9]. Available tools to identify anal cancer precursors or early anal cancer include anal cytology, digital anorectal examination (DARE), hrHPV testing, and high-resolution anoscopy (HRA) [[10], [11], [12], [13]].
Screening practices to detect and treat anal HSIL in high-risk groups are evolving alongside a growing understanding of the natural history and epidemiology of anal cancer. Unlike other cancers with developed screening campaigns, anal cancer lacks national- or international-level guidance for whom, when and how to screen, resulting in heterogenous screening practices, limited uptake among providers and limited awareness among patients considered at increased risk [14,15]. This may be further exacerbated by variability of screening resources available to differing medical specialties who could screen for anal HSIL and cancer. For instance, anal cancer screening could be performed by primary care providers via DARE and anal cytology, by gynecologists who could incorporate HRA into their colposcopy practice, or by designated HRA providers in specialty clinics. Thus far there is insufficient literature to understand what proportion of such specialists currently incorporate elements of anal cancer screening into their practices. Studies evaluating the natural history of anal-associated hrHPV, incidence of HSIL or anal cancer prevention have not addressed screening efficacy per se, and this represents a gap in the literature.
This study evaluated screening recommendations among individual providers and specialists involved in the prevention and/or treatment of anal cancer. We analyzed survey data exploring opinions on populations for anal cancer screening, parameters for age to initiate screening, screening tools or procedures, and referral threshold for abnormal results leading to further evaluation with HRA.
2. Material and methods
2.1. Survey development and distribution
The International Anal Neoplasia Society (IANS) Screening Guidelines Task Force adapted items from a previous 2011 survey which assessed clinical practice recommendations for anal cancer screening in different high-risk populations [16]. Survey items included multiple choice, categorical and short answer queries. The new survey was uploaded to SurveyMonkey® (San Mateo, California). Email invitations containing a link to the survey were sent to 1150 IANS affiliates. The survey remained open for participation for a 14-day period. The study was reviewed and determined to be exempt by the UCLA South Campus Institutional Review Board (IRB# 20-000344).
2.2. Survey variables
Sub-groups considered at high-risk for anal cancer were specified. These included persons (P)LWH, specified by gender and/or sexual behavior: “HIV-positive women,” “HIV-positive MSM,” and “HIV-positive non-MSM men”. Women with a history of LGTD were sub-grouped by disease and anatomical site: “external genital warts,” (EGWs) “history of cervical or vaginal HSIL,” “history of cervical or vaginal cancer,” “history of vulvar HSIL,” and “history of vulvar cancer.” Three additional sub-groups identified were “HIV-negative MSM”, “transgender women” (TGW) and “people with non-HIV immunosuppression”.
To assess recommendations for screening initiation age, respondents selected one option from a list of mutually exclusive choices per sub-group. These included “no age restriction,” four specified ages (”≥25, “≥30, “≥35 and “≥40 years”), “would not recommend [screening]” or “other” with accompanying free text.
To assess recommendations for screening tool(s), respondents selected one or more options from a list of non-mutually exclusive screening tools that included DARE, cytology, hrHPV testing, and HRA. Respondents who would not recommend screening for a particular sub-group were instructed to select none of the listed options.
Last, respondents indicated specifications that would prompt referral for HRA evaluation for immunocompromised versus immunocompetent patients, based on the following mutually exclusive options: “no abnormal test result required for HRA referral,” “any abnormal result on DARE, HPV or cytology,” or a specified abnormality (“abnormal squamous intraepithelial lesions of unknown significance (ASCUS)+,” “low grade squamous intraepithelial lesion (LSIL)+,” “HSIL or abnormal squamous cells that cannot rule out HSIL (ASC-H)+,” “abnormal cytology AND hrHPV+”) and “a different triage (please elaborate)” with accompanying free text.
Additional questions on management of patients with anal dysplasia, follow-up intervals, and age at which to discontinue screening were queried but not included in this analysis.
