Abstract
Free full text

Novel type of murein transglycosylase in Escherichia coli.
Abstract
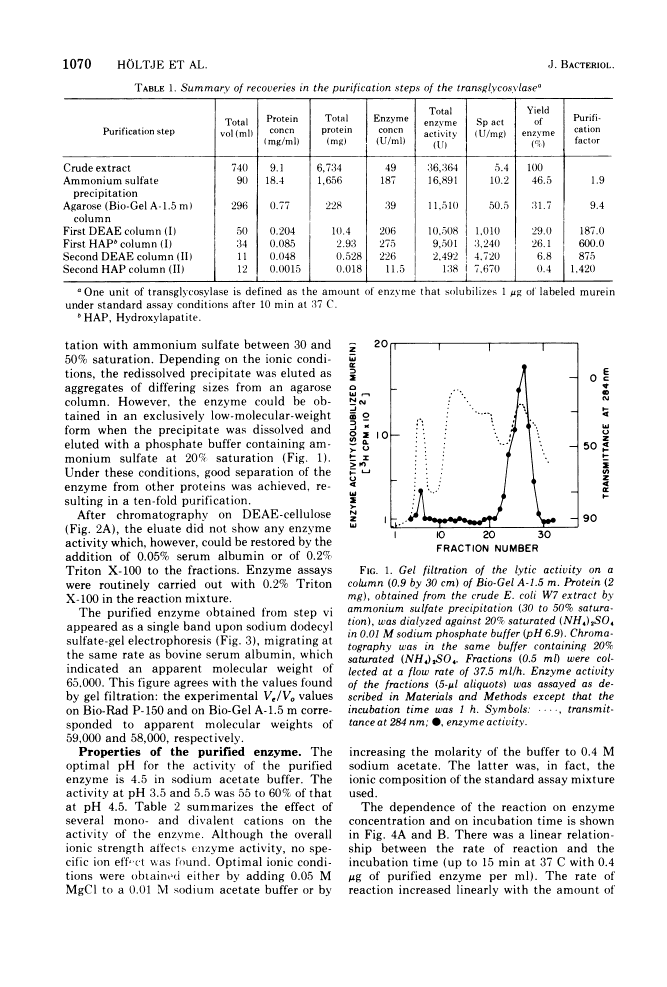
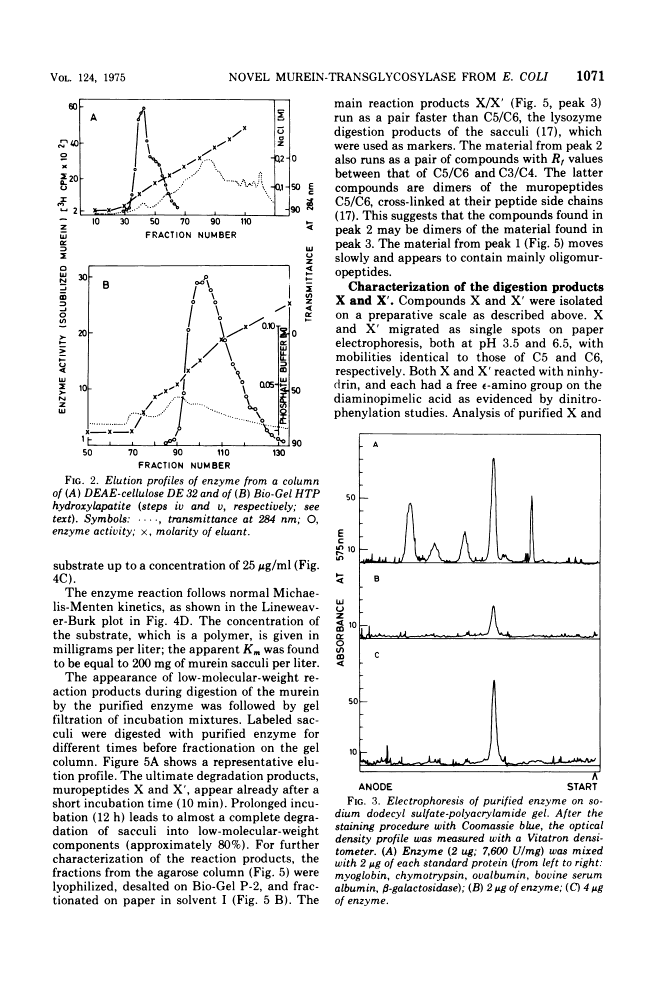
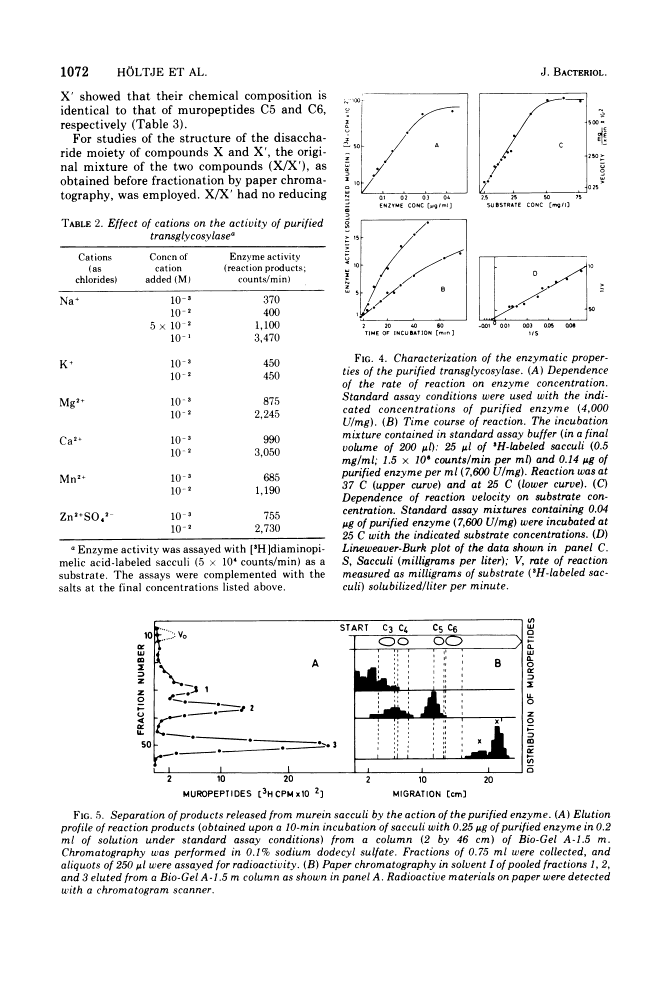
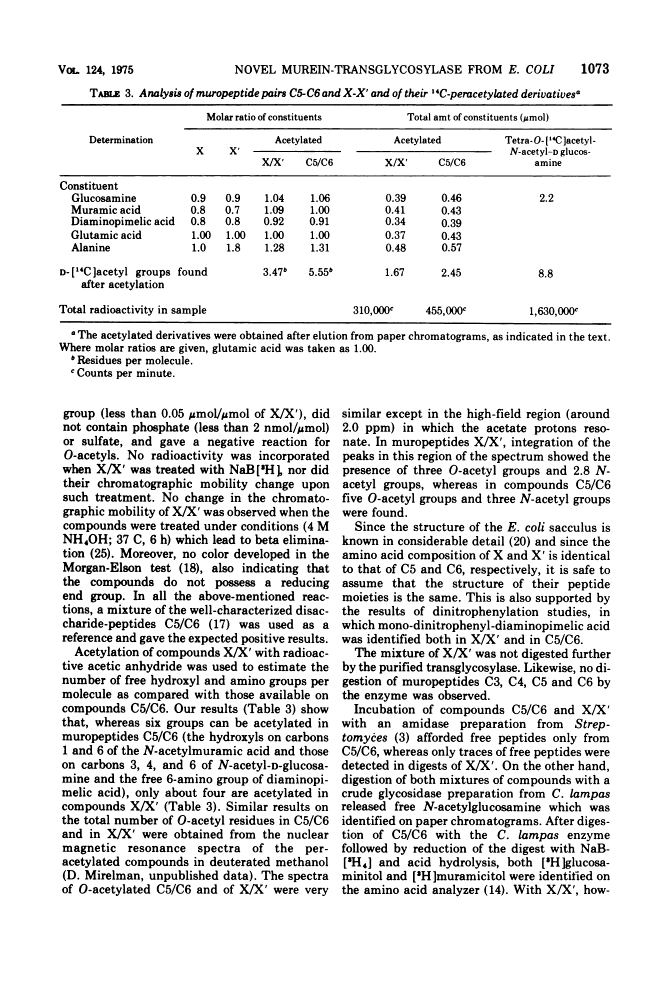
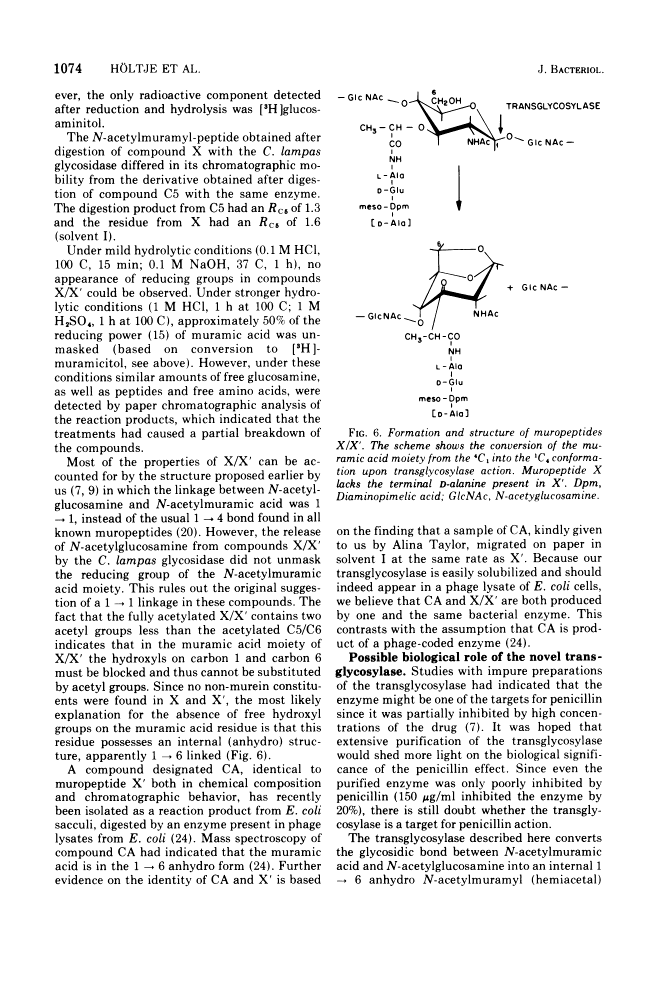
The purification and properties of a novel type of murein transglycosylase from Escherichia coli are described. The purified enzyme appears as a single band on sodium dodecyl sulfate-polyacrylamide gels and has an apparent molecular weight of approximately 65,000 as estimated by gel filtration and gel electrophoresis. It degrades pure murein sacculi from E. coli almost completely into low-molecular-weight products. The two prominent muropeptide fragments in the digest are the disaccharide-tripeptide N-acetylglucosamine-N-acetylmuramic acid-L-alanine-D-iso-glutamic acid-meso-diaminopimelic acid and the corresponding disaccharide-tetrapeptide N-acetylglucosamine-N-acetylmuramic acid-L-alanine-D-iso-glutamic acid-meso-diaminopimelic acid-D-alanine. The unique feature of these compounds is that the disaccharide has no reducing end group and that the muramic acid residue possesses an internal 1 leads to 6 anhydro linkage. The new lytic enzyme is designated as a murein: murein transglycosylase. Its possible role in the rearrangement of murein during cell growth and division is discussed.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. [Europe PMC free article] [Abstract] [Google Scholar]
