Abstract
Objective
To investigate the effects of fever therapy compared with no fever therapy in a wide population of febrile adults.Design
Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.Data sources
CENTRAL, BIOSIS, CINAHL, MEDLINE, Embase, LILACS, Scopus, and Web of Science Core Collection, searched from their inception to 2 July 2021.Eligibility criteria
Randomised clinical trials in adults diagnosed as having fever of any origin. Included experimental interventions were any fever therapy, and the control intervention had to be no fever therapy (with or without placebo/sham).Data extraction and synthesis
Two authors independently selected studies, extracted data, and assessed the risk of bias. Primary outcomes were all cause mortality and serious adverse events. Secondary outcomes were quality of life and non-serious adverse events. Aggregate data were synthesised with meta-analyses, subgroup analyses, and trial sequential analyses, and the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.Results
Forty two trials assessing 5140 participants were included. Twenty three trials assessed 11 different antipyretic drugs, 11 trials assessed physical cooling, and eight trials assessed a combination of antipyretic drugs and physical cooling. Of the participants, 3007 were critically ill, 1892 were non-critically ill, 3277 had infectious fever, and 1139 had non-infectious fever. All trials were assessed as being at high risk of bias. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of death (risk ratio 1.04, 95% confidence interval 0.90 to 1.19; I2=0%; P=0.62; 16 trials; high certainty evidence) and the risk of serious adverse events (risk ratio 1.02, 0.89 to 1.17; I2=0%; P=0.78; 16 trials; high certainty evidence) could be rejected. One trial assessing quality of life was included, showing no difference between fever therapy and control. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of non-serious adverse events could be neither confirmed nor rejected (risk ratio 0.92, 0.67 to 1.25; I2=66.5%; P=0.58; four trials; very low certainty evidence).Conclusions
Fever therapy does not seem to affect the risk of death and serious adverse events.Systematic review registration
PROSPERO CRD42019134006.Free full text

Fever therapy in febrile adults: systematic review with meta-analyses and trial sequential analyses
Abstract
Objective
To investigate the effects of fever therapy compared with no fever therapy in a wide population of febrile adults.
Design
Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Data sources
CENTRAL, BIOSIS, CINAHL, MEDLINE, Embase, LILACS, Scopus, and Web of Science Core Collection, searched from their inception to 2 July 2021.
Eligibility criteria
Randomised clinical trials in adults diagnosed as having fever of any origin. Included experimental interventions were any fever therapy, and the control intervention had to be no fever therapy (with or without placebo/sham).
Data extraction and synthesis
Two authors independently selected studies, extracted data, and assessed the risk of bias. Primary outcomes were all cause mortality and serious adverse events. Secondary outcomes were quality of life and non-serious adverse events. Aggregate data were synthesised with meta-analyses, subgroup analyses, and trial sequential analyses, and the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Results
Forty two trials assessing 5140 participants were included. Twenty three trials assessed 11 different antipyretic drugs, 11 trials assessed physical cooling, and eight trials assessed a combination of antipyretic drugs and physical cooling. Of the participants, 3007 were critically ill, 1892 were non-critically ill, 3277 had infectious fever, and 1139 had non-infectious fever. All trials were assessed as being at high risk of bias. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of death (risk ratio 1.04, 95% confidence interval 0.90 to 1.19; I2=0%; P=0.62; 16 trials; high certainty evidence) and the risk of serious adverse events (risk ratio 1.02, 0.89 to 1.17; I2=0%; P=0.78; 16 trials; high certainty evidence) could be rejected. One trial assessing quality of life was included, showing no difference between fever therapy and control. Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of non-serious adverse events could be neither confirmed nor rejected (risk ratio 0.92, 0.67 to 1.25; I2=66.5%; P=0.58; four trials; very low certainty evidence).
Conclusions
Fever therapy does not seem to affect the risk of death and serious adverse events.
Systematic review registration
PROSPERO CRD42019134006
Introduction
Fever, or pyrexia, can be defined as having a temperature above the normal range owing to an increase in the body’s core temperature setpoint.1 2 The thermoregulatory centre, located in the hypothalamus, contains temperature sensitive neurons, aiming to maintain thermal homoeostasis. Fever is caused by a disturbance in the thermal homoeostasis and thermal setpoint caused by pyrogenic cytokines, pyrogens, as a response to inflammation and infection.3 Pyrogens induce a change from the normal thermal setpoint, leading to an increase or decrease in body core temperature.4 Hyperthermia is different from fever and can be defined as an increase of the body’s core temperature not related to the thermoregulatory centre.5 Changes in body temperature and thermal setpoint evoke physiological and behavioural responses to maintain thermal homoeostasis.6 Fever is an integral part of the inflammatory response, affecting the reproduction of some bacteria and viruses negatively and amplifying the immunological response.7 8 Fever and hyperthermia are also associated with several adverse events including seizures, organ failure, and brain damage.9 10
The origin of treating fever predates 2000 years before the Common Era, and treating fever with antipyretics in patients admitted to hospital is commonly considered standard practice today.11 The aim of treating fever may be to reduce the patient’s discomfort and to decrease physiological stress. However, fever therapy is likewise used in heavily sedated patients in intensive care units, with the rationale for treatment being a mortality and morbidity benefit by reduction of metabolic demand and hypoxic tissue injury.12 Fever is present throughout the animal kingdom, yet the underlying physiology is not fully understood.7 Fever is metabolically costly and increases oxygen demand, but it also induces cellular and immunological mechanisms protecting against microorganisms.12 13 14 In human studies, a positive correlation has been found between febrile temperatures during bacteraemia and survival.15 16 Furthermore, antipyretic drugs have shown to increase the duration of certain illnesses and inhibit antibody response.17 Aggressive treatment with paracetamol has even exposed a tendency towards increased mortality.18 Another study has shown that fever is an independent predictor of mortality.19 Whether fever is an epiphenomenon and merely a marker of illness or a modifiable target affecting important outcomes, such as death, is not known, suggesting an urgent need for robust evidence regarding the benefits and harms of fever therapy.
