Abstract
Free full text

Diagnostic Potential of the Serological Response to Synthetic Peptides from Mycobacterium tuberculosis Antigens for Discrimination Between Active and Latent Tuberculosis Infections
Abstract
Diagnosis and treatment of active tuberculosis (ATB) as well as latent tuberculosis infection (LTBI) are required for effective tuberculosis (TB) control, especially in TB endemic area. The usefulness of conventional tests to distinguish between ATB and LTBI has remained challenging. The present study was aimed to demonstrate the usefulness of the serological response to synthetic peptides from Mycobacterium tuberculosis (Mtb) antigens for discrimination between ATB and LTBI in Warao Amerindians. Serum IgG antibody levels were measured by the indirect ELISA assay using 22 designed and synthesized peptides derived from immunogenic Mtb ESAT-6 and Ag85A proteins. A total of 211 adult Warao Amerindians were included; cases with active TB (ATB, n =
= 75), latent TB infection (LTBI, n
75), latent TB infection (LTBI, n =
= 85) and non-infected (NI, n
85) and non-infected (NI, n =
= 51). The approach’s diagnostic information was compared using receiver operating characteristic (ROC) curves. For ATB diagnostic performance between ATB and NI; ESAT-6; P-12037 had 100% of sensitivity (AUC
51). The approach’s diagnostic information was compared using receiver operating characteristic (ROC) curves. For ATB diagnostic performance between ATB and NI; ESAT-6; P-12037 had 100% of sensitivity (AUC =
= 0.812; 0.733 to 0.891 95% CI); and Ag85A; P-10997 had 100% of specificity (AUC
0.812; 0.733 to 0.891 95% CI); and Ag85A; P-10997 had 100% of specificity (AUC =
= 0.691; 0.597 to 0.785 95% CI); and ATB and LTBI; Ag85A; P-29878 had 100% of sensitivity (AUC
0.691; 0.597 to 0.785 95% CI); and ATB and LTBI; Ag85A; P-29878 had 100% of sensitivity (AUC =
= 0.741; 0.666–0.817 95% CI), and P-29879 had 99% of specificity (AUC
0.741; 0.666–0.817 95% CI), and P-29879 had 99% of specificity (AUC =
= 0.679; 0.593–0.765 95% CI). While that ESAT-6 P-12037 also allowed differentiation between LTBI and NI or healthy ones. It had 98.8% of sensitivity and 98.0% of specificity (AUC
0.679; 0.593–0.765 95% CI). While that ESAT-6 P-12037 also allowed differentiation between LTBI and NI or healthy ones. It had 98.8% of sensitivity and 98.0% of specificity (AUC =
= 0.640; 0.545–0.735 95% CI). The potential of combination-antigen immunoassays with peptides could discriminate between Warao Amerindians with ATB, LTBI and NI. Further validation of this approach could lead to developing a complementary tool for rapid diagnosis of TB infections.
0.640; 0.545–0.735 95% CI). The potential of combination-antigen immunoassays with peptides could discriminate between Warao Amerindians with ATB, LTBI and NI. Further validation of this approach could lead to developing a complementary tool for rapid diagnosis of TB infections.
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) is one of the infectious diseases having the greatest distribution worldwide; it remains a major threat to human health in developing countries. The World Health Organization (WHO) report 2019 stated that 10 million (range 9.0–11.1 million) people were ill with TB, resulting in 1.2 million deaths, accompanied by an increasing amount of new cases (around 87%) occurring in the 30 countries endemic for this disease including Venezuela (WHO, 2019). In Amerindian population tend to have much higher rates of TB in comparison with the general (non-indigenous) population; in Latin America and the Caribbean there are some 40 million indigenous people divided into 400 indigenous groups. Five countries group almost 90% of the regional indigenous population: Bolivia, Guatemala, Peru, Ecuador and Mexico (Montenegro et al. 2006). In 2006, WHO reported that Venezuela had an estimated incidence and prevalence rate of TB that made it occupy the fourth position in South America (INE 2007; MPPS 2007). According to the National Population and Housing Census of 2011 in Venezuela there are 724,592 inhabitants from 52 indigenous groups of Venezuela, corresponding to 2.7% of the national population; the Warao Amerindian population is the second most important ethnic of the country with 48.771 inhabitants (6.7% of indigenous people), they mainly live at the Venezuelan Orinoco delta located in Delta Amacuro state (INE 2015). Data from the Coordination of the Regional Tuberculosis Control Program of the Delta Amacuro state reported that the Delta Amacuro state, in 1998, had the highest TB prevalence rate in Venezuela, with 54.6/100,000 inhabitants, increasing to 93.2/100,000 inhabitants in 1999 (MSAS 1999); so since 1999, the Delta Amacuro state leads with the highest prevalences of TB in the country.
The National Integrated TB Control Program of Venezuela records the variables “indigenous” and “ethnicity” among the patient data; this has allowed the program to know the detailed statistics of the cases registered in indigenous patients. Between 2012 and 2017, the Warao Amerindians people had the highest TB cases among Creole or non-indigenous individuals and different indigenous groups in Venezuela; the latter 82% occurred in the Delta Amacuro state, which mainly live at the Venezuela’s Orinoco delta region and 18% in other states. Averaging the number of cases that occurred between 2012 and 2017, and based on the population registered in the 2011 census, the average TB incidence rate in Delta Amacuro corresponds to 61.8/100,000 inhabitants (INE 2015; MPPS 2018). As mentioned above, in Amerindian population tends to have much higher rates of TB than the general (non-indigenous) population (Narasimhan et al. 2013; Tollefson et al. 2013; Malacarne et al. 2016). Recently it had been reported that in the admixed population of the Brazilian Amazon region; Amerindian ancestry in the 20–60% range was found to be the principal risk factor for increased susceptibility to TB; so the genetic ancestry of Amerindians may be an important factor in the development of infections, which is an important risk factor for susceptibility to TB (Da Leal et al. 2020).
The rapid diagnosis and treatment of TB is essential to contain the disease at an early stage and to lower its prevalence. In addition, diagnosis and treatment of latent tuberculosis infection (LTBI) as well as active tuberculosis (ATB) are required for effective TB control. Latency and active disease are components of the dynamic spectrum of TB (Barry et al. 2009); as latent TB bacilli could reactivate later to cause active TB, diagnosis and treatment of LTBI is also important. The diagnosis of TB is confirmed by a culture of Mtb but is typically delayed because it takes approximately 2–8 weeks to receive the results (Canadian Thoracic Society 2013). Moreover, there are no diagnostic gold standards for LTBI, and all existing tests for LTBI are indirect approaches that provide immunological evidence of host sensitization to TB antigens (Pai et al. 2014). Two tests currently used to diagnose LTBI are the tuberculin skin test (TST) and the blood interferon-gamma release assay (IGRA), both of which do not distinguish between ATB and LTBI (Pai et al. 2014). The little diffusion and the marginalization to which the knowledge of the suffering of the diseases of our indigenous communities has been relegated; this was the reason why we wanted to evaluate diagnostic value and demonstrate the usefulness of serological methods studying the immunoglobulin G (IgG) or B-cell responses to synthetic peptides, which covering the complete sequence of the early secreted antigen-6 (ESAT-6) and the antigen 85A (Ag85A) of Mtb for diagnostic purposes of ATB and LTBI among Warao Amerindians from a highly TB endemic area.
