Abstract
Purpose
To assess the outcome of excess follicle aspiration before intrauterine insemination (EFABI) in intrauterine insemination (IUI) cycles with 4-6 follicles ≥14 mm.Methods
A retrospective case-control study with 1559 patients undergoing IUI (donor and husband's sperm), of whom 86 underwent EFABI. We studied also an historical series of 2213 patients before EFABI implementation. For 3.5 years, all women undergoing IUI developing 4-6 follicles ≥14 mm were offered EFABI on the day of hCG administration. Pregnancy rates (PRs), multiple PRs, and adverse effects were measured.Results
EFABI was associated with a similar multiple PR (17.8% vs 17.5% in non-EFABI cases), with no triplets in EFABI patients. Live birth rates were significantly higher in EFABI cycles in IUI overall (25.5% vs 15.2%). When considered separately, the performance of EFABI resulted in significantly increased live birth rates in IUI-donor cycles (32.5% vs 18.5%), whereas the differences in IUI-husband cycles (19.5% vs 12.9%) did not reach statistical significance. The PR was 21.2% during the EFABI implementation period and 19.4% in the pre-EFABI period.Conclusions
EFABI in cycles in which 4-6 follicles reach ≥14 mm is a simple option that reduces cycle cancellation rates, results in higher PRs than cycles with 1-3 follicles, and lowers the risk of multiple pregnancy.Free full text

Aspiration of excess follicles before intrauterine insemination in high response cycles
Abstract
Purpose
To assess the outcome of excess follicle aspiration before intrauterine insemination (EFABI) in intrauterine insemination (IUI) cycles with 4–6 follicles ≥14 mm.
Methods
A retrospective case–control study with 1559 patients undergoing IUI (donor and husband's sperm), of whom 86 underwent EFABI. We studied also an historical series of 2213 patients before EFABI implementation. For 3.5 years, all women undergoing IUI developing 4–6 follicles ≥14 mm were offered EFABI on the day of hCG administration. Pregnancy rates (PRs), multiple PRs, and adverse effects were measured.
Results
EFABI was associated with a similar multiple PR (17.8% vs 17.5% in non‐EFABI cases), with no triplets in EFABI patients. Live birth rates were significantly higher in EFABI cycles in IUI overall (25.5% vs 15.2%). When considered separately, the performance of EFABI resulted in significantly increased live birth rates in IUI‐donor cycles (32.5% vs 18.5%), whereas the differences in IUI‐husband cycles (19.5% vs 12.9%) did not reach statistical significance. The PR was 21.2% during the EFABI implementation period and 19.4% in the pre‐EFABI period.
Conclusions
EFABI in cycles in which 4–6 follicles reach ≥14 mm is a simple option that reduces cycle cancellation rates, results in higher PRs than cycles with 1–3 follicles, and lowers the risk of multiple pregnancy.
1. INTRODUCTION
Intrauterine insemination (IUI) is one of the most popular infertility treatments worldwide. 1 , 2 In many centers, it is the primary treatment modality in cases of unexplained infertility and mild‐to‐moderate female and male factor infertility, with typical per‐cycle pregnancy rates (PRs) ranging from 10% to 20%. 3 It has the advantage of being a simple, non‐invasive technique and is relatively cheap. 1
In the ESHRE register published in 2020, almost 190 000 IUI with husband's semen (IUIH) and almost 50 000 IUI with donor's semen (IUID) cycles were performed in Europe. The rates of twins and triplets were 8.9% and 0.5% in IUIH and 7.3% and 0.6% in IUID. 4 One of the major challenges in current assisted reproductive techniques (ART) is preventing multiple pregnancies. 5 , 6 , 7 , 8 Nowadays, this can be achieved relatively easily in IVF, since ovarian response, 9 one of the most important favorable prognostic factors, can be completely isolated from multiple pregnancy risk as a result of single embryo transfer policies and frozen embryo programs. 10 In contrast, in IUI, ovarian response is directly related to both PR overall 11 and multiple PR, including high‐order multiple pregnancies. 11 , 12 , 13
In a meta‐analysis, the PR in IUI cycles with monofollicular growth was 8.4% compared with 13.4%, 16.4%, and 16.4% in cycles with two, three, and four follicles. 11 The risk of multiple pregnancy in cycles with monofollicular growth was 3.7% compared with 6%, 14%, and 10% in cycles with two, three, and four follicles. 11
One strategy to regulate the number of follicles is to use lower doses of gonadotropins. 14 , 15 Despite this measure, however, multiple follicular response can still happen occasionally. 16 The standard course of action in IUI cycles with hyperresponse is cycle cancellation. Other options are converting the cycle into IVF 17 , 18 or even continuing the cycle, and, in cases of multiple pregnancy, performing embryo/fetal reduction, 19 with notable ethical and psychological implications.
One possible alternative is excess follicle aspiration before insemination (EFABI). This consists of ultrasound‐guided vaginal puncture of the excess follicles before insemination. A similar approach has been used in women undergoing ovarian stimulation, 20 , 21 , 22 especially those with polycystic ovarian syndrome (PCOS), mainly to prevent multiple pregnancy and ovarian hyperstimulation syndrome (OHSS).
