Abstract
Free full text

Cefiderocol- Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii
ABSTRACT
Cefiderocol may represent a therapeutic option for carbapenem-resistant Acinetobacter baumannii (CRAB) infections, but clinical data are limited. This is an observational retrospective study conducted in the University Hospital of Pisa including consecutive patients with CRAB infections (January 2020 to August 2021). Patients were divided in two study groups according to the antibiotic treatment received: cefiderocol- and colistin-containing regimens. The primary outcome was the 30-day mortality. A Cox regression analysis was performed to identify factors independently associated with 30-day mortality. A propensity score analysis using inverse probability of treatment weighting (IPTW) was also performed. A total of 124 patients were included: 47 (37.9%) received cefiderocol, while 77 (62.1%) colistin-containing regimens. Overall, 79 (63.7%) patients had a bloodstream infection (BSI), 35 (28.5%) a ventilator-associated pneumonia (VAP) and 10 (8.1%) other infections. Thirty-day mortality was higher in patients receiving colistin- compared to those who received cefiderocol-containing regimens (55.8% versus 34%, P =
= 0.018). This difference was confirmed in patients with BSI, but not in those with VAP. On multivariable analysis, septic shock, SOFA score, and age were independently associated with 30-day mortality, while cefiderocol therapy was protective in an IPTW analysis (Hazard ratio 0.44, 95% confidence interval 0.22–0.66, P
0.018). This difference was confirmed in patients with BSI, but not in those with VAP. On multivariable analysis, septic shock, SOFA score, and age were independently associated with 30-day mortality, while cefiderocol therapy was protective in an IPTW analysis (Hazard ratio 0.44, 95% confidence interval 0.22–0.66, P <
< 0.001). Nephrotoxicity was more common in the colistin group. Microbiological failure occurred in 17.4% of patients receiving cefiderocol versus 6.8% of those receiving colistin (P
0.001). Nephrotoxicity was more common in the colistin group. Microbiological failure occurred in 17.4% of patients receiving cefiderocol versus 6.8% of those receiving colistin (P =
= 0.079). Among 8 cases in the cefiderocol group who experienced microbiological failure, 4 (50%) developed resistance to cefiderocol. Cefiderocol represents a promising therapeutic option in patients with severe CRAB infections. Randomized clinical trial in this specific patient population should confirm our findings.
0.079). Among 8 cases in the cefiderocol group who experienced microbiological failure, 4 (50%) developed resistance to cefiderocol. Cefiderocol represents a promising therapeutic option in patients with severe CRAB infections. Randomized clinical trial in this specific patient population should confirm our findings.
INTRODUCTION
In 2017, the World Health Organization (WHO) prioritized carbapenem-resistant Acinetobacter baumannii (CRAB) as one of the species of critical importance for research and development of new antibiotics (1). Patients with CRAB infections have high risk of poor outcome with a mortality rate reported in clinical studies ranging from 40% to 70%, depending on patients conditions, clinical severity and type of infection (2, 3). COVID-19 pandemic further complicated the situation, leading to an increase in the spread of multidrug-resistant organisms in the hospital setting (4, 5). Currently, there is no consensus on the optimal treatment of CRAB infections. Until now, colistin (usually combined to other drugs such as tigecycline, ampicillin/sulbactam, meropenem or fosfomycin) has been considered the backbone therapy despite its considerable nephrotoxic effect (6,–8).
Recently, cefiderocol, a novel catechol-substituted siderophore cephalosporin, has been approved by the Food and Drug Administration for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CR-GNB) (9). However, results from the phase 3 randomized clinical trial (RCT) CREDIBLE-CR comparing cefiderocol to the best available therapy in patients with CR-GNB showed an unexpected increase of mortality in the subset of patients with CRAB (10). Preliminary experience from case series including patients with CR-GNB are promising, but not focused only on CRAB (11, 12). Thus, a gap between results from the CREDIBLE-CR RCT and the real-world observations continues to exist and data on the efficacy of cefiderocol in patients with CRAB infections are still lacking.
The aim of this study was to evaluate the impact of cefiderocol-containing regimens compared to colistin-containing regimens on the outcome of patients with CRAB infection.
RESULTS
Study population.
The study flow chart is represented in Fig. 1. A total 141 patients with CRAB infections were included during the study period. Ten patients who received other treatment regimens, 6 who died before receiving any active antibiotic therapy and 1 with no available clinical data were excluded from the analysis. Overall, 124 patients with CRAB infections were included in the study: 47 (37.9%) patients received cefiderocol, while 77 (62.1%) were controls. Median time from admission to CRAB infection was 15 (IQRs 9–27) days and did not differ between patients who received cefiderocol and those who received colistin-containing regimens (15, IQR 10–40 versus 13, IQR 9–23, P =
= 0.06).
0.06).
Seventy-nine (63.7%) patients had a BSI (30 from respiratory tract, 23 CLABSI, 11 from skin and soft tissue, 10 UTIs, 1 IAI, 4 of unknown source), 35 (28.5%) a VAP and 10 (8.1%) other type of infections (6 surgical site infections, 1 IAI, 1 perianal abscess, 1 central nervous system infection, and 1 UTI) due to CRAB. Thirty (24.2%) patients had polymicrobial infections caused by CRAB plus Gram negative bacilli susceptible to cefiderocol or colistin. Proportion of polymicrobial infections varied according to the type of infection: 11/79 (13.9%) in BSI, 18/35 (51.4%) in VAP and 1/10 (10%) in other infections (P <
< 0.001). Etiology of polymicrobial infections are shown in Fig. S1 in the supplemental material.
0.001). Etiology of polymicrobial infections are shown in Fig. S1 in the supplemental material.
