Abstract
Background
Evidence regarding optimal treatment duration in dogs with aspiration pneumonia (AP) and the role of thoracic radiographs (TXR) and lung ultrasonography (LUS) in the long-term follow-up of affected dogs is lacking. C-reactive protein (CRP) is a reliable acute phase protein to monitor bacterial pneumonia in dogs.Hypothesis
Investigate the safety of antimicrobial discontinuation based on clinical improvement and serum CRP normalization, as well as the usefulness of TXR and LUS for follow-up.Animals
Dogs diagnosed with AP and treated with antimicrobials.Methods
Prospective observational study. Antimicrobials were discontinued based on clinical improvement and serum CRP normalization after 1, 3, or 5 weeks. At each consultation, a quality-of-life questionnaire, physical examination, serum CRP, TXR, and LUS were assessed. Short- (2 weeks) and long-term (>1 month) follow-ups after treatment discontinuation were performed to monitor for possible relapses.Results
Seventeen dogs were included. Antimicrobials were discontinued after 1 week in 12 dogs (70.6%) and 3 weeks in the remaining 5 dogs (29.4%). Short-term relapse was not observed in any dog and long-term relapse was diagnosed in 3 dogs. Thoracic radiographs and LUS were useful for diagnosis, but did not add additional information during follow-up, because image normalization lagged behind clinical improvement and serum CRP normalization.Conclusion and clinical importance
Dogs with AP can be safely and effectively treated using a short-term antimicrobial regimen discontinued after clinical improvement and serum CRP normalization. Imaging might still be useful for complicated cases with a less favorable response to treatment.Free full text

Antimicrobial discontinuation in dogs with acute aspiration pneumonia based on clinical improvement and normalization of C‐reactive protein concentration
Abstract
Background
Evidence regarding optimal treatment duration in dogs with aspiration pneumonia (AP) and the role of thoracic radiographs (TXR) and lung ultrasonography (LUS) in the long‐term follow‐up of affected dogs is lacking. C‐reactive protein (CRP) is a reliable acute phase protein to monitor bacterial pneumonia in dogs.
Hypothesis
Investigate the safety of antimicrobial discontinuation based on clinical improvement and serum CRP normalization, as well as the usefulness of TXR and LUS for follow‐up.
Animals
Dogs diagnosed with AP and treated with antimicrobials.
Methods
Prospective observational study. Antimicrobials were discontinued based on clinical improvement and serum CRP normalization after 1, 3, or 5 weeks. At each consultation, a quality‐of‐life questionnaire, physical examination, serum CRP, TXR, and LUS were assessed. Short‐ (2
weeks. At each consultation, a quality‐of‐life questionnaire, physical examination, serum CRP, TXR, and LUS were assessed. Short‐ (2 weeks) and long‐term (>1 month) follow‐ups after treatment discontinuation were performed to monitor for possible relapses.
weeks) and long‐term (>1 month) follow‐ups after treatment discontinuation were performed to monitor for possible relapses.
Results
Seventeen dogs were included. Antimicrobials were discontinued after 1 week in 12 dogs (70.6%) and 3 weeks in the remaining 5 dogs (29.4%). Short‐term relapse was not observed in any dog and long‐term relapse was diagnosed in 3 dogs. Thoracic radiographs and LUS were useful for diagnosis, but did not add additional information during follow‐up, because image normalization lagged behind clinical improvement and serum CRP normalization.
weeks in the remaining 5 dogs (29.4%). Short‐term relapse was not observed in any dog and long‐term relapse was diagnosed in 3 dogs. Thoracic radiographs and LUS were useful for diagnosis, but did not add additional information during follow‐up, because image normalization lagged behind clinical improvement and serum CRP normalization.
Conclusion and Clinical Importance
Dogs with AP can be safely and effectively treated using a short‐term antimicrobial regimen discontinued after clinical improvement and serum CRP normalization. Imaging might still be useful for complicated cases with a less favorable response to treatment.
Abbreviations
- AP
- aspiration pneumonia
- APP
- acute phase protein
- CRP
- C‐reactive protein
- LUS
- lung ultrasound
- TXR
- thoracic radiographs
1. INTRODUCTION
Aspiration pneumonia (AP) is a common cause of bacterial pneumonia in veterinary medicine and is diagnosed currently based on compatible history, clinical signs, and thoracic radiographic (TXR) lesions. Further differentiation between bacterial pneumonia and pneumonitis with bronchoalveolar lavage fluid analysis rarely is performed in clinical practice and, therefore, AP is primarily treated as a bacterial pneumonia using antimicrobials given the risk for dogs if an infection is left untreated. 1 , 2 , 3 , 4 , 5
C‐reactive protein (CRP) seems to be a reliable acute phase protein (APP) to monitor treatment response in dogs with bacterial pneumonia. 6 , 7 Monitoring serum CRP even allowed a reduction in treatment duration without increasing the number of relapses in a small cohort of dogs with bacterial pneumonia. 6 We sought to evaluate the usefulness of serum CRP monitoring specifically for dogs with suspected AP prospectively, while assessing the use of imaging modalities simultaneously.
