Abstract
Objective
Triple-crossover randomized controlled intervention trial to test whether reduced exposure to household NO2 or fine particles results in reduced symptoms among children with persistent asthma.Methods
Children (n = 126) aged 5-11 years with persistent asthma living in homes with gas stoves and levels of NO2 15 ppb or greater recruited in Connecticut and Massachusetts (2015-2019) participated in an intervention involving three air cleaners configured for: (1) NO2 reduction: sham particle filtration and real NO2 scrubbing; (2) particle filtration: HEPA filter and sham NO2 scrubbing; (3) control: sham particle filtration and sham NO2 scrubbing. Air cleaners were randomly assigned for 5-week treatment periods using a three-arm crossover design. Outcome was number of asthma symptom-days during final 14 days of treatment. Treatment effects were assessed using repeated measures, linear mixed models.Results
Measured NO2 was lower (by 4 ppb, p < .0001) for NO2-reducing compared to control or particle-reducing treatments. NO2-reducing treatment did not reduce asthma morbidity compared to control. In analysis controlling for measured NO2, there were 1.8 (95% CI -0.3 to 3.9, p = .10) fewer symptom days out of 14 in the particle-reducing treatment compared to control.Conclusions
It remains unknown if using an air cleaner alone can achieve levels of NO2 reduction large enough to observe reductions in asthma symptoms. We observed that in small, urban homes with gas stoves, modest reductions in asthma symptoms occurred using air cleaners that remove fine particles. An intervention targeting exposures to both NO2 and fine particles is complicated and further research is warranted.Registration number
NCT02258893.Free full text

Childhood asthma and household exposures to nitrogen dioxide and fine particles: A triple-crossover randomized intervention trial
Abstract
Objective.
Triple-crossover randomized controlled intervention trial to test whether reduced exposure to household NO2 or fine particles results in reduced symptoms among children with persistent asthma.
Methods.
Children (n=126) aged 5–11 years with persistent asthma living in homes with gas stoves and levels of NO2 15 ppb or greater recruited in Connecticut and Massachusetts (2015–2019) participated in an intervention involving three air cleaners configured for: 1) NO2 reduction: sham particle filtration and real NO2 scrubbing; 2) particle filtration: HEPA filter and sham NO2 scrubbing; 3) control: sham particle filtration and sham NO2 scrubbing. Air cleaners were randomly assigned for 5-week treatment periods using a three-arm crossover design. Outcome was number of asthma symptom-days during final 14 days of treatment. Treatment effects were assessed using repeated measures, linear mixed models.
Results.
Measured NO2 was lower (by 4ppb, p<0.0001) for NO2-reducing compared to control or particle-reducing treatments. NO2-reducing treatment did not reduce asthma morbidity compared to control. In analysis controlling for measured NO2, there were 1.8 (95% CI −0.3 – 3.9, p=0.10) fewer symptom days out of 14 in the particle-reducing treatment compared to control.
Conclusions.
It remains unknown if using an air cleaner alone can achieve levels of NO2 reduction large enough to observe reductions in asthma symptoms. We observed that in small, urban homes with gas stoves, modest reductions in asthma symptoms occurred using air cleaners that remove fine particles. An intervention targeting exposures to both NO2 and fine particles is complicated and further research is warranted.
Introduction
Previous research suggests that exposure to nitrogen dioxide (NO2) puts children with asthma, nearly 8.4% of all US children under 18 years of age (1), at increased risk for negative respiratory health outcomes (2–10). NO2 has major sources both outdoors as part of a complex, often traffic-related, air pollution mix (11, 12) and indoors as a byproduct of combustion associated with gas appliances, primarily stoves (12). Levels of indoor NO2 where sources are present and where children spend 70% of their time (13) can be much higher than outdoors. In one study, NO2 levels in homes with gas appliances was 15.6 (10.4) ppb compared to 5.9 (4.7) mean (SD) ppb in homes with electric stoves (2). Some of the highest levels of asthma are found in inner cities (14). where as many as 88% of households cook with gas and where mean levels of NO2 over 30 ppb have been reported (9, 15). In Southern New England, highest indoor NO2 exposures are found in households with the lowest socioeconomic status (2).
Randomized controlled trials of environmental interventions conducted in homes of children with asthma have primarily targeted asthma triggers such as allergen exposure (16–19), and were designed to examine the impact on asthma outcomes in sensitized subjects following attempts to reduce levels of specific allergens. Other trials targeted fine particles using air filtration to examine the impact of reductions in particle levels on asthma outcomes (20–25). None of these trials were conducted with the subject and/or researcher blinded to the intervention.
Except for a randomized trial of school furnace replacement in Australia (26), no environmental intervention trial has specifically targeted indoor NO2 as an asthma trigger. We designed a Phase III clinical trial using an air cleaner to accommodate one of three configurations each containing a particle filter and four media-filled canisters for gas-phase scrubbing: 1) NO2 reduction with sham particle filtration and real NO2 scrubbing (i.e., canisters filled with NO2-reducing media); 2) particle reduction with HEPA filtration and sham NO2 scrubbing (canisters filled with inert media); and 3) control with sham particle filtration and sham NO2 scrubbing. Our intervention trial was designed to test the hypothesis that among children with persistent asthma exposed to household NO2 or fine particles, a reduction in either would result in clinically meaningful reductions in asthma morbidity.