Four previous reports of EFABI exist in the literature, published between 12 and 26 years ago. 23 , 24 , 25 , 26 In them, the timing of the EFABI procedure was markedly heterogeneous, 23 , 24 , 25 , 26 and two of them included both IUI and timed‐intercourse cycles. 23 , 24 , 25 , 26
The aim of our study was to analyze the impact of EFABI on PR, multiple PR, and cancellation rates, when performed systematically on the day of hCG administration, in IUI high responders, in a setting in which cycles of extremely high responders were converted to IVF.
2. MATERIALS AND METHODS
The study population consisted of all the women who underwent EFABI in the reproduction unit of our university hospital, between July 1, 2016, and December 31, 2019. The results of the EFABI population were compared with those of patients in the same period not undergoing EFABI. A second analysis was performed, comparing all IUI results (with and without EFABI) from the period when EFABI was available (July 1, 2016–December 31, 2019) with the 3.5 previous years in which EFABI was not used (January 1, 2013–June 30, 2016).
Institutional board approval was obtained (code CEIC EI8/12), and written informed consent forms were given to all women and, in case of couples, their partners also.
The inclusion criteria for our IUI program were as follows: (1) for IUIH, age <38 years, at least one normal patent tube as assessed by hysterosalpingography (HSG) or laparoscopy, anti‐Müllerian hormone (AMH) >0.1 ng/ml, sperm with >5 million motile sperm recovered and (2) for IUID, age <40 years and at least one normal patent tube regardless of AMH level. Exclusion criteria for our IUI program were as follows: body mass index (BMI) >35 kg/m2; endometriosis III‐IV; myoma type 1–3; and uterine malformation.
The main indications for IUIH were as follows: idiopathic infertility (46%); PCOS (19%); mild‐to‐moderate male factor (14%); low ovarian reserve (12%); unilateral tubal factor (7%); and endometriosis I‐II (2%). The main indications for IUID were azoospermia/cryptozoospermia (23%) and women without a male partner (77%).
Our IUI work‐up has been described previously. 3 , 27 , 28 The female work‐up included at least pelvic examination, blood chemistry, measurement of hormone levels (including AMH levels), HSG, and pelvic ultrasound (US). A laparoscopic study was performed when HSG findings suggested any abnormality (however minor). Women with at least one normal patent tube were considered eligible for IUI. Couples with male factor infertility were considered to be potentially eligible for IUI when, despite some seminogram parameters being subnormal according to World Health Organization standards, 29 it was possible to obtain 5 × 106 motile sperm/ml after semen preparation.
Ovarian stimulation was performed with hMG (Menopur) or recombinant FSH (Gonal; Merck, Spain or Bemfola). Monitoring of ovarian stimulation was performed with plasma estradiol, progesterone, and ultrasound.
The starting dose of gonadotropins was based on AMH, antral follicle count (AFC), the woman's age, BMI, and cause of infertility. The following median starting doses (IU/day) were administered in IUIH patients: 150 [150–206] (interquartile range) when AMH ≤ 0.4 ng/ml; 150 [102–150] when AMH = 0.5–0.8 ng/ml; 110 [81.2; 150] when AMH = 0.9–1.2 ng/ml; 75.0 [75.0; 110] when AMH = 1.3–2.4 ng/ml; and 75.0 [50.0; 75.0] when AMH ≥ 2.5 ng/ml. Starting doses in IUID were somewhat lower: 150 [150; 150], 100 [93.8; 150], 75.0 [75.0; 150], 75.0 [75.0; 100], and 50.0 [37.5; 75.0].
Semen samples for IUI were prepared with density gradients (Origio® Gradient 100, Universal IVF Medium with Phenol Red). A single insemination with 0.3–0.5 ml was performed per cycle 38 h after the administration of 250 mcg of rec‐hCG (Ovitrelle, Merck Laboratories). A total of five cycles of IUI were performed if pregnancy was not obtained earlier. The luteal phase was supplemented with vaginal micronized progesterone (200 mg/12 h for the 14 days following insemination).
2.1. Management of hyperresponder patients
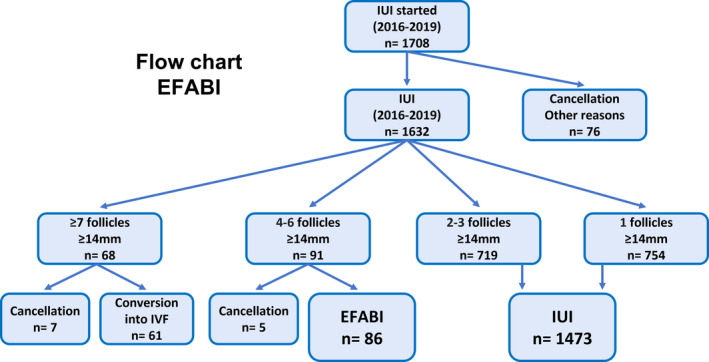
Women with ≥7 follicles ≥14 mm were advised to switch to IVF, and if they declined, the cycle was canceled. Women who had between 4 and 6 follicles between 14 and 18 mm on the day of hCG administration or the day before were advised to undergo EFABI, and if they declined, the cycle was canceled. The exclusion criteria for EFABI were plasma estradiol levels >1500 pg/ml, progesterone levels >1.6 ng/ml, having undergone EFABI previously, and gonadotropins administered at a dose of >150 UI/day (Figure 1).