Methods
Study Population and Study Site
A total of 211 Warao Amerindians aged over 15 years were recruited from indigenous communities living in a remote rural area in Venezuela’s delta region; cases with active pulmonary TB infection (ATB, n =
= 75), latent TB infection (LTBI, n
75), latent TB infection (LTBI, n =
= 85) and non-infected individuals (NI, n
85) and non-infected individuals (NI, n =
= 51). Inclusion criteria took into account recommendation previously reported (Araujo et al. 2019). The group of cases was composed of participants with confirmed pulmonary TB or ATB according to the Regional or Delta State Tuberculosis Control Programme. The control group was composed of indigenous from Warao Amerindians communities in contact with active TB patients with the TST positive, according to international or the World Health Organization (WHO) and the National Tuberculosis Control Program of Venezuela (indigenous positive having
51). Inclusion criteria took into account recommendation previously reported (Araujo et al. 2019). The group of cases was composed of participants with confirmed pulmonary TB or ATB according to the Regional or Delta State Tuberculosis Control Programme. The control group was composed of indigenous from Warao Amerindians communities in contact with active TB patients with the TST positive, according to international or the World Health Organization (WHO) and the National Tuberculosis Control Program of Venezuela (indigenous positive having ≥
≥ 10 mm indurations), which were classified as latent TB infection or LTBI with neither symptoms nor diagnostic confirmed of TB and healthy indigenous without evidence of clinical symptoms suggesting pulmonary TB infection and with the TST negative were included as controls or NI individuals.
10 mm indurations), which were classified as latent TB infection or LTBI with neither symptoms nor diagnostic confirmed of TB and healthy indigenous without evidence of clinical symptoms suggesting pulmonary TB infection and with the TST negative were included as controls or NI individuals.
Clinical Features, Microscopy, the Tuberculin Skin Test and Chest Radiograph
Individuals having evidence of clinical symptoms suggesting pulmonary TB infection were diagnosed as having pulmonary TB using at least one of the following previously applied criteria: X-ray suggestive of TB and positive sputum smear or positive sputum culture. Clinical features consistent with TB, such as recent weight loss or inadequate progress of weight gain, prolonged febrile syndrome, night sweats, coughing or wheezing for more than two weeks, were also taken into account (Araujo et al. 2019).
Regarding confirmatory TB diagnosis, sputum was collected for investigating alcohol/acid-fast bacilli for all Warao Amerindians having respiratory symptoms. The smears were stained using the Ziehl–Neelsen direct method. For each sputum sample; two tubes of modified Ogawa egg medium and Lowenstein–Jensen were inoculated using the Kudoh’s method (Kudoh and Kudoh 1974). The latter was performed by the Laboratory of the Regional or Delta State Tuberculosis Control Programme in the “Dr. Luis Razetti” Hospital located in Tucupita, Delta Amacuro state’s capital; diagnosed cases were confirmed by the Laboratory of Tuberculosis at the “Dr. Jacinto Convit” Biomedicine Institute-Central University of Venezuela in Caracas, Venezuela’s capital. The TST was administered according to the Mantoux method; 2 tuberculin units (0.1 mL) of purified protein derivate (RT23 PPD; Statens Serum Institute, Copenhagen, Denmark) were injected intra-dermally, as previously described (Arnadottir et al. 1996). Trained professionals did the reading between 48 and 72 h after administration. Positive test reactivity criteria were based on transversal diameter measurements of the indurations on the volar surface of the forearm (≥ 10 mm), according to international guidelines.
10 mm), according to international guidelines.
Standard antero-posterior and lateral CXRs were taken from all individuals for TB confirmation; Warao Amerindians’ radiological study was aimed at searching for CXR characteristics regarding lesions suggestive of ATB; the latter performed in the “Luis Razzetti” hospital’s X-ray service in Tucupita, Delta Amacuro state. Inclusion criteria for healthy or control individuals consisted of the absence of a clinical picture suggesting pulmonary TB infection in which HIV and active TB had been ruled out by blood test, microbiological assays and X-ray. Individuals who had been prescribed immunosuppressive drugs (i.e., corticosteroids, azathioprine, and cyclophosphamide) were also excluded, as were participants who did not sign an informed consent agreement.
Treatment was initiated in all identified TB cases where microbiological evidence suggestive of TB, bacteriological confirmation by bacilloscopy or culture were found, and in all CXR studies where findings were consistent with evidence of clinical symptoms suggesting pulmonary TB infection as recommended by the Venezuelan National TB Control Program. TB patients were sampled before beginning anti-TB treatment. Written consent were obtained from each participant and oral explanation about the research work was given to them.
Synthetic Peptides
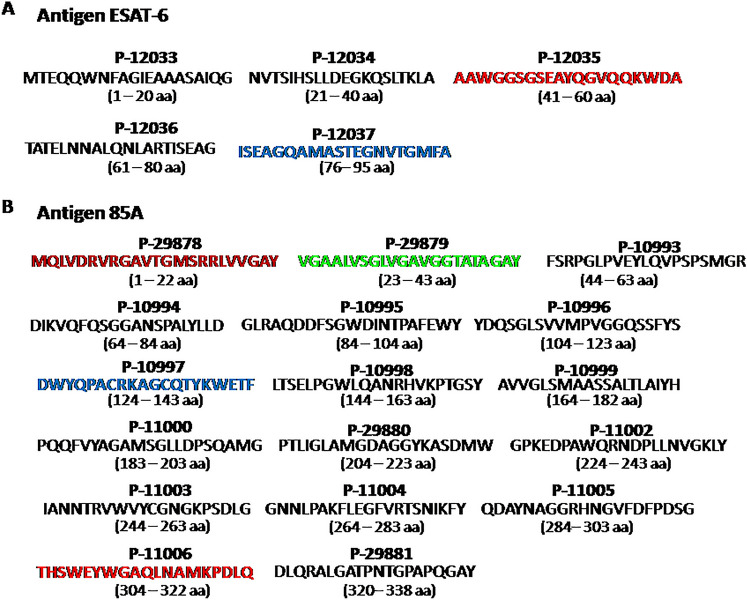
Twenty two synthetic peptide full-length sequences from ESAT-6 (Fig. 1A) and Ag85A (Fig. 1B) antigens were synthesised at the Fundación Instituto de Inmunología de Colombia (FIDIC) in Bogotá, Colombia, and used as single peptides. The solid-phase multiple peptide system was used for synthesising peptides based on M. tuberculosis ESAT-6 and Ag85A amino acid (aa) sequences (Tam et al. 1983; Houghten 1985); of these five synthetic peptides, two ESAT-6 peptides; P-12035 and P-12037 (Fig. 1A), and 4 Ag85A peptides; P-29878, P-29879, P-10997, and P-11006 (Fig. 1B) were selected for discrimination among ATB and LTBI and NI individuals.
Serodiagnosis
Peptide-based indirect indirect enzyme-linked immunosorbent assay (ELISA) was used for determining IgG reactivity against ESAT-6 and Ag85A peptides in sera obtained from Warao Amerindians as previously reported (Araujo et al. 2013). Briefly, sera were obtained from Warao adults with active and latent TB infections and non-infected venous blood. ESAT-6 or Ag85A synthetic peptides at 1 μg/well were then used as antigens to coat 96-well microtitre plates. Indirect ELISA assays were carried out and standardised in our laboratory for measuring Ab (IgG) reactivity against peptides. Standardised serum sample dilution was 1:200. After incubating sera for one hour, the plates were washed four times and then incubated with a peroxidase-conjugated goat anti-human IgG Ab (Promega Corporation, US). The plates were then washed four times and a substrate solution consisting of citrate buffer at pH 5.0, 30% H2O2 and 10 mg o-phenylenediamine dihydrochloride (OPD, Sigma-Aldrich) was added. The plates were incubated for 6 min at room temperature. An ELISA microplate reader was used for measuring colour development at 492 nm.
Statistical Analysis
A Student t-test was used for comparing average age among ATB, LTBI and NI groups. Fisher’s exact test was used for comparing significance between percentages for individuals proving positive and/or negative by TST. GraphPad Prism version 5.00 for Windows (GraphPad Software, La Jolle, CA, USA, http://www.graphpad.com) was used for data analyses. Antibody levels were presented as OD values, the Mann–Whitney U test was used for comparing differences of OD value distributions among groups; additionally the crude p-values were corrected with the Bonferroni method for multiple comparisons (Ranstam 2016), which was used to compare antibody responses among ATB, LTBI, and NI groups; distribution of the data were presented in terms of medians together with their corresponding measures of dispersion (interquartile ranges—IQR). The STATA14® Software was used for these analyzes, ≤
≤ 0.05 p-values were considered statistically significant. Receiver operating characteristic (ROC) curves were constructed for comparing the methods’ overall diagnostic information.