Methods
Study Participants
Families of children with asthma in Connecticut and western Massachusetts were recruited from September 2015-April 2019 (online supplement, Methods). Initial screening determined if the family had a child with asthma, gas stove and home consisting of seven or fewer rooms. The asthmatic child’s eligibility included: 1) asthma symptoms and/or medication use during the previous 12 months consistent with persistent asthma (2); 2) age 5 to 11 years; 3) resident at least 5 days and nights every week in the home. Exclusion criteria included other respiratory co-morbidities or use of steroid medication for conditions other than asthma. Families intending to move within the 15-week study duration were excluded. Families satisfying initial criteria and agreeing to a one-week household NO2 screening were sent a passive NO2 monitor (2, 27) for placement in the main living space. Families with NO2 levels of 15 ppb or higher (as an integrated average over the one-week screening period) were invited to participate. Enrollment of more than one eligible child per family was permitted. All families received payment for participation. The Human Research Protection Program Institutional Review Boards of the University approved this study, and the child’s primary caregiver gave informed consent. The study was registered as a Clinical Trial (Registry Name: Indoor Air Pollution and Children with Asthma: An Intervention Trial (CAPS); Registry Number: NCT02258893.
Study Design
The intervention protocol was a block-randomized, double-blind, triple-crossover design involving three air cleaner configurations (“treatments”). Families were randomized into one of 6 treatment sequences each requiring 3 periods of 5-weeks beginning with a 1-week washout period followed by a 4-week observation period (online supplement, Figure S1). Randomizations were blocked so that for every 18 families randomized there were 3 in each sequence. Families were randomized at the time an enrollment home visit was scheduled. A child (study observational unit) was enrolled at the home visit once the child’s caregiver signed the consent form and accepted delivery of the equipment. All enrolled families, principal investigators and all but three support staff were blind as to the nature and sequence of air cleaner treatment assignments (online supplement, Methods).
Environmental Intervention Protocol
Custom-configured air cleaners were used for three intervention treatments (online supplement, Figure S2). Air cleaner 1: NO2 reduction configured to provide sham particle filtration and real NO2 scrubbing with Purafil® (Doraville, GA) media (28). Air cleaner 2: particle reduction configured to provide fine particle filtration (with a HEPA filter) and sham NO2 scrubbing with inert media. Air cleaner 3: control configured to provide sham particle filtration and sham NO2 scrubbing. All machines were identical in appearance and weight (online supplement, Methods; Tables S1, S2; Figures S2, S3).
At the initial home visit, a research assistant installed an air cleaner; placed passive NO2 and nicotine monitors (29, 30) (to identify passive smoking exposure, a known asthma trigger (20, 23)); interviewed the child’s primary caregiver (designated as the “respondent” for the study) to collect medical history and demographic information; and provided the respondent with a calendar diary to record the child’s daily asthma symptoms, medication use, physician visits, respiratory illnesses, days of restricted activity and missed days of school. At the end of each treatment period, a research assistant replaced the air cleaner with the next one assigned, collected the passive air monitors and placed new ones; or collected all equipment at the end of the study. Data on the child’s asthma symptoms and medication use during each treatment was collected during a phone interview at the end of each period by a blinded interviewer. Asthma-related adverse events were reported regularly to a project Data Safety Monitoring Board.
Information collected at the end of each treatment period was used to determine adherence to study protocol: 1) the air cleaner was running continuously for 90% of the 35-day monitoring period according to the machine hours of operation counter; 2) the air cleaner was in a protocol-acceptable location for 90% of the monitoring period; 3) the child slept in the home for 5 out of every 7 nights.
Outcome Measure
The primary outcome measure was number of days with asthma symptoms reported during the final 14 days of each intervention treatment (24, 31, 32) defined as the maximum of: 1) number of days with wheezing, chest tightness or persistent cough; 2) number of nights of sleep disturbance; 3) number of days when activities were affected.
Statistical Analyses
Power calculations conducted for a clinically meaningful effect size of 0.7 fewer days of symptoms out of 14 days (32) in treatment (NO2-reduction or particle-reduction) compared to control showed 90% power for enrollment of 200 and 80% power for 175. To assess treatment effects on the health outcome, we used a within-subjects, repeated measures, linear mixed model with the covariance matrix defined as “unstructured” (PROC MIXED, in SAS (version 9.4, SAS Institute, Inc.)). Covariates for the adjusted models include mid-point of season of treatment period (Table 2, footnote c), environmental tobacco smoke exposure during treatment period (Table 2, footnote b), allergic status (respondent’s report of physician’s diagnosis), age, gender, race, Hispanic ethnicity, respondent’s education level (Table 1, footnote b), and indicator for second enrolled asthmatic child. The health outcome comparisons of primary importance are those between the NO2-reduction or particle-reduction, and control air cleaners.