- Braun V, Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. [Abstract] [Google Scholar]
- Ghuysen JM, Dierickx L, Coyette J, Leyh-Bouille M, Guinand M, Campbell JN. An improved technique for the preparation of Streptomyces peptidases and N-acetylmuramyl-l-alanine amidase active on bacterial wall peptidoglycans. Biochemistry. 1969 Jan;8(1):213–222. [Abstract] [Google Scholar]
- Hakenbeck R, Goodell EW, Schwarz U. Compartmentalization of murein hydrolases in the envelope of Escherichia coli. FEBS Lett. 1974 Apr 1;40(2):261–264. [Abstract] [Google Scholar]
- Hartmann R, Bock-Hennig SB, Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. [Abstract] [Google Scholar]
- Hartmann R, Höltje JV, Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. [Abstract] [Google Scholar]
- Höltje JV, Schwarz U. Penicillin and the murein-transglycosaminidase of Escherichia coli. Ann N Y Acad Sci. 1974 May 10;235(0):294–299. [Abstract] [Google Scholar]
- LEUTGEB W, WEIDEL W. UBER EIN IN COLI-ZELLWANDPRAEPARATEN ZURUECKGEHALTENES GLYKOGEN. Z Naturforsch B. 1963 Dec;18:1060–1062. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- McLean C, Werner DA, Aminoff D. Quantitative determination of reducing sugars, oligosaccharides, and glycoproteins with (3H)borohydride. Anal Biochem. 1973 Sep;55(1):72–84. [Abstract] [Google Scholar]
- PARK JT, JOHNSON MJ. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [Abstract] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [Abstract] [Google Scholar]