In human medicine, lung ultrasound (LUS) has been increasingly used as a bedside, easy, and rapid tool to diagnose and monitor pneumonia. 8 , 9 , 10 , 11 In veterinary medicine, 1 study described LUS lesions in dogs with various causes of cough and a shred sign was more frequently observed in dogs with AP. 12 In addition, LUS has been described as a sensitive tool for the diagnosis and short‐term follow‐up of AP in dogs. 13
Because clinical improvement precedes TXR resolution of lesions, TXR is no longer recommended in people with pneumonia, 14 , 15 whereas in veterinary medicine, studies about the value of TXR to guide treatment in dogs with AP are lacking.
Current veterinary recommendations advise that bacterial pneumonia should be treated for 3 to 6 weeks with antimicrobials or 1 to 2
weeks with antimicrobials or 1 to 2 weeks beyond the resolution of clinical signs, radiographic lesions or both.
1
These recommendations are largely opinion‐based given the absence of data about the appropriate duration of treatment in affected dogs. In addition, the optimal duration of antimicrobial treatment in dogs with AP, which is mostly treated as bacterial pneumonia in clinical practice, has not been studied yet. The lack of evidence‐based medicine behind these recommendations is problematic given increasing concern over antimicrobial resistance. Studies investigating CRX and LUS findings during the recovery process and their impact on treatment duration also are lacking.
weeks beyond the resolution of clinical signs, radiographic lesions or both.
1
These recommendations are largely opinion‐based given the absence of data about the appropriate duration of treatment in affected dogs. In addition, the optimal duration of antimicrobial treatment in dogs with AP, which is mostly treated as bacterial pneumonia in clinical practice, has not been studied yet. The lack of evidence‐based medicine behind these recommendations is problematic given increasing concern over antimicrobial resistance. Studies investigating CRX and LUS findings during the recovery process and their impact on treatment duration also are lacking.
Our aim was to investigate the safety of antimicrobial discontinuation based on clinical improvement and serum CRP normalization in dogs with a presumptive diagnosis of AP. The second objective was to evaluate the usefulness of TXR and LUS in the follow‐up of these dogs.
2. MATERIAL AND METHODS
We conducted a prospective observational study between January 2020 and June 2021. Owner consent and ethics approval from the University of Liège was obtained before inclusion in the study. Dogs were included if a diagnosis of AP was made based on compatible acute history (eg, vomiting, regurgitation, laryngeal dysfunction), clinical signs (eg, cough, fever, increased respiratory rate), TXR findings (interstitial or alveolar lung lesions), and increased serum CRP concentration. Dogs diagnosed with AP within the previous 30 days, and having received antimicrobials before presentation or those having suspected concomitant diseases were excluded.
days, and having received antimicrobials before presentation or those having suspected concomitant diseases were excluded.
At diagnosis and during each follow‐up visit, a physical examination, 3‐view TXR, LUS, and serum CRP measurements were performed within 60 minutes of each other. Owners also were asked to fill out a quality‐of‐life questionnaire (QoL) at each time point that included 9 questions (scored from 0 to 4, total score of 36) concerning the impact of AP on the daily life of the dog, taking into account the 2
minutes of each other. Owners also were asked to fill out a quality‐of‐life questionnaire (QoL) at each time point that included 9 questions (scored from 0 to 4, total score of 36) concerning the impact of AP on the daily life of the dog, taking into account the 2 days before the consultation. The QoL used was derived from a validated QoL used in dogs with cardiac problems
16
(Appendix S1).
days before the consultation. The QoL used was derived from a validated QoL used in dogs with cardiac problems
16
(Appendix S1).
The TXR and LUS results were reviewed by a board‐certified radiologist and a blinded trained internal medicine resident, respectively. During follow‐up, AP lesions on TXRs were classified as being either resolved, improving, stable, or worsening according to the interpretation of the radiologist. Lung ultrasonography was performed using a portable echography machine and a modification of a protocol described previously
17
in which 3‐second video cineloops were recorded at 9 windows per hemithorax, at the dorsal, mid‐thorax, and ventral levels, and at the 4th, 6th, and 8th intercostal spaces. At the ventral level, a modification of the previously described technique
18
was used, where the probe is turned parallel to the rib within each intercostal space. Abnormalities that could be observed in each of the 9 windows of both hemithoraces were: > 3 B‐lines, coalescent B‐lines, shred sign, tissue‐like sign, air or fluid bronchograms, and pleural effusion.
3 B‐lines, coalescent B‐lines, shred sign, tissue‐like sign, air or fluid bronchograms, and pleural effusion.
Serum CRP concentrations were measured using a particle‐enhanced turbidimetric immunoassay, previously validated in dogs.