Table 1.
Characteristics of children in the Yale Children’s Air Pollution Study (CAPS).
| Analysis Group Inclusion | |||
|---|---|---|---|
| Characteristics | Enrolled N (%) | Intent to Treat N (%) | Compliance N (%) |
| Total Subjects | 126 | 117 | 109 |
| Age (yrs) | |||
 5 – 7 5 – 7 | 74 (58.7) | 67 (57.3) | 62 (56.9) |
 8 – 10a 8 – 10a | 52 (41.3) | 50 (42.7) | 47 (43.1) |
| Gender | |||
 Male Male | 75 (59.5) | 71 (60.7) | 66 (60.6) |
 Female Female | 51 (40.5) | 46 (39.3) | 43 (39.4) |
| Hispanic | 64 (50.8) | 60 (51.3) | 58 (53.2) |
| Race | |||
 American Indian American Indian | 1 (0.8) | 1 (0.9) | 1 (0.9) |
 Asian Asian | 2 (1.6) | 1 (0.9) | 1 (0.9) |
 Pacific Islander Pacific Islander | 1 (0.8) | 0 | 0 |
 Black Black | 44 (34.9) | 41 (35.0) | 35 (32.1) |
 White White | 26 (20.6) | 24 (20.5) | 23 (21.1) |
 Multi-racial Multi-racial | 25 (19.8) | 25 (21.4) | 25 (22.9) |
 Other Other | 27 (21.4) | 25 (21.4) | 24 (22.0) |
| Respondent’sb education (yrs) | |||
 <12 <12 | 15 (11.9) | 14 (12.0) | 11 (10.1) |
 12–15 12–15 | 84 (66.7) | 78 (66.7) | 74 (67.9) |
 ≥ 16 ≥ 16 | 27 (21.4) | 25 (21.4) | 24 (22.0) |
| Allergies (report of MD dx) | 94 (74.6) | 88 (75.2) | 81 (74.3) |
| Asthma severityc | |||
 No symptoms/no medication use No symptoms/no medication use | 0 | 0 | 0 |
 Mild transient Mild transient | 6 (4.8) | 5 (4.3) | 5 (4.6) |
 Mild persistent Mild persistent | 33 (26.2) | 30 (25.6) | 29 (26.6) |
 Moderate persistent Moderate persistent | 40 (31.7) | 36 (30.8) | 33 (30.3) |
 Severe persistent Severe persistent | 47 (37.3) | 46 (39.3) | 42 (38.5) |
| Smoking in the home (self-report at screening) | |||
 None None | 109 (86.5) | 101 (86.3) | 96 (88.1) |
 Tobacco only Tobacco only | 9 (7.1) | 8 (6.8) | 7 (6.4) |
 E-cigarettes only E-cigarettes only | 3 (2.4) | 3 (2.6) | 1 (0.9) |
 Both Both | 4 (3.2) | 4 (3.4) | 4 (3.7) |
 Unknown Unknown | 1 (0.8) | 1 (0.9) | 1 (0.9) |
| Season of first treatment armd | |||
 Summer Summer | 17 (13.5) | 14 (12.0) | 13 (11.9) |
 Fall Fall | 36 (28.6) | 35 (29.9) | 33 (30.3) |
 Winter Winter | 25 (19.8) | 25 (21.4) | 22 (20.2) |
 Spring Spring | 48 (38.1) | 43 (36.8) | 41 (37.6) |
Note: N=126 asthmatic children from 116 families in Connecticut and the Springfield area of Massachusetts.
Table 2.
Distribution of subject characteristics by treatment arms for all subjects included in the intent-to-treat analysis (N=117 subjects completed N=332 treatment arms [observations]).
