Abstract
Background
frailty is common in older adults and associated with poor outcomes following illness. Although stroke is predominantly a disease of older people, our knowledge of frailty in stroke is limited. We aimed to collate the literature on acute stroke and frailty to estimate the prevalence of pre-stroke frailty and its associations with outcomes.Methods
paired researchers searched multidisciplinary electronic databases for papers describing frailty and acute stroke. We assessed risk of bias using Newcastle-Ottawa tools appropriate to study design. We created summary estimates of pre-stroke frailty using random effects models. We collated whether studies reported significant positive associations between frailty and clinical outcomes in adjusted models.Results
we included 14 studies (n = 27,210 participants). Seven studies (n = 8,840) used a frailty index approach, four studies (n = 14,924) used Hospital Frailty Risk Scores. Pooled prevalence of pre-stroke frailty was 24.6% (95% confidence interval, CI: 16.2-33.1%; low quality evidence, downgraded due to heterogeneity, bias). Combining frailty and pre-frailty (nine studies, n = 23,827), prevalence of any frailty syndrome was 66.8% (95%CI: 49.9-83.7%). Seven studies were at risk of bias, from participant selection or method of frailty assessment. Pre-stroke frailty was associated with all adverse outcomes assessed, including longer-term mortality (positive association in 6 of 6 studies reporting this outcome; odds ratio: 3.75 [95%CI: 2.41-5.70]), length of admission (3 of 4 studies) and disability (4 of 6 studies).Conclusions
despite substantial heterogeneity, whichever way it is measured, frailty is common in patients presenting with acute stroke and associated with poor outcomes. This has implications for the design of stroke services and pathways.Free full text

Prevalence and implications of frailty in acute stroke: systematic review & meta-analysis
Associated Data
Abstract
Background
frailty is common in older adults and associated with poor outcomes following illness. Although stroke is predominantly a disease of older people, our knowledge of frailty in stroke is limited. We aimed to collate the literature on acute stroke and frailty to estimate the prevalence of pre-stroke frailty and its associations with outcomes.
Methods
paired researchers searched multidisciplinary electronic databases for papers describing frailty and acute stroke. We assessed risk of bias using Newcastle-Ottawa tools appropriate to study design. We created summary estimates of pre-stroke frailty using random effects models. We collated whether studies reported significant positive associations between frailty and clinical outcomes in adjusted models.
Results
we included 14 studies (n = 27,210 participants).
Seven studies (n =
27,210 participants).
Seven studies (n = 8,840) used a frailty index approach,
four studies (n =
8,840) used a frailty index approach,
four studies (n = 14,924) used Hospital Frailty Risk
Scores. Pooled prevalence of pre-stroke frailty was 24.6% (95% confidence
interval, CI: 16.2–33.1%; low quality evidence, downgraded due to heterogeneity,
bias). Combining frailty and pre-frailty (nine studies,
n =
14,924) used Hospital Frailty Risk
Scores. Pooled prevalence of pre-stroke frailty was 24.6% (95% confidence
interval, CI: 16.2–33.1%; low quality evidence, downgraded due to heterogeneity,
bias). Combining frailty and pre-frailty (nine studies,
n = 23,827), prevalence of any frailty syndrome was
66.8% (95%CI: 49.9–83.7%). Seven studies were at risk of bias,
from participant selection or method of frailty assessment. Pre-stroke frailty was associated
with all adverse outcomes assessed, including longer-term mortality (positive association in 6
of 6 studies reporting this outcome; odds ratio: 3.75 [95%CI: 2.41–5.70]),
length of admission (3 of 4 studies) and disability (4 of 6 studies).
23,827), prevalence of any frailty syndrome was
66.8% (95%CI: 49.9–83.7%). Seven studies were at risk of bias,
from participant selection or method of frailty assessment. Pre-stroke frailty was associated
with all adverse outcomes assessed, including longer-term mortality (positive association in 6
of 6 studies reporting this outcome; odds ratio: 3.75 [95%CI: 2.41–5.70]),
length of admission (3 of 4 studies) and disability (4 of 6 studies).
Conclusions
despite substantial heterogeneity, whichever way it is measured, frailty is common in patients presenting with acute stroke and associated with poor outcomes. This has implications for the design of stroke services and pathways.
Key Points
Prevalence of pre-stroke frailty is around one in four in an acute stroke population.
There is little consistency in the methods used to assess for pre-stroke frailty.
Pre-stroke frailty is independently associated with a variety of poor outcomes.
Although related, pre-stroke frailty is not synonymous with pre-stroke functional ability or comorbidity.
Standardised frailty assessment in stroke may inform treatment decisions, prognostics and design of patient centred services.
