Abstract
Background
Severe forms of SARS-CoV-2 infections are associated with high rates of thromboembolic complications. Professional societies and expert consensus reports have recommended anticoagulants for COVID-19 hospitalized patients. Our study aimed to compare the effect of therapeutic, intermediate and prophylactic doses of heparin on 6-week survival in patients hospitalized for COVID-19.Methods
The study sample is a French cohort of COVID-19 patients hospitalized between Feb 25th and Apr 30th 2020. Patients were assigned to one of 3 anticoagulation dose groups based on the maximum dose they received for at least three days (prophylactic, intermediate or therapeutic). The main outcome was survival up to 42 days after hospital admission. Multivariate Cox regression models were performed to adjust analyses for confounding factors.Results
A total of 323 patients were included. The mean age of the study sample was 71.6 ± 15 years, and 56.3% were men. Treatment with the intermediate versus prophylactic dose of anticoagulation (HR = 0.50, 95%CI = [0.26; 0.99], p = 0.047) and with therapeutic versus prophylactic dose (HR = 0.58 95%CI = [0.34; 0.98], p = 0.044) was associated with a significant reduction in 6-week mortality, after adjustment for potential confounding factors. Comparison of therapeutic versus intermediate doses showed no significant difference in survival.Conclusions
Our results reported a significant positive effect of intermediate and therapeutic doses of heparin on 6-week survival for hospitalized COVID-19 patients compared with a prophylactic dose.Free full text

Association between Heparin Dose and 6-Week Mortality in Patients with COVID-19
Abstract
Background
Severe forms of SARS-CoV-2 infections are associated with high rates of thromboembolic complications. Professional societies and expert consensus reports have recommended anticoagulants for COVID-19 hospitalized patients. Our study aimed to compare the effect of therapeutic, intermediate and prophylactic doses of heparin on 6-week survival in patients hospitalized for COVID-19.
Methods
The study sample is a French cohort of COVID-19 patients hospitalized between Feb 25th and Apr 30th 2020. Patients were assigned to one of 3 anticoagulation dose groups based on the maximum dose they received for at least three days (prophylactic, intermediate or therapeutic). The main outcome was survival up to 42 days after hospital admission. Multivariate Cox regression models were performed to adjust analyses for confounding factors.
Results
A total of 323 patients were included. The mean age of the study sample was 71.6 ± 15 years, and 56.3% were men. Treatment with the intermediate versus prophylactic dose of anticoagulation (HR = 0.50, 95%CI = [0.26; 0.99], p = 0.047) and with therapeutic versus prophylactic dose (HR = 0.58 95%CI = [0.34; 0.98], p = 0.044) was associated with a significant reduction in 6-week mortality, after adjustment for potential confounding factors. Comparison of therapeutic versus intermediate doses showed no significant difference in survival.
Conclusions
Our results reported a significant positive effect of intermediate and therapeutic doses of heparin on 6-week survival for hospitalized COVID-19 patients compared with a prophylactic dose.