0.05 p-values were considered statistically significant. Receiver operating characteristic (ROC) curves were constructed for comparing the methods’ overall diagnostic information.
Ethical Approval and Consent to Participate
This study complied with the Helsinki Declaration’s principles. It was approved by the Biomedicine Institute Dr. Jacinto Convit”-Central University of Venezuela’s Research Ethics Committee (protocol number CDCH-UCV-6256-8007/2011). All participating indigenous signed voluntary informed consent forms.
Results
Characteristics of the Study Participants
A total of 211 Warao Amerindians aged over 15 years were recruited from indigenous communities living in a remote rural area in Venezuela’s delta region. The baseline characteristics of the participants are summarized in Table Table1.1. Ages were not significantly different among groups (ATB, LTBI and NI). Sexes were significantly different between LTBI and NI groups; the proportion of males in the LTBI group was highest (61.2%) compared to females (38.8%), p <
< 0.002; while that the proportion of females in the NI group was highest (64.7%) compared to males (35.3%), p
0.002; while that the proportion of females in the NI group was highest (64.7%) compared to males (35.3%), p <
< 0.0001. Concerning TST
0.0001. Concerning TST +
+ status, it was significantly different between LTBI (79.5%) and ATB (100.0%) groups, p
status, it was significantly different between LTBI (79.5%) and ATB (100.0%) groups, p <
< 0.0001 (Table (Table11).
0.0001 (Table (Table11).
Table 1
Demographic and clinical information of the Warao Amerindians enrolled in the study
NI (n = = 51) 51) | LTBI (n = = 85) 85) | ATB (n = = 75) 75) | |
|---|---|---|---|
| Demographics | |||
Age (years median ± ± SD) SD) | 31.7 ± ± 15.7 15.7 | 36.0 ± ± 15.9 15.9 | 34.5 ± ± 13.6 13.6 |
| Sex | |||
| Male | 18 (35.3%)a | 52 (61.2%)c | 39 (52.0%) |
| Female | 33 (64.7%)b | 33 (38.8%)d | 36 (48.0%) |
| Tuberculin skin test (+) | 79.5e | 100f | |
| Bacteriological examinations | |||
| Acid-fast staining of sputum (%) | n/d | n/d | 68 |
| Positive Mtb culture of sputum (%) | n/d | n/d | 100 |
| Radiographical examinations | n/d | n/d | 100 |
There was a statistically significant difference between females and males (a) and (b) (p <
< 0.002) and (c) and (d) (p
0.002) and (c) and (d) (p <
< 0.0001). Concerning TST+
0.0001). Concerning TST+  status, there was a statistically significant difference among (e) and (f) (p
status, there was a statistically significant difference among (e) and (f) (p <
< 0.0001)
0.0001)
n/d test was not determined
The IgG Reactivity Distribution against M. tuberculosis Synthetic Peptides
Figure 2 shows IgG reactivity distributions among Warao Amerindians with ATB, LTBI and NI individuals. ATB’s IgG reactivities against five peptides were significantly higher P-12035 (0.589 ±
± 0.281), P-12037 (0.711
0.281), P-12037 (0.711 ±
± 0.361), P-10997 (0.741
0.361), P-10997 (0.741 ±
± 0.389), P-29878 (0.645
0.389), P-29878 (0.645 ±
± 0.311) and P-29879 (0.661
0.311) and P-29879 (0.661 ±
± 0.326) than NI individuals; (0.482
0.326) than NI individuals; (0.482 ±
± 0.286) p
0.286) p <
< 0.03 (Fig. 2A), (0.510
0.03 (Fig. 2A), (0.510 ±
± 0.230) p
0.230) p <
< 0.01 (Fig. (Fig.2B), (0.501
0.01 (Fig. (Fig.2B), (0.501 ±
± 0.327)2B), (0.501
0.327)2B), (0.501 ±
± 0.327) p
0.327) p <
< 0.0003 (Fig. 2C), (0.460
0.0003 (Fig. 2C), (0.460 ±
± 0.245) p
0.245) p <
< 0.0001 (Fig. 2E) and (0.523
0.0001 (Fig. 2E) and (0.523 ±
± 0.280) p
0.280) p <
< 0.01, respectively (Fig. 2F), and also than LTBI individuals; (0.442
0.01, respectively (Fig. 2F), and also than LTBI individuals; (0.442 ±
± 0.222) p
0.222) p <
< 0.0003 (Fig. 2A), (0.538
0.0003 (Fig. 2A), (0.538 ±
± 0.358) p
0.358) p <
< 0.0008 (Fig. 2C), (0.401
0.0008 (Fig. 2C), (0.401 ±
± 0.216) p
0.216) p <
< 0.0001 (Fig. 2E), and (0.470
0.0001 (Fig. 2E), and (0.470 ±
± 0.218) p
0.218) p <
< 0.0001, respectively (Fig. 2F). In addition, LTBI’s IgG reactivity against P-11006 was significantly higher (0.493
0.0001, respectively (Fig. 2F). In addition, LTBI’s IgG reactivity against P-11006 was significantly higher (0.493 ±
± 0.251) than ATB (0.326
0.251) than ATB (0.326 ±
± 0.231) p
0.231) p <
< 0.0001 (Fig. 2D); for the P-12037 peptide, a significant difference was also found between LTBI (0.616
0.0001 (Fig. 2D); for the P-12037 peptide, a significant difference was also found between LTBI (0.616 ±
± 0.244) and NI (0.512
0.244) and NI (0.512 ±
± 0.229) p
0.229) p <
< 0.01 (Fig. 2B).
0.01 (Fig. 2B).