At the conclusion of the survey respondent characteristics were collected: HRA provider status; if yes, years practicing HRA, patient volume, practice setting and number of HRA providers in their practice. Provider training, certification, and licensure, as well as clinic details (e.g. location, total patient volume) were collected but not assessed in this analysis.
3. Calculation
Descriptive, tabular, and graphical analyses explored the data. Chi squared goodness of fit tests of significance to compare sub-group dyads (e.g. MSM LWH compared to non-MSM males LWH) were applied for all histograms, with alpha value of 0.05. Kruskal-Wallis tests of significance were performed to determine overall differences in opinion on initiation age among PLWH sub-groups and among LGTD.
We compared recommended screening strategies to “never” recommended screening responses. To calculate respondents who would recommend screening per sub-group, recommendations of “never” were subtracted from the total responses in order to determine which sub-groups were more/less frequently recommended for screening with 95% confidence intervals (CI). Age was treated as a categorical variable: ≥25, ≥30, ≥35 and ≥ 40 years for these analyses were combined to create an “age-specified” variable. Histograms compared “age-specified” versus other response options. Results were compared for PLWH sub-groups, LGTD sub-groups, and MSM by HIV status. TGW and “people with non-HIV immunosuppression” were calculated and reported separately, though not included in a comparison. Additional histograms delineated specified initiation ages and explored consensus versus heterogeneity of responses. Screening strategies including individual screening tools, as well as DARE and cytology combined, were calculated, and tabulated as percentages, using the number of respondents who endorsed screening for each sub-group as the denominator. Recommendations for results prompting HRA referral were compared for immunocompromised and immunocompetent patients. Responses specifying a particular result that was considered a threshold for referral to HRA were combined to create a variable “threshold specified” group, which was compared to other responses. The threshold for referral to HRA was analyzed by responses for immune status and for a specified abnormal result.
4. Results
4.1. Respondent and HRA practice characteristics
Of 1150 survey invitations sent, 140 (12%) respondents from at least 21 countries and one territory participated in the survey and were included. Of 89 respondents (64%) who reported a country, geographic locations spanned 5 continents: North America (49) (Canada, United States, Puerto Rico and Mexico); South America (5) (Argentina, Brazil, Paraguay); Europe (30) (Denmark, Spain, Portugal, Italy, Netherlands, Slovenia, Switzerland, Austria, Greece, France, Belgium, the United Kingdom); Australia and New Zealand (4); and Africa (1) (Kenya).
When asked, “Do you perform HRA?”, most (74%) respondents affirmed practicing HRA; 8% responded they did not – these were researchers, care providers for high-risk patients, or patient advocates; the remaining 18% did not provide an answer. Among those who practice HRA: 66% had practiced between 1 and 10 years; the most common practice settings were infectious disease or HIV clinics (33%), surgical clinics (20%), and specialty HRA practices (12%); finally, most (83%) reported having 1-3 HRA providers in their practice (Table 1).
Table 1
Characteristics of respondents who perform HRA (N = 104).
| Characteristics | n (%) |
|---|---|
| Years practicing HRA | |
| Less than 1 | 11 (11) |
| 1-10 | 69 (66) |
| More than 11-15 | 18 (17) |
| No answer | 6 (6) |
| HRA patients per week | |
| Less than 5 | 22 (21) |
| 5-10 | 40 (38) |
| 11-20 | 24 (23) |
| More than 20 | 12 (12) |
| No answer | 6 (6) |
| Practice Setting | |
| Free standing | 12 (12) |
| Gynecology | 7 (7) |
| Infectious Disease/HIV | 34 (33) |
| Gastroenterology | 6 (6) |
| Surgery Clinic | 22 (21) |
| Specialty STD Clinic | 5 (5) |
| Other | 15 (14) |
| No answer | 3 (3) |
| Years clinic has provided HRA | |
| Less than 1 | 7 (7) |
| 1-5 | 28 (27) |
| 6-10 | 29 (28) |
| 11-20 | 31 (30) |
| More than 20 | 5 (5) |
| No answer | 4 (4) |
| Number HRA providers in clinic | |
| 1-3 | 86 (83) |
| 4-10 | 14 (13) |
| No answer | 4 (4) |
4.2. Anal cancer prevention screening by risk sub-group
For each sub-group, the majority of respondents (mean 92.8%; 95% CI 89%,98%) recommended anal cancer prevention screening. Screening for MSM LWH (99%), women LWH (99%) and patients with a history of vulvar cancer (99%) were recommended more frequently relative to other sub-groups, while MSM not LWH (87%), and women with EGW (76%) were less frequently recommended (Table 2).