Introduction
The coronavirus disease 2019 (COVID-9), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been responsible for the deaths of several million persons worldwide.1 The range of severity of COVID-19 is broad, and most patients who require hospitalization suffer from respiratory failure and/or sepsis. In the most severe cases, acute respiratory distress syndrome (ARDS), multi-organ failure and death can ensue.2
The cytokine storm, an excessive systemic inflammatory response,3 is considered one of the major causes of ARDS in COVID-19 patients.4 It is now well established that interaction between inflammation and coagulation exists.5 It has been observed that severe forms of SARS-CoV-2 infection are associated with elevated levels of D-dimers and fibrinogene with high rates of thromboembolic complications, such as pulmonary embolism.6
In a meta-analysis, Malas et al. estimated that the overall rate of venous thromboembolism was 21%, ranging from 5% among patients hospitalized in conventional wards to 31% in patients admitted to the intensive care unit (ICU).7 In addition, autopsy studies from deceased COVID-19 patients have also shown the presence of fibrinous thrombi in small pulmonary arterioles, confirming the important role of coagulation abnormalities.8
Anticoagulant treatments are used to prevent and treat thromboembolic events.9 Moreover, heparin also has anti-inflammatory effects10 that may benefit patients with severe forms of SARS-CoV-2 infection. Professional societies and expert consensus reports have recommended anticoagulants as part of the treatment of hospitalized COVID-19 patients.11–13 However, vascular complications, occurring even in patients receiving prophylactic doses of anticoagulants, led to intensified prophylactic doses (called intermediate doses) and therapeutic doses,14,15 with changing indications over time. Since April 2020, the French Society of Thrombosis and Haemostasis recommends intensified doses depending on the patient’s state (e.g. requirement for supplemental oxygen therapy, D-dimer levels) and characteristics.16
Observational studies have reported encouraging results regarding the mortality reduction in patients treated with anticoagulants.17–20 However, to the best of our knowledge, no cohort study has investigated the effect of 3 different anticoagulant doses on 6-week mortality. In this context, we aimed to compare the effect of therapeutic, intermediate and prophylactic doses of anticoagulants on 6-week survival among a cohort of patients hospitalized for COVID-19 during the first wave of the pandemic in France.
Methods
Population
The study sample is a prospective cohort of adult patients diagnosed with COVID-19 and admitted to Reims University Hospital, France, between Feb 25th and Apr 30th 2020. This study received approval from the Ethics Committee (number 3838-RM), and informed consent was obtained for each patient. The study was registered on Clinicaltrials.gov under the number NCT04553575.
Patients were included if they were hospitalized with a diagnosis of COVID-19, defined as a positive reverse transcriptase-polymerase chain reaction (RT-PCR) test or the presence of characteristic findings on a computed tomography scan associated with a typical clinical history.
Patients were excluded if they did not receive anticoagulant treatment or were already hospitalized for another condition before their COVID-19 diagnosis. Patients with a length of stay shorter than five days were also excluded to prevent immortality bias21 since they were unlikely to receive the studied doses of treatment in such a short stay.
Anticoagulation treatments were unfractionated heparin (UFH) or low molecular weight heparin (LMWH). Examples of prophylactic, intermediate and therapeutic doses of different anticoagulants are presented in Table S1 (Supplementary material). We assigned each patient to one of the three following anticoagulant dose groups: (1) prophylactic, (2) intermediate or (3) therapeutic. The patient had to receive the corresponding dose for at least three days to be assigned to a group.
Patients who received anticoagulation at different doses during their hospital stay were assigned to the group corresponding to the maximum dose received. If a patient received more than three days of anticoagulation but less than three days of intermediate or therapeutic dose in total, they were assigned to the prophylactic dose group since there is evidence that therapeutic levels are not reached for most patients in this short timeframe.22 Similarly, patients with less than three days of anticoagulation at any dose were excluded from the study since the treatment duration was too short of ensuring a real anticoagulation effect.
Variables and outcome
For each patient, we recorded socio-demographic characteristics, co-morbidities, clinical and biological data regarding the initial severity of COVID-19, as well as treatments and outcomes. We defined cardiovascular disease as the presence of high blood pressure, a history of cerebral stroke, coronary heart disease, cardiac surgery or heart failure of New York Heart Association (NYHA) class III or IV. The updated version of the Charlson co-morbidity index by Quan et al. was used to measure the co-morbidity status.23 To evaluate the severity of infection at admission, we measured and recorded the early warning score (EWS), a modified version of the National Early Warning Score 2 with age ≥ 65 years old as an additional parameter.24 Each patient was classified as low (EWS ≤ 4), medium (EWS > 4 and ≤ 6) or high risk (EWS > 6) of acute deterioration. In addition, we noted whether the patient received systemic corticosteroid therapy, as it is a recommended treatment for severe forms of COVID-19.25 Since Mar 27th 2020, the treatment protocol in our hospital has included corticosteroids for all patients with COVID-19 pneumonia, at a dose of 1 mg/kg equivalent per day of prednisone or methylprednisolone for 3 to 4 weeks, depending on the severity of the disease. Information on potential complications of anticoagulation was extracted from the program for the medicalization of information systems (PMSI) of our hospital. This database contains information on diagnoses, co-morbidities, and complications for each hospital stay.