Table 1 and Table 2 summarize the types of infections and the relative antibiotic regimens in patients treated with cefiderocol and controls, respectively. MIC values for cefiderocol ranged from 0.12 to 2 mg/L. Susceptibility was 100% according to EUCAST and CLSI criteria, while susceptibility was 95.8% according to FDA criteria. Among 47 patients treated with cefiderocol (Table 1), 15 (31.9%) received cefiderocol monotherapy while the remaining 32 patients received cefiderocol associated with tigecycline (n
mg/L. Susceptibility was 100% according to EUCAST and CLSI criteria, while susceptibility was 95.8% according to FDA criteria. Among 47 patients treated with cefiderocol (Table 1), 15 (31.9%) received cefiderocol monotherapy while the remaining 32 patients received cefiderocol associated with tigecycline (n =
= 21), fosfomycin (n
21), fosfomycin (n =
= 8), ertapenem (n
8), ertapenem (n =
= 1), meropenem-vaborbactam (n
1), meropenem-vaborbactam (n =
= 1), and ampicillin/sulbactam (n
1), and ampicillin/sulbactam (n =
= 1). Among 77 controls, colistin was administered as monotherapy in 12 cases (15.6%), while in the remaining cases was combined to other antibiotics as described in Table 2.
1). Among 77 controls, colistin was administered as monotherapy in 12 cases (15.6%), while in the remaining cases was combined to other antibiotics as described in Table 2.
TABLE 1
Type of infection and relative treatment regimens in 47 patients with CRAB infections treated with cefiderocol-containing regimensa
| Type of infection | Treatment regimen | |
|---|---|---|
BSI N = = 27 (57.4%) 27 (57.4%) | Cefiderocol monotherapy Cefiderocol + tigecycline Cefiderocol + fosfomycin Cefiderocol + ertapenem Cefiderocol + ampicillin/sulbactam | n = = 12 12n  = = 10 10n  = = 3 3n  = = 1 1n  = = 1 1 |
VAP N = = 12 (25.5%) 12 (25.5%) | Cefiderocol monotherapy Cefiderocol + tigecycline Cefiderocol + fosfomycin Cefiderocol + meropenem-vaborbactam | n = = 2 2n  = = 6 6n  = = 3 3n  = = 1 1 |
Other infections N = = 8 (17%) 8 (17%) | Cefiderocol monotherapy Cefiderocol + tigecycline Cefiderocol + fosfomycin | n = = 1 1n  = = 5 5n  = = 2 2 |
TABLE 2
Type of infection and relative treatment regimens in 77 patients with CRAB infections treated with colistin-containing regimensa
| Type of infection | Treatment regimen | |
|---|---|---|
BSI N = = 52 (67.5%) 52 (67.5%) | Colistin-containing regimens Colistin alone Colistin + tigecycline Colistin + tigecycline + meropenem Colistin + tigecycline + rifampin Colistin + tigecycline + fosfomycin Colistin + meropenem + fosfomycin Colistin + rifampin Colistin + aminoglycosides | N = = 52 52n  = = 11 11n  = = 30 30n  = = 5 5n  = = 2 2n  = = 1 1n  = = 1 1n  = = 1 1n  = = 1 1 |
VAP N = = 23 (29.9%) 23 (29.9%) | Colistin-containing regimens Colistin alone Colistin + tigecycline Colistin + tigecycline + rifampin Colistin + tigecycline + meropenem Colistin + tigecycline + fosfomycin Colistin + meropenem + fosfomycin Colistin + tigecycline + ampicillin/sulbactam Colistin + ampicillin/sulbactam | N = = 23 23n  = = 1 1n  = = 9 9n  = = 1 1n  = = 2 2n  = = 2 2n  = = 1 1n  = = 6 6n  = = 1 1 |
| Other infections N  = = 2 (2.6%) 2 (2.6%) | Colistin-containing regimens Colistin alone Colistin + tigecycline | N = = 2 2n  = = 1 1n  = = 1 1 |
Clinical features and outcomes of the 2 treatment groups are summarized in Table 3. Patients treated with colistin-containing regimens were more frequently males and more commonly affected by diabetes mellitus. Patients who received cefiderocol-containing regimens were more frequently affected by infections other than BSI or VAP, received more frequently parenteral nutrition and extracorporeal membrane oxygenation. Source control did not differ between the two groups.
TABLE 3
Clinical characteristics and outcomes of patients with CRAB infections by treatment regimensa
| Variable | FDC-containing regimens (N = = 47) 47) | Colistin-containing regimens (N = = 77) 77) | p b |
|---|---|---|---|
| Age, median, IQRs | 63 (53.5–75) | 68 (56–75) | 0.414 |
| Male sex | 29 (61.7%) | 63 (81.8%) | 0.013 |
| Comorbidities | |||
 Diabetes mellitus Diabetes mellitus | 3 (6.4%) | 20 (26%) | 0.006 |
 Cardiovascular disease Cardiovascular disease | 29 (61.7%) | 44 (57.1%) | 0.617 |
 COPD COPD | 4 (8.5%) | 13 (16.9%) | 0.188 |
 Chronic renal disease Chronic renal disease | 2 (4.3%) | 7 (9.1%) | 0.314 |
 Chronic liver disease Chronic liver disease | 2 (4.3%) | 3 (3.9%) | 1.0 |
 Solid cancer Solid cancer | 3 (6.4%) | 5 (6.5%) | 0.981 |
| COVID-19 | 19 (40.4%) | 29 (37.7%) | 0.759 |
| Ward of hospitalization | |||
 Medical wards Medical wards | 4 (8.5%) | 8 (10.4%) | 0.731 |
 Surgery Surgery | 1 (2.1%) | 0 | 0.199 |
 ICU ICU | 42 (89.4%) | 69 (89.6%) | 0.965 |
| Type of infections | |||
 BSI BSI | 27 (57.4%) | 52 (67.5%) | 0.257 |
 VAP VAP | 12 (25.5%) | 23 (29.9%) | 0.603 |
 Other Other | 8 (17%) | 2 (2.6%) | 0.006 |
| Charlson Comorbidity Index, median, IQRss | 3 (1–5) | 3 (1–5) | 0.413 |
 SOFA score, median, IQRs SOFA score, median, IQRs | 9 (6–11) | 9 (4–11) | 0.693 |
 APACHE II score, median, IQRs APACHE II score, median, IQRs | 18 (9–25) | 16 (11–22) | 0.702 |
 Invasive mechanical ventilation Invasive mechanical ventilation | 25 (53.2%) | 45 (55.8%) | 0.459 |
 Intravascular device Intravascular device | 47 (100%) | 77 (100%) | 1.0 |
 Septic shock Septic shock | 30 (63.8%) | 45 (58.4%) | 0.551 |
 AKI at time of sepsis AKI at time of sepsis | 10 (21.3%) | 21 (27.3%) | 0.454 |
 Parenteral nutrition Parenteral nutrition | 20 (42.6%) | 19 (24.7%) | 0.038 |
 ECMO at time of sepsis ECMO at time of sepsis | 7 (14.9%) | 2 (2.6%) | 0.026 |
 CVVH at time of sepsis CVVH at time of sepsis | 6 (12.8%) | 8 (10.5%) | 0.704 |
 Source control Source control | 18 (38.3%) | 31 (40.3%) | 0.828 |
 Polymicrobial infections Polymicrobial infections | 8 (17%) | 22 (28.6%) | 0.145 |
 Duration of targeted antibiotic therapy Duration of targeted antibiotic therapy | 12 (7–14) | 10 (6.5–13) | 0.089 |
 30-day mortality 30-day mortality | 16 (34%) | 43 (55.8%) | 0.018 |
 Microbiological failurec Microbiological failurec | 8/46 (17.4%) | 5/74 (6.8%) | 0.079 |
 Length of hospital stay after CRAB infection, median, IQRs Length of hospital stay after CRAB infection, median, IQRs | 28 (16–34) | 13 (6–23.5) | <0.001 |
Primary outcome.