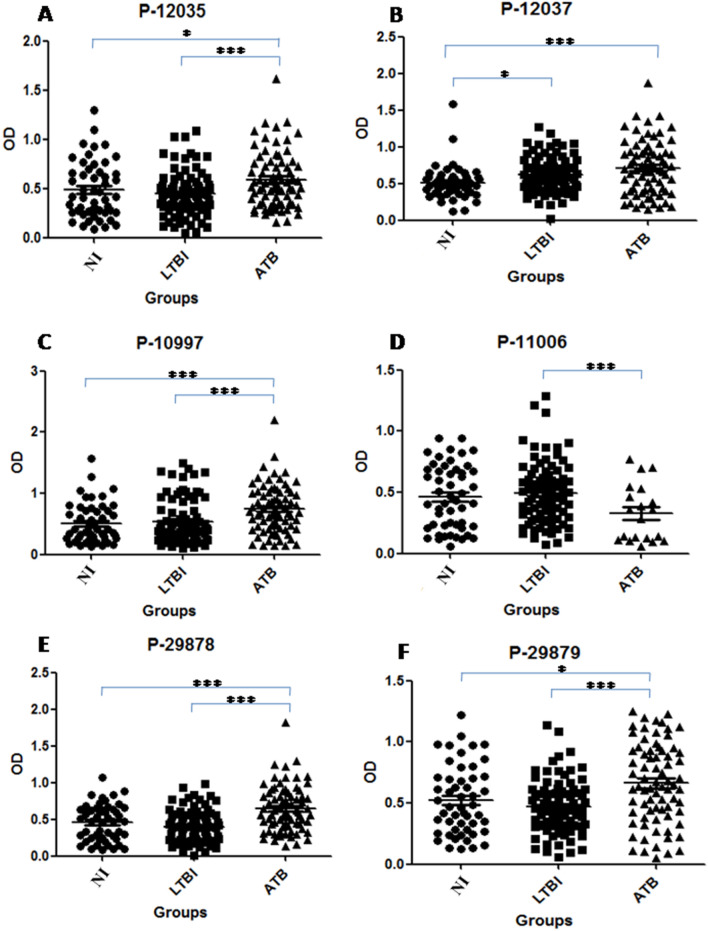
The IgG reactivity distribution against M. tuberculosis synthetic peptides. The optical density (OD) value distribution concerning antibody reactivities between Warao Amerindians with active tuberculosis (ATB, filled triangles), latent tuberculosis infection (LTBI, filled squares) and non infection (NI, filled circles) or healthy individuals. OD of the anti IgG: P-12035 (A), P-12037 (B), P-10997 (C), P-11006 (D), P-29878 (E) and P-29879 (F). *p <
< 0.05; **p
0.05; **p <
< 0.001; ***p
0.001; ***p <
< 0.0001
0.0001
Multiple Comparisons of the IgG Reactivity Against Synthetic Peptides
In order to corrected p values, the Bonferroni method for multiple comparisons was done; the data corresponding for anti-peptide IgG levels was presented in terms of medians along with their corresponding dispersion measures (IQRs). The anti-ESAT-6 IgG levels were significantly higher for P-12035 peptide in ATB (0.531; 0.354–0.753) than LTBI (0.413; 0.317–0.535), and also than NI (0.414; 0.261–0.664) (p <
< 0.0145 Bonferroni correction) (Fig. 3A); for P-12037 peptide in ATB (0.686; 0.401–0.931) than LTBI (0.564; 0.446–0.808), and also than NI (0.483; 0.386–0.583) (p
0.0145 Bonferroni correction) (Fig. 3A); for P-12037 peptide in ATB (0.686; 0.401–0.931) than LTBI (0.564; 0.446–0.808), and also than NI (0.483; 0.386–0.583) (p <
< 0.0085 Bonferroni correction) (Fig. 3B). For anti-Ag85A peptides; median IgG reactivity distribution was significantly higher for P-10997 in ATB (0.697; 0.432–1.016) than LTBI (0.416; 0.262–0.709), and NI (0.369; 0.249–0.719) (p
0.0085 Bonferroni correction) (Fig. 3B). For anti-Ag85A peptides; median IgG reactivity distribution was significantly higher for P-10997 in ATB (0.697; 0.432–1.016) than LTBI (0.416; 0.262–0.709), and NI (0.369; 0.249–0.719) (p <
< 0.0085 Bonferroni correction) (Fig. 3C). For P-11006; there was no significant for multiple IgG reactivity comparisons (p
0.0085 Bonferroni correction) (Fig. 3C). For P-11006; there was no significant for multiple IgG reactivity comparisons (p <
< 0.1235 Bonferroni correction) (Fig. 3D). Median IgG reactivity distribution was significantly higher for P-29878 in ATB (0.595; 0.434–0.855) than LTBI (0.394; 0.227–0.548), and also than NI (0.477; 0.246–0.638) (p
0.1235 Bonferroni correction) (Fig. 3D). Median IgG reactivity distribution was significantly higher for P-29878 in ATB (0.595; 0.434–0.855) than LTBI (0.394; 0.227–0.548), and also than NI (0.477; 0.246–0.638) (p <
< 0.0005 Bonferroni correction) (Fig. 3E). Also median IgG reactivity distribution was significantly higher for P-29879 in ATB (0.638; 0.426–0.926) than LTBI (0.453; 0.321–0.598), and also than NI (0.473; 0.285–0.710) (p
0.0005 Bonferroni correction) (Fig. 3E). Also median IgG reactivity distribution was significantly higher for P-29879 in ATB (0.638; 0.426–0.926) than LTBI (0.453; 0.321–0.598), and also than NI (0.473; 0.285–0.710) (p <
< 0.0025 Bonferroni correction) (Fig. 3F).
0.0025 Bonferroni correction) (Fig. 3F).
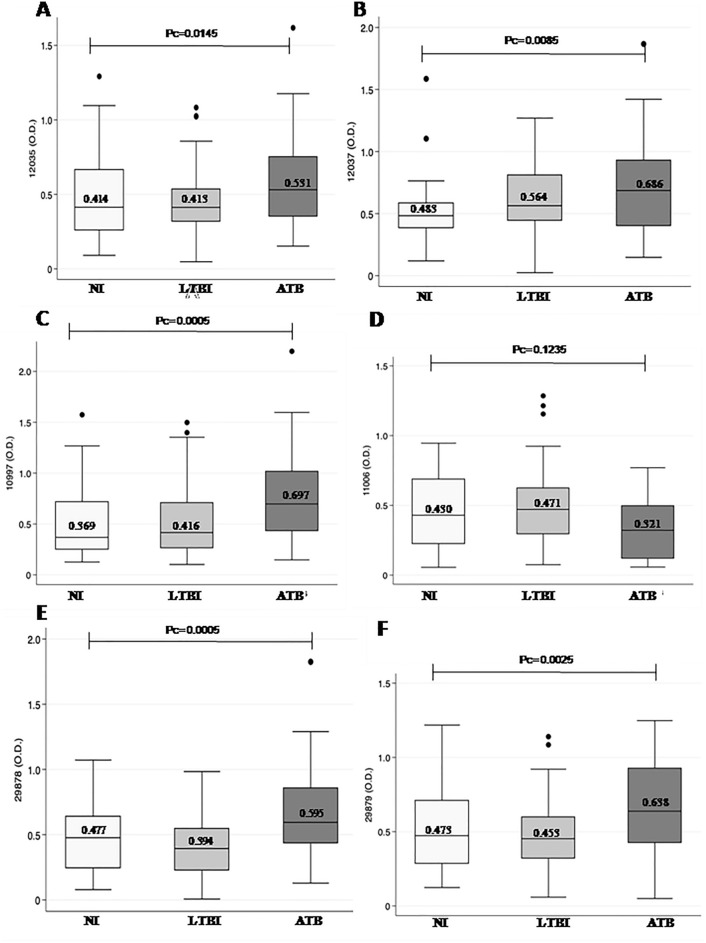
Multiple comparisons of the IgG reactivity against synthetic peptides. Groups of Warao Amerindians with active tuberculosis (ATB, filled triangles), latent tuberculosis infection (LTBI, filled squares) and non infection (NI, filled circles) or healthy individuals were compared by one‐way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons. OD of the anti IgG: P-12035 (A), P-12037 (B), P-10997 (C), P-11006 (D), P-29878 (E) and P-29879 (F). *p <
< 0.05; **p
0.05; **p <
< 0.001; ***p
0.001; ***p <
< 0.0001
0.0001
Diagnostic Performance of B Immune Responses Aga- Synthetic Peptides for Diagnosis of ATB and LTBI
The approach’s diagnostic information was compared using ROC curves. For ATB diagnostic performance, the ROC analysis showed a high diagnostic performance between ATB and NI; P-12037 had 100% of sensitivity (AUC =
= 0.812; 0.733 to 0.891 95% CI) p
0.812; 0.733 to 0.891 95% CI) p <
< 0.0001 (Fig. 4A); while that P-10997 had 100% of specificity (AUC
0.0001 (Fig. 4A); while that P-10997 had 100% of specificity (AUC =
= 0.691
0.691 ±
± 0.048: 0.597–0.785 95% CI) p
0.048: 0.597–0.785 95% CI) p <
< 0.0002 (Fig. 4B). The optimum cutoffs obtained were
0.0002 (Fig. 4B). The optimum cutoffs obtained were >
> 0.125 and
0.125 and >
> 1.585 for ESAT-6 P-12037 and Ag85A P-10997, respectively.
1.585 for ESAT-6 P-12037 and Ag85A P-10997, respectively.