19
The lower detection limit for CRP concentration was 0 mg/L and there was no upper detection limit. The normal reference range for CRP was between 0 and ≤9 mg/L. Samples were either analyzed within 24 hours or stored at −80°C until analysis. The QoL scores and serum CRP concentrations in all dogs included at each time point were reported as median and range.
hours or stored at −80°C until analysis. The QoL scores and serum CRP concentrations in all dogs included at each time point were reported as median and range.
Follow‐up consultations were scheduled 1 week after diagnosis and every 2 weeks thereafter. Treatment was discontinued at 1 of these time points based on a normal physical examination, a decrease of at least 25% in the QoL score compared to the time of diagnosis and normal serum CRP concentration. The cut‐off of 25% in the QoL score was arbitrarily decided by the investigators. If after 5
weeks thereafter. Treatment was discontinued at 1 of these time points based on a normal physical examination, a decrease of at least 25% in the QoL score compared to the time of diagnosis and normal serum CRP concentration. The cut‐off of 25% in the QoL score was arbitrarily decided by the investigators. If after 5 weeks the dog had not reached the endpoints, further information about outcome was not included in the study.
weeks the dog had not reached the endpoints, further information about outcome was not included in the study.
Relapse was defined as the reoccurrence of clinical signs observed by the owner before the scheduled consultation (eg, cough, increased respiratory rate or respiratory distress, anorexia, lethargy), which was quantified by an increase in QoL score, abnormal physical examination findings (eg, increased temperature, crackles or increased lung sounds at auscultation), increased serum CRP concentration, or some combination of these. Based on this definition, relapse could be a consequence of inappropriate antimicrobial treatment, relapse of AP, a new occurrence of pneumonia or an increase in serum CRP concentration caused by another concomitant disease. Evolution of imaging findings was not taken into account when considering the occurrence of relapse. Clinical improvement was defined as an improvement in clinical signs observed by the owners at home and was quantified by a decrease of at least 25% in the QoL score compared to the time of diagnosis and the absence of abnormalities on physical examination. If relapse was present in the short‐term, antimicrobial treatment was re‐implemented. Long‐term relapse was investigated by follow‐up consultation or phone call. Owners were advised to contact an internal medicine resident before the scheduled consultation if necessary.
Empirical antimicrobial treatment with amoxicillin/clavulanic acid (approximately 22 mg/kg PO q12h) was implemented in all dogs at diagnosis. Dogs were hospitalized for at least 24
mg/kg PO q12h) was implemented in all dogs at diagnosis. Dogs were hospitalized for at least 24 hours after diagnosis. Treatments other than antimicrobials or other diagnostic tests were performed at the discretion of the attending clinician. In severe cases with a clinical diagnosis of systemic inflammatory response syndrome or sepsis as previously defined,
20
or that worsened within the first 48
hours after diagnosis. Treatments other than antimicrobials or other diagnostic tests were performed at the discretion of the attending clinician. In severe cases with a clinical diagnosis of systemic inflammatory response syndrome or sepsis as previously defined,
20
or that worsened within the first 48 hours, another antimicrobial (enrofloxacin, 5 mg/kg PO q24h) was added.
hours, another antimicrobial (enrofloxacin, 5 mg/kg PO q24h) was added.
3. RESULTS
3.1. Study population
Seventeen dogs of various breeds (4 French Bulldogs, 3 Beagles, and 1 Leonberger, Dachshund, Bloodhound, Swiss White Shepherd, Belgian Malinois, Beauceron, Maltese, Cocker Spaniel, West Highland Terrier, and Great Dane) were included. Median weight was 12 kg (range, 4‐60
kg (range, 4‐60 kg). One dog was included twice in the study, because it was presented with another episode of AP 6 months after the first occurrence of AP and had demonstrated complete resolution of clinical signs and normalization of serum CRP concentrations between episodes, according to the owners. Median age at inclusion was 5
kg). One dog was included twice in the study, because it was presented with another episode of AP 6 months after the first occurrence of AP and had demonstrated complete resolution of clinical signs and normalization of serum CRP concentrations between episodes, according to the owners. Median age at inclusion was 5 years (range, 2 months‐14
years (range, 2 months‐14 years). Eight dogs were females (4 neutered) and 9 were males (5 neutered). Fourteen of the 17 dogs with AP had a recent history of vomiting, 2 dogs had reported regurgitation, and 1 suffered from laryngeal paralysis.
years). Eight dogs were females (4 neutered) and 9 were males (5 neutered). Fourteen of the 17 dogs with AP had a recent history of vomiting, 2 dogs had reported regurgitation, and 1 suffered from laryngeal paralysis.