The main outcome of our analysis was survival up to 6 weeks (42 days) after hospital admission.
Statistical analysis
Quantitative variables are described as mean ± standard deviation (SD) or median and interquartile range (IQR), and qualitative variables as numbers (percentage). Comparisons by anticoagulant dose group were performed using the ANOVA test for quantitative variables and the Chi2 test for qualitative variables. In addition, validity conditions for these tests were verified.
We constructed survival curves using the Kaplan Meier method to compare the effect of the 3 anticoagulant doses on 6-week survival. Curves were compared using the log-rank test.
Multivariate Cox regression analysis was performed to adjust for potential confounders, which were identified in the bivariate analyses or recognized confounding or prognostic factors from the literature. The choice of variables also took into account the risk of multicollinearity. Thus, the following adjustment variables were chosen for our analyses: sociodemographic characteristics (age and sex), co-morbidities evaluated with the Charlson co-morbidity index, anticoagulant as part of regular treatment, the severity of COVID-19 (O2 therapy needed at hospital arrival and hospitalization in ICU) and treatment of SARS-CoV-2 infection with systemic corticosteroids. Results are expressed as hazard ratios (HR) with a 95% confidence interval (95% CI).
All analyses were performed using R software, version 4.0.5 (R Core Team (2019). R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
Results
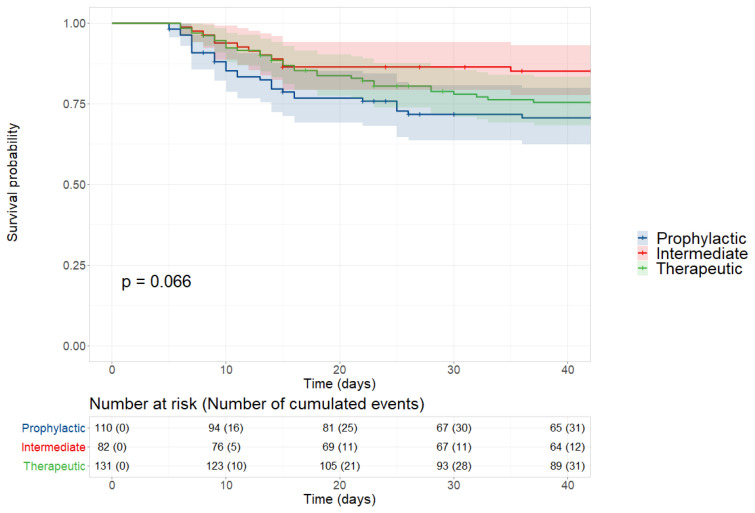
A total of 479 patients were included in the cohort between Feb 25th and Apr 30th. Thirty-seven patients were not treated with anticoagulants, 111 patients were hospitalized for other reasons before their COVID-19 diagnosis or were hospitalized for less than five days, and eight patients did not receive at least three days of any anticoagulant dose. Therefore, 323 patients were included in our analysis: 34.1% in the prophylactic group, 25.4% in the intermediate group and 40.6% in the therapeutic group (Figure 1).