Table 2
Proportion of respondents who recommend routine screening for anal cancer prevention, by risk sub-group (N = 140).
| Risk Sub-group | n (%) |
|---|---|
| People Living with HIV (PLWH), by gender and sexual partner | |
| HIV-positive Men who have sex with men (MSM)a | 139 (99) |
| HIV-positive Womena | 138 (99) |
| HIV positive Men who have sex with women (MSW) | 128 (91) |
| Non-HIV Immunocompromised | 131 (94) |
| MSM and Transgender Women | |
| HIV-negative MSMa | 122 (87) |
| Transgender women (HIV status not specified)a | 121 (86) |
| Lower Genital Tract Disease (LGTD) | |
| Women with external genital wartsa | 106 (76) |
| Cervicovaginal HSIL | 133 (95) |
| Cervicovaginal Cancer | 137 (98) |
| Vulvar HSIL | 137 (98) |
| Vulvar Cancera | 139 (99) |
Mean respondents who answered “yes”: 130, 95% CI (123, 137).
4.3. Age of screening initiation
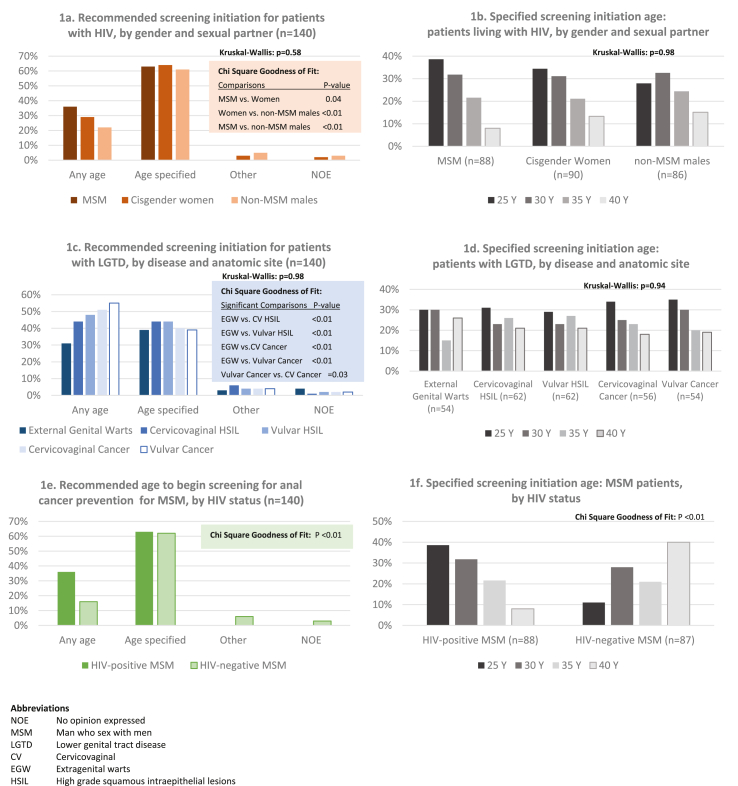
The majority (>60%) of respondents recommended a specific screening initiation age for each sub-group LWH. When all three PLWH sub-groups were compared, overall statistically significant differences were not seen (p = 0.58). Yet, in comparing individual sub-groups to one another via dyads, participants’ recommendations differed significantly when goodness-of-fit comparisons were performed: MSM versus women (p = 0.04), MSM versus non-MSM males (P < 0.001), and cisgender women versus non-MSM males (p = 0.009). Of note, although more respondents recommended “no age restriction” for screening MSM LWH (36%) versus other PLWH, differences were not statistically significant (Fig. 1a). Among respondents who specified a screening initiation age for PLWH, consensus was lacking as to which specific age was appropriate, and proportions were similar across sub-groups (p = 0.70) (Fig. 1b).