| Treatment Arms Completed Air Cleaner Configuration | ||||||
|---|---|---|---|---|---|---|
| Covariates | N (%) (Ss) | N (obs) | NO2-reduction N (%) (Ss) | Particle-reduction N (%) (Ss) | Control N (%) (Ss) | p-valueb |
| Total | 117 | 332 | 109 | 113 | 110 | |
| Age (yrs) | 0.97 | |||||
 5 – 7 5 – 7 | 67 (57.3) | 192 | 64 (58.7) | 65 (57.5) | 63 (57.3) | |
 8 – 10 8 – 10 | 50 (42.7) | 140 | 45 (41.3) | 48 (42.5) | 17 (42.7) | |
| Gender | 0.98 | |||||
 Male Male | 71 (60.7) | 200 | 65 (59.6) | 68 (60.2) | 67 (60.9) | |
 Female Female | 46 (39.3) | 132 | 44 (40.4) | 45 (39.8) | 43 (39.1) | |
| Hispanic | 0.96 | |||||
 No No | 57 (48.7) | 161 | 54 (49.5) | 54 (47.8) | 53 (48.2) | |
 Yes Yes | 60 (51.3) | 171 | 55 (50.5) | 59 (52.2) | 57 (51.8) | |
| Race | 0.99 | |||||
 Black Black | 41 (35.0) | 114 | 38 (34.9) | 38 (33.6) | 38 (34.5) | |
 White White | 24 (20.5) | 70 | 23 (21.1) | 24 (21.2) | 23 (20.9) | |
 Multi-racial Multi-racial | 25 (21.4) | 68 | 21 (19.3) | 24 (21.2) | 23 (20.9) | |
 Other Other | 27 (23.1) | 80 | 27 (24.8) | 27 (23.9) | 26 (23.6) | |
| Respondent’s education (yrs) | 0.99 | |||||
 <12 <12 | 14 (12.0) | 42 | 14 (12.8) | 14 (12.4) | 14 (12.7) | |
 12–15 12–15 | 78 (66.7) | 221 | 73 (67.0) | 74 (65.5) | 74 (67.3) | |
 ≥ 16 ≥ 16 | 25 (21.4) | 69 | 22 (20.2) | 25 (22.1) | 22 (20.0) | |
| Allergies (report of MD dx) | 0.99 | |||||
 No No | 29 (24.8) | 82 | 27 (24.8) | 28 (24.8) | 27 (24.5) | |
 Yes Yes | 88 (75.2) | 250 | 82 (75.2) | 85 (75.2) | 83 (75.5) | |
| Enrolled child number | 0.98 | |||||
 1 1 | 107 (91.4) | 304 | 100 (91.7) | 103 (91.2) | 101 (91.8) | |
 2 2 | 10 ( 8.6) | 28 | 9 (8.3) | 10 (8.8) | 9 (8.2) | |
| Smoking in the home during treatment armc | 0.34 | |||||
 No No | 237 | 83 (76.2) | 80 (70.8) | 74 (67.3) | ||
 Yes Yes | 95 | 26 (23.8) | 33 (29.2) | 36 (32.7) | ||
| Season of treatment armd | 0.60 | |||||
 Summer Summer | 74 | 27 (24.8) | 22 (19.5) | 25 (22.7) | ||
 Fall Fall | 62 | 16 (14.7) | 25 (22.1) | 21 (19.1) | ||
 Winter Winter | 96 | 37 (33.9) | 31 (27.4) | 28 (25.4) | ||
 Spring Spring | 100 | 29 (26.6) | 35 (31.0) | 36 (32.7) | ||
Note: N=9 subjects of 126 enrolled were excluded from the intent-to-treat analysis including 7 who withdrew before completing the first treatment arm due to moving out of the study area (n=1), electricity cost concerns (n=2), machine noise (n=3), and children damaging machine (n=1); and 2 subjects completing study prior to adoption of final outcome measure.
All observations were used in an intent-to-treat analysis. Observations that did not adhere to study protocol for machine hours of operation or location, or duration of subject’s presence in the home were excluded from the compliance analysis. Measured NO2 levels, i.e., the 5-week integrated average concentration resulting from analysis of the passive monitor placed in the main living area during each treatment, were used in repeated measures models to examine the NO2-reducing efficacy in the NO2-reducing treatment compared to other treatments. The association of NO2 concentration and health outcome was examined with repeated measures regression analysis.
Results
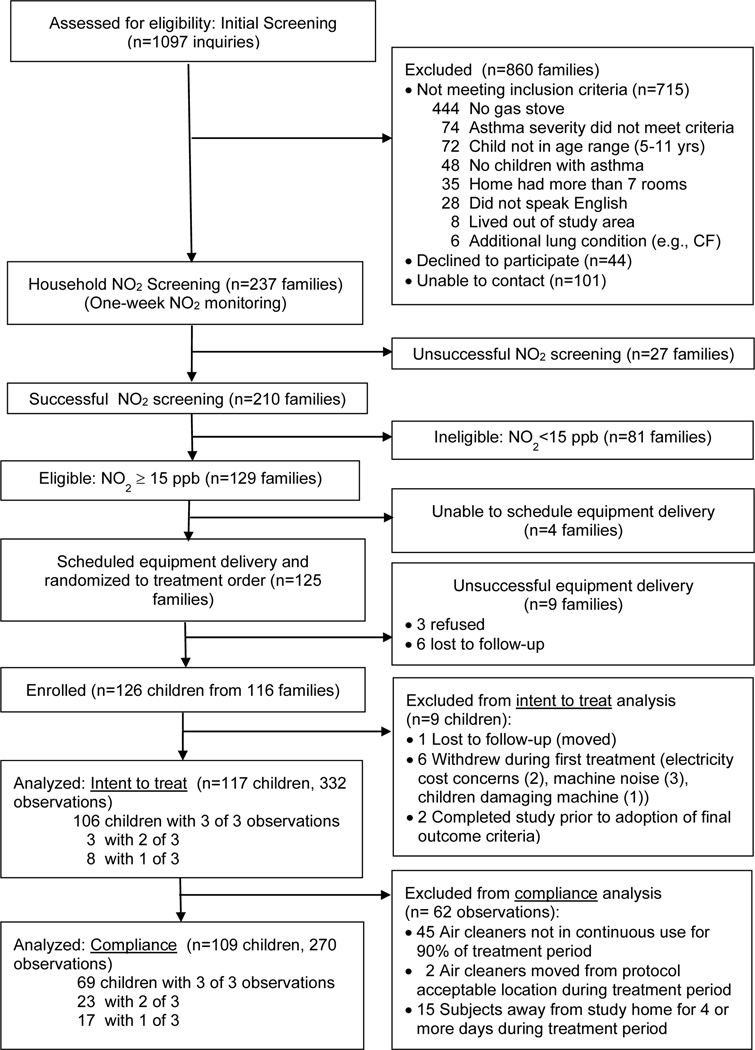
From October 2015 through April 2019, nearly 2,000 inquiries were generated by our recruitment efforts (online supplement Table S3). Initial screening interviews resulted in 237 invitations to participate in household NO2 screening (22% of those who inquired) (Figure 1). Nearly 90% of those invited completed the one-week NO2 monitoring, and 61% of these families had household NO2 levels of 15 ppb or greater and were invited to participate in the study. A total of 126 children from 116 families were successfully enrolled (i.e., randomized, scheduled, consented, and accepted equipment delivery) (Figure 1). Two eligible asthmatic children were enrolled from 9% of the families (10/116).