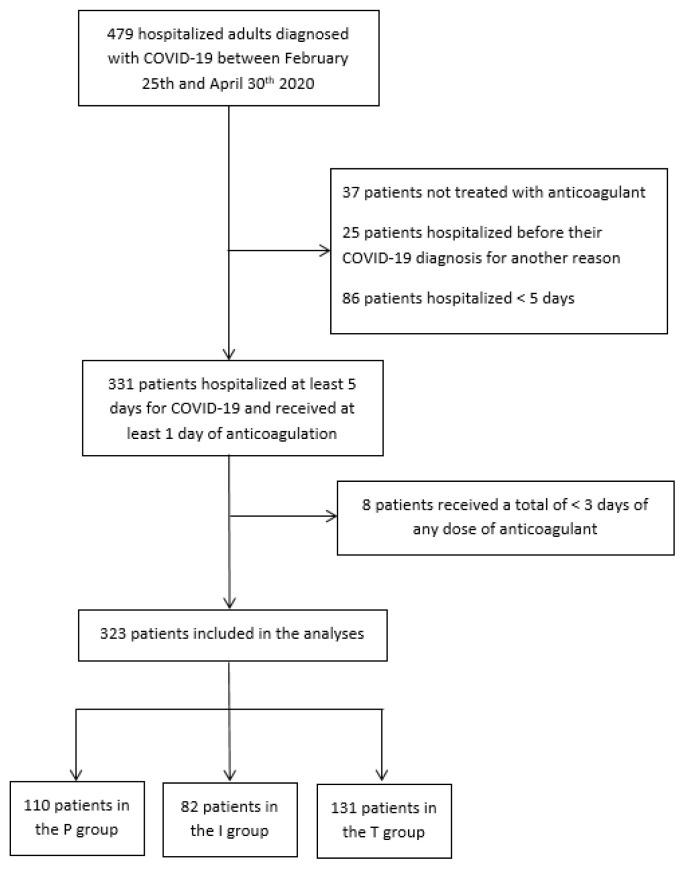
Flow chart of the study population. Abbreviations: P, prophylactic; I, intermediate; T, therapeutic.
Overall, the mean age of the population was 71.6 ± 15 years, and 56.3% were men. Fifty-nine (18.3%) patients received anticoagulants, and 114 (35.3%) had an antiplatelet agent in their regular treatment before admission.
Information on baseline D-dimer levels was missing for 91 patients. Among those with available data, the median D-dimer level was 0.9 mg/L (IQR = [0.7; 2.0]) in the prophylactic group, 0.8 mg/L (IQR = [0.4; 1.1]) in the intermediate group and 1.6 mg/L (IQR = [0.6; 5.7]) in the therapeutic group. Nineteen patients (5.9%) suffered pulmonary embolism during their hospital stay. The characteristics of the three groups are presented in Table 1.
Table 1
Characteristics of the study population according to the dose of anticoagulant received (prophylactic, intermediate or therapeutic) during hospitalization for COVID-19.
| Missing data (n) | Total (n = 323) | Prophylatic (n = 110) | Intermediate (n = 82) | Therapeutic (n = 131) | p | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Clinical | ||||||
 Male gender, n (%) Male gender, n (%) | 182 (56.3) | 55 (50.0) | 49 (59.8) | 78 (59.5) | 0.255 | |
 Age, mean (SD), years Age, mean (SD), years | 71.6 (15.0) | 73.7 (15.9) | 67.7 (16.9) | 72.2 (12.4) | 0.018 | |
 Living in a nursing home, n (%) Living in a nursing home, n (%) | 1 | 72 (22.4) | 32 (29.1) | 18 (22.0) | 22 (16.9) | 0.078 |
| Biological | ||||||
 Fibrinogen, mean (sd) Fibrinogen, mean (sd) | 64 | 6.3 (1.5) | 5.9 (1.6) | 6.2 (1.3) | 6.6 (1.5) | 0.011 |
 CRP, n (%) CRP, n (%) | 14 | 0.092 | ||||
  < 40 mg/L < 40 mg/L | 97 (31.4) | 41 (39.0) | 26 (32.5) | 30 (24.2) | ||
  ≥ 40 and < 150 mg/L ≥ 40 and < 150 mg/L | 137 (44.3) | 43 (41.0) | 38 (47.5) | 56 (45.2) | ||
  ≥ 150 mg/L ≥ 150 mg/L | 75 (24.3) | 21 (20.0) | 16 (20.0) | 38 (30.6) | ||
| Co-morbidities | ||||||
 Charlson comorbidity index > 2, n (%) Charlson comorbidity index > 2, n (%) | 107 (33.1) | 37 (33.6) | 21 (25.6) | 49 (37.4) | 0.203 | |
 Cardiovascular disease, n (%) Cardiovascular disease, n (%) | 226 (70.0) | 80 (72.7) | 47 (57.3) | 99 (75.6) | 0.014 | |
 Diabetes mellitus, n (%) Diabetes mellitus, n (%) | 103 (31.9) | 32 (29.1) | 19 (23.2) | 52 (39.7) | 0.031 | |
