Abstract
Free full text

Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long‐term outcomes in COVID‐19 patients
year after recovery: Assessing the long‐term outcomes in COVID‐19 patients
Abstract
Sustained hypercoagulability and endotheliopathy are present in convalescent COVID‐19 patients for up to 4 months from recovery. The hemostatic, endothelial, and inflammatory profiles of 39 recovered COVID‐19 patients were evaluated up to 16
months from recovery. The hemostatic, endothelial, and inflammatory profiles of 39 recovered COVID‐19 patients were evaluated up to 16 months after recovery from COVID‐19. These values were compared with a control group of healthy volunteers (n = 124). 39 patients (71.8% males, median age 43
months after recovery from COVID‐19. These values were compared with a control group of healthy volunteers (n = 124). 39 patients (71.8% males, median age 43 years) were reviewed at a mean of 12.7
years) were reviewed at a mean of 12.7 ±
± 3.6
3.6 months following recovery. One patient without cardiovascular risk factors had post COVID‐19 acute ischaemic limb. Elevated D‐dimer and Factor VIII levels above normal ranges were noted in 17.9% (7/39) and 48.7% (19/39) of patients respectively, with a higher median D‐dimer 0.34 FEU
months following recovery. One patient without cardiovascular risk factors had post COVID‐19 acute ischaemic limb. Elevated D‐dimer and Factor VIII levels above normal ranges were noted in 17.9% (7/39) and 48.7% (19/39) of patients respectively, with a higher median D‐dimer 0.34 FEU μg/mL (IQR 0.28, 0.46) (p
μg/mL (IQR 0.28, 0.46) (p <
< .001) and Factor VIII 150% (IQR 171, 203) (p = .004), versus controls. Thrombin generation (Thromboscreen) showed a higher median endogenous thrombin potential (ETP) of 1352
.001) and Factor VIII 150% (IQR 171, 203) (p = .004), versus controls. Thrombin generation (Thromboscreen) showed a higher median endogenous thrombin potential (ETP) of 1352 nM*min (IQR 1152, 1490) (p = .002) and a higher median peak height of 221.4
nM*min (IQR 1152, 1490) (p = .002) and a higher median peak height of 221.4 nM (IQR 170.2, 280.4) (p = 0.01) and delayed lag time 2.4
nM (IQR 170.2, 280.4) (p = 0.01) and delayed lag time 2.4 min (1.42–2.97) (p = 0.0002) versus controls. Raised vWF:Ag and ICAM‐1 levels were observed in 17.9% (7/39) and 7.7% (3/39) of patients respectively, with a higher median VWF:Ag 117% (IQR 86, 154) (p = 0.02) and ICAM‐1 54.1
min (1.42–2.97) (p = 0.0002) versus controls. Raised vWF:Ag and ICAM‐1 levels were observed in 17.9% (7/39) and 7.7% (3/39) of patients respectively, with a higher median VWF:Ag 117% (IQR 86, 154) (p = 0.02) and ICAM‐1 54.1 ng/mL (IQR 43.8, 64.1) (p = .004) than controls. IL‐6 was noted to be raised in 35.9% (14/39) of patients, with a higher median IL‐6 of 1.5
ng/mL (IQR 43.8, 64.1) (p = .004) than controls. IL‐6 was noted to be raised in 35.9% (14/39) of patients, with a higher median IL‐6 of 1.5 pg/mL (IQR 0.6, 3.0) (p = 0.004) versus controls. Subgroup analysis stratifying patients by COVID‐19 severity and COVID‐19 vaccination preceding SARS‐CoV‐2 infection did not show statistically significant differences. Hypercoagulability, endothelial dysfunction, and inflammation are still detectable in some patients approximately 1
pg/mL (IQR 0.6, 3.0) (p = 0.004) versus controls. Subgroup analysis stratifying patients by COVID‐19 severity and COVID‐19 vaccination preceding SARS‐CoV‐2 infection did not show statistically significant differences. Hypercoagulability, endothelial dysfunction, and inflammation are still detectable in some patients approximately 1 year after recovery from COVID‐19.
year after recovery from COVID‐19.
1. INTRODUCTION
The increased post‐COVID‐19 burden of cardiovascular disease and thrombotic events is an emerging global health concern, 1 , 2 where the occurrence of thrombotic events in survivors without known cardiovascular risk factors is significant. 3 , 4
COVID‐19 associated coagulopathy (CAC) 5 , 6 , 7 is characterized by platelet activation, endothelial dysfunction, neutrophil extracellular traps, and activation of the coagulation cascade causing hypercoagulability and hypofibrinolysis, and immune dysregulation of those results in a poorly controlled pro‐inflammatory response. During acute illness, this culminates in an increased clinical manifestation of macro‐ and microvascular thromboembolic events, particularly in severe and critical COVID‐19 patients. 8 , 9 Mildly elevated levels of Factor VIII and von Willebrand factor antigen (vWF:Ag) were detected in mild, non‐hypoxic COVID‐19 patients in an age‐ and gender‐matched prospective observational study we performed, although no features of hypercoagulability were present with evaluation by global hemostatic tests such as Thromboelastography (TEG) and clot waveform analysis (CWA). 10
However, there is a paucity of information on sustained systemic inflammation, hypercoagulability, and endotheliopathy beyond a 6‐month period. Given our initial findings from a previous study performed in Singapore on post‐COVID‐19 survivors 11 showing elevated levels of circulating endothelial cells and raised pro‐inflammatory cytokines implying cytokine driven endothelial dysfunction, we hypothesized that a persistent hypercoagulable state, endothelial dysfunction, and inflammation may be present in recovered COVID‐19 patients, resulting in rare but catastrophic manifestations of acute thrombosis even during the post‐convalescent phase of illness. Secondly, we hypothesized that hemostatic, endothelial, and inflammatory marker derangements would be more marked in those who were more critically ill.