Table 1 describes the characteristics of children enrolled in the study (n=126) and included in the intent-to-treat (n=117) and compliance (n=109) analyses. The mean (SD) age of children enrolled was 7 (2) years with a median (IQR) age of 7 (3) years. Over half (60%) were male, 80% non-white and 51% Hispanic. Nearly 80% of the respondents had less than 16 years of education. Three-quarters of the children had been diagnosed with atopy, and 95% entered the study with the target disease severity, i.e., mild persistent to severe persistent asthma. Asthma severity at enrollment was based on asthma symptoms and medication use in each of the previous 12 months using data collected at the time of the enrollment home interview, and adapted from Global Initiative for Asthma guidelines (2, 33). Smoking in the home was self-reported at the time of screening by 13% of respondents. Most subjects began their first treatment in spring (38%) and fewest began in summer (14%).
Table 2 displays the distribution of characteristics across treatments for subjects included in the intent-to-treat analysis (N=117). Of 126 subjects enrolled, 9 were excluded from the intent-to-treat analysis including 7 who withdrew before completing the first treatment due to moving out of the study area (n=1), electricity cost concerns (n=2), machine noise (n=3), and children damaging machine (n=1); and 2 subjects completing the study prior to adoption of final outcome measure. Analyses included all observations from treatments completed by these 117 subjects (n=332 observations from NO2-reduction (n=109), particle-reduction (n=113) and control (n=110) treatments). Household smoking during each treatment was determined by measured nicotine levels (at or above 0.07 μg/m3 nicotine) (34) for 98.5% of the observations. For homes missing nicotine measurements (1.4%), household smoking was determined by self-report on the treatment follow-up interview. No significant differences in distribution were observed for any of the covariates. Table 3 displays the association of individual study covariates with the health outcome for subjects included in the intent-to-treat analysis. Mean reported symptoms were significantly associated with respondent’s education level: mean (SD) symptom days reported for children of parents with less than a high school education (5 (5) days out of 14) was significantly higher than days reported for children of parents with 16 or more years of education (2 (4) days, p = 0.03). None of the asthma-related adverse events reported were deemed to be study-related (online supplement, Table S4).
Table 3.
Distribution of subject characteristics by number of symptom days in final 14 days of treatment arm for all subjects included in the intent-to-treat analysis (N=117 subjects completed N=332 treatment arms [observations]).