2.2. EFABI method
The aspiration of excess follicles was performed on the day of the hCG administration or, at weekends, 24 h earlier. When more than 3 follicles were confirmed, women were told about the procedure and what it involved, as well as the alternative, namely cancellation. Blood was drawn to confirm estradiol, and progesterone levels were adequate, and while waiting for the results (approximately 2–3 h), the woman had time to read and sign the informed consent form. Even if written informed consent was given, the technique was not performed if the levels of estradiol or progesterone were not right.
As a safety measure, a peripheral line was put in place. Before starting the ultrasound‐guided vaginal puncture, the presence of excess follicles was confirmed, and the best site for the aspiration was decided (aiming to aspirate the smallest follicles and leave the larger ones, if possible, with a single ovarian puncture). The EFABI procedure was very similar to oocyte pick‐up performed with local anesthesia. 30 The vagina was disinfected with povidone‐iodine and saline solution. For local anesthesia, 20 ml of paracervical mepivacaine 1% were applied on one or both sides, depending on where the puncture was to be performed. If the woman experienced pain, she was administered metamizole IV, 1 ampoule of 0.4 g/ml in 500 ml of saline. The puncture was performed with the usual COOK® 18‐gauge Ovum Aspiration Needle, single lumen (K‐PPS‐6035‐RWH‐B‐ET, G20943). If the patient had good ovarian access and good tolerance, one 18–20 mm follicle was left in each ovary or two in the same ovary in the case of unilateral tubal factor. The objective of the EFABI was to leave two follicles in IUID and two to three follicles in IUIH (depending on accessibility, personal preferences, and clinical parameters).
The follicular fluids were checked for oocytes under a stereoscopic microscope (40×–100×). When oocytes were found, the protocol of carrying out IUI on the same day as the puncture was followed. After the puncture, vaginal hemostasis and pain were assessed, and the line was removed. Women were prescribed a single 1g dose of oral azithromycin to take at home, advised to rest at home, and given hCG administration guidelines. IUI was performed 38 h later, and the patient was asked about possible side effects.
2.3. Definitions
Biochemical pregnancy was defined as a positive urine pregnancy test 15 days after insemination. Clinical pregnancy was defined as the presence of a gestational sac at 7–8 weeks of gestation. Multiple pregnancy was defined as the presence of more than 1 gestational sac at 7–8 weeks. High‐order pregnancy was defined as the presence of >3 gestational sacs at 7–8 weeks. Live birth was defined as live birth at ≥24 weeks of gestational age.
2.4. Statistical analysis
Continuous variables were presented as mean (standard deviation), if the data were normally distributed; and otherwise, as median (interquartile range). Furthermore, for normally distributed data, comparisons were made with t‐tests for two groups and analysis of variance for more than two groups. The p‐value was corrected with Tukey's test for multiple comparisons. If data were not normally distributed, the Mann–Whitney U test was used for two groups and the Kruskal–Wallis test for more than two, using Benjamin and Hochberg's correction. Categorical variables were described using frequency and percentages, and the chi‐squared test was used to compare groups.
The analyses were performed with the statistical software R, version 3.6.1. (R: a language and environment for statistical computing. R Foundation for Statistical Computing).
3. RESULTS
3.1. Flow chart
During the study period, 1708 IUI processes were started (Figure 1).
Among these, 1632 IUI cycles were performed. Of them, 68 women had ≥7 follicles >14 mm and were advised to convert to IVF, 61 agreed, and the other 7 cycles being cancelled (because they did not agree). Moreover, 91 women had 4–6 follicles and were recommended EFABI, which ended up being performed on 86 of them, and 5 cycles were cancelled (2 due to elevated progesterone levels and 3 because they declined EFABI). In 76 cases, the cycle was cancelled for lack of response, premature luteinization, or social problems. The remaining 1473 cycles had between 1 and 3 follicles (1 follicle in 754 cases and 2–3 follicles in 719 cases), and IUI was performed without EFABI.
3.2. EFABI‐IUI vs IUI without EFABI
A total of 1559 IUI cycles were performed, including 927 IUIH and 632 IUID. 1473 of them were non‐EFABI cases, while EFABI was performed in 86, amounting to 5.5% of all IUI cycles. Before aspiration, the mean number of follicles ≥14 mm was 4.7 ± 1.0 (4.2 ± 1.0 >17 mm and 0.5 ± 0.9 of 14–17 mm). After aspiration, the mean number of follicles ≥14 mm was 2.4 ± 0.7, similar than in the non‐EFABI group (2.3 ± 1.1), but the mean number of follicles >17 mm was significantly higher (2.2 ± 0.6 vs 1.6 ± 0.8) and the mean number of follicles 14–17 mm was significantly lower (0.2 ± 0.5 vs 0.6 ± 0.7) (Table 1).