2. METHODS
We performed a prospective observational study at the National Centre for Infectious Diseases (NCID), Singapore. The study was approved by the National Healthcare Group Domain Specific Research Board (DSRB) and written informed consent was obtained. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for cohort studies in the preparation of this manuscript. Patients ≥21 years of age who were admitted to NCID from January 2020 to July 2021 with laboratory‐confirmed SARS‐CoV‐2 infection confirmed by polymerase chain reaction (PCR) were screened. Patients were recruited if they were more than 6
years of age who were admitted to NCID from January 2020 to July 2021 with laboratory‐confirmed SARS‐CoV‐2 infection confirmed by polymerase chain reaction (PCR) were screened. Patients were recruited if they were more than 6 months and less than 15
months and less than 15 months after recovery from COVID‐19 infection. Patients with congenital bleeding diathesis, thrombophilia, established chronic liver failure, severe anemia with hemoglobin <7.5
months after recovery from COVID‐19 infection. Patients with congenital bleeding diathesis, thrombophilia, established chronic liver failure, severe anemia with hemoglobin <7.5 g/dL, on full‐dose anticoagulation, cognitive impairment, or pregnancy were excluded. For the control cohort, 124 asymptomatic healthy volunteers (age range 21–65
g/dL, on full‐dose anticoagulation, cognitive impairment, or pregnancy were excluded. For the control cohort, 124 asymptomatic healthy volunteers (age range 21–65 years old, median age 43
years old, median age 43 years, 61 males and 63 females) provided written informed consent and underwent venesection between June 2019 and October 2019 (preceding the SARS‐CoV‐2 outbreak) for the preservation of their double spun plasma stored at −70°C to −80°C for the establishment of laboratory reference ranges and other coagulation studies. Freezer temperatures were recorded and monitored daily. All frozen aliquots were thawed at 37°C and used immediately, with residual plasma discarded. The following tests (prothrombin time [PT], activated partial thromboplastin time [aPTT], D‐dimer and fibrinogen) were performed on fresh samples (primary tubes) on the same day of blood collection, while all other tests were performed on plasma stored under the above‐mentioned conditions.
years, 61 males and 63 females) provided written informed consent and underwent venesection between June 2019 and October 2019 (preceding the SARS‐CoV‐2 outbreak) for the preservation of their double spun plasma stored at −70°C to −80°C for the establishment of laboratory reference ranges and other coagulation studies. Freezer temperatures were recorded and monitored daily. All frozen aliquots were thawed at 37°C and used immediately, with residual plasma discarded. The following tests (prothrombin time [PT], activated partial thromboplastin time [aPTT], D‐dimer and fibrinogen) were performed on fresh samples (primary tubes) on the same day of blood collection, while all other tests were performed on plasma stored under the above‐mentioned conditions.
Baseline patient characteristics included age, sex, ethnicity, comorbidities, and month of COVID‐19 infection from blood sampling at the point of enrolment. Disease severity upon recruitment was classified using the Clinical Spectrum of SARS‐CoV‐2 Infection by the National Institutes of Health.
12
Clinical data were prospectively collected including oxygen requirement, intensive care unit (ICU) stay, COVID‐19 vaccination status, and thrombotic events during acute SARS‐COV‐2 infection as well as during recovery, were available from the clinical records. A one‐off collection of 50 ml of whole blood was obtained from each participant via venepuncture for a point prevalence assessment of the inflammatory, hemostatic, and endothelial profile parameters. In addition, full blood count, fasting glucose, HBA1c, and lipid panel were performed.
ml of whole blood was obtained from each participant via venepuncture for a point prevalence assessment of the inflammatory, hemostatic, and endothelial profile parameters. In addition, full blood count, fasting glucose, HBA1c, and lipid panel were performed.
ICAM‐1 and IP‐10 (R&D Systems, Abington, England) and high sensitivity IL‐6 (Interleukin‐6) (ThermoFisher, Waltham, MA) were assayed by enzyme‐linked immunosorbent assay, C‐reactive protein (CRP) was quantified using CRP Latex reagent (Beckman Coulter AU System). Coagulation tests were performed on platelet‐poor plasma using the STA R Max Series coagulation analyzer (Diagnostica Stago, France) and Sysmex CN‐6000 automated coagulation analyzer (Sysmex Corporation, Kobe, Japan). The PT was measured with Innovin (Siemens Healthcare, Marburg, Germany), aPTT with Dade Actin FSL (Siemens Healthcare), fibrinogen (modified Clauss) with STA Liquid FIB, D‐dimer with STA Liatest D‐Dimer (Fibrinogen equivalent units [FEU]). Clotting factor levels (Factor V and VIII) were measured with STA Deficient V and VIII respectively. vWF antigen was assayed with an immunoturbidimetric method using STA Liastest vWF: Ag kit. Thrombin generation was performed using the ST‐Genesia (STG) (Stago, Asnières‐sur‐Seine, France). The CWA was performed with Sysmex CN‐6000 with parameters obtained from aPTT and for PT, as per the International Society of Hemostasis and Thrombosis Scientific and Standardization Committee recommendation. 13
Descriptive analyses were used to summarize the variables. Normality of the data was first assessed before determining the appropriate summary statistic to be used for continuous variables. Numbers and frequencies were used to describe categorical variables. Due to the skewed data distribution for most tests, median (interquartile range [IQR]) was used; tests of association between the tests and the participants/healthy controls were conducted using the Wilcoxon Rank‐sum test. Statistical significance was declared if p <
< .05. Analyses were performed using STATA 17 (StataCorp 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.).
.05. Analyses were performed using STATA 17 (StataCorp 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.).