Introduction
An understanding and consideration of frailty is of fundamental importance in the care of the unwell older adult [1]. In older adults presenting with acute illness, frailty is common, quantifiable and strongly associated with poor outcomes [2]. In recognition of this, many international healthcare systems are incorporating measures of frailty into their acute care pathways [3]. Stroke is one of the more common acute presentations seen in older adults, yet assessment of frailty is not yet standard in stroke-care, and best practice guidance on frailty rarely mentions stroke [3]. A first step in understanding the relationship between frailty and stroke is to have a benchmark figure for the prevalence of frailty in people presenting with stroke.
Despite advances in stroke-care, stroke has a persistently high mortality and can leave survivors with disability or requiring care-home admission. Predicting outcomes at the time of acute stroke is difficult [4, 5]. However, frailty is associated with many of the poor outcomes seen in stroke [6] and assessment of frailty may aid prognostication.
There is a strong association between pre-stroke function, usually measured using the modified Rankin Scale (mRS), and stroke outcomes [7]. Assessment of pre-stroke mRS is recommended to inform decisions around hyperacute therapies such as intravenous thrombolysis or mechanical thrombectomy, but is an imperfect tool [8]. Plausibly a measure of frailty may have greater prognostic utility and be less prone to the limitations of the mRS. Conversely, it could be argued that pre-stroke function is already a suitable proxy for frailty, or that describing pre-stroke frailty adds little to the mRS assessment.
Our overall aim was to describe frailty in an acute stroke population.
Specific objectives were to estimate the prevalence of pre-stroke frailty; describe the association between pre-stroke frailty and clinical outcomes and assess relationships between pre-stroke frailty and pre-stroke function or morbidity.
Methods
This review was reported following Preferred Reporting of Items in Systematic Reviews and Meta-Analyses (PRISMA) guidance. The protocol was registered in 2019 (CRD: 42019154402: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=154402).
Each aspect of the review process was performed by at least two experienced reviewers with access to a third party as required.
Eligibility criteria
We placed no restrictions on date or language of publication. If abstracts were identified, we searched for subsequent full-text publications using author names and abstract titles.
Population
We included studies of adults (aged 16 years and older) with stroke presentations (any stroke subtype, but not including transient ischaemic attack (TIA), subarachnoid or subdural haemorrhage). We accepted any definition of ‘acute’ stroke, but did not include studies that recruited from rehabilitation or community settings.
Exposure
We included any measure that the authors described as a frailty assessment, provided that the intention was to quantify frailty status before the stroke event. We included studies if they reported either frailty prevalence or quantitative data describing association between frailty and our prespecified outcomes.
Outcomes
Our pre-specified outcomes of interest were mortality (in-hospital, 30 days and 1 year), length of hospital stay, functional outcome and discharge destination. Where other clinical outcomes were described, we noted these.
Study types
We included prospective and cross-sectional studies and adapted data extraction accordingly.
Information sources
We searched MEDLINE (Ovid); EMBASE (Ovid), CINAHL (EBSCOhost), PsycInfo (EBSCOhost) and Health Management Information Consortium (Ovid) from 1980 to September 2020. The search was developed using the Cochrane search filter for stroke studies and a bespoke syntax for frailty (Appendix 1, Supplementary data are available in Age and Ageing online). We included papers that were published after the initial search but were identified through forward searching or other strategies.
Study selection and data collection
We used Covidence software for de-duplication and title searching [9]. We developed a data extraction form and piloted this against two papers, to ensure authors were familiar with the required data content and format. As a further quality control step, after paired review, a third author double-extracted a random sample (25%) of papers.
Data were extracted on study design, frailty assessment and outcomes, noting any data regarding pre-stroke function or quantitative measures of comorbidity.
Risk of bias assessment
Risk of bias was assessed based on the Newcastle-Ottawa Scale, using the tool for cohort studies or cross-sectional studies as required and modifying the anchoring statements to suit the review question (Appendix 2, Supplementary data are available in Age and Ageing online; [10]). In keeping with best practice, we present a narrative assessment of risk of bias, rather than a summary score [11].
Synthesis of results
We categorised frailty as a binary variable to enable calculation of summary prevalence estimates. Where a scale used hierarchical categorisation, for example mild, moderate and severe frailty, for the primary analysis we classified those in the most severe category as frail. Where available we also collected data on pre-frailty and for nominal, hierarchical scales we used the category below that which was used to define frank frailty. Where uncertainty in the prevalence estimate was not reported in the original paper, we derived a 95% confidence interval (95%CI) using prevalence and sample size.
We used Comprehensive Meta-Analysis software [12] to generate a summary estimate of frailty and pre-frailty prevalence pooled across the included studies using random effects models.
We ran subgroup analyses limited to commonly employed frailty measures and sensitivity analyses where we excluded highly selected populations. As a post-hoc exploratory analysis, we created a summary estimate of strength of association between frailty and mortality.