TABLE 1
EFABI vs non‐EFABI in the IUI population
|
EFABI N:86 |
Non‐EFABI N:1473 |
All IUI N:1559 (EFABI + non‐EFABI) | OR (95% CI) | p (EFABI vs non‐EFABI) | |
|---|---|---|---|---|---|
| Woman's age (years) | 34.5 ± 3.1 | 34.5 ± 3.4 | 34.5 ± 3.4 | 0.87 | |
| Man's age (years) | 36.4 ± 4.8 | 36.7 ± 5.1 | 36.7 ± 5.0 | 0.62 | |
| Duration of infertility (years) | 3.0 ± 1.7 | 3.1 ± 1.5 | 3.1 ± 1.5 | 0.44 | |
| AMH (ng/ml) | 3.1 ± 2.6 | 2.8 ± 2.7 | 2.8 ± 2.7 | 0.32 | |
| Starting dose of gonadotropins (UI/day) | 98.6 ± 38.4 | 107.0 ± 52.1 | 107.0 ± 51.5 | 0.05 | |
| AFC | 15.1 ± 7.6 | 13.4 ± 6.7 | 13.5 ± 6.7 | 0.06 | |
| BMI (kg/m2) | 24.3 ± 4.2 | 24.6 ± 4.6 | 24.6 ± 4.6 | 0.51 | |
| No. of follicles >17 mm | 2.2 ± 0.6 a (4.2 ± 1.0) b | 1.6 ± 0.8 | 1.7 ± 0.8 c (1.8 ± 1.0) d | <0.001 vs a; <0.001 vs b | |
| No. of follicles 14–17 mm | 0.2 ± 0.5 a (0.5 ± 0.9) b | 0.6 ± 0.7 | 0.6 ± 0.8 c (0.6 ± 0.8) d | <0.001 vs a; 0.24 vs b | |
| No. of follicles ≥14 mm | 2.4 ± 0.7 a (4.7 ± 1.0) b | 2.3 ± 1.1 | 2.3 ± 1.1 c (2.4 ± 1.2) d | 0.12 vs a; <0.001 vs b | |
| OHSS (%) | 0 (0/86) | 0.1 (2/1473) | 0.1 (2/1559) | 3.4 (0.2–71.4) | 1 |
| Estradiol on the day of hCG (pg/ml) | 1031.9 (497.1) | 483.9 (313.0) | 748.9 (405.0) | <0.01 | |
| Biochemical PR (%) | 34.8 (30/86) | 22.2 (327/1473) | 22.9 (357/1559) | 1.9 (1.2–3.0) | 0.01 |
| Clinical PR (%) | 32.5 (28/86) | 20.6 (303/1473) | 21.2 (331/1559) | 1.9 (1.2–3.0) | 0.01 |
| Multiple pregnancies (%) e | 17.8 (5/28) | 17.5 (53/303) | 17.5 (58/331) | 1.0 (0.4–2.8) | 0.96 |
| Triplet pregnancies (%) e | 0.0 (0/28) | 1.3 (4/303) | 1.2 (4/331) | 1.2 (0.1–22.2) | 0.91 |
| Live births (%) f | 25.5 (22/86) | 15.2 (224/1473) | 15.8 (246/1559) | 1.9 (1.2–3.2) | 0.01 |
| Singleton births (%) g | 90.9 (20/22) | 84.4 (189/224) | 85.0 (209/246) | 1.9 (0.4–8.3) | 0.42 |
| Twin births (%) g | 9.1 (2/22) | 15.6 (35/224) | 15.0 (37/246) | 0.5 (0.1–2.4) | 0.42 |
Demographic parameters and clinical results. Values are mean ± SD, unless specified otherwise.
Abbreviations: AFC, antral follicle count; EFABI, excess follicle aspiration before intrauterine insemination; BMI, body mass index; CI, confidence interval; IUI, intrauterine insemination; OR, odds ratio; PR, pregnancy rate.
The clinical PR per cycle was 21.2%. The live birth rate (LBR) per cycle in IUI was 15.8%. The singleton birth rate was 85%. No significant differences were found in IUI cycles with and without EFABI in terms of woman's age, man's age, duration of infertility, AMH levels, starting dose of gonadotropins, or AFC, although there was a trend toward higher AFC and lower gonadotropin doses in EFABI patients.
The clinical PR in the EFABI group was 32.5% (28/86), significantly higher than the 20.6% (303/1473) found in the non‐EFABI group (OR = 1.86, CI = 1.16–2.97; p = 0.0076). The LBR was also significantly higher: 25.5% (22/86) vs 15.2% (303/1473) (OR = 1.91, CI = 1.15–3.17; p 0.0120), as well as the biochemical PR.
The multiple pregnancy rate was very similar in both groups (17.8% and 17.5%). There were no triplets in the EFABI group (0/28), while there were several in the non‐EFABI population (1.3%; 4/303). Differences in the singleton birth rate were not significant (90.9% and 84.4%).
Among the 61 patients converted to IVF, the per‐transfer pregnancy rate was 40.98% and the LBR was 26.6%. A mean of 8.89 (4.54 DS) oocytes was obtained.
Most EFABIs were performed in the first (44.2%) or second (26.7%) cycle of IUI; however, clinical PRs were higher than usual in all attempts. Specifically, the pregnancy rate was 29.3% in the first cycle, and 36.2%, 42.7%, 26.4%, and 18.5% in the second, third, fourth, and fifth cycle, respectively. EFABI was not performed in the sixth cycle.