3. RESULTS
Thirty‐nine COVID‐19 patients with a median age of 43 years (IQR 32, 56) were recruited at NCID at a mean of 12.7+/−3.6
years (IQR 32, 56) were recruited at NCID at a mean of 12.7+/−3.6 months following their COVID‐19 diagnosis confirmed by SARS‐CoV‐2 PCR testing. Twenty‐eight (71.8%) were males, with a raised median BMI of 26.3
months following their COVID‐19 diagnosis confirmed by SARS‐CoV‐2 PCR testing. Twenty‐eight (71.8%) were males, with a raised median BMI of 26.3 kg/m2 (IQR 22.3, 30.2) and median waist circumference of 91.5
kg/m2 (IQR 22.3, 30.2) and median waist circumference of 91.5 cm (IQR 82, 99). The ethnicity ratios of patients were representative of the Singapore population. The majority of these patients did not have significant cardiovascular risk factors (diabetes mellitus, n = 11 (28.2%), hypertension, n = 7 (17.9%), hyperlipidemia, n = 10 (25.6%)) and only 3 had the previous history of acute myocardial infarction, with no patients with previous stroke or venous thromboembolism (VTE). On recruitment for this study, an assessment of their metabolic profile revealed good glycaemic control and cholesterol levels, with a median HBA1c of 5.5% (IQR 5.3, 6.2), median fasting glucose of 5.5
cm (IQR 82, 99). The ethnicity ratios of patients were representative of the Singapore population. The majority of these patients did not have significant cardiovascular risk factors (diabetes mellitus, n = 11 (28.2%), hypertension, n = 7 (17.9%), hyperlipidemia, n = 10 (25.6%)) and only 3 had the previous history of acute myocardial infarction, with no patients with previous stroke or venous thromboembolism (VTE). On recruitment for this study, an assessment of their metabolic profile revealed good glycaemic control and cholesterol levels, with a median HBA1c of 5.5% (IQR 5.3, 6.2), median fasting glucose of 5.5 mmol/L (IQR 5.0, 6.0), a median total cholesterol level of 4.9
mmol/L (IQR 5.0, 6.0), a median total cholesterol level of 4.9 mmol/L (IQR 4.1, 5.5) and mean low‐density lipoprotein of 3.0
mmol/L (IQR 4.1, 5.5) and mean low‐density lipoprotein of 3.0 ±
± 1.0
1.0 mmol/L. Full blood count showed normal median white blood cell, hemoglobin, and platelet counts.
mmol/L. Full blood count showed normal median white blood cell, hemoglobin, and platelet counts.
All 39 patients were hospitalized as per previous public health policy in Singapore where all confirmed COVID‐19 patients (including those with asymptomatic or mild COVID‐19) were quarantined in hospitals or decanted to community care facilities. Of the patient cohort, 11 had asymptomatic COVID‐19, 19 had mild to moderate COVID‐19, 2 had severe COVID‐19 and 7 had critical COVID‐19 requiring intensive care support. As per our Singapore NCID treatment guidelines for COVID‐19,
14
pharmacological VTE prophylaxis is recommended for patients with critical or severe COVID‐19; mild/moderate COVID‐19 patients are risk‐stratified for the requirement for thromboprophylaxis using the PADUA prediction score. Five patients requiring oxygen supplementation were initiated on pharmacological thromboprophylaxis with low molecular weight heparin (enoxaparin) while the remaining 4 who were oxygen‐dependent were not started as per the managing ICU physician's discretion. The other 29 patients with asymptomatic or mild COVID‐19 had PADUA scores <4 and were not initiated on pharmacological thromboprophylaxis. Nine patients were started on IV remdesivir, 3 patients on low dose IV dexamethasone, 3 on Tocilizumab, and 3 on lopinavir/ritonavir. As per the criteria at recruitment for the study, none of the patients were on anticoagulation or extended thromboprophylaxis at the time of recruitment which would have affected coagulation test results. None of the patients had active malignancy or autoimmune disease. Two male patients with critical COVID‐19 that required mechanical ventilation had a segmental pulmonary embolism (PE) during their ICU admission, while 1 male patient aged 37 years old with prior asymptomatic COVID‐19 in May 2020 (SARS‐CoV‐2 PCR negative, raised Immunoglobulin G for SARS‐CoV‐2 [Roche Elecsys Anti‐SARS‐CoV‐2]) and no significant cardiovascular risk factors suffered from post‐COVID‐19 right acute ischaemic limb embolism in August 2021 requiring embolectomy, with CT angiography showing a mural aortic thrombus, and thrombi involving the right popliteal artery and right external iliac artery. The patient had no radiological evidence of vasculitis, aortitis, dissection, or aneurysm in the arterial system, and his thrombophilia screen was negative. Patients' demographics and clinical data are summarized in Table 1.
years old with prior asymptomatic COVID‐19 in May 2020 (SARS‐CoV‐2 PCR negative, raised Immunoglobulin G for SARS‐CoV‐2 [Roche Elecsys Anti‐SARS‐CoV‐2]) and no significant cardiovascular risk factors suffered from post‐COVID‐19 right acute ischaemic limb embolism in August 2021 requiring embolectomy, with CT angiography showing a mural aortic thrombus, and thrombi involving the right popliteal artery and right external iliac artery. The patient had no radiological evidence of vasculitis, aortitis, dissection, or aneurysm in the arterial system, and his thrombophilia screen was negative. Patients' demographics and clinical data are summarized in Table 1.
TABLE 1
Demographic and baseline characteristics of recovered COVID‐19 patients
| Participants (n = 39) | ||
|---|---|---|
| Patient characteristics | Age at recruitment (years); median (IQR) | 43 (32, 56) |
| Males; n (%) | 28 (71.8) | |
| Ethnicity; n (%) | ||
| Chinese | 12 (30.8) | |
| Malay | 2 (5.1) | |
| Indian | 17 (20.5) | |
| Others | 8 (20.5) | |
| Months from infection to first blood sampling; mean (SD) | 12.7 (3.6) | |
| COVID‐19 vaccinated a | 29 (74.4) | |