| Treatment Arms | Symptom Daysa | |||
|---|---|---|---|---|
| Covariates | N (obs) | Mean (SD) | Median (IQR) | p-valueb |
| Age (yrs) | 0.16 | |||
 5 – 7 5 – 7 | 192 | 2.81 (3.83) | 1.0 (4.0) | |
 8 – 10 8 – 10 | 140 | 3.61 (4.74) | 2.0 (5.0) | |
| Gender | 0.45 | |||
 Male Male | 200 | 2.96 (4.22) | 1.0 (4.0) | |
 Female Female | 132 | 3.43 (4.34) | 2.0 (5.5) | |
| Hispanic | 0.51 | |||
 No No | 161 | 2.93 (4.10) | 1.0 (4.0) | |
 Yes Yes | 171 | 3.35 (4.41) | 2.0 (5.0) | |
| Race | 0.18 | |||
 Black Black | 114 | 3.54 (4.47) | 2.0 (4.0) | |
 White White | 70 | 2.06 (3.16) | 1.0 (3.0) | |
 Multi-racial Multi-racial | 68 | 2.72 (4.00) | 0 (4.0) | |
 Other Other | 80 | 3.90 (4.84) | 2.0 (6.5) | |
| Respondent’s education (yrs) | 0.03 | |||
 <12 <12 | 42 | 4.95 (5.24) | 3.0 (8.0) | |
 12–15 12–15 | 221 | 3.14 (4.16) | 2.0 (4.0) | |
 ≥ 16 ≥ 16 | 69 | 2.06 (3.58) | 0 (3.0) | |
| Allergies (report of MD dx) | 0.75 | |||
 No No | 82 | 3.00 (3.90) | 1.5 (4.0) | |
 Yes Yes | 250 | 3.20 (4.39) | 2.0 (4.0) | |
| Enrolled child number | 0.18 | |||
 1 1 | 304 | 3.01 (4.14) | 1.0 (4.0) | |
 2 2 | 28 | 4.68 (5.28) | 2.5 (8.5) | |
| Smoking in the home during treatment armc | 0.55 | |||
 No No | 237 | 3.05 (4.23) | 1.0 (4.0) | |
 Yes Yes | 95 | 3.38 (4.36) | 2.0 (5.0) | |
| Season of treatment armd | 0.14 | |||
 Summer Summer | 74 | 2.15 (3.07) | 1.0 (3.0) | |
 Fall Fall | 62 | 3.61 (4.46) | 1.5 (6.0) | |
 Winter Winter | 96 | 3.51 (4.74) | 1.5 (6.0) | |
 Spring Spring | 100 | 3.25 (4.37) | 2.0 (4.0) | |
Note: N=9 subjects of 126 enrolled were excluded from the intent-to-treat analysis including 7 who withdrew before completing the first treatment arm due to moving out of the study area (n=1), electricity cost concerns (n=2), machine noise (n=3), and children damaging machine (n=1); and 2 subjects completing study prior to adoption of final outcome measure.
Of the subjects (n=117; 332 observations) in the intent-to-treat analysis, an additional 8 subjects (62 observations) were excluded from the compliance analysis including 45 observations where the air cleaner was not in continuous use for 90% of the treatment; 2 air cleaners were moved from protocol acceptable locations; and 15 subjects were away from the study home for 4 or more days.
Results of analysis of effect of intervention treatment on number of symptom days in final 14 days of treatment are shown for unadjusted and adjusted models in Table 4 both for intent-to-treat (A) and compliance (B) analyses and revealed no statistically significant effect of air cleaner configuration.
Table 4.
Effect of treatment on number of symptom days in final 14 days of treatment shown for intent-to-treat (A) and compliance (B) analyses.
| Treatment arms | Unadjusted | Adjusteda | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Analysis | N (Ss) | N (obs) | df | Estimate (SE) | p-valueb | df | Estimate (SE) | p-valueb |
| A. Intent-to-treat c | 117 | 332 | ||||||
| Air Cleaner Configurations | 2, 116 | 0.84 | 2, 106 | 0.77 | ||||
 NO2-reduction vs Control NO2-reduction vs Control | 116 | 0.20 (0.45) | 0.65 | 106 | 0.31 (0.45) | 0.49 | ||
 Particle-reduction vs Control Particle-reduction vs Control | 116 | 0.25 (0.44) | 0.58 | 106 | 0.24 (0.44) | 0.59 | ||
| B. Compliance d | 109 | 270 | ||||||
| Air Cleaner Configurations | 2, 108 | 0.86 | 2, 98 | 0.92 | ||||
 NO2-reduction vs Control NO2-reduction vs Control | 108 | −0.27 (0.50) | 0.59 | 98 | −0.19 (0.51) | 0.71 | ||
 Particle-reduction vs Control Particle-reduction vs Control | 108 | −0.20 (0.50) | 0.69 | 98 | −0.16 (0.51) | 0.75 | ||
Note: Effect estimates shown for pairwise air cleaner filter configuration contrasts show differences in symptom days between treatment arms.
Household levels of NO2 measured in each 5-week treatment period for observations included in the compliance analysis were significantly different. Estimated mean (SEM) concentrations were 17.1 (1.2) ppb in NO2-reducing, 20.9 (1.3) ppb in particle-reducing, and 21.0 (1.3) ppb in control treatments. Analysis estimated concentration differences between treatments to be 3.8 ppb (95% CI 2.3 – 5.3 ppb) higher for control compared to NO2-reducing, 3.8 ppb (95% CI 2.3 – 5.2 ppb) higher for particle-reducing compared to NO2-reducing, and −0.07 ppb (95% CI −1.6 – 1.4 ppb) for particle-reducing compared to control (online supplement, Table S5).
Table 5 shows the distribution of symptom outcomes in the compliance analysis by NO2 quartiles of concentration measured in the home during each treatment. Median NO2 concentration was 15.8 ppb, close to the eligibility criteria of 15 ppb; three-quarters of all measurements were at or below 22 ppb. A repeated measures analysis of the association between household NO2 concentration and the symptom outcome showed a positive association (p=0.0025), i.e., an increase of 0.7 symptom days in 14 days for every 10 ppb increase in NO2.
Table 5.