We assessed heterogeneity using the I2 statistic and created a funnel plot to visually inspect for publication bias.
We described certainty in our estimate of pre-stroke frailty using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework. We modified the GRADE approach for observational epidemiology assessing for bias, consistency (based on I2), directness (applicability of included studies to pre-stroke frailty), precision (based on uncertainty of the summary estimate) and publication bias (funnel plot).
We described associations of pre-stroke frailty as a narrative, tabulated strength of association and complemented this with a data visualisation approach where we displayed all studies that assessed adjusted associations of pre-stroke frailty against clinical outcomes.
We tabulated data on the prevalence of pre-stroke functional impairment or quantitative measures of comorbidity as described by the authors. If not already reported in the paper, and where data allowed, we compared proportional prevalence of pre-stroke disability or comorbidity with pre-stroke frailty.
Results
We screened 2,504 records by title and abstract after de-duplication [9]. We assessed 51 studies in full and included 16 publications representing 14 studies [13–28]. We identified an additional 12 conference abstracts that were potentially eligible for inclusion, but with no corresponding full-text publication (Appendix 3, Supplementary data are available in Age and Ageing online; [29–40]). We excluded 23 studies with main reasons for exclusion including, no frailty data and not an acute stroke population (Appendix 4, Supplementary data are available in Age and Ageing online).
Included study characteristics
Included study characteristics are summarised in Table 1. Included studies were mainly cohort designs, with two cross-sectional studies. Two studies had multiple publications using the same underlying dataset and data from all were used as needed [17, 18, 25, 26]. All studies were hospital based, seven were single-centre and all recruited from industrialised countries. Sample sizes varied from 156 to 12,019 participants with mean sample size 2,221 participants. The average age of participants was between 64 and 87 years. Men accounted for 39–63% of participants. Five studies only included ischaemic stroke and three included only haemorrhagic stroke. Ten of the included studies provided baseline stroke severity using National Institutes of Health Stroke Scale (NIHSS) with scores ranging from 1 to 20.
Table 1
Characteristics of included studies
| Study ID | Country | Sample size | Setting | Age | Male sex (%) | Stroke subtype | Stroke severity |
|---|---|---|---|---|---|---|---|
| Evans 2020 [13] | England | 433 | Single hospital | Median Non-frail 83 [77–86] Frail 87 [83–92] | 43.8 | Ischaemic stroke only | Median baseline NIHSS Non-frail 3 [1–7] Frail 4.5 [1–12] |
| Fearon 2012 [14] | Scotland | 231 | Single hospital | Median 74 [63–82] | NR | NR | TACS 20% PACS 27% LACS 40% POCS 10% |
| Imaoka 2018 [15] | Japan | 156 | Multiple hospitals | Mean 66.0 ± 13.4 | 58.9 | Haemorrhagic stroke only | NR |
| Joyce 2021 [16] | Northern Ireland | 175 | Single hospital | Mean 72 ± 13 | 43 | Ischaemic stroke only | Median Baseline NIHSS 16 [10–20] |
| Kanai 2020 [17] & Noguchi 2021 [18] | Japan | 234 | Single hospital | Median 76 [11] | 63.6 | 81.2% ischaemic | Median Baseline NIHSS 2 [3] |
| Kilkenny 2021 [19] | Australia | 12,019 | Multiple hospitals | Median 75.8 | 53.6 | 82.6% ischaemic | NR |
| Kim 2020 [20] | USA | 240 | Single hospital | Mean 68.5 ± 14.2 | 53.8 | Haemorrhagic stroke only | NR |
| Myint 2017 [21] | England | 2,388 | Multiple hospitals | Mean 76.9 ± 12.7 | 47.3 | 87% ischaemic | TACS 21.5% PACS 39.6% LACS 24.8% POCS 14.1% |
| Pinho 2021 [22] | Germany | 489 | Multiple hospitals | Median 75.6 [65–82] | 49.5 | Ischaemic stroke only | Median Baseline NIHSS 15 [11–19] |
| Schnieder 2021 [23] | Germany | 318 | Single hospital | Median 80.1 [9.58] | 39.6 | Ischaemic stroke only | Median Baseline NIHSS 15 [10] |
| Seamon 2019 [24] | USA | 7,258 | Multiple hospitals | Mean 79.4 ± 8.4 | 43.3 | Ischaemic stroke only | SASI Severe 22.1% Moderate 32.9% Mild 45% |
| Taylor-Rowan 2019 [25, 26] | Scotland | 546 | Single hospital | Mean 64 ± 14 | 54 | NR | Median Baseline NIHSS 3 [1–5] |
| Waehler 2021 [27] | Norway | 625 | Multiple hospitals | Mean Robust 66 ± 11.6 Pre-frail 73 ±10.6 Frail 81 ± 7.2 | 57.9 | 91.8% ischaemic | Mean Baseline NIHSS Robust 1.8 ± 3.9 Prefrail 3 ±4 Frail 4 ± 3.9 |
| Zhang 2020 [28] | Australia | 2098 | Multiple hospitals | Median 76 [65,84] | 52 | Haemorrhagic stroke only | NR |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; LACS, Lacunar strokes; NIHSS, National Institute for Health Stroke Scale; NR, not reported; PACS, partial anterior circulation strokes; POCS, posterior circulation strokes; PVD, peripheral vascular disease; SASI, Stroke Administrative Severity Index; TACS, total anterior circulation strokes.