| BMI (kg/m2); median (IQR) | 26.3 (22.3, 30.2) | |
| Waist circumference (cm); median (IQR) | 91.5 (82, 99) | |
| Average systolic blood pressure (mmHg); median (IQR) | 121 (114, 139) | |
| Average diastolic blood pressure (mmHg); median (IQR) | 72 (66, 82) | |
| Patient comorbidities | Diabetes mellitus; n (%) | 11 (28.2) |
| Hypertension; n (%) | 7 (18.0) | |
| Hyperlipidemia; n (%) | 10 (25.6) | |
| Previous cardiac AMI; n (%) | 3 (7.7) | |
| Previous Stroke; n (%) | 0 (0.0) | |
| Previous deep vein thrombosis; n (%) | 0 (0.0) | |
| COVID‐19 severity | Asymptomatic COVID‐19 | 11 (30.8) |
| Mild to moderate COVID‐19 | 19 (48.7) | |
Severe COVID‐19 (PaO2/FiO2 < < 300 300 mmHg) mmHg) | 2 (5.1) | |
| Critical COVID‐19 (requiring intensive care) | 7 (17.9) | |
| Treatment | Thromboprophylaxis | 5 (12.8) |
| Remdesivir | 9 (23.1) | |
| Tocilizumab | 3 (7.7) | |
| Dexamethasone | 3 (7.7) | |
| Lopinavir/ritonavir | 3 (7.7) | |
| Thrombotic complications | COVID‐19 associated thrombosis; n (%) | 2 (5.1) |
| Post Covid‐19 thrombosis; n (%) | 1 (2.6) | |
| Laboratory parameters | Fasting glucose; median (IQR) | 5.5 (5.0, 6.0) |
| HbA1c (%); median (IQR) | 5.5 (5.3, 6.2) | |
| Total cholesterol; median (IQR) | 4.9 (4.1, 5.5) | |
| HDL; median (IQR) | 1.3 (1.1, 1.4) | |
| LDL; mean (SD) | 3.0 (1.0) | |
| C‐reactive protein (mg/L); median (IQR) b | 3.7 (0.9, 20.4) | |
| Hemoglobin (g/dL); median (IQR) | 14.5 (12.8, 15.2) | |
| White Blood Cells (109/L); median (IQR) | 6.2 (5.2, 7.0) | |
| Platelets; median (IQR) | 228 (194, 273) | |
Recovered COVID‐19 patients showed significantly higher biomarkers of hypercoagulability, endotheliopathy, and inflammation compared with the control group (Table 2, Figure 1). There were significantly higher levels of D‐dimer and Factor VIII, with elevated D‐dimer and Factor VIII levels above the normal ranges were noted in 17.9% (7/39) and 48.7% (19/39) of recovered COVID‐19 patients respectively, with a higher median D‐dimer (FEU) 0.34 μg/mL (IQR 0.28, 0.46) (p
μg/mL (IQR 0.28, 0.46) (p <
< .001) and Factor VIII 150% (IQR 171, 203) (p = .004), versus controls. This correlated with thrombin generation tests where thrombin generation (Thromboscreen) showed a higher median endogenous thrombin potential (ETP) 1352
.001) and Factor VIII 150% (IQR 171, 203) (p = .004), versus controls. This correlated with thrombin generation tests where thrombin generation (Thromboscreen) showed a higher median endogenous thrombin potential (ETP) 1352 nM*min (IQR 1152, 1490) (p = .002), peak height 221.4
nM*min (IQR 1152, 1490) (p = .002), peak height 221.4 nM (IQR 170.2, 280.4) (p = .01) and a delayed lag time 2.4
nM (IQR 170.2, 280.4) (p = .01) and a delayed lag time 2.4 min (1.42–2.97) (p = .0002) versus controls. The median anti‐thrombin levels were lower in the patient group 97% (IQR 94, 102) (p
min (1.42–2.97) (p = .0002) versus controls. The median anti‐thrombin levels were lower in the patient group 97% (IQR 94, 102) (p <
< .001) versus controls. Other parameters of hemostasis in recovered COVID‐19 patients, including thrombin clotting time, fibrinogen, Factor V, Protein C, Protein S, and clot waveform analysis on both PT and aPTT had normal median levels with no statistically significant differences with the control group. Raised endothelial biomarkers such as vWF:Ag and ICAM‐1 were observed in 17.9% (7/39) and 7.7% (3/39) of recovered COVID‐19 patients respectively, with a higher median vWF:Ag 117% (IQR 86, 154) (p = .02) and ICAM‐1 54.1
.001) versus controls. Other parameters of hemostasis in recovered COVID‐19 patients, including thrombin clotting time, fibrinogen, Factor V, Protein C, Protein S, and clot waveform analysis on both PT and aPTT had normal median levels with no statistically significant differences with the control group. Raised endothelial biomarkers such as vWF:Ag and ICAM‐1 were observed in 17.9% (7/39) and 7.7% (3/39) of recovered COVID‐19 patients respectively, with a higher median vWF:Ag 117% (IQR 86, 154) (p = .02) and ICAM‐1 54.1 ng/mL (IQR 43.8, 64.1) (p = .004) than controls. The inflammatory marker IL‐6 was noted to be raised in 35.9% (14/39) of recovered COVID‐19 patients, with a higher median IL‐6 of 1.5
ng/mL (IQR 43.8, 64.1) (p = .004) than controls. The inflammatory marker IL‐6 was noted to be raised in 35.9% (14/39) of recovered COVID‐19 patients, with a higher median IL‐6 of 1.5 pg/mL (IQR 0.6, 3.0) (p = .001) versus controls. C‐reactive protein was raised in 43.6% (17/39) of recovered patients with a raised median level of 20.4
pg/mL (IQR 0.6, 3.0) (p = .001) versus controls. C‐reactive protein was raised in 43.6% (17/39) of recovered patients with a raised median level of 20.4 mg/L; however, CRP was unable to be performed on the stored plasma of historical controls. The median levels for IP‐10 were normal, with only 1 patient having a raised level, and no statistically significant difference compared with controls. Subgroup analysis of results in selected hemostatic, endothelial, and inflammatory biomarkers did not show statistically significant differences in patients who had prior COVID‐19 mRNA vaccination before their COVID‐19 infection or patients who had variation in COVID‐19 severity (Table 3).
mg/L; however, CRP was unable to be performed on the stored plasma of historical controls. The median levels for IP‐10 were normal, with only 1 patient having a raised level, and no statistically significant difference compared with controls. Subgroup analysis of results in selected hemostatic, endothelial, and inflammatory biomarkers did not show statistically significant differences in patients who had prior COVID‐19 mRNA vaccination before their COVID‐19 infection or patients who had variation in COVID‐19 severity (Table 3).