Distribution of symptom outcomes in the compliance analysis. Mean (SD) number of symptom days in final 14 days of treatment by quartile of measured household NO2 for each 5-week treatment.
| Treatment Arms Completed | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | NO2-reduction | Particle-reduction | Control | |||||
|
|
|
|
| |||||
| NO2 (ppb)a | N (obs) | Mean (SD) | N (obs) | Mean (SD) | N (obs) | Mean (SD) | N (obs) | Mean (SD) |
| 0 to ≤ 12.3 | 68 | 2.88 (3.95) | 37 | 3.16 (4.03) | 15 | 1.60 (2.41) | 16 | 3.44 (4.82) |
| > 12.3 to ≤ 15.8 | 64 | 2.56 (4.01) | 24 | 2.83 (3.94) | 23 | 2.74 (4.13) | 17 | 1.94 (3.70) |
| > 15.8 to ≤ 21.7 | 69 | 2.93 (4.09) | 18 | 3.11 (4.20) | 27 | 2.70 (4.06) | 24 | 3.04 (4.21) |
| > 21.7 | 66 | 4.08 (4.62) | 14 | 2.86 (3.55) | 23 | 5.39 (5.67) | 29 | 3.62 (4.00) |
To further explore potential health effects of treatment since household NO2 level did not appear to be a factor in treatment efficacy, measured NO2 concentration was included as a factor in the compliance analysis (Table 6). Both the unadjusted and adjusted analyses show significant effects for measured NO2 and treatment arm interaction. Pairwise comparisons of treatments on symptom days during the final 14 days of treatment showed no difference between NO2-reduction and control treatments (0.33 symptom days [95% CI −1.7 – 2.5 days]). Particle-reduction had 1.8 (95% CI −0.32 – 3.9 days, p=0.10) fewer symptom days out of 14 compared to control.
Table 6.
Effect of treatment including measured NO2 concentration as a factor on number of symptom days in final 14 days of treatment for adjusted and unadjusted compliance analysis.
| Treatment arms | Unadjusted | Adjusteda | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
| |||||||
| Factors | N (Ss) | N (obs) | df | Estimate (SE) | p-value | df | Estimate (SE) | p-value |
| Compliance including NO 2 b | 106 | 267 | ||||||
 Measured NO2 (ppb) Measured NO2 (ppb) | 1, 105 | 0.01 | 1, 95 | 0.04 | ||||
 Treatment arm Treatment arm | 2, 105 | 0.03 | 2, 95 | 0.03 | ||||
 Measured NO2 x Treatment Measured NO2 x Treatment | 2, 105 | 0.01 | 2, 95 | 0.009 | ||||
| Treatment Arm Contrasts | ||||||||
 NO2-reduction vs Control NO2-reduction vs Control | 105 | 0.33 (1.03) | 0.75 | 95 | 0.41 (1.05) | 0.70 | ||
 Particle-reduction vs Control Particle-reduction vs Control | 105 | −1.81 (1.06) | 0.09 | 95 | −1.80 (1.08) | 0.10 | ||
Note: N=106 subjects in the compliance analysis completed 267 treatment arms with non-missing NO2 measurements. Effect estimates shown for pairwise air cleaner filter configuration contrasts show differences in symptom days between treatment arms.
Discussion
We designed a randomized, blinded intervention trial to examine the effect of reducing household NO2 or fine particles on asthma symptoms in children while allowing for a control condition designed to have minimal influence on particles or NO2. Neither NO2-reducing nor particle-reducing treatments resulted in reductions in asthma morbidity compared to control. However, in analysis controlling for measured household NO2, there were 1.8 (95% CI −0.32 – 3.9, p=0.10) fewer symptom days out of 14 in the particle-reducing treatment compared to control.
Although we observed a difference of over 3 ppb between the NO2-reduction treatment and other treatments (online supplement, Table S5), over one-third of household NO2 measurements in each 5-week treatment fell at or below the study participation eligibility concentration of 15 ppb: 38% of control, 43% of particle-reduction, and 66% of NO2-reduction observations (Table 5). A previous study(2) found an apparent respiratory effect threshold at 6 ppb for the association between NO2 concentration and asthma severity. Clinically meaningful differences in reported symptoms related to reductions in NO2 proved difficult to discern where NO2 levels in all three treatments were close to this NO2 effect threshold.
The impact of interventions designed to reduce indoor NO2 on respiratory morbidity in children with asthma was the focus of a randomized, double-blind trial of school furnace replacement in Australia.(26) Eight of 18 schools were randomly selected to have unflued gas heaters replaced with flued gas or electric heat. Ten schools with unflued gas heat served as controls. NO2 exposure was 31 ppb lower in schools with replacement heaters (mean (SD) 15.5 (6.6) ppb) compared to control schools (47.0 (26.8) ppb); asthma symptoms were significantly reduced among children in intervention schools. This study suggests that reductions of NO2 large enough to result in clinically meaningful reductions in symptoms might only be possible with removal of the source e.g., when a gas stove is replaced with an electric stove.