3.3. EFABI‐IUIH vs IUIH without EFABI
There were no significant differences in woman's age, man's age, duration of infertility, AMH, starting dose, or AFC in women with and without EFABI. There was a trend to a higher clinical PR in EFABI patients (32.6% [13/46] vs 17.0% [150/881] in non‐EFABI patients), but the difference did not reach significance (OR = 1.91, CI = 0.98–3.72, p = 0.054), and the pattern in biochemical PR was somewhat similar. The LBR was 19.5% in EFABI patients and 12.9% in non‐EFABI patients, which was not significantly different. The multiple PR was 7.7% in the EFABI group vs 22% in the non‐EFABI group, OR = 0.29 (CI = 0.03–2.35), (p = 0.20). There were no triplet pregnancies in the EFABI group (0/13) vs 2% (3/150) in the non‐EFABI group (p = 0.77). There were no differences in the singleton birth rate (Table 2).
TABLE 2
EFABI vs non‐EFABI in IUIH
|
EFABI N:46 |
Non‐EFABI N:881 |
All IUI N:927 (EFABI + non‐EFABI) | OR (95% CI) | p (EFABI vs non‐EFABI) | |
|---|---|---|---|---|---|
| Woman's age (years) | 35.9 ± 2.8 | 34.1 ± 3.4 | 34.1 ± 3.4 | 0.16 | |
| Man's age (years) | 36.4 ± 4.5 | 36.5 ± 4.6 | 36.5 ± 4.6 | 0.21 | |
| Duration of infertility (years) | 3.3 ± 1.6 | 3.2 ± 1.4 | 3.2 ± 1.4 | 0.37 | |
| AMH (ng/ml) | 3.6 ± 3.2 | 3.1 ± 3.1 | 3.1 ± 3.1 | 0.39 | |
| Starting dose of gonadotropins (UI/day) | 103.6 ± 42.3 | 110.4 ± 53.0 | 110.0 ± 52.5 | 0.24 | |
| AFC | 15.9 ± 8.8 | 14.0 ± 7.0 | 14.1 ± 7.2 | 0.19 | |
| Estradiol on the day of hCG (pg/ml) | 1104.9 ± 519.0 | 532.1 ± 328.9 | 818.5 ± 424.4 | <0.01 | |
| BMI (kg/m2) | 23.9 ± 4.2 | 23.7 ± 4.5 | 23.7 ± 4.5 | 0.78 | |
| No. of follicles >17 mm | 2.3 ± 0.2 a (4.4 ± 1.1) b | 1.7 ± 0.8 | 1.7 ± 0.8 c (1.9 ± 1.0) d | <0.001 vs a; <0.01 vs b | |
| No. of follicles 14–17 mm | 0.2 ± 0.4 a (0.6 ± 0.9) b | 0.7 ± 0.7 | 0.7 ± 0.8 c (0.7 ± 0.8) d | <0.001 vs a; 0.39 vs b | |
| No. of follicles ≥14 mm | 2.5 ± 0.6 a (5.0 ± 0.9) b | 2.4 ± 1.1 | 2.4 ± 1.1 c (2.6 ± 1.2) d | <0.001 vs a; <0.001 vs b | |
| OHSS (%) | 0 (0/46) | 0.23 (2/881) | 0.22 (2/927) | 3.8 (0.2–79.9) | 1 |
| Biochemical PR (%) | 30.4 (14/46) | 18.9 (167/881) | 19.5 (181/927) | 1.9 (1.0–3.5) | 0.06 |
| Clinical PR (%) | 28.3 (13/46) | 17.0 (150/881) | 17.6 (163/927) | 1.9 (1.0–3.7) | 0.05 |
| Multiple pregnancies (%) e | 7.7 (1/13) | 22.0 (33/150) | 20.8 (34/163) | 0.3 (0.0–2.4) | 0.24 |
| Triplet pregnancies (%) e | 0.0 (0/13) | 2.0 (3/150) | 1.8 (3/163) | 1.6 (0.1–1.8) | 0.77 |
| Live births (%) f | 19.5 (9/46) | 12.9 (114/881) | 13.2 (123/927) | 1.6 (0.1–3.5) | 0.20 |
| Singleton births (%) g | 100.0 (9/9) | 81.6 (93/114) | 82.9 (102/123) | 4.4 (0.2–7.8) | 0.31 |
| Twin births (%) g | 0.0 (0/9) | 18.4 (21/114) | 17.1 (21/123) | 0.2 (0.0–4.1) | 0.31 |
Demographic parameters and clinical results. Values are mean ± SD, unless specified otherwise.
Abbreviations: AFC, antral follicle count; EFABI, excess follicle aspiration before insemination; BMI, body mass index; CI, confidence interval; IUI, intrauterine insemination; OR, odds ratio; PR, pregnancy rate; OHSS, ovarian hyperstimulation syndrome.
3.4. EFABI‐IUID vs. IUID without EFABI
EFABI and non‐EFABI populations were very similar in clinical and demographic characteristics, although there was a trend in EFABI patients toward lower doses of gonadotropins (the difference nearly reached statistical significance). In IUID, the LBR rate was 32.5% (13/40) in EFABI patients vs 18.5% (110/592) in non‐EFABI (OR = 2.10; CI = 1.05–4.22; p = 0.03). Clinical PRs were 37.5% and 25.8% (p = 0.10). Multiple PR was 26.6% (4/15) in the EFABI group compared with 13.1% (20/153) in the non‐EFABI group, but the difference did not reach significance (p = 0.16) (Table 3).