3.2. Clinical findings at admission
Presenting complaints reported by the owner were lethargy in all dogs, and increased respiratory rate (> 40 breaths/min) in 14, anorexia in 10, and coughing in 2 dogs. At admission based on physical examination findings, 11, 4, and 2 dogs had expiratory, mixed, and normal respiratory patterns, respectively. The expiratory pattern was further classified as being restrictive. Mixed inspiratory and expiratory effort was observed in the 4 French Bulldogs included in the study. Bilateral or unilateral increased lung sounds and bilateral crackles were detected on auscultation in 7, 2, and 2 dogs, respectively. Hyperthermia (>39.3°C) was present in 6 dogs with a median of 39.9°C (range, 39.4‐40°C).
40 breaths/min) in 14, anorexia in 10, and coughing in 2 dogs. At admission based on physical examination findings, 11, 4, and 2 dogs had expiratory, mixed, and normal respiratory patterns, respectively. The expiratory pattern was further classified as being restrictive. Mixed inspiratory and expiratory effort was observed in the 4 French Bulldogs included in the study. Bilateral or unilateral increased lung sounds and bilateral crackles were detected on auscultation in 7, 2, and 2 dogs, respectively. Hyperthermia (>39.3°C) was present in 6 dogs with a median of 39.9°C (range, 39.4‐40°C).
Changes in QoL scores at presentation, at 1 and 3 weeks, and short‐ and long‐term follow‐ups are presented in Figure 1. Median QoL score was 15 (range, 8‐30) at admission.
weeks, and short‐ and long‐term follow‐ups are presented in Figure 1. Median QoL score was 15 (range, 8‐30) at admission.
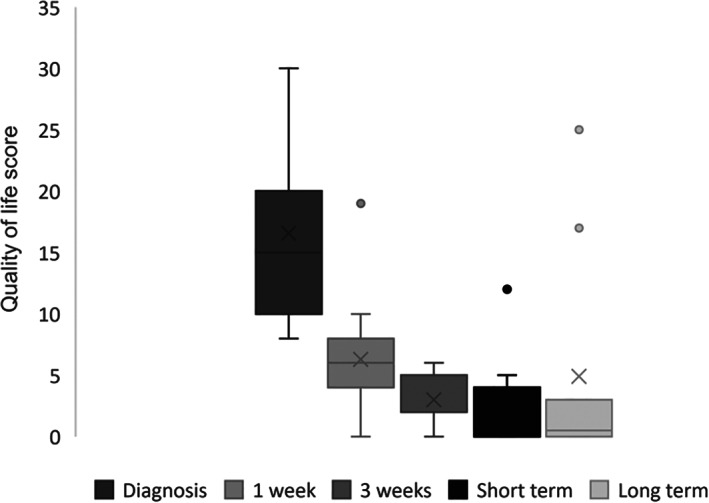
Box plot showing quality‐of‐life scores (QoL) in dogs at diagnosis (n = 17 dogs, mean (X): 16; median (‐): 15; range, 8‐30), 1 week (n = 17 dogs, mean (X): 6; median (‐): 6; range, 0‐19), 3 weeks (n = 5 dogs, mean (X): 3; median (‐): 2; range, 0‐6), short‐term (n = 17 dogs, mean (X): 3; median (‐): 3; range, 0‐12), and long‐term follow‐ups (n = 10 dogs, mean (X): 5; median (‐): 1; range, 0‐25). Outliers at each time point are represented as circles
weeks (n = 5 dogs, mean (X): 3; median (‐): 2; range, 0‐6), short‐term (n = 17 dogs, mean (X): 3; median (‐): 3; range, 0‐12), and long‐term follow‐ups (n = 10 dogs, mean (X): 5; median (‐): 1; range, 0‐25). Outliers at each time point are represented as circles
Information regarding the evolution of serum CRP concentration at admission and during follow‐up is presented in Figure 2. Median serum CRP concentration was 82.8 mg/L (range, 24‐267 mg/L) at admission.
mg/L) at admission.