- PRIMOSIGH J, PELZER H, MAASS D, WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. [Abstract] [Google Scholar]
- Ryter A, Hirota Y, Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. [Abstract] [Google Scholar]
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. [Europe PMC free article] [Abstract] [Google Scholar]
- Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. [Abstract] [Google Scholar]
- SHARON N, JEANLOZ RW. The diaminohexose component of a polysaccharide isolated from Bacillus subtilis. J Biol Chem. 1960 Jan;235:1–5. [Abstract] [Google Scholar]
- SHARON N, SEIFTER S. A TRANSGLYCOSYLATION REACTION CATALYZED BY LYSOZYME. J Biol Chem. 1964 Jul;239:PC2398–PC2399. [Abstract] [Google Scholar]
- VOGEL HJ, BONNER DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [Abstract] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [Abstract] [Google Scholar]
- WEIDEL W, PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.124.3.1067-1076.1975
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/124/3/1067.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/124/3/1067
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/124/3/1067
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jb.124.3.1067-1076.1975
Article citations
A lytic transglycosylase connects bacterial focal adhesion complexes to the peptidoglycan cell wall.
Elife, 13:RP99273, 01 Oct 2024
Cited by: 0 articles | PMID: 39352247 | PMCID: PMC11444678
Control of bacterial cell wall autolysins by peptidoglycan crosslinking mode.