TABLE 3
EFABI vs non‐EFABI in IUID
|
EFABI N:40 |
Non‐EFABI N:592 |
All IUI N:632 (EFABI + non‐EFABI) | OR (95% CI) | p (EFABI vs non‐EFABI) | |
|---|---|---|---|---|---|
| Woman's age (years) | 35.2 ± 3.3 | 35.0 ± 3.3 | 35.0 ± 3.3 | 0.88 | |
| Man's age (years) | 36.0 ± 5.6 | 37.9 ± 7.0 | 37.8 ± 7.0 | 0.79 | |
| Duration of infertility (years) | 1.3 ± 1.3 | 2.7 ± 1.8 | 2.6 ± 1.8 | 0.59 | |
| AMH (ng/ml) | 2.5 ± 1.6 | 2.3 ± 1.8 | 2.3 ± 1.8 | 0.60 | |
| Starting dose of gonadotropins (UI/day) | 88.3 ± 32.8 | 101.0 ± 50.4 | 100.2 ± 49.6 | 0.06 | |
| AFC | 14.0 ± 5.6 | 12.6 ± 5.9 | 12.6 ± 5.9 | 0.38 | |
| Estradiol on the day of hCG (pg/ml) | 906.2 ± 451.8 | 415.4 ± 274.9 | 660.8 ± 363.3 | <0.01 | |
| No. of follicles >17 mm | 2.1 ± 0.6 a (4.0 ± 1.0) b | 1.5 ± 0.7 | 1.5 ± 0.7 c (1.7 ± 0.9) d | <0.001 vs a; <0.01 vs b | |
| No. of follicles 14–17 mm | 0.3 ± 0.6 a (0.4 ± 0.9) b | 0.5 ± 0.7 | 0.5 ± 0.7 c (0.5 ± 0.8) d | <0.001 vs a; 0.49 vs b | |
| No. of follicles ≥14 mm | 2.4 ± 0.8 a (4.5 ± 1.2) b | 2.1 ± 1.1 | 2.1 ± 1.6 c (2.2 ± 1.2) d | 0.04 vs a; <0.001 vs b | |
| OHSS (%) | 0 (0/40) | 0 (0/592) | 0 (0/632) | NC | NC |
| BMI (kg/m2) | 24.3 (4.2) | 25.4 (5.5) | 25.4 (5.4) | 0.17 | |
| Biochemical PR (%) | 40.0 (16/40) | 27.0 (160/592) | 27.8 (176/632) | 1.8 (0.9–3.5) | 0.08 |
| Clinical PR (%) e | 37.5 (15/40) | 25.8 (153/592) | 26.6 (168/632) | 1.7 (0.9–3.4) | 0.10 |
| Multiple pregnancies (%) e | 26.7 (4/15) | 13.1 (20/153) | 14.3 (24/168) | 2.4 (0.7–8.3) | 0.16 |
| Triplet births (%) e | 0.0 (0/15) | 0.7 (1/153) | 0.6 (1/168) | 3.3 (0.1–84.0) | 0.47 |
| Live births (%) f | 32.5 (13/40) | 18.5 (110/592) | 19.4 (123/632) | 2.1 (1.1–4.2) | 0.03 |
| Singleton births (%) g | 84.6 (11/13) | 87.2 (96/110) | 87.0 (107/123) | 0.8 (0.2–4.0) | 0.78 |
| Twin births (%) g | 15.4 (2/13) | 12.7 (14/110) | 13.0 (16/123) | 1.2 (0.2–6.2) | 0.78 |
Demographic parameters and clinical results. Values are mean ± SD, unless specified otherwise.
Abbreviations: AFC, antral follicle count; EFABI, excess follicle aspiration before insemination; BMI, body mass index; CI, confidence interval; IUI, intrauterine insemination; OR, odds ratio; PR, pregnancy rate; NC, not calculable.
3.5. IUI results before and after EFABI implementation
There were no significant differences in man's age, duration of infertility, AMH, or AFC between cases from the years 2013 to 2016 without EFABI and 2016 to 2019 with EFABI. However, in the pre‐EFABI years, women were significantly older (35.8 ± 3.08 years vs 35.5 ± 3.37 years). Moreover, the percentage of IUI performed in the fifth or sixth cycle was significantly higher in the pre‐EFABI period than in the EFABI period (12% vs 6.2%, p < 0.0001). The dose of gonadotropins used was also significantly higher in the pre‐EFABI period (115.7 ± 46.5 vs 107 ± 51.5, p < 0.0001).
The clinical PR was somewhat higher in the EFABI period (21.2% vs 19.4%), although the difference did not reach statistical significance. The multiple PR was very similar in both groups, while the decrease in triplets (2.32% to 1.2%) did not reach significance. The singleton birth rate was significantly higher in the EFABI period (82.3% vs 77.2%). The rate of cancellation due to hyperresponse significantly decreased (3.4% to 0.7%), and the rate of transformation to IVF remained similar. The rate of OHSS was 0.2% (6/2439) in the pre‐EFABI period (only 1 case requiring hospital admission) vs 0.1% (2/1632) in the EFABI period (none requiring hospitalization). There was no case of OHSS in EFABI patients (p > 0.05).