 BMI ≥ 40 kg/m2, n (%) BMI ≥ 40 kg/m2, n (%) | 20 (6.2) | 6 (5.5) | 4 (4.9) | 10 (7.6) | 0.665 | |
 GFR < 60 mL/min/1.73m2, n (%) GFR < 60 mL/min/1.73m2, n (%) | 4 | 116 (36.4) | 50 (46.3) | 17 (20.7) | 49 (38.0) | 0.001 |
| Severity | ||||||
 O2 therapy at hospital arrival, n (%) O2 therapy at hospital arrival, n (%) | 3 | 173 (54.1) | 45 (41.3) | 45 (55.6) | 83 (63.8) | 0.002 |
 ARDS or severe pneumonia, n (%) ARDS or severe pneumonia, n (%) | 1 | 124 (38.5) | 34 (31.2) | 34 (41.5) | 56 (42.7) | 0.153 |
 EWS score, n (%) EWS score, n (%) | 35 | 0.262 | ||||
  ≤ 4 ≤ 4 | 65 (22.6) | 26 (27.1) | 13 (17.1) | 26 (22.4) | ||
  > 4 and ≤ 6 > 4 and ≤ 6 | 58 (20.1) | 21 (21.9) | 19 (25.0) | 18 (15.5) | ||
  > 6 > 6 | 165 (57.3) | 49 (51.0) | 44 (57.9) | 72 (62.1) | ||
 Hospitalized in ICU, n (%) Hospitalized in ICU, n (%) | 89 (27.6) | 22 (20.0) | 20 (24.4) | 47 (35.9) | 0.017 | |
| Treatment | ||||||
 Treated with corticosteroids, n (%) Treated with corticosteroids, n (%) | 1 | 212 (65.8) | 59 (54.1) | 55 (67.1) | 98 (74.8) | 0.003 |
Abbreviations: BMI, Body mass index; GFR, Glomerular filtration rate; ARDS, acute respiratory distress syndrome; EWS, Early warning score; ICU, Intensive care unit.
Independently of the treatment duration, and thus of the assigned group, we also explored the different anticoagulant doses received by each patient in chronological order. Nearly two thirds (209) of included patients received only one type anticoagulant regimen, 79 (24.5%) received two regimens, 15 (4.6%) received three regimens, and 20 (6.2%) had more complicated treatment regiments comprising several dose changes that did not fit with any other regimen (Table 2).
Table 2
Schemes of anticoagulant doses received by each patient.
| Scheme of doses | n (%) |
|---|---|
| T | 67 (20.7) |
| T-I | 3 (0.9) |
| T-I-P | 5 (1.5) |
| T-P | 11 (3.4) |
| I | 42 (13.0) |
| I-T | 9 (2.8) |
| I-T-P | 3 (0.9) |
| I-P | 12 (3.7) |
| P | 100 (31.0) |
| P-T | 20 (6.2) |
| P-T-I | 7 (2.2) |
| P-T-P | 3 (0.9) |
| P-I | 21 (6.5) |
| Complicated regimen | 20 (6.2) |
Note: for each regimen, the different doses are written in the same order as received by the patient, independently of duration and assigned group. Abbreviations: P, prophylactic dose; I, intermediate dose; T, therapeutic dose.