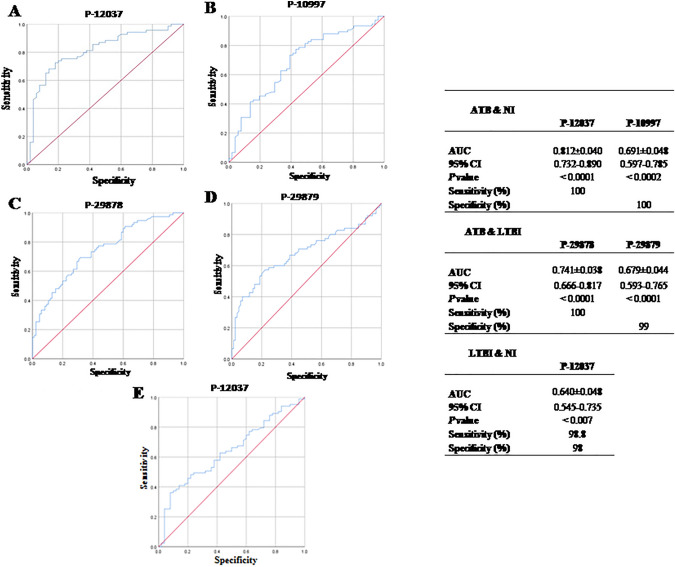
Diagnostic performance of B immune responses against synthetic peptides for diagnosis of ATB and LTBI. Receiver operating characteristic (ROC) curve analysis were used to evaluate the discriminating power of synthetic peptides between active tuberculosis (ATB) and non‐infected (NI); P-12037 (A) and P-10997 (B), and ATB and latent tuberculosis infection (LTBI); P-29878 (C) and P-29879 (D), and LTBI and NI; P-12037 (E). Area under the curve (AUC), 95% confidence interval (95% CI), sensitivity, specificity and p value
For ATB diagnostic performance between ATB and LTBI; P-29878 had 100% of sensitivity (AUC =
= 0.741
0.741 ±
± 0.038: 0.666–0.817 95% CI) p
0.038: 0.666–0.817 95% CI) p <
< 0.0001(Fig. 4C); while that P-29879 had 99.0% of specificity (AUC
0.0001(Fig. 4C); while that P-29879 had 99.0% of specificity (AUC =
= 0.679
0.679 ±
± 0.044: 0.593–0.765 95% CI), p
0.044: 0.593–0.765 95% CI), p <
< 0.0001 (Fig. 4D). The optimum cutoffs obtained for Ag85A P-29878 and P-29879 were;
0.0001 (Fig. 4D). The optimum cutoffs obtained for Ag85A P-29878 and P-29879 were; >
> 0.128 and
0.128 and >
> 1.150, respectively.
1.150, respectively.
Regarding ROC curve analysis performed for LTBI diagnostic performance between LTBI and NI; P-12037 had 98.8% of sensitivity and 98.0% of specificity (AUC =
= 0.640
0.640 ±
± 0.048: 0.545–0.735 95% CI), p
0.048: 0.545–0.735 95% CI), p <
< 0.007 (Fig. 4E). The optimum cutoffs obtained for ESAT-6 P-12037 were;
0.007 (Fig. 4E). The optimum cutoffs obtained for ESAT-6 P-12037 were; >
> 0.171 and
0.171 and >
> 1.428, respectively.
1.428, respectively.
Discussion
The TB diagnosis is usually based on clinical signs and symptoms, chest radiography, tuberculin testing and history of contact with an open case of TB. A quick, sensitive and specific diagnostic modality is therefore, desirable for the TB diagnosis. A series of independent studies has shown that B-cells and antibodies (Abs) may contribute significantly to reduce the mycobacterial burden. It had been reported current evidence on the contribution of B-cells and Abs to immunity toward Mtb, their potential utility as biomarkers, and their functional contribution to Mtb control (Rijnink et al. 2021). The majority of Ab studies have been largely concentrated on their utility as diagnostic tools; with much attention for serological tests based on the detection of circulating Abs against Mtb specific antigens, because these have showed several advantages, as they are simple, cheap and feasible for point of care diagnostics. Many attempts have been made to develop a serologic TB test, which include the need to discriminate ATB from LTBI and finally able to perform consistently in genetically and immunologically diverse populations (Abebe et al. 2007; Ireton et al. 2010). Many studies have suggested the utility of antibody responses to TB antigens for the diagnosis of TB including the attention for dissection of Abs at the isotype and subclass level in relation to TB diagnosis; additionally, many reports proposed to perform the serological TB diagnosis using several combinations of antigens or specific antibodies (Araujo et al. 2004, Araujo et al. 2008, Araujo et al. 2013; Abebe et al. 2007; He et al. 2011).
There has been renewed interest in the development of antibody-based diagnostic assays that utilize multiple antigens to achieve high sensitivity and specificity. In the present study, IgG antibody levels were measured by the indirect ELISA assay using 22 designed and synthesized peptides derived from immunogenic Mtb ESAT-6 and Ag85A proteins, these levels were compared among ATB, LTBI and non-infected or NI Warao Amerindians. Results showed that six of these peptides could be used to distinguish among Warao Amerindians with ATB, LTBI and NI individuals; in the present work, the anti-ESAT-6 IgG levels against P-12035 and P-12037 peptides were significantly higher in Warao Amerindians with ATB; 0.589 ±
± 0.281 (P-12035), and 0.711
0.281 (P-12035), and 0.711 ±
± 0.362 (P-12037) than NI individuals; 0.482
0.362 (P-12037) than NI individuals; 0.482 ±
± 0.286, and 0.512
0.286, and 0.512 ±
± 0.229, respectively and also Warao with LTBI; 0.442
0.229, respectively and also Warao with LTBI; 0.442 ±
± 0.222, and 0.616
0.222, and 0.616 ±
± 0.244, respectively. Additionally, IgG against P-12037 was a significant biomarker, independently related to both ATB diagnosis and discrimination between LTBI (0.616
0.244, respectively. Additionally, IgG against P-12037 was a significant biomarker, independently related to both ATB diagnosis and discrimination between LTBI (0.616 ±
± 0.244) and NI (0.512
0.244) and NI (0.512 ±
± 0.229). The latter showed that ESAT-6 could be used to distinguish among patients with ATB, individuals with LTBI, and NI individuals. The ESAT-6 antigen is TB-specific secreted protein encoded by the RD1 gene of Mtb (Berthet et al. 1998); this is not present in Bacillus Calmette-Guérin (BCG) strains and in most other non-TB mycobacterial species (Andersen et al. 2000). ESAT-6 has been identified as a promising component for vaccine development with regard to human T cell recognition and protective efficacy (Williams et al. 2005; Langermans et al. 2005). On the other hand, Legesse et al. reported that IgA levels for ESAT-6 could be used to distinguish among patients with ATB, patients with LTBI, and non-infected subjects (Legesse et al. 2013). Similarly, like ESAT-6 antigen; 85 antigen (Ag85) has been investigated as a major antigen in candidate vaccines; due to its adaptability and ability to induce CD4 and CD8 T lymphocyte responses in a wide range of vertebrate hosts (Langermans et al. 2005). The Ag85 antigen is also a secreted protein of TB and is highly immunogenic, resulting in specific humoral and cell-mediated immune responses in both LTBI and ATB patients, and has been shown to induce partial protection in murine models of infection (Boesen et al. 1995; Belisle et al. 1997; Smith 2000). Recently Lee, et al., demonstrated the utility of the serological tests based on the detection of circulating antibodies against Mtb specific antigens; they reported that IgG levels against Mtb chorismate mutase (TBCM) were significantly higher in LTBI than non-infected subjects; while that IgG against Ag85B could distinguish between LTBI and ATB groups (Lee et al. 2020). Present results in relation to IgG levels against anti-Ag85A peptides were significantly higher between ATB and NI individuals; ATB: 0.741
0.229). The latter showed that ESAT-6 could be used to distinguish among patients with ATB, individuals with LTBI, and NI individuals. The ESAT-6 antigen is TB-specific secreted protein encoded by the RD1 gene of Mtb (Berthet et al. 1998); this is not present in Bacillus Calmette-Guérin (BCG) strains and in most other non-TB mycobacterial species (Andersen et al. 2000). ESAT-6 has been identified as a promising component for vaccine development with regard to human T cell recognition and protective efficacy (Williams et al. 2005; Langermans et al. 2005). On the other hand, Legesse et al. reported that IgA levels for ESAT-6 could be used to distinguish among patients with ATB, patients with LTBI, and non-infected subjects (Legesse et al. 2013). Similarly, like ESAT-6 antigen; 85 antigen (Ag85) has been investigated as a major antigen in candidate vaccines; due to its adaptability and ability to induce CD4 and CD8 T lymphocyte responses in a wide range of vertebrate hosts (Langermans et al. 2005). The Ag85 antigen is also a secreted protein of TB and is highly immunogenic, resulting in specific humoral and cell-mediated immune responses in both LTBI and ATB patients, and has been shown to induce partial protection in murine models of infection (Boesen et al. 1995; Belisle et al. 1997; Smith 2000). Recently Lee, et al., demonstrated the utility of the serological tests based on the detection of circulating antibodies against Mtb specific antigens; they reported that IgG levels against Mtb chorismate mutase (TBCM) were significantly higher in LTBI than non-infected subjects; while that IgG against Ag85B could distinguish between LTBI and ATB groups (Lee et al. 2020). Present results in relation to IgG levels against anti-Ag85A peptides were significantly higher between ATB and NI individuals; ATB: 0.741 ±
± 0.389 (P-10997), 0.645
0.389 (P-10997), 0.645 ±
± 0.311 (P-29878) and 0.661
0.311 (P-29878) and 0.661 ±
± 0.327 (P-29879) than NI: 0.501
0.327 (P-29879) than NI: 0.501 ±
± 0.327, 0.460
0.327, 0.460 ±
± 0.246 and 0.523
0.246 and 0.523 ±
± 0.281, respectively and also between ATB and LTBI individuals; LTBI: 0.538
0.281, respectively and also between ATB and LTBI individuals; LTBI: 0.538 ±
± 0.358, 0.401
0.358, 0.401 ±
± 0.216 and 0.470
0.216 and 0.470 ±
± 0.218, respectively.