When the analysis was limited to IUIH, there were no significant differences in IUI outcomes. When the study was focused on IUID, a significantly higher singleton birth rate was observed (70.4%–87%), as well as a trend to a lower multiple PR (22.7% vs 14.3%).
3.6. Adverse effects and costs
Among all the EFABIs performed, two patients experienced a vagal reaction (2.3%), while other patients reported slight pain, and none developed complications. All follicular fluids were analyzed, and an oocyte was found in only one sample (which was vitrified), in the case of a woman with a progesterone level of 1.6 ng/ml on the day of the puncture; IUI was therefore performed on the same day. There were no cases of OHSS in any of the women who underwent EFABI.
The average cost of an EFABI procedure in our center is 225 euros.
4. DISCUSSION
IUI is a low‐complexity, low‐cost technique, which has well‐known efficacy in certain conditions such as idiopathic infertility, PCOS, failing to conceive with ovulation induction and timed‐intercourse, mild‐moderate male factor, and donor insemination. 31 , 32 , 33 , 34 However, in recent years, although the number of IUI cycles has risen in absolute terms, the increase in IVF cycles has been more pronounced, 4 , 14 and the main factor responsible for this relative decrease in IUI is the risk of multiple pregnancy. 35 In IVF, rates of multiple pregnancies, especially high‐order pregnancies, have markedly decreased due to the almost complete disappearance of transfers of three or more embryos and the increasing adoption of single embryo transfer policies. The adoption of these restrictive practices in terms of the number of embryos transferred has had little influence on per‐cycle PRs, due to improvements in methods of embryo selection, incubation, culture, and freezing, among others. 4 , 14 , 36
In contrast, to date, there are only two widespread strategies to prevent multiple pregnancy in IUI. The first is the use of milder stimulation; however, with milder stimulation, PRs are significantly lower 15 , 37 , 38 and monofollicular cycles are associated with a pregnancy rate of 8.4% 39 compared with 15% in multi‐follicular cycles. 11 Furthermore, even with smaller doses, the hyperresponse risk cannot be avoided completely. In an Italian retrospective study, despite initial doses of 50 IU, the multiple PR was still 9.5% and 5% of cycles were cancelled due to hyperresponse. 16
The second prevention strategy is the cancellation of the IUI cycle. This cancellation may be complete, that is to say, the IUI cycle is cancelled, and no other reproductive treatment is used. Cancellation rates due to hyperresponse in IUI with gonadotropins are not always reported, but they seem to range from 5% to 12.7%, varying with population, starting dose, and cancellation criteria. 16 , 40 , 41 Logically, this leads to considerable frustration among couples and also requires patients not to have sexual relations or to use barrier contraceptive methods to avoid a multiple pregnancy through natural intercourse. Additionally, there may be some risk of OHSS. Moreover, the money invested in the cycle and the medication has been spent with no chance of success.
A further form of cancellation is conversion of the IUI cycle into an IVF cycle, which has been reported to have good results. 7 , 17 , 18 , 37 Nonetheless, this strategy poses some problems. On the one hand, there is the unanticipated additional cost to the infertile couple, and the requirement for an ongoing IVF program, which limits the use of this approach to IVF centers. 7 Additionally, there is an issue in cases that reach a number of follicles considered too high for an IUI cycle, but still relatively few for an IVF cycle. For example, 4–6 follicles of 14–16 mm will often yield markedly fewer mature oocytes than what is deemed desirable for a standard IVF cycle, taking into account that the ideal response in IVF is between 10 and 15 oocytes, and that fewer than 4 is considered insufficient. 42 , 43 Therefore, such converted cycles would presumably have significantly lower PRs than if these patients had undergone IVF from the outset, using higher stimulation doses, and thus greater follicular responses, larger numbers of embryos, and the possibility of cryotransfers later on.
Selective follicular aspiration has been applied in ovarian stimulation cycles followed by intercourse, especially in PCOS patients, to prevent multiple pregnancy and OHSS. 20 , 21 , 22 Previous reports on EFABI differ in methodology from the one we used: None of them considered the option of IVF conversion for extremely high responders; in two cases, EFABI was performed in nearly 50% of all women undergoing IUI 23 , 24 vs nearly 5% in our series, and EFABI was performed 36 h after hCG administration 25 or on the day of the first IUI, 23 , 24 while the only study in which aspiration was performed on the day of hCG administration included only 26 cases (followed by IUI or intercourse). 26 There were also differences regarding the number of follicles left, as well as the performance of flushing.
Our protocol restricted the use of EFABI to women with 4–6 follicles ≥14 mm, thereby offering IVF if they had >7 follicles. Moreover, we performed EFABI on the day of hCG administration in order to avoid prior ovulation of any of the oocytes, posing a risk of multiple pregnancy. Furthermore, we did not flush the follicles, and we did not recover oocytes (except in one case with premature progesterone rise). Therefore, the decrease in multiple PR should be due to the inability of follicles to ovulate after aspiration of the follicular fluid, and not to the aspiration of oocytes. Moreover, the aim of our protocol was to convert cycles with ≥4 mature follicles to bi‐trifollicular cycles, while cycles with 3 follicles remained unpunctured.