Thirty-day all-cause mortality was 47.6% (59/124 patients). Thirty-day mortality was higher in patients treated with colistin-containing regimens (55.8%) compared to those who received cefiderocol (34%, P =
= 0.018). This difference persists in the sensivity analysis when only patients with monomicrobial CRAB infections were considered (54.5% versus 33.3%, P
0.018). This difference persists in the sensivity analysis when only patients with monomicrobial CRAB infections were considered (54.5% versus 33.3%, P =
= 0.04).
0.04).
In the subgroup of patients with BSI, both 14-days and 30-days mortality rates were lower in patients treated with cefiderocol compared to those treated with colistin-containing regimens (7.4% versus 42.3%, P <
< 0.001; and 25.9% versus 57.7%, P
0.001; and 25.9% versus 57.7%, P =
= 0.007 respectively, Fig. 2). Conversely, in the subset of patients with VAP no differences in 14-days and 30-days mortality were detected between the 2 study groups (Fig. 2).
0.007 respectively, Fig. 2). Conversely, in the subset of patients with VAP no differences in 14-days and 30-days mortality were detected between the 2 study groups (Fig. 2).
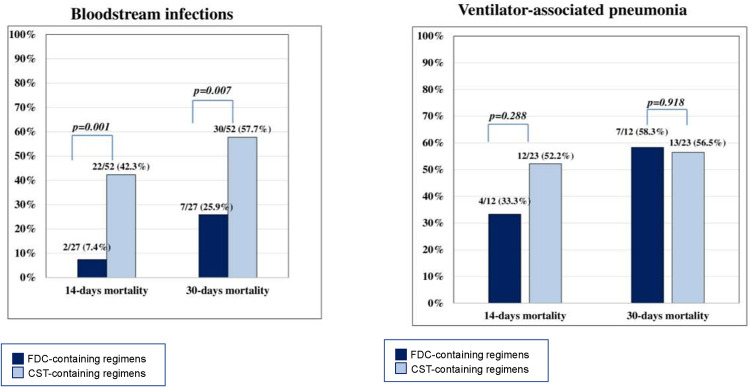
Fourteen- and 30-days mortality in patients with bloodstream infection (BSI) and ventilator-associated pneumonia (VAP). FDC cefiderocol.
Comparison of patients who died or not within 30 days from the CRAB infection is shown in Table 4. ID consultation did not differ between the two groups (55.9% versus 61.5%, P
days from the CRAB infection is shown in Table 4. ID consultation did not differ between the two groups (55.9% versus 61.5%, P =
= 0.526). On multivariable analysis (Table 5), septic shock (hazard ratio [HR] 2.56, 95% confidence interval [CI] 1.11–5.94, P
0.526). On multivariable analysis (Table 5), septic shock (hazard ratio [HR] 2.56, 95% confidence interval [CI] 1.11–5.94, P =
= 0.028), SOFA score (HR 1.15, 95% CI 1.05–1.27, P
0.028), SOFA score (HR 1.15, 95% CI 1.05–1.27, P =
= 0.003) and age (HR 1.05, 95% CI 1.02–1.07, P
0.003) and age (HR 1.05, 95% CI 1.02–1.07, P =
= 0.001) were factors independently associated with 30-day mortality, while cefiderocol (compared to colistin-based regimens) was a protective factor (HR 0.32, 95% CI 0.18–0.57, P
0.001) were factors independently associated with 30-day mortality, while cefiderocol (compared to colistin-based regimens) was a protective factor (HR 0.32, 95% CI 0.18–0.57, P <
< 0.001). Sensitivity analysis after excluding patients with polymicrobial infections confirmed that cefiderocol was independently associated with 30
0.001). Sensitivity analysis after excluding patients with polymicrobial infections confirmed that cefiderocol was independently associated with 30 day mortality (adjusted HR 0.31, 95% CI 0.16–0.62, P
day mortality (adjusted HR 0.31, 95% CI 0.16–0.62, P <
< 0.001).
0.001).