Regarding patients with LGTD, fewer respondents (39-44%) identified a specific screening initiation age, compared to ‘no age restriction’ for patients with a history of vulvar or cervicovaginal cancer (55% and 51% respectively) (Fig. 1c). While in overall comparison, recommendations for sub-groups were not statistically significant (p = 0.98), paired comparisons revealed that recommendations for screening initiation age differed significantly when comparing women with EGW to every other sub-group (p < 0.01 for all sub-group comparisons); history of vulvar cancer versus cervicovaginal HSIL was also significantly different (p = 0.03). Similar to PLWH, there was no consensus among those who specified an initiation age, and distributions of ages recommended were similar for all groups (p > 0.99) (Fig. 1d).
Finally, for both MSM LWH and MSM not LWH, the majority of respondents specified a screening initiation age. Although, more respondents recommended screening at any age for MSM LWH (36%) compared to 16% for their counterparts not LWH (p < 0.01) (Fig. 1e). More respondents recommended younger ages for MSM LWH (39% and 32% for 25 and 30 years respectively), while older ages were more frequently recommended for MSM not LWH (e.g. 40% for 40 years) (p < 0.01).
Approximately half of respondents recommended a specific screening initiation age for both TGW (46%) and people with non-HIV immunosuppression (54%); specified ages were equally distributed for both, similar to PLWH and LGTD sub-groups (data not shown).
4.4. Screening tools
Screening tool preferences are listed by sub-group (Table 3). There were no significant differences in screening tool recommendations when comparing sub-groups, with the exception of HRA as a baseline screening tool. Cytology was the most commonly recommended tool (77-90%) followed by DARE (76-86%); the combination of both cytology and DARE – with or without additional testing – was recommended by most respondents (65-76%). Recommendations for hrHPV screening ranged from 40 to 50% across all sub-groups. HRA as an initial screening tool varied significantly by sub-group (p < 0.01), recommended more commonly for MSM LWH (58%) and patients with a history of vulvar cancer (52%), and least frequently for people with non-HIV immunosuppression, (33%), MSM not LWH (36%), and TGW (36%).
Table 3
Recommended screening tool, by anal cancer risk sub-group.
| Screening Tool | People Living with HIV | HIV Negative | HIV Not specified | EGW | HSIL | Cancer | P-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSM (n = 139) n (%) | Women (n = 138) n (%) | MSW (n = 128) n (%) | MSM (n = 122) n (%) | Non-HIV immuno- suppression (n = 131) n (%) | TGW (n = 121) n (%) | Genital n = 106 n (%) | Cervico-vaginal (n = 133) n (%) | Vulvar (n = 137) n (%) | Cervico-vaginal (n = 137) n (%) | Vulvar (n = 139) n (%) | ||
| Anal Cytology | 125 (90) | 123 (89) | 115 (90) | 107 (88) | 101 (77) | 102 (84) | 86 (81) | 110 (83) | 114 (83) | 114 (83) | 119 (83) | 0.06 |
| DARE | 116 (83) | 110 (80) | 105 (82) | 102 (84) | 99 (76) | 98 (81) | 91 (86) | 100 (75) | 102 (74) | 104 (76) | 105 (76) | 0.38 |
| HrHPV | 65 (47) | 67 (49) | 64 (50) | 62 (51) | 52 (40) | 54 (45) | 51 (48) | 66 (50) | 66 (48) | 67 (49) | 68 (49) | 0.87 |
| HRAa | 81 (58) | 69 (50) | 61 (40) | 44 (36) | 43 (33) | 43 (36) | 45 (42) | 58 (44) | 64 (47) | 64 (47) | 72 (52) | <0.01 |
| Cytology plus DARE | 106 (76) | 101 (73) | 93 (73) | 89 (73) | 82 (63) | 86 (71) | 71 (67) | 87 (65) | 91 (66) | 93 (68) | 96 (69) | 0.40 |
4.5. Threshold for HRA referral
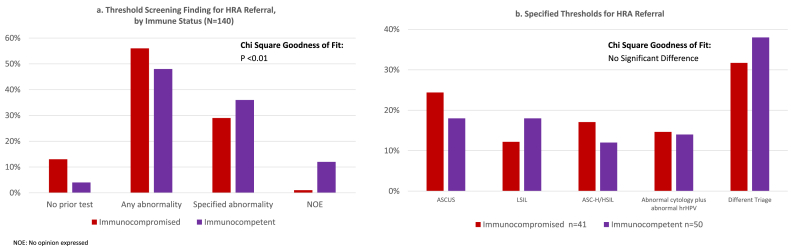