TABLE 2
Summary of hemostatic, endothelial, and inflammatory parameters of 39 COVID‐19 survivors
| Reference range | COVID‐19 survivors (n = 39) | Controls (n = 124) | p value | ||
|---|---|---|---|---|---|
| Hemostatic Tests | PT (sec); median (IQR) | 11.7–14.0 | 13.1 (12.7, 13.5) | 12.7 (12.4, 13.1) | .002 |
| APTT (sec); median (IQR) | 27.0–37.0 | 29.6 (28.0, 30.5) | 31.2 (29.6, 32.7) | .001 | |
| TCT (sec); median (IQR) | 15.0–18.0 | 16.5 (16.2, 17.0) | 16.6 (15.7, 17.2) | .90 | |
| Fibrinogen (g/L); median (IQR) | 1.8–4.5 | 3.3 (2.7, 3.8) | 3.2 (2.6, 3.6) | .37 | |
| D‐dimer (ug/mL); median (IQR) | <0.50 | 0.34 (0.28, 0.46) | 0.25 (0.17, 0.33) | <.001 | |
| Factor VIII (%); median (IQR) | 60–150 | 150 (119, 190) | 114 (103, 145) | .004 | |
| Factor V (%); median (IQR) | 70–120 | 95 (82, 108) | 97 (89, 117) | .21 | |
| AT (%); median (IQR) | 80–130 | 97 (94, 102) | 109.5 (104, 116) | <.001 | |
| PC (%); median (IQR) | 70–150 | 121 (100, 133) | 114 (104, 128) | .41 | |
| PS (%); median (IQR) | 55–130 | 94 (80, 103) | 89.8 (81.5, 98.5) | .15 | |
| Endothelial markers | ICAM‐1 (ng/mL); median (IQR) | <95 | 54.1 (43.8, 64.1) | 62.3 (54.1, 68.8) | .004 |
| vWF:Ag (%); median (IQR) | 56–160 | 117 (86, 154) | 99 (77.5, 122.5) | .02 | |
| Inflammatory markers | Interleukin‐6 (pg/ml); median (IQR) | <2 | 1.5 (0.6, 3.0) | 0.5 (0.4, 0.7) | .001 |
| IP‐10 (pg/ml); median (IQR) | <152.1 (IQR 9.1–40.2) | 12.5 (7.0, 25.2) | 22.1 (9.4, 38.1) | .07 | |
| Thrombo‐screen – TM | Lag time (min); median (SD) | 1.42–2.97 | 2.4 (2.2, 2.8) | 2.1 (1.9, 2.4) | .0002 |
| Peak height (nM); median (IQR) | 97–290 | 221.4 (170.2, 280.4) | 194.8 (160.4, 219.4) | .01 | |
| Endogenous thrombin potential (ETP) (nM*min); median (IQR) | 741–1654 | 1352 (1152, 1490) | 1193 (1080.5, 1327) | .002 | |
Thrombo‐screen + + TM TM | Lag time (min); median (IQR) | 1.45–3.38 | 2.5 (2.3, 2.8) | 2.3 (2.1, 2.6) | .008 |
| Peak height (nM); median (IQR) | 11–193 | 102.8 (72.6, 150.3) | 94.2 (68.4, 131.2) | .24 | |
| ETP (nM*min); median (IQR) | 50–806 | 431.4 (307.2, 673.4) | 404.2 (300.6, 542.7) | .13 | |
| Clot Waveform Analysis | Min1 (%/s) (PT); median (IQR) | 1.95–5.67 | 3.9 (3.3, 4.7) | 3.6 (3.2, 4.1) | .14 |
| Min2 (%/s2) (PT); median (IQR) | 0.97–2.93 | 2.0 (1.6, 2.6) | 1.9 (1.6, 2.1) | .08 | |
| Max2 (%/s2) (PT); median (IQR) | 0.75–2.35 | 1.6 (1.3, 2.0) | 1.5 (1.3, 1.7) | .06 | |
| (dH/bH)*100 (%) (PT); median (IQR) | 6.52–17.28 | 12.1 (10.2, 14.0) | 11.4 (10.1, 12.8) | .33 | |
| Min1 (%/s) (aPPT); median (IQR) | 2.86–6.78 | 5.1 (4.4, 5.9) | 4.7 (4.1, 5.4) | .07 | |
| Min2 (%/s2) (aPPT); median (IQR) | 0.46–1.10 | 0.8 (0.7, 1.0) | 0.8 (0.6, 0.9) | .12 | |
| Max2 (%/s2) (aPPT); median (IQR) | 0.37–0.93 | 0.7 (0.6, 0.8) | 0.6 (0.5, 0.7) | .055 | |
| (dH/bH)*100 (%) (aPPT); median (IQR) | 25.21–63.09 | 45.9 (39.2, 53.1) | 43.0 (37.8, 49.5) | .10 |
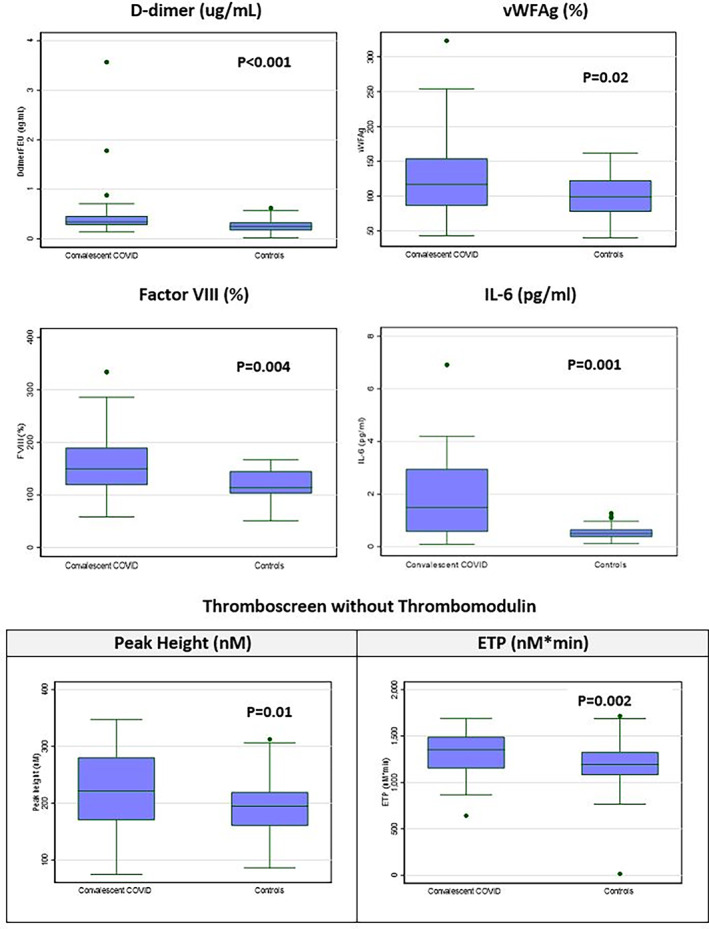
Box and whisker plots of statistically significant hemostatic, endothelial and inflammatory parameters [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3
Median (IQR) of hemostatic, endothelial, and inflammatory parameters, stratified by COVID‐19 severity and COVID‐19 vaccination
| COVID‐19 severity a , b | COVID‐19 vaccinated a | |||||
|---|---|---|---|---|---|---|
| Parameters | Asymptomatic mild/moderate (non‐oxygen dependent) | Severe/critical (oxygen dependent) | p value | No | Yes | p value |
| Fibrinogen (g/L) | 3.2 (2.8, 3.8) | 3.3 (2.7, 3.8) | .14 | 3.4 (2.7, 3.8) | 3.1 (2.7, 3.8) | .93 |
| D‐dimer (ug/mL) | 0.30 (0.28, 0.43) | 0.40 (0.36, 0.61) | .14 | 0.34 (0.31, 0.40) | 0.33 (0.28, 0.50) | .93 |
| FVIII (%) | 152 (116, 182) | 130 (122, 202) | .81 | 147.5 (101, 202) | 150 (120, 184) | .97 |
| FV (%) | 94 (81, 107) | 100.5 (91, 109) | .32 | 92 (75, 105) | 95 (85, 109) | .37 |
| vWFAg (%) | 111 (84, 142) | 120 (104, 197) | .31 | 118 (84, 218) | 111 (90, 148) | .73 |
| ICAM1 (ng/mL) | 51.8 (43.8, 56.8) | 63.0 (46.1, 75.9) | .16 | 51.2 (42.7, 63.6) | 54.4 (43.9, 65.0) | .60 |
| ETP (nM*min) (Thromboscreen without TM) | 1352 (1187, 1510) | 1294.5 (1137, 1434) | .44 | 1287 (1137, 1352) | 1394 (1170, 1510) | .19 |
| Peak height (nM) (Thromboscreen without TM) | 230.8 (187.7, 280.4) | 188.4 (116.4, 254.5) | .11 | 211.4 (177.1, 254.4) | 230.8 (170.2, 280.4) | .38 |
| Delayed lag time (min) (Thromboscreen without TM) | 2.43 (2.05, 2.63) | 2.64 (2.20, 2.94) | .20 | 2.13 (1.99, 2.33) | 2.45 (2.34, 2.75) | .054 |
4. DISCUSSION
The unique pathophysiology of SARS‐CoV‐2 with its affinity to ACE‐2 receptors on endothelial cells leads to direct and indirect endothelial injury and has been shown to cause endothelial dysfunction and endotheliitis in multiple vascular beds.