TABLE 4
Clinical characteristics and outcomes of patients with CRAB infections who died and who did not within 30 days from infectiona
days from infectiona
| Variable | 30-day mortality (N = = 59) 59) | Survivors (N = = 65) 65) | p b |
|---|---|---|---|
| Age, median, IQRs | 71 (65–77) | 59 (42–73) | <0.001 |
| Male sex | 49 (83.1%) | 43 (66.2%) | 0.032 |
| Comorbidities | |||
 Diabetes mellitus Diabetes mellitus | 12 (20.3%) | 11 (16.9%) | 0.625 |
 Cardiovasculardisease Cardiovasculardisease | 42 (71.2%) | 31 (47.7%) | 0.008 |
 COPD COPD | 12 (20.3%) | 5 (7.7%) | 0.041 |
 Chronicrenaldisease Chronicrenaldisease | 7 (11.9%) | 2 (3.1%) | 0.060 |
 Chronicliverdisease Chronicliverdisease | 4 (6.8%) | 1 (1.5%) | 0.138 |
 Solid cancer Solid cancer | 3 (5.1%) | 5 (7.7%) | 0.555 |
| COVID-19 | 31 (52.5%) | 17 (26.2%) | 0.003 |
 Ward of hospitalization Ward of hospitalization | |||
 Medicalwards Medicalwards | 6 (10.2%) | 6 (9.2%) | 0.860 |
 Surgery Surgery | 0 | 1 (1.5%) | 1.0 |
 ICU ICU | 53 (89.8%) | 58 (89.2%) | 0.913 |
| Type of infections | |||
 BSI BSI | 37 (62.7%) | 42 (64.6%) | 0.826 |
 VAP VAP | 20 (33.9%) | 15 (23.1%) | 0.181 |
 Other Other | 2 (3.4%) | 8 (12.3%) | 0.069 |
| Charlson Comorbidity Index, median, IQRs | 4 (3–5) | 2 (1–5) | 0.002 |
| SOFA score, median, IQRs | 10 (9–11) | 6 (2.25–9) | <0.001 |
| APACHE II score, median, IQRs | 21 (16–25) | 12 (7–20) | <0.001 |
| Invasive mechanical ventilation | 41 (71.9%) | 29 (44.6%) | 0.002 |
| Intravascular device | 59 (100%) | 65 (100%) | |
| Septic shock | 50 (84.7%) | 25 (38.5%) | <0.001 |
| AKI at time of sepsis | 24 (40.7%) | 7 (10.8%) | <0.001 |
| Parenteral nutrition | 19 (32.2%) | 20 (30.8%) | 0.864 |
| ECMO at time of sepsis | 5(8.5%) | 4(6.2%) | 0.619 |
| CVVH at time of sepsis | 9 (15.5%) | 5 (7.7%) | 0.173 |
| Source control | 20 (33.9%) | 29 (44.6%) | 0.223 |
| Polymicrobial infections | 16 (27.1%) | 14 (21.5%) | 0.469 |
| Duration of targeted antibiotic therapy | 8 (5–12) | 8 (12–14) | 0.03 |
| Cefiderocol-based regimen | 16 (27.1%) | 31 (47.7%) | 0.018 |
| Cefiderocol monotherapy | 1 (1.7%) | 14 (21.5%) | 0.001 |
| Microbiological failurec | 0/59 | 13/61 (21.3%) | <0.001 |
TABLE 5
Cox regression multivariable analysis of factors independently associated with 30-day mortality
| Analysis and factor | aHRa (95% CI) | p b |
|---|---|---|
| Cox regression multivariable analysis | ||
 Septic shock Septic shock | 2.56 (1.11–5.94) | 0.028 |
 SOFA score SOFA score | 1.15 (1.05–1.27) | 0.003 |
 Age Age | 1.05 (1.02–1.07) | 0.001 |
 Cefiderocol-containing regimens (colistin-containing regimens as reference variable) Cefiderocol-containing regimens (colistin-containing regimens as reference variable) | 0.32 (0.18–0.57) | <0.001 |
| Propensity score analysis | ||
 Cefiderocol-containing regimens (IPTW-adjusted) Cefiderocol-containing regimens (IPTW-adjusted) | 0.44 (0.22–0.66) | <0.001 |
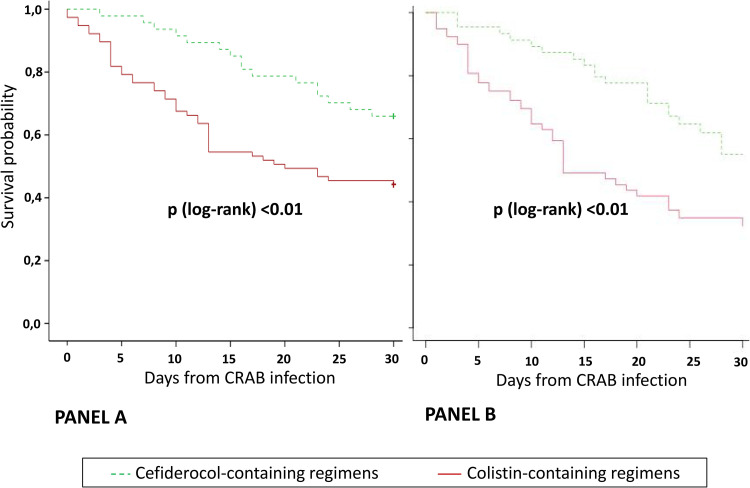
Balance tables before and after the PS weighting were reported Tables S3 and and44 in the supplemental material, respectively. Inverse probability of treatment weighting (IPTW) using PS yielded 2 well-balanced groups with no differences between the groups. On IPTW-adjusted multivariable analysis (Table 5), cefiderocol-containing regimens was associated with lower 30-day mortality (HR 0.44, 95% CI 0.22–0.66, P <
< 0.001). Fig. 3 shows unadjusted and IPTW-adjusted Kaplan Meier curves (P
0.001). Fig. 3 shows unadjusted and IPTW-adjusted Kaplan Meier curves (P <
< 0.01, log-rank).
0.01, log-rank).
Secondary outcomes.