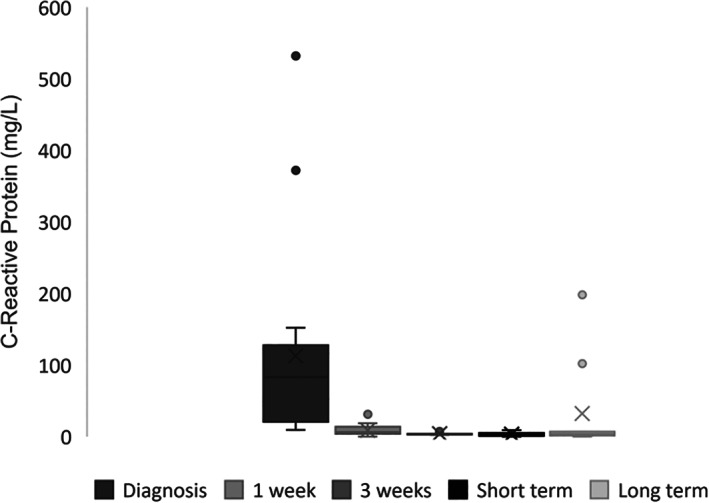
Box plot showing C‐reactive protein (CRP) concentrations in dogs at diagnosis (n = 17 dogs, mean (X): 112.7 mg/L; median (‐): 82.8 mg/L; range, 9.4‐532 mg/L), 1 week (n = 17 dogs, mean (X): 8.2 mg/L; median (‐): 6. mg/dL; range, 0‐31.3 mg/L), 3
mg/L), 1 week (n = 17 dogs, mean (X): 8.2 mg/L; median (‐): 6. mg/dL; range, 0‐31.3 mg/L), 3 weeks (n = 5 dogs, mean (X): 4 mg/L; median (‐): 3.4 mg/L; range, 2.7‐3.4 mg/L), short‐term (n = 17 dogs, mean (X): 3.2 mg/L; median (‐): 2.9 mg/L; range, 0‐9 mg/L), and long‐term follow‐ups (n = 10 dogs, mean (X): 32.4 mg/L; median (‐): 3.5 mg/L; range, 0‐198
weeks (n = 5 dogs, mean (X): 4 mg/L; median (‐): 3.4 mg/L; range, 2.7‐3.4 mg/L), short‐term (n = 17 dogs, mean (X): 3.2 mg/L; median (‐): 2.9 mg/L; range, 0‐9 mg/L), and long‐term follow‐ups (n = 10 dogs, mean (X): 32.4 mg/L; median (‐): 3.5 mg/L; range, 0‐198 mg/L). Outliers at each time point are represented as circles
mg/L). Outliers at each time point are represented as circles
3.2.1. Thoracic radiographic findings
At admission on TXR, all dogs had localized ventral alveolar consolidation that was bilateral in 9 and unilateral in 8 dogs (left or right lung consolidation in 5 and 3 dogs, respectively). The cranial and middle lung lobes were affected in 13 and 8 dogs, respectively, and in 12 dogs an alveolar pattern was observed in multiple lobes. Pleural effusion was observed in 2 dogs. Other findings on TXR included generalized megaesophagus in 1 dog, bronchiectasis of the consolidated lung lobe in 1 dog, and bronchiectasis affecting multiple bronchi in another dog. Information regarding the evolution of TXR lesions during follow‐up is presented in Table 1.
TABLE 1
Classification of TXR lesions during follow‐up
| 1 week (n = 17) | 3 weeks (n = 5) weeks (n = 5) | Short term (n = 17) | Long term (n = 10) | |
|---|---|---|---|---|
| Resolution | 2 | 2 | 14 | 8 |
| Improving | 15 | 2 | 2 | 0 |
| Stable | 0 | 1 | 1 | 0 |
| Relapse | 0 | 0 | 0 | 2 |
3.2.2. Lung ultrasound findings
At admission on LUS, all dogs had lung consolidation characterized by a shred sign. The lung consolidations were observed in the same hemithorax (left, right, or bilaterally) as the radiographic consolidations. Fluid and air bronchograms were observed in at least 1 of the 18 regions evaluated per dog in 1 and 9 dogs, respectively. Coalescent numbers of B‐lines were detected in all dogs and >3 B‐lines in 11 dogs. Pleural effusion was only observed in 1 dog. Table 2 gives the percentage of regions that had LUS lesions at admission and during follow‐up.
TABLE 2
Percentage of regions (total regions = n ×
× 18 regions/dog) showing LUS lesions at diagnosis, 1 week, 3
18 regions/dog) showing LUS lesions at diagnosis, 1 week, 3 weeks, short‐, and long‐term follow‐ups
weeks, short‐, and long‐term follow‐ups
| Diagnosis (n = 17) (%) | 1 week (n = 17) (%) | 3 weeks (n = 5) (%) weeks (n = 5) (%) | Short term (n = 17) (%) | Long term (n = 10) (%) | |
|---|---|---|---|---|---|
| Shred sign | 20.6 | 6.5 | 4 | 0 | 1.6 |
| Fluid bronchogram | 5.9 | 1.6 | 0 | 0 | 0 |
| Air bronchogram | 15.3 | 2.9 | 0 | 0 | 1 |
| >3 B‐lines | 8.8 | 10.1 | 12 | 6.2 | 3.6 |
| Coalescent numbers of B‐lines | 26.1 | 6.8 | 1 | 0 | 1.6 |
| Pleural effusion | 1.3 | 0 | 0 | 0 | 0 |
3.3. Antimicrobial treatment
All dogs were treated with amoxicillin/clavulanic acid. Enrofloxacin was added in only 1 dog. Treatment was discontinued after 1 and 3 weeks in 12 (70.6%) and 5 dogs (29.4%), respectively, based on unremarkable physical examination, a decrease of at least 25% in the QoL score and normal serum CRP concentrations. The 5 dogs that received 3
weeks in 12 (70.6%) and 5 dogs (29.4%), respectively, based on unremarkable physical examination, a decrease of at least 25% in the QoL score and normal serum CRP concentrations. The 5 dogs that received 3 weeks of antimicrobials already demonstrated QoL score improvement >25%, a normal physical examination, and all had improved but still mildly increased serum CRP concentrations (median, 15
weeks of antimicrobials already demonstrated QoL score improvement >25%, a normal physical examination, and all had improved but still mildly increased serum CRP concentrations (median, 15 mg/L; range, 14‐31.3 mg/L). No dogs needed 5
mg/L; range, 14‐31.3 mg/L). No dogs needed 5 weeks of antimicrobial treatment.