15
,
16
Activated endothelial cells play a key role in contributing to the hypercoagulable and inflammatory state in COVID‐19 patients and are also implicated in the pathogenesis of long COVID‐19.
17
This study, to the best of our knowledge, is the first to evaluate the longer‐term hemostatic, endothelial and inflammatory profile of patients at a mean of 12.7 ±
± 3.6
3.6 months after recovery from COVID‐19 infection. We demonstrated that approximately 1
months after recovery from COVID‐19 infection. We demonstrated that approximately 1 year after COVID‐19, there was the persistence of hypercoagulability, endotheliopathy, and inflammation in some recovered COVID‐19 patients, where most of our patients did not have significant cardiovascular risk factors or previous thromboembolic events. Subgroup analysis of COVID‐19 severity did not show increased severity of these biomarkers in the severe or critical COVID‐19 group. Besides patients who experienced severe to critical COVID‐19, there was a proportion of patients with mild or asymptomatic COVID‐19 that also demonstrated raised biomarkers of hypercoagulability, endothelial dysfunction, and inflammation (Supplementary File, Figure S1 showing heatmap of hemostatic, endothelial and inflammatory parameters). While physiological response versus pathogenic response has been challenging to define even in literature, our findings are in keeping with the ROADMAP‐PostCOVID‐19 study
18
that showed persisting endothelial cell activation and hypercoagulability in a cohort of 208 COVID‐19 survivors that had a mild COVID‐19 disease trajectory (severity not described) who were assessed at a median of 62
year after COVID‐19, there was the persistence of hypercoagulability, endotheliopathy, and inflammation in some recovered COVID‐19 patients, where most of our patients did not have significant cardiovascular risk factors or previous thromboembolic events. Subgroup analysis of COVID‐19 severity did not show increased severity of these biomarkers in the severe or critical COVID‐19 group. Besides patients who experienced severe to critical COVID‐19, there was a proportion of patients with mild or asymptomatic COVID‐19 that also demonstrated raised biomarkers of hypercoagulability, endothelial dysfunction, and inflammation (Supplementary File, Figure S1 showing heatmap of hemostatic, endothelial and inflammatory parameters). While physiological response versus pathogenic response has been challenging to define even in literature, our findings are in keeping with the ROADMAP‐PostCOVID‐19 study
18
that showed persisting endothelial cell activation and hypercoagulability in a cohort of 208 COVID‐19 survivors that had a mild COVID‐19 disease trajectory (severity not described) who were assessed at a median of 62 days from recovery. These survivors had no pre‐existing cardiovascular disease, autoimmune disease, or cancer and were not on long‐term medication. In addition, several previous studies evaluating COVID‐19 patients at a short‐term follow‐up of 2.5–4
days from recovery. These survivors had no pre‐existing cardiovascular disease, autoimmune disease, or cancer and were not on long‐term medication. In addition, several previous studies evaluating COVID‐19 patients at a short‐term follow‐up of 2.5–4 months after COVID‐19
19
,
20
,
21
showed similar findings in the earlier phase of COVID‐19 recovery. Von Meijenfeldt et al.
19
demonstrated raised plasma levels of Factor VIII and plasminogen activator inhibitor type 1 (PAI‐1) which supported a prothrombotic state driven by increased thrombin generation and decreased plasma fibrinolysis in 52 recovered COVID‐19 patients assessed at 4‐month follow‐up. Similar to our study, thrombin generation performed showed enhanced thrombin generating capacity, as evidenced by increased ETP and higher median peak height. Von Meijenfeldt et al. also showed similar findings of delay in lag time as seen in our study, which we postulate may be associated with increased levels of tissue factor pathway inhibitor (TFPI) and thrombomodulin which is synthetized by and released by an activated endothelium. The release of TFPI and thrombomodulin (TM) deprives the vascular microenvironment of important inhibitors of thrombin generation and tissue factors, resulting in delayed lag time in thrombin generation. While our study did not evaluate for these biomarkers, raised levels of TFPI and TM were found to be significantly higher in the ROADMAP‐Post COVID‐19 study.
18
Fogarty et al.