Microbiological failure rates were calculated on 120/124 (96.8%) patients with available follow-up cultures. Microbiological failure occurred more frequently in patients receiving cefiderocol although the difference was not statistically significant (8/46, 17.4% versus 5/74, 6.8%, P =
= 0.079). The 8 patients of the cefiderocol group with microbiological failure included seven burn patients and one COVID-19 patient. All had a BSI, and six received cefiderocol monotherapy. The rate of microbiological failure was significantly higher in patients who received monotherapy compared to those who received combination therapy (6/14, 42.9% versus 2/32, 6.3%, P
0.079). The 8 patients of the cefiderocol group with microbiological failure included seven burn patients and one COVID-19 patient. All had a BSI, and six received cefiderocol monotherapy. The rate of microbiological failure was significantly higher in patients who received monotherapy compared to those who received combination therapy (6/14, 42.9% versus 2/32, 6.3%, P =
= 0.006). All relapsing A. baumannii strains isolated from patients receiving cefiderocol were re-tested, and 4 strains (50%) resulted resistant (MIC values range from 4 to ≥32
0.006). All relapsing A. baumannii strains isolated from patients receiving cefiderocol were re-tested, and 4 strains (50%) resulted resistant (MIC values range from 4 to ≥32 mg/L); resistance emerged during treatment in 2 cases while in the remaining 2 cases after the discontinuation of cefiderocol therapy.
mg/L); resistance emerged during treatment in 2 cases while in the remaining 2 cases after the discontinuation of cefiderocol therapy.
Adverse events were more commonly detected in patients who received colistin-containing regimens compared to those who received cefiderocol (16/76, 21.1% versus 1/47, 2.1%, P =
= 0.003). All the adverse events recorded in the colistin group were AKI during colistin treatment. One patient in the cefiderocol group had a skin rash.
0.003). All the adverse events recorded in the colistin group were AKI during colistin treatment. One patient in the cefiderocol group had a skin rash.
DISCUSSION
This is the first study evaluating the clinical outcome of patients with nosocomial CRAB infections treated with cefiderocol or with colistin-based regimens. Our most noteworthy finding is that patients with CRAB infections who received cefiderocol have a lower risk of 30-day mortality compared to controls. This advantage of cefiderocol was evident in patients with BSI but not in those with VAP. Moreover, higher microbiological failure and occurrence of cefiderocol resistance represent important reasons of concern. Our findings represent real-life data about patients with severe nosocomial CRAB infections and advocate some important considerations.
First, we focused on the specific setting of hospitalized patients with CRAB infections, a complex population of patients with reported mortality rates as high as 70% (2, 3, 6,–8, 13). The optimal treatment of patients with CRAB infections is not well established. Colistin-containing regimens (e.g., colistin plus tigecycline or ampicillin/sulbactam) are usually administered in these patients, despite high nephrotoxicity and clinical failure rates (8). Although the RCT CREDIBLE-CR showed promising results of cefiderocol in the treatment of patients with CR-GNB infections, more deaths occurred in the subgroup of patients with CRAB infections (10). Patients with CRAB infections had a higher frequency of septic shock and were more frequently hospitalized in ICU, factors suggesting a higher baseline risk of mortality of these patients (10). It is noteworthy that our observational study is the first comparative study evaluating the role of cefiderocol in a cohort of patients with CRAB infections. We detected a 30-day mortality rate of 34% in patients treated with cefiderocol with a reduction of the risk of dying by 60% compared to colistin-based regimens. Despite the limitation of the study design, these data are promising and suggest the need of RCTs evaluating this new drug.
Second, we highlighted a significant benefit of cefiderocol in patients with BSI but not in those with VAP due to CRAB. This may be due to several reasons: i) the management of VAP remains a critical unmet need and mortality of these patients is affected by several factors including the severity of clinical conditions; ii) most of patients with VAP in our study had COVID-19; thus, the role of antibiotic therapy in influencing the risk of mortality in these patients is difficult to assess; and iii) it may be possible that at current dosages penetration of cefiderocol in the epithelial lining fluid (ELF) is sub-optimal. Recent data suggest ELF penetration comparable to that of ceftazidime (14). In a recent study on critically ill patients with pneumonia cefiderocol reached ELF concentration that were sufficient to treat Gram-negative bacteria with an MIC of 4 mg/L (15). However, the ELF penetration of antibiotics is variable after systemic administration and in the clinical practice several factors may influence its pharmacokinetics (altered binding protein, increased volume of distribution, and augmented renal clearence). Pharmacokinetic data are necessary to evaluate the usefulness of therapeutic drug monitoring in this specific category of patients.
mg/L (15). However, the ELF penetration of antibiotics is variable after systemic administration and in the clinical practice several factors may influence its pharmacokinetics (altered binding protein, increased volume of distribution, and augmented renal clearence). Pharmacokinetic data are necessary to evaluate the usefulness of therapeutic drug monitoring in this specific category of patients.
Third, we observed an increased rates of microbiological failure in patients who received cefiderocol compared to those who received colistin. Microbiological failure occurred more frequently in patients who received cefiderocol monotherapy compared to combination therapy. As general rule, a combination therapy reduces the risk of in vivo selection of antimicrobial resistance (16). It has been reported an association between overall mortality among patients treated with cefiderocol and presence of heteroresistance, suggesting that heteroresistance might have contributed to cefiderocol treatment failure in the CREDIBLE-CR study (17). Antibiotic combinations targeting multiple heteroresistance determinants may be more effective compared to monotherapies in reducing the risk of failure of antibiotic therapy (16). However, our study was not designed to compare efficacy of cefiderocol monotherapy versus combination therapy and it is difficult to ascertain the true influence of cefiderocol versus the other antibiotics used in combination regimens. Thus, our findings should be interpreted with caution and the role of cefiderocol in monotherapy or combined to other antibiotics should be better studied in larger cohort of patients.