Respondents were asked to recommend a test result threshold for HRA referral for immunosuppressed and immunocompetent patients who they deemed eligible for anal cancer screening (e.g. “For an immunocompromised patient in a risk group you recommend for anal cancer screening, what test result(s), if any, would you recommend be the threshold for referral to HRA?”). Participants’ recommendations differed significantly (p < 0.01), with more preferring no prior screening test for referral to HRA for immunocompromised patients (13%) compared to immunocompetent patients (4.4%). Likewise, referral to HRA for any abnormal physical finding or lab result was higher for immunocompromised (57%) compared to immunocompetent (38%) (Fig. 2a). Nevertheless, when queried explicitly, “Would your answer for what test result(s) should be referred to HRA be different for an immunocompetent patient?” 58% of respondents indicated they would not triage patients differently based on immune status. For those who indicated a particular abnormal finding or test/cytology result as a threshold for HRA referral, there was no significant difference between immunocompromised versus immunocompetent for which abnormal result indicated referral to HRA (Fig. 2b). Notably, among respondents who identified a specific threshold, more indicated “a different triage” (32%, 38%) than either abnormal cytology or hrHPV. (Fig. 2b). Responses for “A different triage” included examples such as “repeatedly abnormal ASCUS or LSIL (two separated by six months) or one HSIL/ASC-H warrants HRA.”
6. Discussion
This study reports a detailed survey of opinions regarding current international anal cancer prevention screening approaches. Respondents from 21 countries, the majority of whom were HRA providers, represented a wide range of views by healthcare professionals, medical specialties, and clinic settings. This survey, evaluating current practices and opinions, may inform future screening recommendations.
Overall, respondents endorsed prevention screening in all queried sub-groups, which were those considered at-risk for anal cancer by the authors. Variances in endorsement are interpreted as the respondents' presumed risk stratification for anal cancer in these sub-groups, which differed slightly from recently published risk estimates [7]. Anal cancer incidence was higher in women with vulvar HSIL (42/100,000) compared to women LWH (22/100,000) or non-MSM LWH (32/100,000), yet respondents’ most frequently recommended screening for women LWH.
Anal cancer risk increases with age, which suggests that age may be an appropriate component for future guidance [4]. Among MSM LWH, Clifford et al. noted an increased incidence from 17/100,000 PY (<30 years-old) to 66/100,000 PY (30-44 years), at which point incidence among MSM LWH surpassed those of all other high-risk sub-groups; at ≥60 years, incidence reached 108/100,000 PY. In sub-groups receiving stronger recommendations for screening, respondents consistently recommended initiation at younger ages, reflecting known increased anal cancer incidences in these groups. Ages trended youngest for MSM and women LWH, followed by non-MSM males LWH. Respondents would initiate screening younger in MSM LWH than those not LWH. However, nearly 1/3 of respondents would initiate screening at any age for MSM LWH and women with LGTD. “No age threshold” may have been interpreted by respondents to suggest that a sub-group's risk factor alone was an indication for screening irrespective of age, or in order to include <25 years since that was not an option. Thus, most respondents recommended initiating screening by age 30 for all PLWH sub-groups: MSM 81%, women 71%, and non-MSM males 59%. A more significant difference was seen when comparing MSM by HIV status, where 75% recommended MSM LWH begin screening at age 25 compared with only 27% of MSM not LWH. Likewise, in LGTD sub-groups “any age” or younger ages were more frequently recommended for conditions more strongly associated with anal cancer (e.g. vulvar cancer).