Q1–Q3 or interquartile range presented in square brackets based on reporting within individual papers with median results; standard deviation of mean results denoted by ±.
Characteristics of the 12 studies reported in abstract format alone are summarised in Appendix 3, Supplementary data are available in Age and Ageing online. There was duplication of reporting with data from the same underlying study cohorts in 3 of the 12. Sample sizes varied from 33 to 3,965 participants, with most studies describing ischaemic stroke populations.
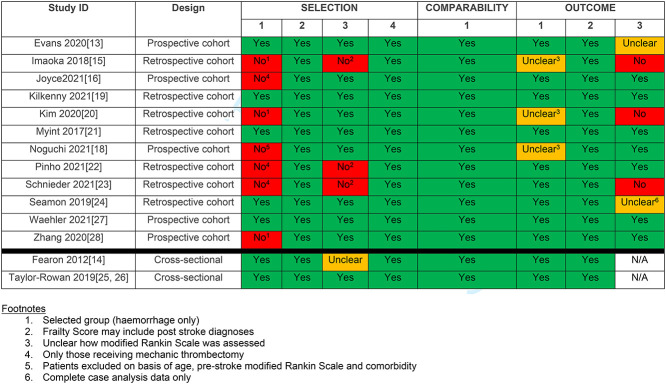
Risk of bias within studies
Complete risk of bias assessment is presented in Table 2. Seven studies had at least one domain graded as high risk. All of these studies had problems with patient selection due to unnecessary exclusions [17]; a focus on intracerebral haemorrhage patients only [15, 20, 28] and recruitment limited to those offered mechanical thrombectomy [12, 18, 19]. In three studies [11, 18, 19] the method used to assess for pre-stroke frailty may have included post stroke complications. In three studies [11, 16, 19], there may not have been sufficient time for outcomes to develop, these were generally studies that assessed in-hospital outcomes only.
Table 2
Comparing frailty prevalence by measure to define frailty
| Study ID | Frailty tool | Frailty definition | Frailty prevalence | Pre-frailty prevalence |
|---|---|---|---|---|
| Evans 2020 [13] | Clinical Frailty Scale (CFS) | Frailty (CFS = = 5–8) 5–8) | 54.0% | Not reported |
| Fearon 2012 [14] | 4-item Rockwood | At least one marker on the 4-item score | 32.0% | Not reported |
| Imaoka 2018 [15] | 11-item Modified Frailty index | Treated as a continuous variable, score between 0 and 11 | Not reported | Not reported |
| Joyce 2021 [16] | 33-item Frailty index (FI) | Frailty defined by FI score of ≥0.24 | Frail 28% | Not reported |
| Kanai 2020 [17] & Noguchi 2021 [18] | 5-item Simplified frailty index | Pre-frailty = = 1 or 2 1 or 2Frailty >  = = 3 3 | Frail 12.4% | Pre-frail 55.1% |
| Kilkenny 2021 [19] | Hospital Frailty Risk Score | Low risk 1–4 Intermediate risk 5–15 High risk >15 | High risk 24.1% | Intermediate risk 49.3% |
| Kim 2020 [20] | 11-item Modified Frailty index | Treated as a continuous variable, score between 0 and 11 | 31.3% (moderately frail) | Pre-frail 11.3% |
| Myint 2017 [21] | Pre-stroke modified Rankin scale | Not defined | 20.5% (if mRS  > > 2 2 = = frail) frail) | Not reported |
| Pinho 2021 [22] | Hospital Frailty Risk Score | Low risk <5 Intermediate risk 5–15 High risk >15 | High risk 29.7% | Intermediate risk 68.3% |
| Schnieder 2021 [23] | Hospital Frailty Risk Score | Low risk <5 Moderate risk 5–15 High risk >15 | High risk 2.2% | Moderate risk 22.7% |
| Seamon 2019 [24] | Faurot Frailty Index | Non-frail <0.1 Pre-frail 0.1–4.9 Frail >5.0 | Frail 24.9% | Pre-frail 36.0% |
| Taylor-Rowan 2019 [25, 26] | 33-item Frailty Index & Fried Frailty Phenotype | Robust <0.08 Pre-frail 0.08–0.24 Frail >0.24 | Frail 28% Phenotype frail 28%* | Pre-frail 50.5% |
| Waehler 2021 [27] | Fried Frailty Phenotype (adjusted for stroke) | Robust no Fried criteria Pre-frail 1 or 2 Fried criteria Frail 3 or more Fried criteria | Frail 11% | Pre-frail 58.6% |
| Zhang 2020 [28] | Hospital Frailty Risk Score | Low risk <5 Intermediate risk 5–15 High risk >15 | High risk 27% | Intermediate risk 47.9% |
mRS, modified Rankin scale; *Phenotype data only available for 258/347 participants.