With the use of EFABI and the strategy outlined in the flow chart, we managed to overcome several of the aforementioned problems. Firstly, we were able to prevent cancellation of cycles, which would otherwise have amounted to 9.7% of all the IUI cycles, with the psychological and economic implications this poses. This allows the use of stimulation protocols aimed at the development of 1–3 follicles, which was achieved in 90.3% of cases in our population. Among the other cases, only 3.7% were converted into an IVF cycle, resulting in ovarian responses that were not far from optimal, although EFABI was performed in 5.2% of the cycles, compared with nearly 50% in other reports. 23 , 24
Regarding EFABI outcomes, it should be noted that after EFABI (practiced in patients at high risk of multiple pregnancy because they had 4 to 6 follicles), the rates of twin birth were the same as in non‐EFABI patients (17.8% vs 17.5%). Concerning triplets, although there were no triple pregnancies after EFABI, the sample size was too small for differences to reach statistical significance. Indeed, EFABI transformed cycles with high risk of multiple pregnancy into standard risk IUI cycles. In addition, LBRs were significantly higher in EFABI cycles considering all IUI (25.5% vs 15.2%) and IUID (32.5% vs 18.5%), while in IUIH, although higher, the difference did not reach significance (19.5% vs 12.9%). This means that in patients with hyperresponse in IUI, which would typically require the cancellation of the cycle, not only was the cycle not cancelled, but also the LBR achieved was higher than in the usual cycles. Presumably, this could be explained because after EFABI, there were still 3–2 follicles in IUIH and 2 in IUID, while the number of follicles in standard IUI ranged between 1 and 3. It could also be due to the fact that higher IUI responders have better oocyte quality, which is unaffected by EFABI.
We found an overall multiple PR in IUI of 17.8%, which, despite still being high, is considerably lower than the rate of 31.8% in IUI with gonadotropins described in the most recent meta‐analysis. 38 Furthermore, the triplet pregnancy rate was 1% of pregnancies, representing just 0.2% of all cycles.
When comparing our results since the implementation of EFABI to the historical results prior to the use of EFABI, the following aspects should be considered. First of all, this is not a randomized study, and hence, there are many possible confounding factors, including diagnostic, selection, and cancellation criteria. Nonetheless, we would like to point out that despite the current use of lower doses for initial stimulation, the LBRs were similar in both groups, while current data indicate a significantly higher rate of single births, fewer cancellations due to hyperresponse, and a lower rate of triple pregnancies.
EFABI is a low‐cost, simple, fast, and well‐tolerated technique. Although more studies are needed, EFABI seems to be a good option for minimizing cancellation rates in IUI cycles with hyperresponse in which 4 to 6 mature follicles are produced, converting them into standard IUI cycles in terms of risk of multiple pregnancy, but with higher PRs than standard IUI cycles.
CONFLICT OF INTEREST
B Prieto, M Diaz‐Núñez, L Lainz, A Rabanal, M Iglesias, T Jauregui, B Corcostegui, A Matorras, S Pérez, and R Matorras declare that they have no conflict of interest.
ETHICS STATEMENT
Human rights statements and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Institutional board approval was obtained (code CEIC EI8/12), and written informed consent was given to women and, in case of couples, also to their partners.
Notes
Prieto B, Diaz‐Nuñez M, Lainz L, et al. Aspiration of excess follicles before intrauterine insemination in high response cycles. Reprod Med Biol. 2022;21:e12470. 10.1002/rmb2.12470 [CrossRef] [Google Scholar]
REFERENCES
Articles from Reproductive Medicine and Biology are provided here courtesy of John Wiley & Sons Australia, Ltd on behalf of Japan Society for Reproductive Medicine.
Full text links
Read article at publisher's site: https://doi.org/10.1002/rmb2.12470
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/rmb2.12470
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/140846853
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/rmb2.12470
Article citations
Aspiration of excess follicles before intrauterine insemination in high response cycles.
Reprod Med Biol, 21(1):e12470, 01 Jan 2022
Cited by: 1 article | PMID: 35781922 | PMCID: PMC9241166
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Intrauterine insemination versus intracervical insemination in donor sperm treatment.
Cochrane Database Syst Rev, 1:CD000317, 25 Jan 2018
Cited by: 8 articles | PMID: 29368795 | PMCID: PMC6491301
Review Free full text in Europe PMC
Effect of small follicles on clinical pregnancy and multiple pregnancy rates in intrauterine insemination: a cohort study.
Hum Reprod, 39(2):335-345, 01 Feb 2024
Cited by: 0 articles | PMID: 38148021
Effect of prematurely elevated late follicular progesterone on pregnancy outcomes following ovarian stimulation-intrauterine insemination for unexplained infertility: secondary analysis of the AMIGOS trial.
Hum Reprod, 39(8):1684-1691, 01 Aug 2024
Cited by: 0 articles | PMID: 38822675 | PMCID: PMC11291944
Controlled ovarian stimulation should not be preferred for male infertility treated with intrauterine insemination: a retrospective study.
Reprod Biol Endocrinol, 19(1):45, 19 Mar 2021
Cited by: 2 articles | PMID: 33740990 | PMCID: PMC7977560

 3
3