We also detected the development of resistance to cefiderocol in the 8.5% of patients treated with this drug. Although the siderophore drugs represent a new and innovative therapeutic strategy against multidrug resistant Gram-negative bacilli (18), some of these compounds may also encounter bacterial resistance mechanisms (19). For example, the siderophore-conjugated monocarbam SMC-3176 was not tested in the clinical setting because it demonstrated attenuated efficacy due to adaptive resistance in Pseudomonas aeruginosa strains (19). Recently, PER-like β-lactamases and, to a lesser extent, NDM-like β-lactamases were found to be associated with reduced susceptibility to cefiderocol in CRAB (20). Cefiderocol resistance has been also related to reduced expression of the siderophore receptor gene pirA and to mutations involving penicillin-binding proteins (21). These findings suggest the need of a careful monitoring of the susceptibility profiles of CRAB strains exposed to cefiderocol. However, it should be acknowledged that patients of the cefiderocol group had higher survival rates and, consequently, a longer length of hospital stay after the CRAB infection compared to those in the colistin group. Thus, patients receiving cefiderocol had more possibilities to have a recurrent infection compared to controls. Future comparative studies should assess risk factors for microbiological failure and in vivo development of resistance in patients receiving cefiderocol.
Finally, the safety profile of cefiderocol was better than that observed in the group of patients treated with colistin-containing regimens. As expected, a considerable proportion of patients treated with colistin developed nephrotoxicity, while only a skin rash was detected in the cefiderocol group.
Our study has several limitations: i) the sample size is relatively low, but sufficient to highlight differences in the primary outcome in our cohort of patients; ii) the retrospective and non-randomized nature of the study are major limitations, especially because findings are in contrast with available RCT (10); iii) since the decision to start cefiderocol or colistin-containing regimens was based on clinical judgment, it is possible that prescribing physicians were biased in the selection of therapy for patients, generating the so-called “channeling bias” (that occurs when drug therapies with similar indications are preferentially prescribed to groups of patients with different baseline prognoses). To overcome this bias, we performed a PS analysis also including the variable “ID consultation” (22); iv) findings about the role of cefiderocol monotherapy or in combination with other drugs should be read with caution and cannot be used to prefer combination regimens instead of monotherapy; v) this is a single-center study and results may be not generalizable to all settings; vi) the dose of ampicillin-sulbactam 3 g (2 g ampicillin/1 g sulbactam) every 6 h is a low dose for CRAB infections, but this is the maximum dosage authorized by regulatory agencies; vii) the majority of patients in the colistin group received a combination therapy including tigecycline, that is known to be associated with increased risk of inadequate serum levels in patients with BSI and increased mortality in those with VAP (23, 24). However, debate continues to exists since contrasting results are published (25) and in a field of limited treatment options, tigecycline represents a commonly used partner of colistin in the treatment of CRAB infections (8); viii) in line with a recent meta-analysis (26), some patients included in our study had a polymicrobial infection. Since the pathogenetic role of A. baumannii in polymicrobial infections is debated (26), this may have influenced the study outcome. To overcome this potential bias we excluded patients with polymicrobial infections caused by Gram positive and fungi and also performed a sensitivity analysis excluding patients with polymicrobial infections; ix) the study was conducted during the COVID-19 pandemic, fact that may have influenced both the diagnosis of VAP and the outcome of patients with VAP. Although we used the CDC/NHSH criteria for HAP/VAP diagnosis, the diagnosis of VAP in COVID-19 patients may be challenging due to shared clinical and radiological findings between severe COVID-19 without superinfection and VAP in COVID-19 (27). Moreover, the mortality may be a challenging outcome measure in patients with COVID-19 since several factors and therapies may influence the outcome of these patients. Thus, further studies evaluating the role of cefiderocol in a more homogeneous group of patients with VAP and without COVID-19 are warranted.
In conclusions, in patients with CRAB infections cefiderocol-containing regimens were associated with lower 30-day mortality and risk of toxicity compared to colistin-containing regimens, but detection of resistance to cefiderocol in some cases is concerning. Moreover, the benefit of cefiderocolwas highlighted in patients with BSI but not confirmed in those with VAP. Further trials are warranted to confirm our findings and to clarify the role of cefiderocol in patients with VAP and its use in combination with partner antibiotics.
MATERIALS AND METHODS
Study design and definitions.
This observational retrospective cohort study was performed in a tertiary care hospital in Pisa, Italy, between June 2019 and August 2021. All consecutive hospitalized patients with infections due to CRAB during the study period were eligible for the study. Inclusion criteria were i) patients ≥
≥ 18
18 years old; ii) documented infections caused by CRAB; and iii) receipt of therapy with colistin-containing or cefiderocol-containing regimens (demonstrating in vitro activity against the CRAB strain) for at least 48
years old; ii) documented infections caused by CRAB; and iii) receipt of therapy with colistin-containing or cefiderocol-containing regimens (demonstrating in vitro activity against the CRAB strain) for at least 48 h.
h.
An infection was defined as polymicrobial if another organism was detected by the same site of CRAB infection within 2 days from the CRAB culture (28). Patients with polymicrobial infections caused by CRAB plus Gram-positive bacteria or fungi were excluded. Patients with polymicrobial infections caused by CRAB plus Gram negative bacilli susceptible to cefiderocol or colistin were included.
days from the CRAB culture (28). Patients with polymicrobial infections caused by CRAB plus Gram-positive bacteria or fungi were excluded. Patients with polymicrobial infections caused by CRAB plus Gram negative bacilli susceptible to cefiderocol or colistin were included.
According to CDC/NHSN criteria infections were classified in the following categories: ventilator-associated pneumonia (VAP), bloodstream infections (BSI) and others (urinary tract infections [UTI], skin and soft tissue infections, intra-abdominal infections [IAI]) (29). HAP/VAP was defined as pneumonia fulfilling CDC/NHSN Surveillance definition of health care-associated infections for pneumonia with specific laboratory findings as reported in Table S1 in the supplemental material (29).
Each individual contributed to one type of infection. In case of BSI, source of infection was defined using the CDC criteria as previously described (30). Day 1 was the first calendar day CRAB was isolated from blood, or respiratory specimens, or urine, or other fluids or specimens and associated with clinical infections according to above-mentioned CDC/NHSN criteria (29). Clinical data, including ward of hospitalization, SOFA score, APACHE score, septic shock, on Day 1 were collected by trained sub-investigators. Each patient was followed-up until 30 day from Day 1. For hospitalized patients, follow-up was performed daily through patients’ records. In the rare instancesin which patients were discharged before day 30, follow-up was completed via phone visits. All patients had complete 30-day follow-up.
day from Day 1. For hospitalized patients, follow-up was performed daily through patients’ records. In the rare instancesin which patients were discharged before day 30, follow-up was completed via phone visits. All patients had complete 30-day follow-up.