There was no difference in screening tool recommendations for most sub-groups. However, HRA as the initial screening modality was endorsed significantly more for MSM and women LWH and women with a history of vulvar cancer compared to all other sub-groups. While HRA has better sensitivity for identifying HSIL, it is more invasive and expensive with variable accessibility. Respondents may weigh these considerations against benefits of disease detection, reserving HRA for those at higher risk. Cytology was the most commonly endorsed tool. There is robust evidence for detection of anal dysplasia (though not necessarily HSIL) with cytology [17]. In recent metanalyses, cytology had moderate sensitivity (83%) and suboptimal specificity (45%) for MSM LWH; moderate sensitivity (71%) and specificity (73%) for women with history of anogenital neoplasia [18,19]. Data for anal cancer screening tools – including cytology – in other persons are less available. Existing guidelines reflect this gap, recommending anal cytology for MSM LWH, but not other at-risk groups with known increased anal cancer incidences. Despite these limitations, cytology remains the best-established and available option for detection of asymptomatic anal dysplasia. DARE, though commonly selected as a screening tool, has only been shown to detect early anal cancers [20,21] rather than precancerous lesions for anal cancer prevention (not specified in the survey). DARE may be performed by patients, partners or clinicians within routine clinical care [22]. It is well tolerated, sensitive for lesions as small as 3 mm [23]. Despite these advantages and its endorsement by most respondents, DARE is infrequently performed in clinical practice [24]. Interestingly, hrHPV testing was recommended by nearly 50% in most sub-groups. This was unexpected given high hrHPV prevalence among MSM LWH inferring excellent positive predictive value of true hrHPV infection, but correspondingly poor specificity [25]. Nonetheless, Wang et al. reported a 92% negative predictive value of hrHPV positivity for anal HSIL among 156 PLWH, with no anal cancers detected [26]. In a separate prospective study, when incorporated into anal cancer screening algorithms for high risk groups, hrHPV testing increased sensitivity for histologic HSIL and anal cancer, though decreased specificity when compared to anal cytology alone [27]. This raises the possibility that hrHPV as an initial screening test could play a role if followed by more specific modalities such as cytology and HRA as indicated. HPV genotyping might have a role in screening given associations of HPV-16 and -18 with anal HSIL and cancer [28], although MSM LWH have a lower fraction of HPV-16-associated anal cancer than other high-risk groups [29]. Conversely, women have a lower prevalence of HPV-16 and higher fraction of HPV-16-associated anal cancer [30].
Finally, HRA is considered to be the gold standard for diagnosis and treatment of anal HSIL in light of the ability to visualize and biopsy specific lesions in the anal canal under high-magnification. However, it is resource-intensive for healthcare systems and providers and can be invasive and time-consuming for patients. Despite these limitations, a large number of respondents recommend HRA as an initial screening test. More respondents recommended referring immunocompromised patients either directly to HRA as an initial screening or to HRA for any abnormality on any screening tool (total 60%), compared to HRA referral for non-immunocompromised patients with the same parameters (42%). Notwithstanding, among respondents who chose a particular result (e.g. abnormal cytology), answers were evenly distributed, reflecting lack of consensus regarding the abnormality for referral to an HRA, and did not differ by immune status. Most respondents selected “other” and elaborated with individualized triage protocols that considered additional clinical factors (e.g. repeated abnormal cytology results, low CD4 counts, etc.). Several indicated HRA referral was dependent on available resources, or that HRA and/or cytology was not yet available in their practice. While much of the published literature uses ASCUS+ as the standard for what is considered an abnormal cytology, practitioners in the field indicated a more nuanced approach perhaps reflecting their familiarity with limitations in available resources.