At 6 weeks post-admission, 31 (28.2%) patients in the prophylactic group, 12 (14.6%) in the intermediate group and 31 (23.7%) in the therapeutic group had died. The Kaplan-Meier curves depicting survival across the three groups are shown in Figure 2. There was no significant difference between groups (p = 0.066) (Figure 2). The results of the bivariate analysis investigating the impact of patient characteristics on 6-week survival are presented in Table S2 (Supplementary material).
A total of 319 patients were included in the multivariate analyses; 4 were excluded because of missing data on one of the adjustment variables.
Treatment with intermediate dose of anticoagulation was associated with a significant reduction in the risk of 6-week mortality, compared to prophylactic dose (HR = 0.50, 95%CI = [0.26; 0.99], p = 0.047). Similarly, therapeutic dose of heparin, compared to prophylactic dose, was associated with a significant reduction in the risk of mortality (HR = 0.58, 95%CI = [0.34; 0.98], p = 0.044). When comparing the therapeutic versus the intermediate dose, no significant difference was found (HR = 1.14, 95%CI = [0.57; 2.29], p = 0.704).
Four patients, 1 in the prophylactic group, 1 in the intermediate group and 2 in the therapeutic group, presented a minor complication of anticoagulant treatment (i.e. asymptomatic overdose, oral or nasal bleeding) and 3 patients of the therapeutic group presented an intramuscular hematoma.
Discussion
The results of our study highlight the positive impact of intermediate and therapeutic doses of heparin on 6-week survival among hospitalized COVID-19 patients, as compared with prophylactic dose of anticoagulant therapy.
It has been well established that severe SARS-CoV-2 infection is associated with a high prevalence of pulmonary thrombosis and macro-vascular thromboembolic events. Besides its anticoagulant activity, heparin also has anti-inflammatory and immunomodulatory properties that may explain its beneficial effects on the outcomes of COVID-19 patients.26
Our results are consistent with other observational studies22,27 reporting that patients receiving therapeutic doses had a higher survival probability than those receiving only prophylactic doses. A further study by Meizlish et al.28 reported a significantly lower cumulative incidence of in-hospital death for patients receiving intermediate doses of anticoagulant compared to prophylactic doses.
Due to the observational design of our study, our results remain subject to hidden confounding factors. The association between anticoagulation and survival in SARS-CoV-2 infected patients warrants evaluation in interventional studies. Randomized controlled trials (RCTs) in hospitalized COVID-19 patients have found contrasting results. Two RCTs testing the effects of full dose anticoagulant therapy showed no beneficial effect on clinical outcomes compared to prophylactic doses.29,30 One trial had to stop enrolment of critically ill patients prematurely due to futility and safety concerns.31 Nevertheless, results in non-critically ill patients showed that therapeutic doses of heparin increased the probability of survival to hospital discharge, with reduced use of cardiovascular and respiratory organ support compared to prophylactic doses.32 Another recent study concluded that therapeutic doses of heparin reduced major thromboembolism and death among in-patients with elevated D-dimers, but no treatment effect was seen in ICU patients.33 Regarding the comparison between intermediate and prophylactic doses of anticoagulants, two RCTs, including only patients with severe COVID-19, found no significant difference.34,35 There are still many on-going RCTs that will provide further evidence to determine the optimal anticoagulation doses.36
To the best of our knowledge, this study is the first prospective, observational study to compare the effect of 3 types of anticoagulant doses on 6-week mortality. Our cohort included adult patients hospitalized with COVID-19 independently of the severity of the disease, the need for mechanical ventilation and the presence of coagulation disorders. Moreover, patients were followed for 6 weeks, whereas most published observational studies and trials focused only on in-hospital or short-term mortality.