The study was conducted according to the principles stated in the Declaration of Helsinki. The local ethics committee approved the study protocol (Number 19462).
Study outcome.
The aim of the study was to compare outcomes of patients with CRAB infections treated with cefiderocol compared to colistin-containing regimens. The main outcome variable was the 30-day all-cause mortality which was defined as the occurrence of death within 30 days from the initial CRAB infection.
days from the initial CRAB infection.
Secondary outcomes were:
1. microbiological failure, defined as the isolation of the CRAB from the same site of infection following ≥7 days of end of antibiotic treatment (11). Microbiological failure was evaluated for patients who had available follow-up cultures. According to clinical practice in our hospital, all patients hospitalized in ICU underwent periodic microbiological surveillance through the collection of weekly blood cultures and respiratory cultures;
days of end of antibiotic treatment (11). Microbiological failure was evaluated for patients who had available follow-up cultures. According to clinical practice in our hospital, all patients hospitalized in ICU underwent periodic microbiological surveillance through the collection of weekly blood cultures and respiratory cultures;
2. occurrence of adverse events during the antibiotic treatment. Nephrotoxicity was considered as the occurrence of acute kidney injury (AKI), defined according to the KDOQI guidelines (31).
Bacterial isolates identification and susceptibility testing.
Isolates identification was performed by Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry MALDI-ToF MS (MALDI Biotyper, Bruker Daltonics). Antimicrobial susceptibility tests were performed with the BD PhoenixTM (Becton, Dickinson) and/or Micronaut AST systems (Merlin Diagnostika GmbH), according to the manufacturer’s instructions (32). MICs were classified according to breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (33). Cefiderocol MIC values were determined by a broth microdilution method using iron-depleted and cation-adjusted Mueller–Hinton broth (SensiTitre Cefiderocol MIC panel EUMDROXF, Thermo Fisher) according to the manufacturer’s instructions. Growth inhibition zone was tested by disk diffusion method (30 μg cefiderocol disks, Liofilchem). EUCAST pharmacokinetic/pharmacodynamic (PK/PD) breakpoint MIC values of ≤2
μg cefiderocol disks, Liofilchem). EUCAST pharmacokinetic/pharmacodynamic (PK/PD) breakpoint MIC values of ≤2 mg/L was used for A. baumannii if inhibition zone diameter for cefiderocol disk was ≥17
mg/L was used for A. baumannii if inhibition zone diameter for cefiderocol disk was ≥17 mm (32). Cefiderocol CLSI (34) breakpoints (≤4/8/≥16
mm (32). Cefiderocol CLSI (34) breakpoints (≤4/8/≥16 mg/L as susceptible/intermediate/resistant) and FDA (35) breakpoints (≤1/2/≥4
mg/L as susceptible/intermediate/resistant) and FDA (35) breakpoints (≤1/2/≥4 mg/L as susceptible/intermediate/resistant) were also used to evaluate cefiderocol susceptibility.
mg/L as susceptible/intermediate/resistant) were also used to evaluate cefiderocol susceptibility.
Antibiotic therapy.
Patients were treated with targeted antibiotic regimens chosen by an infectious disease (ID) consultant or by an intensivist on the basis of the phenotypic profile of isolate.
The patients were divided according to the received definitive antibiotic treatment: 1) cefiderocol-containing regimens; 2) colistin-containing regimens (controls). Cefiderocol was administered as monotherapy or combined to other antibiotics (tigecycline or fosfomycin) according to clinical judgment. The drug was administered as a 3 h IV infusion of a standard dose of 2 g, diluted in 100
h IV infusion of a standard dose of 2 g, diluted in 100 mL of saline solution, intravenously every 8
mL of saline solution, intravenously every 8 h, with adjustments for renal impairment made according to manufacturer recommendations. A dose of 2 g every 6
h, with adjustments for renal impairment made according to manufacturer recommendations. A dose of 2 g every 6 h was used for patients with glomerular filtration rate ≥120 mL/min.
h was used for patients with glomerular filtration rate ≥120 mL/min.
Control group included patients who received colistin differently combined with other antibiotics according to clinical judgment. The usual antimicrobial dosages, adopted for the most used antibiotics were the following: colistin, loading dose of 9 million IU followed by 4.5 million IU every 12 h; tigecycline, loading dose of 200 mg followed by 100
mg followed by 100 mg every 12 h; gentamicin, dosage of 5
mg every 12 h; gentamicin, dosage of 5 mg/kg every 24 h; intravenous fosfomycin 12–24 g/day divided every 6–8 h; ampicillin/sulbactam 3 g every 6 h; meropenem 1–2 g i.v every 8
mg/kg every 24 h; intravenous fosfomycin 12–24 g/day divided every 6–8 h; ampicillin/sulbactam 3 g every 6 h; meropenem 1–2 g i.v every 8 h in extended infusion (36) .
h in extended infusion (36) .
Statistical analysis.
A comparison between patients who received cefiderocol and those who received colistin was performed. Continuous variables were reported as mean ± standard deviation or median and interquartile range according to their distribution. The normality of distributions was assessed by the Kolmogorov-Smirnov test. Continuous variables were compared by the Student's t test or the Mann-Whitney U test, as appropriate. Categorical data were expressed as frequency distributions, and the χ 2 test or Fisher exact test was used to determine if differences existed between groups.
A multivariable analysis was performed using Cox proportional hazards regression to identify factors independently associated with the 30-day mortality. All patients had a complete 30-day follow-up. Only patients who died were censored. Variables that resulted statistically significant at univariate analysis were considered for the multivariable model. The final multivariable model was chosen according to the Akaike information criterion. Adjusted HR and 95% CIs were reported. As sensitivity analysis, the primary outcome was also evaluated in a Cox regression model excluding patients with polymicrobial infections.