This study has several limitations. First, survey distribution was limited to individuals affiliated with IANS. The opinions and recommendations expressed therefore may reflect participation in an IANS-sponsored educational program. As a convenience sample, there may be volunteer bias in submitted recommendations. We queried experts on multiple levels of screening practices relevant to important, sometimes intersecting, affected populations, yet this analysis does not distinguish patient who may have multiple risk factors, (e.g. a PLWH with a history of EGW). Terminology including ‘immunocompromised’ vs. ‘non-immunocompromised’ that was based on the prior survey, may have been inconsistent with the sub-groups used in this survey. Querying respondents for their recommendations rather than current practice obviated real-world constraints from their consideration of screening preferences. Our respondents, who were primarily HRA providers, might more readily choose HRA as an initial screening test, versus the opinions of those without immediate access to the procedure. Finally, this analysis would have been strengthened by a higher response rate, as well as a survey available to non-English speaking IANS members.
7. Conclusion
Effective anal cancer prevention via screening hinges on identifiable groups at-risk, an ability to detect asymptomatic precursors, and effective interventions to prevent progression. The reported data indicate provider willingness to screen persons considered at increased risk for anal cancer. Providers designated younger screening age, and more invasive and costly but specific screening tools for populations with higher anal cancer incidences, particularly MSM LWH and women with a history of vulvar cancer. However, evidence-based guidance is needed to clarify specifically who to screen, when to screen, and how to screen.
Sources of funding support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
NJ received $750 as a Merck Advisory Board participant. All other contributing authors confirm they have no conflicts of interest.
CRediT author statement
All co-authors contributed to the Conceptualization, Methodology, Analytic approach, and Writing of this manuscript. RP performed data management, formal analysis, and data visualization; NJ provided additional supervision.
References
Articles from Tumour Virus Research are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/123419213
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.tvr.2022.200235
Article citations
Barriers and facilitators to anal cancer screening among people living with HIV in Puerto Rico.
BMC Public Health, 23(1):1940, 06 Oct 2023
Cited by: 2 articles | PMID: 37803344 | PMCID: PMC10559598
Anal cancer screening results from 18-to-34-year-old men who have sex with men living with HIV.
Int J Cancer, 154(1):21-27, 20 Sep 2023
Cited by: 2 articles | PMID: 37728489
Anal Cancer and Anal Cancer Screening.
Clin Obstet Gynecol, 66(3):516-533, 13 Jul 2023
Cited by: 2 articles | PMID: 37439541 | PMCID: PMC10524277
Evaluating the performance of anal cytology and high-risk HPV genotyping for detecting anal HSIL in a clinic-based sample of people living with and without HIV in Puerto Rico.
Cancer Cytopathol, 131(10):655-664, 26 Jun 2023
Cited by: 0 articles | PMID: 37358055 | PMCID: PMC10650567
Digital Anal Rectal Examination Usage Among Individuals at Increased Risk for Anal Cancer.
J Low Genit Tract Dis, 27(3):242-247, 23 Apr 2023
Cited by: 2 articles | PMID: 36961479
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
International Anal Neoplasia Society's consensus guidelines for anal cancer screening.
Int J Cancer, 154(10):1694-1702, 31 Jan 2024
Cited by: 29 articles | PMID: 38297406
Racial Disparities in Anal Cancer Screening Among Men Living With HIV: Findings From a Clinical Cohort Study.
J Acquir Immune Defic Syndr, 84(3):295-303, 01 Jul 2020
Cited by: 8 articles | PMID: 32097251
Facilitators of and barriers to high-resolution anoscopy adherence among men who have sex with men: a qualitative study.
Sex Health, 15(5):431-440, 01 Nov 2018
Cited by: 7 articles | PMID: 30244691
Anal cancer screening and prevention: a review for dermatologists.
J Eur Acad Dermatol Venereol, 35(8):1622-1627, 11 Jun 2021
Cited by: 3 articles | PMID: 33797819
Review