weeks of antimicrobial treatment.
3.4. Response to treatment
Physical examination was normal in all dogs at 1 week, 3 weeks and at short‐term follow‐up visits.
weeks and at short‐term follow‐up visits.
At 1 week follow‐up, the number of LUS lesions per region across all dogs decreased (Table 2), although each dog still displayed at least 1 abnormality. The lesions identified were a shred sign in 7 dogs, air bronchograms in 4 dogs, fluid bronchograms in 3 dogs, coalescent numbers of B‐lines in 8 dogs, and >3 B‐lines in 10 dogs.
For the 5 dogs that received 3 weeks of antimicrobials, 1 dog displayed complete resolution of LUS lesions at follow‐up, whereas abnormalities were persistently detected in all of the others (a shred sign in 2 dogs, coalescent numbers of B‐lines in 1 dog, >
weeks of antimicrobials, 1 dog displayed complete resolution of LUS lesions at follow‐up, whereas abnormalities were persistently detected in all of the others (a shred sign in 2 dogs, coalescent numbers of B‐lines in 1 dog, > 3 B‐lines in 2 dogs).
3 B‐lines in 2 dogs).
Short‐term follow‐up was performed 3 (12/17) and 5 (5/17) weeks after diagnosis depending on the time of antimicrobial discontinuation, and failed to identify any relapse. There was no need for consultation outside of the scheduled consultation in any dog. At the short‐term follow‐up visit, the only abnormality that was identified was >3 B‐lines in 4 dogs.
Long‐term follow‐up consultation was performed in 10 of 17 dogs at least 1 month after antimicrobial discontinuation (range, 1‐5 months) and a phone call follow‐up was performed in the remaining 7 dogs (range, 1‐10 months). Long‐term relapses occurred in 3 dogs (17%) after 1, 1.5, and 3 months and information was obtained by follow‐up consultation in 2 dogs and by phone call in 1 dog.
At long‐term follow‐up, all 8 dogs that were reportedly normal did not show any lesions on LUS, whereas the 2 dogs that relapsed had a shred sign with coalescent numbers of B‐lines and air bronchograms in the right or left lung lobes.
Two dogs of the 3 relapsing dogs during the long‐term follow‐up were presented for consultation. These 2 dogs did not show any acute gastrointestinal signs before the onset of dyspnea 1 and 1.5 months after their last follow‐up. Both had bronchiectasis on TXR that persisted despite complete resolution of lung consolidations during follow‐up. At relapse, both had acute onset of lethargy and dyspnea with hyperthermia (41°C and 40.1°C), a restrictive respiratory pattern and bilateral crackles on auscultation. The QoL score was increased again at 17 and 25. Thoracic radiography showed an alveolar pattern in the right ventral lung lobe in 1, and a left ventral alveolar pattern in the other dog, with bronchiectasis in the affected lobe and other lung lobes in the other dog. A thoracic computed tomography scan showed diffuse bronchiectasis and ventral lung consolidations compatible with AP in 1 dog. Both were treated again with amoxicillin/clavulanic acid that was discontinued after 4 weeks based on the absence of relapse, normal physical examination, and normal serum CRP concentration. Despite the absence of predictive signs, 1 dog relapsed again 2 months after the last follow‐up and was euthanized. The other dog was still alive and did not show any signs of relapse at the time of writing. Eight other dogs were presented for long‐term follow‐up consultation without any report of a possible relapse, and all had normal physical examination findings.
weeks based on the absence of relapse, normal physical examination, and normal serum CRP concentration. Despite the absence of predictive signs, 1 dog relapsed again 2 months after the last follow‐up and was euthanized. The other dog was still alive and did not show any signs of relapse at the time of writing. Eight other dogs were presented for long‐term follow‐up consultation without any report of a possible relapse, and all had normal physical examination findings.