To address the non-randomized treatment assignment of antibiotics, we developed a multivariable gradient boosted logistic regression model to estimate, for each patient, the propensity score (PS) of receiving cefiderocol. Covariates included to generate the PS were determined referring to all potential risk factors for death reported in previous studies and included: age, sex, COVID-19, ICU stay, Charlson comorbidity index, type of infection (BSI, VAP, or others), SOFA score, invasive mechanical ventilation, septic shock, extracorporeal membrane oxygenation and source control. ID consultation and presence of polymicrobial infection were also included due to their clinical relevance (Table S2 in the supplemental material). A PS weighting using IPTW was then performed to assess the causal effect (precisely, the average treatment effect) of cefiderocol compared to colistin, under an assumption of selection on observables. A patient who received cefiderocol-containing regimens was weighted by the inverse probability of treatment that he or she would be treated with cefiderocol, and a patient who received a colistin-containing regimens was weighted by the inverse probability that he or she would be treated with colistin, equivalent to 1 minus his or her PS. Weighting created a pseudo-population, which increased the influence of patients receiving a treatment they would not be expected to receive (37). More specifically, weighting mathematically increases the representation of “rare” patients in each exposure group. Standardized differences were used to compare balance in baseline covariates between treated and control subjects before and after weighing by the inverse probability of treatment, as appropriate (38). Cox regression analysis was performed on the weighted sample to compare the outcome between the 2 treatment groups, and HR (95% CI) were calculated. Finally, both unadjusted and IPTW-weighted Kaplan-Meier curves of 30-day survival according to treatment assignment were estimated. Nonparametric (log-rank) tests were used to compare survival functions in the two groups.
Statistical significance was established at P ≤
≤ .05. All reported P values are 2-tailed. The results obtained were analyzed using commercially available statistical software packages (IBM SPSS version 22.0, Armonk, New York; and R version 4.1.1, Vienna, Austria).
.05. All reported P values are 2-tailed. The results obtained were analyzed using commercially available statistical software packages (IBM SPSS version 22.0, Armonk, New York; and R version 4.1.1, Vienna, Austria).
ACKNOWLEDGMENTS
M.F. received grants and/or speaker honoraria from Merck Sharp & Dohme (MSD), Angelini, Shionogi, Pfizer, Gilead, Menarini, and Nordic Pharma. F.M. has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, MSD, Nordic Pharma, Pfizer, Astellas, Gilead, Bristol-Myers Squibb, Janssen, ViiV, bioMérieux, Biotest, Becton, Dickinson, Pfizer, and Shionogi. Declared conflicts of interest are outside the submitted work and did not affect the scientific objectivity of this study. The other authors have none to declare.
Footnotes
[This article was published on 21 March 2022 with three incorrect values in Table 2. These values were corrected in the current version, posted on 28 March 2022.]
For a commentary on this article, see https://doi.org/10.1128/AAC.00065-22.
Supplemental material is available online only.
Supplemental file 1
Tables S1 to S4 and Fig. S1. Download aac.02142-21-s0001.pdf, PDF file, 0.9 MB
REFERENCES
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.02142-21
Read article for free, from open access legal sources, via Unpaywall:
https://art.torvergata.it/bitstream/2108/304237/1/cefiderocol.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/125086271
Article citations
Effectiveness and Safety of Cefiderocol in Clinical Practice for Treatment of Patients with Gram-Negative Bacterial Infections: US Interim Results of the PROVE Study.
Infect Drug Resist, 17:4427-4443, 15 Oct 2024
Cited by: 0 articles | PMID: 39431212 | PMCID: PMC11490232
Analysis of antimicrobial resistance and genetic diversity of Acinetobacter baumannii in a tertiary care hospital in Haikou City.
Sci Rep, 14(1):22068, 27 Sep 2024
Cited by: 0 articles | PMID: 39333332 | PMCID: PMC11437051
Challenges Facing Two Outbreaks of Carbapenem-Resistant Acinetobacter baumannii: From Cefiderocol Susceptibility Testing to the Emergence of Cefiderocol-Resistant Mutants.
Antibiotics (Basel), 13(8):784, 21 Aug 2024
Cited by: 0 articles | PMID: 39200084 | PMCID: PMC11350900
Prognostic Factors That Affect Mortality Patients with Acinetobacter baumannii Bloodstream Infection.
Infect Drug Resist, 17:3825-3837, 03 Sep 2024
Cited by: 0 articles | PMID: 39247754 | PMCID: PMC11380481
Comparison of cefiderocol and colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii: a systematic review with meta-analysis and trial sequential analysis.
BMC Infect Dis, 24(1):967, 13 Sep 2024
Cited by: 1 article | PMID: 39271977 | PMCID: PMC11395218
Review Free full text in Europe PMC
Go to all (72) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparison of cefiderocol and colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii: a systematic review with meta-analysis and trial sequential analysis.
BMC Infect Dis, 24(1):967, 13 Sep 2024
Cited by: 1 article | PMID: 39271977 | PMCID: PMC11395218
Review Free full text in Europe PMC
Clinical effectiveness of cefiderocol for the treatment of bloodstream infections due to carbapenem-resistant Acinetobacter baumannii during the COVID-19 era: a single center, observational study.
Eur J Clin Microbiol Infect Dis, 43(6):1149-1160, 18 Apr 2024
Cited by: 2 articles | PMID: 38634975 | PMCID: PMC11178648
Efficacy of cefiderocol- vs colistin-containing regimen for treatment of bacteraemic ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19.
Int J Antimicrob Agents, 62(1):106825, 21 Apr 2023
Cited by: 19 articles | PMID: 37088438 | PMCID: PMC10121149
Should we, and how to, optimize cefiderocol administration during severe nosocomial pneumonia due to carbapenem-resistant Acinetobacter baumanii? A viewpoint.
J Glob Antimicrob Resist, 38:140-145, 05 Jun 2024
Cited by: 0 articles | PMID: 38844258
Review
 a
a