Measurement of frailty
There was considerable heterogeneity across the included studies in the tool used to assess frailty and how this was defined and classified (Table 3). Seven studies used an approach based on frailty index (varying number of items included, range: 4–33; [14–17, 20, 24, 25]) four studies used the Hospital Frailty Risk Score [19, 22, 23, 28], albeit with differing terminology for the included categories; two used the Fried phenotype approach [25, 27], one used pre-stroke mRS [21] and one study used the Clinical Frailty Scale (CFS; [13]). One study compared two measures of frailty in the same cohort, a 33-item frailty index and the Fried frailty phenotype, although there was substantial missing phenotype data [25]. The other author team who adapted the Fried phenotype criteria for application in stroke, also reported problems with completion [27].
Table 3
Summary table of quality assessment of included studies
|
|
The definition used to classify an individual’s frailty status was not explicitly
provided in three of the included studies [15, 20, 21]. For two
of these studies, a modified frailty index was treated as a continuous variable [15, 20], we were
able to extrapolate a frailty prevalence for one of these. The other study defined frailty
based on pre-stroke mRS assessment [21]. For the
purpose of the analysis, we assumed that frailty status would be based on a
mRS >
> 2.
2.
Among the abstracts, four used a frailty index approach [29, 30, 38, 39], three used the CFS [32, 36, 37], one used pre-stroke mRS [40], one used Fried phenotype criteria [33], one used comprehensive geriatric assessment (CGA [34]), one used the Canadian Study on Health and Ageing Scale [35] and one provided their own definition of frailty [31].
Frailty prevalence
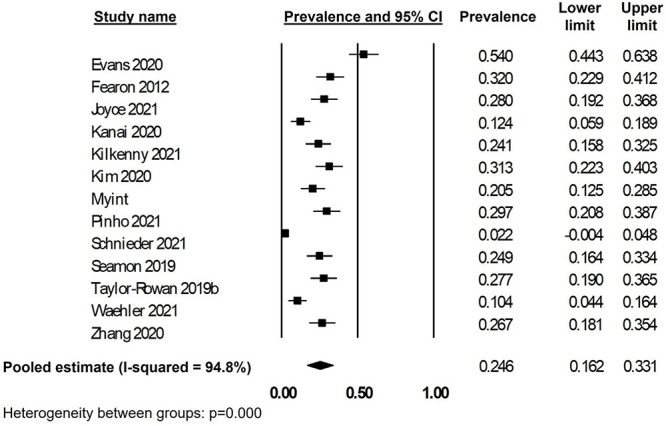
Treating frailty as a binary construct, the prevalence of frailty varied from 2.2 to 54% of included participants. The pooled summary estimate for the prevalence of frailty in stroke was 24.6% (95%CI: 18.1–35.4%; I2: 94.8%; 13 studies; 27,054 participants; Figure 1). Funnel plot suggested no publication bias (Appendix 5, Supplementary data are available in Age and Ageing online). Using the GRADE framework, certainty of evidence was low due to problems with heterogeneity and risk of bias.
Pre-frailty prevalence was described in nine of the included studies, ranging from 11.3 to 68.3%. The pooled summary estimate for pre-frailty was 45.5% (95%CI: 34.6–56.4%; I2: 92.1%; nine studies; 23,827 participants). Combining frailty and pre-frailty, prevalence of any frailty syndrome pre-stroke was 66.8% (95%CI: 49.9–83.7%; I2: 98.0%; nine studies; 23,827 participants). Combining those studies where only frailty was described and those with frailty and pre-frailty, the summary estimate was 56.6% (95%CI: 39.0–74.1%; I2: 98.7%; 13 studies; 27,054 participants).
Frailty prevalence using any form of frailty index was 25.7% (95%CI: 19.0–32.4%; I2: 74.0%; six studies; 8,684 participants); whereas for those studies using HFRS the prevalence was 20.4% (95%CI: 3.9–36.8% I2: 96.0%; four studies; 14,924 participants).