Seven dogs only had long‐term follow‐up information available by phone. Six of these dogs (range, 1‐10 months afterward) were reported to be normal, but 1 had been diagnosed with megaesophagus secondary to focal myasthenia gravis, and the owners observed recurrent regurgitation and relapse 3 months after treatment discontinuation. This dog was further treated by its referring veterinarian with amoxicillin/clavulanic acid for 2 weeks with complete resolution of clinical signs. The dosage of pyridostigmine bromide was increased by 20% resulting in complete resolution of the megaesophagus 6 months after diagnosis. No further relapse had been reported at the time of writing.
weeks with complete resolution of clinical signs. The dosage of pyridostigmine bromide was increased by 20% resulting in complete resolution of the megaesophagus 6 months after diagnosis. No further relapse had been reported at the time of writing.
4. DISCUSSION
Our results suggest that, in dogs with suspected AP, antimicrobial discontinuation can be safely based on clinical improvement and normal serum CRP concentration. This approach resulted in a treatment duration of 1 week (70.6%) or 3 weeks (29.4%) in this population, because none of the dogs required >3
weeks (29.4%) in this population, because none of the dogs required >3 weeks of antimicrobial treatment. Based on our findings, current opinion‐based veterinary recommendations to treat bacterial pneumonia for 3 to 6
weeks of antimicrobial treatment. Based on our findings, current opinion‐based veterinary recommendations to treat bacterial pneumonia for 3 to 6 weeks with antimicrobials or 1 to 2
weeks with antimicrobials or 1 to 2 weeks beyond resolution of clinical signs or radiographic lesions or both should be reconsidered, specifically for AP.
1
weeks beyond resolution of clinical signs or radiographic lesions or both should be reconsidered, specifically for AP.
1
C‐reactive protein is a reliable APP to monitor treatment response in dogs with bacterial pneumonia,
6
,
7
but treatment discontinuation based on serum CRP normalization only has been described in a small number of dogs. In the previous study, a significant decrease in treatment duration was achieved without increasing the number of relapses.
6
Our study reinforces the concept that clinical and biomarker‐guided antimicrobial discontinuation is useful in decreasing treatment duration in dogs with AP. Whether the persistent mild increase in serum CRP concentration in 5 dogs and therefore the continued antimicrobial treatment for 3 weeks was really necessary was not assessed in our study.
weeks was really necessary was not assessed in our study.
In addition to physical examination findings, we also used QoL score to assess clinical improvement. The QoL assessment is a tool that previously has been described in veterinary medicine 16 and seems to be useful in assessing the health of patients even if information about its usefulness to modulate treatment is lacking. Although our suggested QoL score has not been validated, it appeared useful during the follow‐up of dogs with AP, and only relapsing dogs had increasing QoL scores.
Diagnostic imaging modalities such as TXR and LUS are useful in the diagnosis of AP in dogs, 1 , 2 , 3 , 4 , 12 but information about their impact on treatment modulation is lacking. We showed that the follow‐up of both TXR and LUS might not be useful in dogs with AP given that, despite improvement, lesions persisted in 88% (15/17) and 71% (12/17) of dogs 1 week after initiation of antimicrobials based on TXR and LUS, respectively, whereas treatment was discontinued based on clinical improvement and normalized serum CRP concentration in 70.6% (12/17) of dogs, without any short‐term relapse. During serial follow‐up of AP, LUS lesions seemed to resolve faster than TXR lesions and to follow more closely clinical improvement and serum CRP normalization.
There is a lack of information in the veterinary literature about short‐ and long‐term relapse in dogs with AP. A single prospective study described a long‐term relapse rate of 17.1% in dogs treated for uncomplicated AP. 21 We found a similar long‐term complication rate of 17% (3/17) despite a shorter treatment duration. Given that all relapses occurred in the long‐term, it is probable they were caused by a new AP event, rather than a complication of the previous AP. Long‐term relapse occurred in a dog with megaesophagus, which is not surprising because AP is a common complication of this disease, being described in 38% of cases. 22 The 2 other relapsing dogs had evidence of bronchiectasis in the same region as the new lesions compatible with pneumonia that could predispose to recurrent or new infections. Bacterial pneumonia is a common cause and complication of bronchiectasis. Bronchiectasis is irreversible and requires effective treatment to attempt to slow the cycle of destruction and preserve the integrity of unaffected bronchi. 23 Information about appropriate duration of antimicrobial treatment in both veterinary and human medicine is lacking in cases of bronchiectasis with secondary pneumonia. 23 , 24 , 25 In our opinion, given the complete resolution of imaging lesions at short‐term follow‐up in addition to serum CRP normalization, longer antimicrobial treatment may not necessarily be warranted in these dogs. In dogs with predisposing (eg, megaesophagus) or aggravating (eg, bronchiectasis) factors, owners should be made aware of the increased risk of relapse so as to encourage fast and appropriate action. It seems unlikely that other long‐term relapses were missed and, therefore, clinical and serum CRP‐guided shorter treatment duration appears to be safe, without increased occurrence of relapse.