20
showed in 50 COVID‐19 patients assessed at a median of 68
months after COVID‐19
19
,
20
,
21
showed similar findings in the earlier phase of COVID‐19 recovery. Von Meijenfeldt et al.
19
demonstrated raised plasma levels of Factor VIII and plasminogen activator inhibitor type 1 (PAI‐1) which supported a prothrombotic state driven by increased thrombin generation and decreased plasma fibrinolysis in 52 recovered COVID‐19 patients assessed at 4‐month follow‐up. Similar to our study, thrombin generation performed showed enhanced thrombin generating capacity, as evidenced by increased ETP and higher median peak height. Von Meijenfeldt et al. also showed similar findings of delay in lag time as seen in our study, which we postulate may be associated with increased levels of tissue factor pathway inhibitor (TFPI) and thrombomodulin which is synthetized by and released by an activated endothelium. The release of TFPI and thrombomodulin (TM) deprives the vascular microenvironment of important inhibitors of thrombin generation and tissue factors, resulting in delayed lag time in thrombin generation. While our study did not evaluate for these biomarkers, raised levels of TFPI and TM were found to be significantly higher in the ROADMAP‐Post COVID‐19 study.
18
Fogarty et al.
20
showed in 50 COVID‐19 patients assessed at a median of 68 days, similar findings of increased endogenous thrombin potential and peak thrombin, and raised endothelial markers that included von Willebrand factor antigen (vWF:Ag), vWF propeptide and factor VIII. A cohort study by Willems et al.
21
on 203 recovered COVID‐19 patients at 3
days, similar findings of increased endogenous thrombin potential and peak thrombin, and raised endothelial markers that included von Willebrand factor antigen (vWF:Ag), vWF propeptide and factor VIII. A cohort study by Willems et al.
21
on 203 recovered COVID‐19 patients at 3 months showed sustained endothelial dysfunction, hypercoagulability, and inflammation, with higher endothelin‐1, increased activated coagulation factor: inhibitor complexes, and inflammatory cytokines (Interleukin[IL]‐18, IL‐6, IL‐1ra) supporting this. Our study showed that cytokine production was persistent even after recovery, with raised IL‐6 levels in 36% of our patients, which were also observed in a study of 121 recovered COVID‐19 patients at early (<90
months showed sustained endothelial dysfunction, hypercoagulability, and inflammation, with higher endothelin‐1, increased activated coagulation factor: inhibitor complexes, and inflammatory cytokines (Interleukin[IL]‐18, IL‐6, IL‐1ra) supporting this. Our study showed that cytokine production was persistent even after recovery, with raised IL‐6 levels in 36% of our patients, which were also observed in a study of 121 recovered COVID‐19 patients at early (<90 days) and late (>90
days) and late (>90 days) timepoints by Peluso et al.,
22
and in a cross‐sectional study evaluating neuropsychiatric and medical findings in 60 recovered COVID‐19 patients at the 6‐to‐8‐month recovery period, where 27 (45%) of patients had raised IL‐6 levels.
23
days) timepoints by Peluso et al.,
22
and in a cross‐sectional study evaluating neuropsychiatric and medical findings in 60 recovered COVID‐19 patients at the 6‐to‐8‐month recovery period, where 27 (45%) of patients had raised IL‐6 levels.
23
Immunothrombotic mechanisms may persist well into recovery, reflected by the derangements seen in hemostatic and inflammatory biomarkers in our study. This is reflected in the occurrence of rare cardiovascular and thrombotic events in post‐COVID‐19 patients we observed earlier on in the pandemic 4 , 24 as well as in one of our patients who later developed an acute ischaemic limb. Further real‐world evidence includes a large retrospective cohort study of national healthcare databases from the US Department of Veterans Affairs evaluating recovered COVID‐19 patients, which suggests increased risk and a significant 1‐year burden of thromboembolic and cardiovascular disease. 25 These risks were evident regardless of age, race, sex, and cardiovascular risk factors, including patients without preceding cardiovascular disease before COVID‐19 infection. The underlying factors driving persistently raised markers of hemostasis and inflammation are yet to be established; these could include SARS‐CoV‐2 viral antigen persistence in tissues 26 and in monocytes 27 causing sustained endothelial activation, inflammation, and hypercoagulability. Other plausible mechanisms include immune dysregulation 28 with failure to return to baseline homeostasis, dysfunctional endothelium, 29 or acquired autoimmunity. 30
Given our findings, thromboprophylaxis may need to be considered for high‐risk recovered COVID‐19 patients who have elevated biomarkers of hypercoagulability, endothelial dysfunction, and inflammation. However, presently the role of extended duration of thromboprophylaxis post‐hospital discharge is controversial with no consensus established. The ACTION trial
31
showed that therapeutic‐dose rivaroxaban (in‐hospital and post‐discharge) for 30 days was not superior to prophylactic‐dose heparin given inpatient and was associated with higher bleeding risk. In contrast, a large registry of 4906 patients (the CORE‐19 registry)
32
showed that post‐discharge anticoagulation was significantly associated with a reduction in composite risk of VTE, arteriothrombotic embolism, and all‐cause mortality by 46%. The MICHELLE trial
33
showed that in hospitalized patients with high VTE and low bleeding risk, post‐discharge low‐dose rivaroxaban was efficacious in reducing thrombotic events and thrombotic‐related death with a low risk of major bleeding. Our study provides evidence of elevated biomarkers that may predispose to post COVID‐19 thrombosis as well as possible accelerated arteriosclerosis in our population of patients. As most patients will survive and recover from COVID‐19 infection, further studies are required to provide mechanistic insights on COVID‐19 vascular pathobiology to enable stratification of patients with the highest pro‐thrombotic risk for data‐driven risk stratification for extended post‐discharge thromboprophylaxis to mitigate thrombotic and cardiovascular disease.
days was not superior to prophylactic‐dose heparin given inpatient and was associated with higher bleeding risk. In contrast, a large registry of 4906 patients (the CORE‐19 registry)
32
showed that post‐discharge anticoagulation was significantly associated with a reduction in composite risk of VTE, arteriothrombotic embolism, and all‐cause mortality by 46%. The MICHELLE trial
33
showed that in hospitalized patients with high VTE and low bleeding risk, post‐discharge low‐dose rivaroxaban was efficacious in reducing thrombotic events and thrombotic‐related death with a low risk of major bleeding. Our study provides evidence of elevated biomarkers that may predispose to post COVID‐19 thrombosis as well as possible accelerated arteriosclerosis in our population of patients. As most patients will survive and recover from COVID‐19 infection, further studies are required to provide mechanistic insights on COVID‐19 vascular pathobiology to enable stratification of patients with the highest pro‐thrombotic risk for data‐driven risk stratification for extended post‐discharge thromboprophylaxis to mitigate thrombotic and cardiovascular disease.