The precision of the data collected enabled us to study the different dose regimens received by the patients. Accordingly, we noted that more than a third of the study population received at least two types of regimen (from among prophylactic, intermediate and therapeutic doses). This could be explained by the lack of consensus on anticoagulant treatment before April 2020 in France, but above all, by the constant adaptation of the treatment doses by the clinicians in response to the patient’s state and any potential complications of the disease.
Our results also provide interesting findings as regards the comparison of therapeutic versus intermediate doses. Indeed, we found no significant advantage of therapeutic anticoagulation in terms of survival. While therapeutic and intermediate doses were both found to be superior to prophylactic doses, the use of intermediate anticoagulation may have the advantage of reducing the risk of bleeding complications. Indeed, it has previously been shown that therapeutic doses of anticoagulant increase the risk of bleeding in COVID-19 patients.37
Our study presents several limitations. Firstly, we did not have precise data on the start and end dates of each dose for each patient. As a result, we were not able to perform a time-dependent analysis, which would have been a more appropriate approach to correctly classify the immortal time in pharmacoepidemiology.38 We partially took this risk of bias into account by excluding patients with a length of stay less than 5 days, because there was a high risk that these patients were not hospitalized long enough to receive intermediate or therapeutic doses of anticoagulant. Secondly, the fact that patients with less than 3 days of intermediate or therapeutic doses were assigned to the prophylactic group could induce classification bias. Since the reason for the short duration of treatment was unknown, we may have overlooked potential complications due to higher doses of anticoagulation. However, as highlighted by Ionescu et al., assigning patients with less than 3 days of treatment in the intermediate or therapeutic group could induce other biases, as therapeutic levels are often not reached in such a short timeframe.27 Thirdly, our study may suffer from a lack of statistical power due to the small sample size. Finally, the external validity of our results might be affected by the fact that this was a single-centre study.
Conclusions
Our study reports a significant positive effect of intermediate and therapeutic doses of heparin, compared to prophylactic doses, on 6-week survival in hospitalized COVID-19 patients. Results of on-going RCTs will be helpful to determine the optimal anticoagulation doses.
Supplementary data
Table S1
Examples of anticoagulant doses
| Class of anticoagulant | Name | Maximum prophylactic dose | Intermediate dose | Minimum therapeutic dose |
|---|---|---|---|---|
| UFH | Calciparine | 0.2 ml twice daily | 0.3 ml three times daily | |
| Heparin sodium | 10 000 IU per day | 500 IU/kg per day | ||
| LMWH | Enoxaparin | 4000 IU per day | 6 000 IU per day or 4 000 IU twice daily | 100 IU/kg per 12h |
| Tinzaparin | 3500 IU per day | 175 IU/kg per day | ||
| Fondaparinux | 2:5 mg per day | 5 mg per day |
Abbreviations: UFH, unfractionated heparin; LMWH, low molecular weight heparin.
Table S2
Bivariate analysis of the effects of patient characteristics on 6-week survival.