0.218, respectively.
Regarding ROC curve analysis performed for ATB diagnostic performance distinguished between Warao Amerindians with ATB and non-infected or NI individuals; findings about ROC curves analysis showed that synthetic peptides from two different antigens; ESAT-6 P-12037 and Ag85A P-10997 reached ATB diagnostic performance distinguished between ATB and NI individuals; ESAT-6; P-12037 had 100% of sensitivity (AUC =
= 0.812); while that Ag85A; P-10997 had 100% of specificity (AUC
0.812); while that Ag85A; P-10997 had 100% of specificity (AUC =
= 0.691). Potential Mtb antigen targets were reviewed in a recent meta-analysis; a total of 254 studies were identified, encompassing 9 native and 27 recombinant proteins, 15 lipid-derived antigens and 30 combined antigen targets; the results showed that the tests with high specificities frequently exhibited poor sensitivity, especially when a single antigen is used (Steingart et al. 2009). In this context, there has been renewed interest in developing antibody-based diagnostics combining multiple antigens to achieve high levels of sensitivity and specificity (Abebe et al. 2007; Lagrange et al. 2014); the latter was obtained in the present study with the combination of ESAT-6 P-12037 and Ag85A P-10997, which reached ATB diagnostic performance distinguished between ATB and NI individuals. However, synthetic peptides from an unique antigen Ag85A, reached ATB diagnostic performance distinguished between Warao Amerindians with ATB and LTBI individuals; Ag85A P-29878 had 100% of sensitivity (AUC
0.691). Potential Mtb antigen targets were reviewed in a recent meta-analysis; a total of 254 studies were identified, encompassing 9 native and 27 recombinant proteins, 15 lipid-derived antigens and 30 combined antigen targets; the results showed that the tests with high specificities frequently exhibited poor sensitivity, especially when a single antigen is used (Steingart et al. 2009). In this context, there has been renewed interest in developing antibody-based diagnostics combining multiple antigens to achieve high levels of sensitivity and specificity (Abebe et al. 2007; Lagrange et al. 2014); the latter was obtained in the present study with the combination of ESAT-6 P-12037 and Ag85A P-10997, which reached ATB diagnostic performance distinguished between ATB and NI individuals. However, synthetic peptides from an unique antigen Ag85A, reached ATB diagnostic performance distinguished between Warao Amerindians with ATB and LTBI individuals; Ag85A P-29878 had 100% of sensitivity (AUC =
= 0.741); while that Ag85A P-29879 had 99% of specificity (AUC
0.741); while that Ag85A P-29879 had 99% of specificity (AUC =
= 0.679). Current immune-based diagnostics, including the TST or the IGRA, can detect individuals with ATB but cannot distinguish individuals with ATB from latent TB infection or LTBI, which accounts for
0.679). Current immune-based diagnostics, including the TST or the IGRA, can detect individuals with ATB but cannot distinguish individuals with ATB from latent TB infection or LTBI, which accounts for ~
~ 95% of world cases, therefore limiting the ability to identify disease that requires immediate treatment. Furthermore, current immune diagnostics cannot distinguish those who have successfully completed therapy from those with ATB and actively replicating Mtb.
95% of world cases, therefore limiting the ability to identify disease that requires immediate treatment. Furthermore, current immune diagnostics cannot distinguish those who have successfully completed therapy from those with ATB and actively replicating Mtb.
The global resurgence of TB has made it imperative that improved diagnostics be devised for the control of this epidemic; then as mentioned above, many attempts have been made to develop a serologic TB test, which include the need to discriminate LTBI from non-infected individuals, and also to avoid cross-reactivity to BCG or non-tuberculous mycobacterial. Several studies recommend either a dual screening strategy TST followed by IGRA or a single one IGRA only for LTBI, the former largely based on claims that it is more cost-effective. It was compared two commercially available versions of the IGRA: the Quantiferon-TB-Gold-In-Tube (QFT-GIT) and T-SPOT.TB. Cost effectiveness values were sensitive to changes in LTBI prevalence, IGRA test sensitivities/specificities and IGRA test costs. A dual strategy is more cost effective than a single strategy but this conclusion is sensitive to screening test assumptions and LTBI prevalence (Pooran 2010). As regards to ROC curve analysis performed for LTBI diagnostic performance, one exception was found in relation to LTBI diagnostic performance, an unique peptide; the P-12037 synthetic peptide was able to distinguish between Warao Amerindians with LTBI and non-infected or NI individuals; ESAT-6; P-12037 had 98.8% of sensitivity and 98.0% of specificity (AUC =
= 0.640). Present results of these immunodiagnostic tests show promise, which permit us to suggest that are useful as a complementary test in excluding and short the time of diagnosis of Warao Amerindians with LTBI.
0.640). Present results of these immunodiagnostic tests show promise, which permit us to suggest that are useful as a complementary test in excluding and short the time of diagnosis of Warao Amerindians with LTBI.