When studies looking only at haemorrhagic stroke were excluded, prevalence was 23.9% (95%CI: 14.6–33.1%; I2: 95.2%; 11 studies; 24,716 participants). When studies looking only at patients receiving thrombectomy were excluded prevalence was 26.1% (95%CI: 18.7–33.5%; I2: 88.1%; 10 studies; 26,072 participants).
Only half of the included abstracts reported the prevalence of frailty among their included participants, ranging from 24.8 to 68.4%. Re-running the frailty prevalence analysis including the abstract data suggested a prevalence of 28.4% (95%CI: 20.1–36.7%; I2: 95.8%; 19 reports; 29,722 participants).
Association between frailty and outcomes
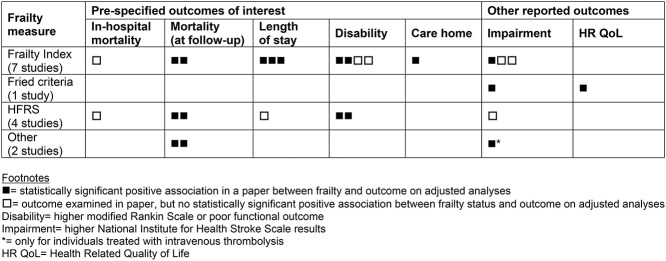
The relationship between frailty status and outcomes after stroke was inconsistently reported across the included studies, we sought data from all 16 of the identified papers. Three papers did not report any of our pre-specified outcomes of interest [14, 25, 27]. In a fourth paper the outcomes for those experiencing stroke were not reported separately from those with TIA [19]. A tabular summary of the association between frailty and outcomes is reported in Table 4, with additional information in Appendix 6 (Supplementary data are available in Age and Ageing online) quantifying the associations between frailty and mortality and length of stay.
Table 4
Summary of association between pre-stroke frailty and outcomes (published papers)
|
|
Mortality: seven studies examined the association between frailty status and mortality risk over a range of follow-up periods [13, 15, 16, 20, 21, 23, 28]. Two studies specifically considered in-hospital mortality and found no association with frailty, using a frailty index and the HFRS [20, 23]. Another reported on mortality at 28 days and found those who were frail had a higher rate of mortality and that increasing frailty remained independently associated with mortality after adjustment for cardiovascular risk factors and stroke severity, using CFS [13]. Mortality at 90 days was higher in those who were frail compared to the non-frail participants in two studies, using frailty index and HFRS respectively [16, 23]. Mortality at 180 days was higher in a single study using HFRS [28]. Mortality at 6–8 months follow-up was associated with increased frailty, odds ratio (OR) 2.97 (95%CI: 1.52–5.8), in one study, using a frailty index [15]. The study, which used mRS as a measure of frailty reported that mortality increased as mRS increased [21]. For seven papers [13, 15, 16, 19, 21, 23, 26], data were suitable to allow a summary analysis, the estimate of association between frailty and mortality was OR: 3.71 (95%CI: 2.41–5.70).
Length of hospital stay: four studies examined the association between frailty status and hospital length of stay, three of which identified frailty (measured by frailty index) as associated with longer stays [18, 20, 24] and one found no difference in length of stay between low, moderate and high frailty risk individuals using HFRS [23].
Functional outcome/disability: six studies evaluated the association between frailty status and functional outcome or level of disability [15, 16, 18, 20, 22, 23]. Four studies found a statistically significant association between frailty and poorer functional outcome (mRS; [15, 16, 22, 23]). However, one study found no association [18] and the final study reported that the association was not present after adjustment [20].
Discharge destination: only one study looked at discharge destination, with an increasing likelihood of discharge to long-term care as frailty increased (non-frail 18.5% versus pre-frail 28% versus frail 46.9%) [24].
Other outcomes reported: there was limited examination of other outcomes’ association with frailty status. Impairment, measured using NIHSS, was associated with frailty in two studies [17, 27] but in three studies [16, 23, 25] there was no association after adjustment. One study suggested that frailty may moderate acute treatment effects, reporting that every one-point increase in CFS was independently associated with a one-point reduction in post-thrombolysis improvement, using NIHSS [13]. One study reported health-related quality of life, measured using EQ-5D, at 18 months of follow-up, reporting lower scores in those who were frail compared to those who were pre-frail or robust [27]. Post-stroke cognitive impairment was associated with frailty in one study [26].
The abstracts reported associations with in-hospital mortality, poorer recovery or neurological outcome, reduced chance of returning home/increased risk of new institutionalisation, longer length of stay and complications after stroke. The mRS was commonly used as an outcome measure associated with frailty (Appendix 7, Supplementary data are available in Age and Ageing online).