The limitations of our study include the use of plasma samples from a historical healthy cohort (while having the benefit that all samples were collected before November 2019, hence individuals would not have prior SARS‐CoV‐2 infection) which may not be fully representative of a prospective age and gender‐matched cohort during this COVID‐19 pandemic. In addition, a separate control group of patients with non‐COVID‐19 viral infections such as influenza or other human coronavirus types will help provide a more robust study methodology. We also recognize the lack of specific evaluation of ADAMTS13 and a functional assay for von Willebrand Factor (vWF), which would have both strengthened the findings. vWF:Ag is noted to be raised, but it is unclear if the raised vWF:Ag represents specifically a more active vWF (e.g., High molecular weight vWF), which could have been assessed using a functional assay such as vWF:RCo, vWF:GPIbR, VWF:GPIbM and/or vWF:CB, and further reported as vWF activity/Ag ratio. Similarly, as vWF:Ag is raised, the contribution of ADAMTS13 (perhaps relative reduction) could have been assessed, and furthermore reported as vWF/ADAMTS13 ratio. While our study is still enrolling patients, we acknowledge our present sample size is small and our selection of the limited number of parameters and biomarkers was based on existing published evidence during acute SARS‐CoV‐2 infection, where recruitment of a larger cohort and a broader panel of biomarker testing may yield further information.
5. CONCLUSION
Hypercoagulability, Endotheliopathy, and inflammation extend well beyond acute SARS‐CoV‐2 infection, where recovered COVID‐19 patients may develop long‐term thrombotic and cardiovascular complications. This underpins the need for robust public health measures and sound primary prevention policies in preventing SARS‐CoV‐2 infection as well as appropriate follow‐up strategies for recovered patients.
AUTHOR CONTRIBUTIONS
Dr. Bingwen Eugene Fan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Bingwen Eugene Fan, Yew Woon Chia, Eng Soo Yap, Chuen Wen Tan, Barnaby Edward Young, David Chien Lye; Performing laboratory tests: Christina Lai Lin Sum, Bernard PuiLam Leung; Statistical analysis: Gek Hsiang Lim; and Drafting of the manuscript: Bingwen Eugene Fan. Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content: All authors (Bingwen Eugene Fan, Shiun Woei Wong, Christina Lai Lin Sum, Gek Hsiang Lim, Bernard PuiLam Leung, Chuen Wen Tan, Kollengode Ramanathan, Rinkoo Dalan, Christine Cheung, Xin Rong Lim, Mucheli Shravan Sadasiv, David Chien Lye, Barnaby Edward Young, Eng Soo Yap, Yew Woon Chia).
Supporting information
Data S1. Supporting information.
ACKNOWLEDGMENTS
The authors greatly appreciate the efforts of our fellow healthcare workers during this pandemic. Special thanks to Sysmex Corporation (Japan), Diagnostica Stago (France), and All Eights (Singapore) Pte Ltd. for their technical support.
Notes
Fan BE, Wong SW, Sum CLL, et al. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long‐term outcomes in COVID‐19 patients. Am J Hematol. 2022;97(7):915‐923. 10.1002/ajh.26575
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
year after recovery: Assessing the long‐term outcomes in COVID‐19 patients. Am J Hematol. 2022;97(7):915‐923. 10.1002/ajh.26575
[Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Funding information National Healthcare Group‐National Centre for Infectious Diseases (NHG‐NCID) COVID‐19 Centre Grant, Grant/Award Number: COVID19 CG008
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1002/ajh.26575
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9073976
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/127472566
Article citations
Psychosocial Aspects of the Lived Experience of Long COVID: A Systematic Review and Thematic Synthesis of Qualitative Studies.
Health Expect, 27(5):e70071, 01 Oct 2024
Cited by: 0 articles | PMID: 39445819 | PMCID: PMC11500211
Review Free full text in Europe PMC
COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase.
JACC Adv, 3(8):101107, 17 Jul 2024
Cited by: 0 articles | PMID: 39113913 | PMCID: PMC11304887
Review Free full text in Europe PMC
Thromboinflammation in COVID-19: Unraveling the interplay of coagulation and inflammation.
Medicine (Baltimore), 103(28):e38922, 01 Jul 2024
Cited by: 2 articles | PMID: 38996158 | PMCID: PMC11245273
Review Free full text in Europe PMC
Endothelial dysfunction and persistent inflammation in severe post-COVID-19 patients: implications for gas exchange.
BMC Med, 22(1):242, 13 Jun 2024
Cited by: 0 articles | PMID: 38867241
Effects of Pycnogenol® in people with post-COVID-19 condition (PYCNOVID): study protocol for a single-center, placebo controlled, quadruple-blind, randomized trial.
Trials, 25(1):385, 15 Jun 2024
Cited by: 0 articles | PMID: 38879571 | PMCID: PMC11179231
Go to all (36) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Global haemostatic tests demonstrate the absence of parameters of hypercoagulability in non-hypoxic mild COVID-19 patients: a prospective matched study.
J Thromb Thrombolysis, 53(3):646-662, 28 Sep 2021
Cited by: 8 articles | PMID: 34581945 | PMCID: PMC8476716
Persistent endotheliopathy in the pathogenesis of long COVID syndrome.
J Thromb Haemost, 19(10):2546-2553, 12 Sep 2021
Cited by: 158 articles | PMID: 34375505 | PMCID: PMC8420256
Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction.
J Thromb Haemost, 20(10):2429-2438, 04 Aug 2022
Cited by: 30 articles | PMID: 35875995 | PMCID: PMC9349977
Long-term hypercoagulability, endotheliopathy and inflammation following acute SARS-CoV-2 infection.
Expert Rev Hematol, 16(12):1035-1048, 01 Jul 2023
Cited by: 7 articles | PMID: 38018136
Review

 1
,
2
,
3
,
4
1
,
2
,
3
,
4