| Missing data (n) | HR [95% CI] | p | |
|---|---|---|---|
| Baseline characteristics | |||
| Clinical | |||
| Male gender | 1.5 [0.9; 2.4] | 0.120 | |
| Age > 70 years old | 3.0 [1.7; 5.4] | <0.01 | |
| Living in a nursing home | 1 | 1.8 [1.1; 2.9] | 0.017 |
| Biological | |||
| Baseline CRP | 14 | ||
 < 40 mg/L < 40 mg/L | 1 | ||
 ≥ 40 and < 150 mg/L ≥ 40 and < 150 mg/L | 2.5 [1.3; 4.8] | <0.01 | |
 ≥ 150 mg/L ≥ 150 mg/L | 2.2 [1.1; 4.5] | 0.033 | |
| Co-morbidities | |||
| Charlson score > 2 | 2.0 [1.2; 3.1] | <0.01 | |
| Cardiovascular disease | 1.9 [1.1; 3.3] | 0.032 | |
| Diabetes mellitus | 1.8 [1.1; 2.8] | 0.017 | |
| BMI ≥ 40 kg/m2 | 2.0 [1.0; 4.1] | 0.067 | |
| GFR < 60 mL/min/1.73m2 | 4 | 2.1 [1.3; 3.4] | <0.01 |
| Severity | |||
| O2 therapy at admission | 3 | 2.4 [1.4; 3.9] | <0.01 |
| ARDS or severe pneumonia | 1 | 2.3 [1.5; 3.7] | <0.01 |
| EWS score | 35 | ||
 ≤ 4 ≤ 4 | 1 | ||
 > 4 and ≤ 6 > 4 and ≤ 6 | 1.6 [0.6; 4.3] | 0.320 | |
 > 6 > 6 | 2.8 [1.3; 6.2] | 0.012 | |
| Hospitalized in ICU | 1.6 [1.0; 2.6] | 0.046 | |
| Treatment | |||
| Treated with corticosteroids | 1 | 1.0 [0.6; 1.6] | 0.950 |
| Anticoagulant dose group | |||
 intermediate vs prophylactic intermediate vs prophylactic | 0.5 [0.2; 0.9] | 0.022 | |
 therapeutic vs prophylactic therapeutic vs prophylactic | 0.8 [0.5; 1.3] | 0.293 | |
 therapeutic vs intermediate therapeutic vs intermediate | 1.7 [0.9; 3.2] | 0.134 | |
Abbreviations: BMI, Body mass index; GFR, Glomerular filtration rate; ARDS, acute respiratory distress syndrome; EWS, Early warning score; ICU, Intensive care unit
Acknowledgements
Reims COVID Study Group: Ailsa ROBBINS, Kévin DIDIER, Pauline ORQUEVAUX, Violaine NOEL, Paola MARIANETTI, Juliette ROMARU, Dorothée LAMBERT, Jean Luc BERGER, Sandra DURY, Maxime DEWOLF, Jean Hugues SALMON, Jérôme COSTA, Julia SIMON, Natacha NOEL, Sara BARRAUD, Marion BARROIS, Hédia BRIXI, Quentin LAURENT-BADR, Manuelle VIGUIER, Clélia VANHAECKE, Laurence GUSDORF, Isabelle QUATRESOUS, Aline CARSIN-VU, Véronique BRODARD, Antoine HUGUENIN, Morgane BONNET, Aurore THIERRY.
We extend our sincere thanks to Fiona CAULFIELD for her assistance in editing this manuscript.
References
Articles from Mediterranean Journal of Hematology and Infectious Diseases are provided here courtesy of Catholic University in Rome
Full text links
Read article at publisher's site: https://doi.org/10.4084/mjhid.2022.036
Read article for free, from open access legal sources, via Unpaywall:
https://www.mjhid.org/index.php/mjhid/article/download/4957/4170
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04553575
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Randomised controlled trial comparing efficacy and safety of high versus low Low-Molecular Weight Heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol.
Trials, 21(1):574, 26 Jun 2020
Cited by: 27 articles | PMID: 32586394 | PMCID: PMC7316577
Coagulopathy of hospitalised COVID-19: A Pragmatic Randomised Controlled Trial of Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic (RAPID COVID COAG - RAPID Trial): A structured summary of a study protocol for a randomised controlled trial.
Trials, 22(1):202, 10 Mar 2021
Cited by: 14 articles | PMID: 33691765 | PMCID: PMC7943934
The Effect of Heparin Full-Dose Anticoagulation on Survival of Hospitalized, Non-critically Ill COVID-19 Patients: A Meta-analysis of High Quality Studies.
Lung, 201(2):135-147, 04 Feb 2023
Cited by: 7 articles | PMID: 36738324 | PMCID: PMC9899107
Review Free full text in Europe PMC

 1,2
1,2