Many attempts have been made to develop a serologic TB test, which include the need to discriminate active from latent infection, and to perform consistently in genetically and immunologically diverse populations. In Amerindian population tend to have much higher rates of TB in comparison with the general (non-indigenous) population. Studies have reported that this disparity has been attributed to multiple factors, including environmental and socioeconomic variables, co-infections with other pathogens, and the genetics of the host. The profile of genomic ancestry is known to be a determining factor in the susceptibility of some groups to certain diseases, as well as their response to treatment (Narasimhan et al. 2013; Tollefson et al. 2013; Malacarne et al. 2016). In regard to the profile of genomic ancestry; among the non-indigenous or Creole population, the TB average rate was 14.9/100,000, while in the Warao Amerindians population it was equivalent to 201.8/100,000 inhabitants; 13 times higher than the average rate in the non-indigenous population and 19 times higher than the national rate. The TB situation in Venezuela highlighted the Delta Amacuro state as having the highest morbility (65.6 ×
× 100,000) and mortality (6.1
100,000) and mortality (6.1 ×
× 100,000) rates in 2015 (MPPS 22018. Warao Amerindians remained largely isolated for millennia and largely unknown due to its their difficult swampland habitat, so it is possible to think that their contact with infections has been very limited until recently, when their contacts with individuals of European descent increased and when Creoles or “Criollos”, members of the Venezuelan population, and missionaries have entered their habitat in sizable numbers. Since 1980 it was described the existence of deaths among Warao Amerindians derived from epidemics of TB as well as smallpox, measles, plague and gastrointestinal problems (Araujo García 2020). The incidence of TB still occurs at relatively high rates in Amerindian populations; this suggests that the genetic ancestry of Amerindians may be an important factor in the development of infections, and may account for at least some of the variation in infection rates in the different populations. Leal et al. reported that a recent study indicate that Amerindian ancestry is an important risk factor for susceptibility to TB in the admixed population of the Brazilian Amazon region (Da Leal et al. 2020). The disparity attributed to infections with pathogens, and the genetics of the host, which could be associated with genetic differences, specifically at the level of the immune system of this Amerindian population compared to the Creole, this based on the findings derived from immunogenetic studies carried out in the Warao indigenous people and the Creole population, which were carried out by Zulay Layrisse published in the years 1988 and 1997, which showed that there are immunogenetic differences between both populations; inheritance and segregation of DW, a class II antigen, of the human leukocyte antigens (HLA or human leukocyte antigens), currently known as major histocompatibility complex (MHC), HLA haplotypes, defined in linkage disequilibrium, only with homozygous cells of Warao origin and DR/DQ associations not seen or previously described in other human populations (Layrisse et al. 1988; Makhatadze 1997). This condition found in the Warao indigenous people could predispose them to susceptibility to infections, including that due to Mtb. Results of the present study showed that IgG antibody responses to ESAT-6 and Ag85A synthetic peptides show potential in diagnosing TB. Further, these responses could significantly discriminate between ATB, LTBI and NI Warao Amerindian individuals. These results suggest that TB antigen-specific antibodies could be used to develop reliable ELISA tests for the diagnosis of TB; however, further studies for validation of the diagnostic usefulness of each comparison groups are recommended.
100,000) rates in 2015 (MPPS 22018. Warao Amerindians remained largely isolated for millennia and largely unknown due to its their difficult swampland habitat, so it is possible to think that their contact with infections has been very limited until recently, when their contacts with individuals of European descent increased and when Creoles or “Criollos”, members of the Venezuelan population, and missionaries have entered their habitat in sizable numbers. Since 1980 it was described the existence of deaths among Warao Amerindians derived from epidemics of TB as well as smallpox, measles, plague and gastrointestinal problems (Araujo García 2020). The incidence of TB still occurs at relatively high rates in Amerindian populations; this suggests that the genetic ancestry of Amerindians may be an important factor in the development of infections, and may account for at least some of the variation in infection rates in the different populations. Leal et al. reported that a recent study indicate that Amerindian ancestry is an important risk factor for susceptibility to TB in the admixed population of the Brazilian Amazon region (Da Leal et al. 2020). The disparity attributed to infections with pathogens, and the genetics of the host, which could be associated with genetic differences, specifically at the level of the immune system of this Amerindian population compared to the Creole, this based on the findings derived from immunogenetic studies carried out in the Warao indigenous people and the Creole population, which were carried out by Zulay Layrisse published in the years 1988 and 1997, which showed that there are immunogenetic differences between both populations; inheritance and segregation of DW, a class II antigen, of the human leukocyte antigens (HLA or human leukocyte antigens), currently known as major histocompatibility complex (MHC), HLA haplotypes, defined in linkage disequilibrium, only with homozygous cells of Warao origin and DR/DQ associations not seen or previously described in other human populations (Layrisse et al. 1988; Makhatadze 1997). This condition found in the Warao indigenous people could predispose them to susceptibility to infections, including that due to Mtb. Results of the present study showed that IgG antibody responses to ESAT-6 and Ag85A synthetic peptides show potential in diagnosing TB. Further, these responses could significantly discriminate between ATB, LTBI and NI Warao Amerindian individuals. These results suggest that TB antigen-specific antibodies could be used to develop reliable ELISA tests for the diagnosis of TB; however, further studies for validation of the diagnostic usefulness of each comparison groups are recommended.
Conclusions
Findings demonstrated the usefulness of enzyme-linked immunosorbent assay for serodiagnosis of ATB and LTBI, using synthetic peptides, covering the complete sequence of the ESAT-6 and Ag85A antigens of Mtb. Peptide based antibody assay is cost effective and simple and may be interchangeably with native proteins based ELISA assays for effective diagnosis of TB in developing world. The potential of multi-antigen immunoassay with ESAT-6 and Ag85A peptides highly sensibles and specifics, especially in combinations as a complementary test could provide a screening strategy for shorting the time to diagnosis of ATB and LTBI.
Funding
This work was supported by funds from the Central University of Venezuela; CDCH/UCV No. PG-09-6256-8007/2011.
Declarations
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zaida Araujo, Email: moc.oohay@aicragojuaraz.
Manuel A. Patarroyo, Email: [email protected].
References
- Abebe F, et al. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol. 2007;66(2–3):176–191. 10.1111/j.1365-3083.2007.01978.x. [Abstract] [CrossRef] [Google Scholar]
- Andersen P, et al. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356(9235):1099–1104. 10.1016/S0140-6736(00)02742-2. [Abstract] [CrossRef] [Google Scholar]
- Araujo Z, et al. Study of the antibody response against Mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem Inst Oswaldo Cruz. 2004;99(5):517–524. 10.1590/S0074-02762004000500011. [Abstract] [CrossRef] [Google Scholar]
- Araujo Z, et al. Comparison of serological responses in two different populations with pulmonary tuberculosis. Mem Inst Oswaldo Cruz. 2008;103(7):661–667. 10.1590/S0074-02762008000700006. [Abstract] [CrossRef] [Google Scholar]
- Araujo Z, et al. Immunologic evaluation and validation of methods using synthetic peptides derived from Mycobacterium tuberculosis for the diagnosis of tuberculosis infection. Mem Inst Oswaldo Cruz. 2013;108(2):131–139. 10.1590/0074-0276108022013001. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Araujo Z, et al. Evaluation of the transcriptional immune biomarkers in peripheral blood from Warao indigenous associate with the infection by Mycobacterium tuberculosis. Rev Soc Bras Med Trop. 2019;52:e20180516. 10.1590/0037-8682-0516-2018. [Abstract] [CrossRef] [Google Scholar]