Nat Commun, 15(1):7937, 11 Sep 2024
Cited by: 0 articles | PMID: 39261529 | PMCID: PMC11390936
Breaking barriers: pCF10 type 4 secretion system relies on a self-regulating muramidase to modulate the cell wall.
mBio, 15(8):e0048824, 28 Jun 2024
Cited by: 0 articles | PMID: 38940556 | PMCID: PMC11323569
Peptidoglycan fragment release and NOD activation by commensal <i>Neisseria</i> species from humans and other animals.
Infect Immun, 92(5):e0000424, 02 Apr 2024
Cited by: 1 article | PMID: 38563734 | PMCID: PMC11075463
A peptidoglycan N-deacetylase specific for anhydroMurNAc chain termini in Agrobacterium tumefaciens.
J Biol Chem, 300(2):105611, 28 Dec 2023
Cited by: 1 article | PMID: 38159848 | PMCID: PMC10838918
Go to all (204) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Exoenzymatic activity of transglycosylase isolated from Escherichia coli.
Eur J Biochem, 116(2):355-358, 01 May 1981
Cited by: 33 articles | PMID: 7018908
Subcellular distribution of the soluble lytic transglycosylase in Escherichia coli.
J Bacteriol, 173(18):5668-5676, 01 Sep 1991
Cited by: 25 articles | PMID: 1885544 | PMCID: PMC208296
Purification and properties of a membrane-bound lytic transglycosylase from Escherichia coli.
J Bacteriol, 176(2):338-343, 01 Jan 1994
Cited by: 32 articles | PMID: 8288527 | PMCID: PMC205055
Enzymology of elongation and constriction of the murein sacculus of Escherichia coli.
Biochimie, 83(1):103-108, 01 Jan 2001
Cited by: 30 articles | PMID: 11254982
Review