Frailty and pre-stroke function or comorbidity
Six studies reported on the distribution of pre-stroke mRS in their cohorts (Appendix 8, Supplementary data are available in Age and Ageing online; [13, 14, 17, 22, 25, 27]). Differing approaches were used to assess for the association of mRS with frailty. Two studies found no statistical association between frailty and pre-stroke mRS [13, 22]. Correlation between the frailty measure and pre-stroke mRS was moderate [14, 25]. In one study there was increasing mean pre-stroke mRS across robust, pre-frail and frail states [27]. The proportions classified as having pre-stroke disability and pre-stroke frailty differed in three studies [14, 17, 25], whereas there was no significant difference in one study [22].
Of the six studies reporting quantitative measures of comorbidity, correlation was moderate. Four studies showed differences in comorbidity with increasing frailty [19, 24, 27] but the differences were not all in a ‘dose response’ pattern (Appendix 8, Supplementary data are available in Age and Ageing online; [23]).
Discussion
Through a synthesis of the published evidence, we have shown that frailty is common in acute stroke. We estimate that at least one in four people presenting with a stroke are living with frailty, this increases to two in three if we include pre-frailty. We have also shown that pre-stroke frailty is associated with poor outcomes including death, disability and increased length of stay. Accepting all the caveats of our post hoc analysis, we estimated that the presence of frailty increased odds of death from stroke by almost four times. Finally, we have shown that while pre-stroke frailty is related to the concepts of pre-stroke function and pre-stroke comorbidity, the three are not equivalent and frailty measures seem to be a distinct construct.
Findings in context
For a condition as common and prognostically important as frailty, the number of relevant studies was disproportionately limited. A similar pattern is seen for studies looking at related older adult syndromes in stroke, for example delirium [41] and sarcopenia [42]. Our limited understanding of frailty and cerebrovascular disease in general was highlighted in a recent narrative review that outlined important future directions for frailty research in stroke [43]. The areas highlighted align with the data gaps seen in our review: a better understanding of how to assess for frailty and the challenges of assessment in stroke; more data on the effect of frailty on multimodal outcomes and studies assessing how we can use frailty assessment to improve stroke-care. We would hope the landscape around frailty and stroke is changing and we note that 12 of the 14 included studies were published in the last three years, suggesting that the topic is of increasing interest.
Our focus was pre-stroke frailty, as we recognised that an understanding of frailty status could help in hyperacute treatment decisions and support person centred care. A review of the epidemiology of frailty in a post-stroke population suggested a population prevalence of 22% frailty and 49% pre-frailty [44]. These figures are similar to our data. One may expect the prevalence to increase, particularly as the stroke event and related impairments could move someone from the pre-frail state to frank frailty. However, as pre-stroke frailty was so strongly associated with mortality, it seems plausible that many people living with pre-stroke frailty do not survive their stroke event and so will not feature in the community estimates of post stroke frailty.
Clinical implications
At the time of writing, pre-stroke frailty is not routinely measured in national stroke audits and registers such as the UK sentinel Stroke National Audit Programme (SSNAP), RiksStroke in Sweden or SITS-MOST (Safe Implementation of Thrombolysis in Stroke Monitoring Study). However, these registers all collect pre-stroke function as measured by the mRS. Our data suggest that assessment of frailty may offer additional information to the pre-stroke mRS assessment. Problems with pre-stroke mRS are well recognised [14]. One could even argue that pre-stroke frailty assessment could replace pre-stroke mRS. Pre-stroke mRS is used as exclusion criterion, case-mix adjuster and prognostic tool—our data suggest that pre-stroke frailty assessment could fulfil all these roles. A frailty tool such as the CFS offers a similar ordinal, hierarchical structure to mRS and could be incorporated into systems that already use pre-stroke.
A motivation for this review was the changing treatment landscape in stroke. A key component of evidence-based stroke-care is the acute stroke unit (ASU; [45]). ASUs offer multidisciplinary assessment and intervention that has many similarities to the CGA that can be effective for people living with frailty [46]. Recent developments in acute and hyperacute stroke place an increasing emphasis on interventions such as mechanical thrombectomy. Although these treatments are lifesaving or life changing for many, their safety and efficacy in people living with advanced frailty is less certain. Our review included three studies describing frailty and thrombectomy and all came to the same conclusion that outcomes were considerably poorer in people living with frailty. An unanswered question is whether these interventions offer any relative benefit in a frail population who tend to have poor outcomes regardless of treatment.