- Araujo García Z. Tuberculosis: An endemic and neglected disease among the Warao indigenous people of the Venezuelan delta. Rev EntreRios. 2020;3(2):50–71. 10.26694/rer.v3i02.10887. [CrossRef] [Google Scholar]
- Arnadottir T, et al. Guidelines for conducting tuberculin skin test surveys in high prevalence countries. Tuberc Lung Dis. 1996;77(Suppl 1):1–19. 10.1016/S0962-8479(96)90127-6. [Abstract] [CrossRef] [Google Scholar]
- Barry CE, III, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. 10.1038/nrmicro2236. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Belisle JT, et al. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276(5317):1420–1422. 10.1126/science.276.5317.1420. [Abstract] [CrossRef] [Google Scholar]
- Berthet FX, et al. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144(11):3195–3203. 10.1099/00221287-144-11-3195. [Abstract] [CrossRef] [Google Scholar]
- Boesen H, et al. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63(4):1491–1497. 10.1128/iai.63.4.1491-1497.1995. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Canadian Thoracic Society . Canadian tuberculosis standards. 7. Ottawa: The Public Health Agency of Canada; 2013. [Google Scholar]
- Da Leal DFVB, et al. Amerindian genetic ancestry as a risk factor for tuberculosis in an Amazonian population. PLoS ONE. 2020;15(7):e0236033. 10.1371/journal.pone.0236033. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- He XY, et al. Assessment of five antigens from Mycobacterium tuberculosis for serodiagnosis of tuberculosis. Clin Vaccine Immunol. 2011;18(4):565–570. 10.1128/CVI.00507-10. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen–antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82(15):5131–5135. 10.1073/pnas.82.15.5131. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Instituto Nacional de Estadística (INE) (2007) Población Indígena por Entidad Federal. Tasa bruta de mortalidad corregida, según entidad federal, 2002–2007. Instituto Nacional de Estadística
- Instituto Nacional de Estadística (INE) (2015) Censo Nacional de Población y Vivienda 2011. Empadronamiento de la Población Indígena”. República Bolivariana de Venezuela, Ministerio del Poder Popular de Planificación, Caracas
- Ireton GC, et al. Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. 2010;17(10):1539–1547. 10.1128/CVI.00198-10. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kudoh S, Kudoh T. A simple technique for culturing tubercle bacilli. Bull World Health Organ. 1974;51(1):71–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Lagrange PH, et al. A toolbox for tuberculosis (TB) diagnosis: an Indian multi-centric study (2006–2008); evaluation of serological assays based on PGL-Tb1 and ESAT-6/CFP10 antigens for TB diagnosis. PLoS ONE. 2014;9(5):e96367. 10.1371/journal.pone.0096367. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Langermans JA, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23(21):2740–2750. 10.1016/j.vaccine.2004.11.051. [Abstract] [CrossRef] [Google Scholar]
- Layrisse Z, et al. Unique HLA-DR/DQ associations revealed by family studies in Warao Amerindians. Haplotype and homozygosity frequencies. Hum Immunol. 1988;23(1):45–57. 10.1016/0198-8859(88)90017-1. [Abstract] [CrossRef] [Google Scholar]
- Lee JY, et al. Diagnostic potential of IgG and IgA responses to Mycobacterium tuberculosis antigens for discrimination among active tuberculosis, latent tuberculosis infection, and non-infected individuals. Microorganisms. 2020;8(7):979–996. 10.3390/microorganisms8070979. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Legesse M, et al. IgA response to ESAT-6/CFP-10 and Rv2031 antigens varies in patients with culture-confirmed pulmonary tuberculosis, healthy Mycobacterium tuberculosis-infected and non-infected individuals in a tuberculosis endemic setting, Ethiopia. Scand J Immunol. 2013;78(3):266–274. 10.1111/sji.12080. [Abstract] [CrossRef] [Google Scholar]
- Makhatadze NJ. HLA class I and class II allele and haplotype distribution in the Venezuelan population. Hum Immunol. 1997;55(1):53–58. 10.1016/S0198-8859(97)00088-8. [Abstract] [CrossRef] [Google Scholar]
- Malacarne J, et al. Prevalence and factors associated with latent tuberculosis infection in a indigenous population in the Brazilian Amazon. Rev Soc Bras Med Trop. 2016;49(4):456–464. 10.1590/0037-8682-0220-2016. [Abstract] [CrossRef] [Google Scholar]
- Ministerio de Sanidad y Asistencia Social (MSAS) (1999) Seminario Técnico-Administrativo. Programa Integrado de Control de la Tuberculosis, Caracas
- Ministerio del Poder Popular para la Salud (MPPS) (2007) Evaluación del programa nacional de control de la tuberculosis año 2007. Caracas
- Ministerio del Poder Popular para la Salud (MPPS) (2018) Programa Nacional Integrado de Control de la TB. Tuberculosis en población indígena Warao. Venezuela 2012–2017. Dirección General de Programas de Salud, Caracas
- Montenegro RA, Stephens C. Indigenous Health part 2: Indigenous health in Latin America and the Caribbean. Lancet. 2006;367(9525):1859–1869. 10.1016/S0140-6736(06)68808-9. [Abstract] [CrossRef] [Google Scholar]
- Narasimhan P, et al. Risk factors for tuberculosis. Pulm Med. 2013;2013(828939):1–11. 10.1155/2013/828939. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pai M, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. 10.1128/CMR.00034-13. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pooran A. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med. 2010;10(7):2–14. [Europe PMC free article] [Abstract] [Google Scholar]
- Ranstam J. Multiple P-values and Bonferroni correction. Osteoarthr Cartil. 2016;24(5):763–764. 10.1016/j.joca.2016.01.008. [Abstract] [CrossRef] [Google Scholar]
- Rijnink WF, et al. B-cells and antibodies as contributors to effector immune responses in tuberculosis. Front Immunol. 2021;12:e640168. 10.3389/fimmu.2021.640168. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Smith SM, et al. Human CD8(+) T cells specific for Mycobacterium tuberculosis secreted antigens in tuberculosis patients and healthy BCG-vaccinated controls in The Gambia. Infect Immun. 2000;68(12):7144–7148. 10.1128/IAI.68.12.7144-7148.2000. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Steingart KR, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16(2):260–276. 10.1128/CVI.00355-08. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tam JP, et al. SN 1 and SN 2 mechanisms for the deprotection of synthetic peptides by hydrogen fluoride. Studies to minimize the tyrosine alkylation side reaction. Int J Pept Protein Res. 1983;21(1):57–65. 10.1111/j.1399-3011.1983.tb03078.x. [Abstract] [CrossRef] [Google Scholar]
- Tollefson D, et al. Burden of tuberculosis in indigenous peoples globally: a systematic review. Int J Tuberc Lung Dis. 2013;17(9):1139–1150. 10.5588/ijtld.12.0385. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Williams A, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a Guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb) 2005;85(1–2):29–38. 10.1016/j.tube.2004.09.009. [Abstract] [CrossRef] [Google Scholar]
- World Health Organization . Global Tuberculosis Report. Geneva: WHO; 2019. [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1007/s10989-022-10392-3
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s10989-022-10392-3.pdf
Citations & impact
Impact metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s10989-022-10392-3
Article citations
Impact of in-utero exposure to HIV and latent TB on infant humoral responses.
Front Immunol, 15:1423435, 27 Jun 2024
Cited by: 1 article | PMID: 38994354 | PMCID: PMC11236605
Serological analysis reveals differential antibody responses between TB patients and latently infected individuals from the TB endemic country of Mozambique.
Front Med (Lausanne), 10:1286785, 09 Oct 2023
Cited by: 0 articles | PMID: 37877025 | PMCID: PMC10591198
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prediction of Th1 and Cytotoxic T Lymphocyte Epitopes of Mycobacterium tuberculosis and Evaluation of Their Potential in the Diagnosis of Tuberculosis in a Mouse Model and in Humans.
Microbiol Spectr, 10(4):e0143822, 08 Aug 2022
Cited by: 5 articles | PMID: 35938824 | PMCID: PMC9430503
Mycobacterium tuberculosis latency-associated antigen Rv1733c SLP improves the accuracy of differential diagnosis of active tuberculosis and latent tuberculosis infection.
Chin Med J (Engl), 135(1):63-69, 01 Jan 2022
Cited by: 6 articles | PMID: 34802023 | PMCID: PMC8850866
Evaluation of Mycobacterium tuberculosis-specific antibody responses for the discrimination of active and latent tuberculosis infection.
Int J Infect Dis, 70:1-9, 02 Feb 2018
Cited by: 11 articles | PMID: 29410147
[Evolution of IGRA researches].
Kekkaku, 83(9):641-652, 01 Sep 2008
Cited by: 6 articles | PMID: 18979999
Review
Funding
Funders who supported this work.
Consejo de Desarrollo Científico y Humanístico, Universidad Central de Venezuela (1)
Grant ID: CDCH/UCV No. PG- 09-6256-8007/2011

 1
1