The major limitation of our study is the lack of lower airway sampling to accurately differentiate bacterial pneumonia from pneumonitis, which does not necessarily warrant antimicrobial treatment. Additional prospective studies in which supportive treatment for pneumonitis is implemented pending culture results from bronchoalveolar lavage fluid are needed. Another limitation is the absence of long‐term follow‐up in all dogs. Long‐term follow‐up was not achieved in some dogs because of lack of owner compliance. Nevertheless, given the follow‐up information obtained by phone, it seems unlikely that any long‐term relapse was missed. Serum CRP concentration can be increased as a result of any inflammatory systemic disease. Consequently, increased serum concentrations of CRP may have been caused by other systemic inflammatory conditions. That being said, dogs were reportedly healthy before developing clinical signs suggestive of AP. Moreover, complementary examinations were performed based on clinical suspicion by the attending clinician. All dogs had imaging findings suggestive of AP, without any other indication of a different systemic inflammatory disease. Finally, all dogs also had resolution of their clinical signs, which appeared to be associated with normalization of serum CRP concentrations. All of these findings make it unlikely that another systemic inflammatory condition was missed in these patients.
In conclusion, AP seems to be safely and effectively treated in most cases with 1 to 3 weeks of antimicrobials based on clinical resolution and serum CRP normalization without increased risk of short‐ or long‐term relapses. In this study population, TXR and LUS do not seem to add valuable information during follow‐up because resolution of imaging abnormalities appears to lag behind clinical improvement and serum CRP normalization.
weeks of antimicrobials based on clinical resolution and serum CRP normalization without increased risk of short‐ or long‐term relapses. In this study population, TXR and LUS do not seem to add valuable information during follow‐up because resolution of imaging abnormalities appears to lag behind clinical improvement and serum CRP normalization.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Approved by the small animal ethics committee at the University of Liège, number 1610012.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Quality of life questionnaire.
ACKNOWLEDGMENT
Study financed by the European College of Veterinary Internal Medicine ‐ Companion Animals (ECVIM‐CA) and Purina Institute Resident Research Award (2019_Purina_05). Presented as an oral abstract at the 2021 ECVIM‐CA Online Congress.
Notes
Fernandes Rodrigues N, Giraud L, Bolen G, et al. Antimicrobial discontinuation in dogs with acute aspiration pneumonia based on clinical improvement and normalization of C‐reactive protein concentration. J Vet Intern Med. 2022;36(3):1082‐1088. 10.1111/jvim.16405 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Funding information Purina Institute Resident Research Award, Grant/Award Number: 2019_Purina_05; European College of Veterinary Internal Medicine‐Companion Animals (ECVIM‐CA)
REFERENCES
Articles from Journal of Veterinary Internal Medicine are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1111/jvim.16405
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/jvim.16405
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/125685951
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/jvim.16405
Article citations
Recognition and Diagnosis of Underlying Disease Processes in Bacterial Pneumonia.
Animals (Basel), 14(11):1601, 29 May 2024
Cited by: 0 articles | PMID: 38891647 | PMCID: PMC11171252
Review Free full text in Europe PMC
In-hospital mortality in dogs with protein-losing enteropathy and associated risk factors.
J Vet Intern Med, 38(4):2265-2272, 31 May 2024
Cited by: 1 article | PMID: 38819636 | PMCID: PMC11256150
Nursing strategies for the mechanically ventilated patient.
Front Vet Sci, 10:1145758, 28 Jul 2023
Cited by: 1 article | PMID: 37576838 | PMCID: PMC10421733
Review Free full text in Europe PMC
Antimicrobial discontinuation in dogs with acute aspiration pneumonia based on clinical improvement and normalization of C-reactive protein concentration.
J Vet Intern Med, 36(3):1082-1088, 29 Mar 2022
Cited by: 4 articles | PMID: 35348224 | PMCID: PMC9151469
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparison of lung ultrasound, chest radiographs, C-reactive protein, and clinical findings in dogs treated for aspiration pneumonia.
J Vet Intern Med, 36(2):743-752, 05 Mar 2022
Cited by: 13 articles | PMID: 35247005 | PMCID: PMC8965265
Serial evaluation of thoracic radiographs and acute phase proteins in dogs with pneumonia.
J Vet Intern Med, 36(4):1430-1443, 26 May 2022
Cited by: 5 articles | PMID: 35616241 | PMCID: PMC9308444
The Utility of Acute-Phase Proteins in the Assessment of Treatment Response in Dogs With Bacterial Pneumonia.
J Vet Intern Med, 31(1):124-133, 29 Dec 2016
Cited by: 27 articles | PMID: 28032360 | PMCID: PMC5259651
Aspiration Pneumonia in the Dog: A Review.
Top Companion Anim Med, 32(1):1-7, 01 Mar 2017
Cited by: 10 articles | PMID: 28750782
Review
Funding
Funders who supported this work.
European College of Veterinary Internal Medicine-Companion Animals (ECVIM-CA)
Purina Institute Resident Research Award (1)
Grant ID: 2019_Purina_05

 1
1