Research implications
Our review has highlighted many important evidence gaps around frailty and stroke. If frailty is to become part of the baseline stroke assessment, we need further research comparing the different frailty measures in stroke populations. We note the issues with applying the frailty phenotype in acute stroke and future studies would need to consider feasibility and applicability. Use of frailty index in a clinical setting, requires collection of multiple domains, some of which may not be readily available in routine care systems. The Clinical Frailty Scale, popular as a short assessment tool in practice, was only used in a single study. A key question to explore is around electronic frailty scoring approaches, which are based on prior healthcare utilisation, such as HFRS. Different diagnoses and conditions are recorded within these datasets and the extent to which these determine an individual’s pre-stroke frailty is yet to be established. There is potential for bias around prior engagement with healthcare services in the region, jurisdiction or digital system drawing together data. Further work exploring the relative properties of pre-stroke frailty tools and the established mRS seems warranted [47]. Finally, there are also important questions around when to assess the impact of a stroke event on an individual’s frailty status and how to use these data.
Strengths and limitations
We performed a comprehensive review conducted as per our registered protocol and following best practice in conduct and reporting. The timeliness of our review is evidenced by the inclusion of many recently published studies. Although there was heterogeneity in our included cohorts, the mix of ages and stroke severities suggest that our aggregate group are representative of contemporary acute stroke populations. Our summary estimate of prevalence was robust across various subgroup and sensitivity analyses.
The available data show a bias towards industrialised nations in typically affluent countries. The search was designed to be inclusive and non-English language publications could be included, so this evidence gap suggests that interest in frailty and stroke is limited to higher income countries. Although our aggregate summary estimate is potentially biased by inclusion of selected patient cohorts, this allows us to explore frailty in groups of particular interest, for example those considered for thrombectomy. Frailty is a continuum, and we recognise that a dichotomous approach is reductionist. However, this was necessary to allow us to offer summary estimates and the inclusion of pre-frailty hopefully offers information on the frailty spectrum.
Conclusions
Pre-stroke frailty is common and prognostically important. Assessment of pre-stroke frailty could be used to inform hyperacute treatment decisions and may have greater utility than commonly employed measures such as pre-stroke mRS.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
JKB is supported by a Joint NHS Education for Scotland/Chief Scientist Office (NES/CSO) Postdoctoral Clinical Lectureship. The funders played no part in the conduct of this review.
References
Articles from Age and Ageing are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/ageing/afac064
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ageing/article-pdf/51/3/afac064/43491622/afac064.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/125729770
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/ageing/afac064
Article citations
The association of multidimensional frailty with metabolic syndrome and low-grade inflammation in community-dwelling older adults in the Netherlands: a Lifelines cohort study.
Immun Ageing, 21(1):78, 13 Nov 2024
Cited by: 0 articles | PMID: 39538284 | PMCID: PMC11558828
Association between pre-stroke frailty status and stroke risk and impact on outcomes: a systematic review and meta-analysis of 1,660,328 participants.
Aging Clin Exp Res, 36(1):189, 11 Sep 2024
Cited by: 0 articles | PMID: 39259235 | PMCID: PMC11390839
Review Free full text in Europe PMC
Association of platelet-to-HDL cholesterol ratio with frailty and all-cause mortality.
Lipids Health Dis, 23(1):344, 23 Oct 2024
Cited by: 0 articles | PMID: 39443978 | PMCID: PMC11515673
Effects of multicomponent exercise nursing intervention in elderly stroke patients with frailty: a randomized controlled trial.
Front Med (Lausanne), 11:1450494, 02 Oct 2024
Cited by: 0 articles | PMID: 39416863 | PMCID: PMC11479928
Pre-Stroke Frailty and Outcomes following Percutaneous Endoscopic Gastrostomy Tube Insertion.
Healthcare (Basel), 12(16):1557, 06 Aug 2024
Cited by: 0 articles | PMID: 39201117 | PMCID: PMC11353361
Go to all (27) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Cochrane Database Syst Rev, 2(2022), 01 Feb 2022
Cited by: 12 articles | PMID: 36321557 | PMCID: PMC8805585
Review Free full text in Europe PMC
Implications of frailty in acute ischemic stroke receiving endovascular treatment: systematic review and meta-analysis.
Aging Clin Exp Res, 35(5):969-978, 25 Mar 2023
Cited by: 2 articles | PMID: 36964867
Review
Association between pre-stroke frailty status and stroke risk and impact on outcomes: a systematic review and meta-analysis of 1,660,328 participants.
Aging Clin Exp Res, 36(1):189, 11 Sep 2024
Cited by: 0 articles | PMID: 39259235 | PMCID: PMC11390839
Review Free full text in Europe PMC
The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis.
Age Ageing, 47(6):793-800, 01 Nov 2018
Cited by: 68 articles | PMID: 30084863
Review
Funding
Funders who supported this work.
Chief Scientist Office (1)
Using and improving Scotland’s care home data: a mixed methods programme of data linkage research and consensus gathering
Dr Jenni Burton, University of Glasgow
Grant ID: PCL/21/01

 1
1