Along with NO2, household exposure to fine particles is another important trigger for asthma exacerbation and has been examined in intervention studies enrolling urban asthmatic children with socioeconomic characteristics similar to our study subjects (20, 21, 23). Although funding constraints precluded inclusion of particle measurements in our study, previous studies demonstrate that air cleaners with HEPA filters are effective at significantly reducing levels of fine particles (20, 21, 23). All three trials reported significant health benefits: fewer clinic visits in the HEPA air cleaner group (23); reduction in daytime symptoms (21); and a 1.36 day increase in symptom-free days in a 14-day period for the HEPA intervention compared to control (20). We observed a similar result. In the analysis controlling for NO2 concentration (Table 6), during the final 14 days of treatment there was a statistically non-significant 1.8 day reduction in symptom-days in particle-reduction compared to control treatment.
A major factor contributing to the trial’s failure to detect any statistically significant health benefit associated with household NO2 or fine particle reduction was that the trial was underpowered. Our effective sample size for the intent-to-treat analysis was 117, which fell well short of our target enrollment. Recruitment was a challenge. Previous studies (2, 3) found NO2 levels were significantly associated with asthma severity, and highest NO2 levels were found in small homes (7 or fewer rooms) with gas stoves and were associated with multifamily housing (an indicator of lower socioeconomic status). For this trial, the target population was children with persistent asthma residing in urban homes with gas stoves, typically families living in larger cities. Home-based, environmental intervention studies tend to have high subject burden, and our study was no exception. Families that agreed to participate had to commit to having a relatively large, non-silent appliance running continuously placed in the main living area of their relatively small home for 15 weeks. Efforts to recruit these families included repeated use of many different methods (online supplement, Table S3), but we were unable to reach our target before the end of funding.
Conclusions
Although the NO2-reduction treatment was more effective at reducing household NO2 than the other treatment and control, it remains unknown if it is possible to configure an air cleaner to achieve a level of NO2 reduction large enough to observe a clinically meaningful effect on asthma symptoms. It has been shown that larger levels of NO2 reduction are possible with an intervention that targets and removes the source (26). We observed that in smaller, urban homes with gas stoves modest reductions in symptoms are possible with the use of air cleaners that remove fine particles. An intervention targeting exposures to both NO2 and fine particles is complicated and further research is warranted.
Supplementary Material
Online Supplemental Materials (PDF)
Online Supplemental Materials (Word)
Acknowledgments
We gratefully acknowledge our Harvard colleagues Stephen T. Ferguson and J. M. Wolfson for their engineering and laboratory expertise in the development phase of this project; and the support and encouragement provided during the trial by our Data Safety Monitoring Board: Drs. George O’Connor, Meyer Kattan and Judith Goldberg.
Funding:
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) under Grant R01ES023505-05; and the Connecticut Department of Public Health (CTDPH) under Contract RFP# 2016-0087.
Abbreviations:
| CI | confidence interval |
| HEPA | high-efficiency, particle-arresting |
| NO2 | nitrogen dioxide |
| ppb | parts per billion |
| RCT | randomized controlled trial |
| SD | standard deviation |
| SE | standard error |
| SEM | standard error of the mean |
| μg/m3 | micrograms per cubic meter |
Footnotes
Registration Number: NCT02258893
Declaration of interest statement: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Online Data Supplement: This article has an online data supplement.
References
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/135015175
Article citations
Early life exposures of childhood asthma and allergies-an epidemiologic perspective.
Front Allergy, 5:1445207, 23 Aug 2024
Cited by: 0 articles | PMID: 39247214 | PMCID: PMC11377413
Recent Insights into the Environmental Determinants of Childhood Asthma.
Curr Allergy Asthma Rep, 24(5):253-260, 18 Mar 2024
Cited by: 0 articles | PMID: 38498229
Review
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT02258893
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Home interventions are effective at decreasing indoor nitrogen dioxide concentrations.
Indoor Air, 24(4):416-424, 11 Jan 2014
Cited by: 26 articles | PMID: 24329966 | PMCID: PMC4909253
Principal stratification analysis to determine health benefit of indoor air pollution reduction in a randomized environmental intervention in COPD: Results from the CLEAN AIR study.
Sci Total Environ, 868:161573, 18 Jan 2023
Cited by: 2 articles | PMID: 36669663 | PMCID: PMC9975085
Household levels of nitrogen dioxide and pediatric asthma severity.
Epidemiology, 24(2):320-330, 01 Mar 2013
Cited by: 37 articles | PMID: 23337243 | PMCID: PMC3686297
Evaluating heterogeneity in indoor and outdoor air pollution using land-use regression and constrained factor analysis.
Res Rep Health Eff Inst, (152):5-80; discussion 81-91, 01 Dec 2010
Cited by: 8 articles | PMID: 21409949
Funding
Funders who supported this work.
Connecticut Department of Public Health (1)
Grant ID: RFP# 2016–0087
NIEHS NIH HHS (1)
Grant ID: R01 ES023505
National Institutes of Health/National Institute of Environmental Health Sciences (1)
Grant ID: R01ES023505-05