Whether the benefits of fever therapy outweigh the risks is unknown, and previous randomised clinical trials and meta-analyses have not included sufficient information to confirm or reject the effect of fever therapies.20 21 22 23 24 In 2019 Young and colleagues did a meta-analysis comparing more active fever management with less active fever management in critically ill patients.25 This study showed no statistically significant difference between the compared groups. In 2015 Zhang and colleagues did a meta-analysis with trial sequential analysis comparing fever therapy with control in critically ill patients with sepsis.26 This study failed to identify any beneficial effect of antipyretic therapy. In 2021 Sakkat and colleagues did a meta-analysis comparing antipyretics with placebo in critically ill patients.27 This study concluded that antipyretic therapy does not reduce mortality.
To overcome the limitations of these previous studies and to increase statistical power, we aimed to include both a wide population base and multiple fever therapies. We aimed to answer the question of whether the evidence supports the use of fever therapy compared with no fever therapy in adult patients in relation to outcomes important to patients, such as mortality, adverse events, and quality of life.
Methods
This systematic review with meta-analyses and trial sequential analyses of randomised clinical trials was conducted in accordance with a pre-specified protocol registered on the international prospective registry of systematic reviews (PROSPERO CRD42019134006).28
We searched all relevant databases from their inception to 2 July 2021 and included randomised clinical trials including adults diagnosed as having fever of any origin. Trials had to compare fever therapy with no fever therapy (with or without placebo/sham). Primary outcomes were all cause mortality and serious adverse events. Secondary outcomes were quality of life and non-serious adverse events. In accordance with the instructions in the Cochrane Handbook for Systematic Reviews of Interventions, two authors independently reviewed each trial, using the second version of the Cochrane risk of bias tool for randomised trials (RoB2) to assess the risks of bias.29 30 We calculated risk ratios with 95% confidence intervals by using meta-analyses for dichotomous outcomes. We handled missing data by following the eight step procedure suggested by Jakobsen and colleagues. We did meta-analyses by following the Cochrane Handbook of Systematic Reviews of Interventions, Keus and colleagues, and Jakobsen and colleagues.29 31 32 We used Stata version 16 to analyse the data. We combined a visual inspection of forest plots with a statistical analysis to identify potential heterogeneity. We did subgroup analyses to further investigate heterogeneity and to inspire hypotheses for future studies. Aiming to reduce the risk of type I and II errors, we used a multiplicity adjusted P value and did trial sequential analyses.32 33 34 35 36 37 38 39 40 41 We used the approach proposed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group for rating the certainty of the evidence.42 43 44 A comprehensive description of the methods is provided in the supplementary methods.
Differences between protocol and review
We revised our planned methods of assessing the risk of bias of the included trials and used the Cochrane risk of bias assessment tool 2 (RoB2 tool)30 instead of the first version of the tool as stated in the protocol.28 Research in the field has progressed, and RoB2 reflects current understanding of how the causes of bias may influence trial results and is the most appropriate way to assess the risk of bias. Furthermore, we revised our planned methods for confirming or rejecting a fixed intervention effect of 25% to instead use trial sequential analyses to define the lowest intervention effects threshold we could confirm or reject.
Patient and public involvement
This study was originally planned as a background for the design, rationale, and interpretation of the TTM cardiac arrest trials investigating hypothermia and fever management,45 46 for which the organisation uniting people in Sweden with cardiovascular and lung disease, the Swedish Heart and Lung Association, has been part of the planning and has a member on the steering committee.
Results
The search strategy defined in the protocol found 3273 publications that were evaluated to identify trials matching our inclusion criteria.28 We included a total of 42 trials randomising 5140 participants (supplementary figure S1). Twenty three trials assessed 11 different antipyretic drugs, 11 trials assessed physical cooling, and eight trials assessed a combination of antipyretic drugs and physical cooling. Of the participants, 3007 were critically ill, 1892 were non-critically ill, 3277 had infectious fever, and 1139 had non-infectious fever; 3062 participants were admitted to hospital, and 2078 were outpatients. We assessed 21 trials as being of some concern and 21 trials as being at high risk of bias (fig 1). The 42 included trials provided a total of 75 comparisons (supplementary table S1). Seventeen trials were included in meta-analyses. Missing data constituted ≤5% of the overall data, and we deemed the impact of missing data to be low; therefore, we did not do sensitivity analyses
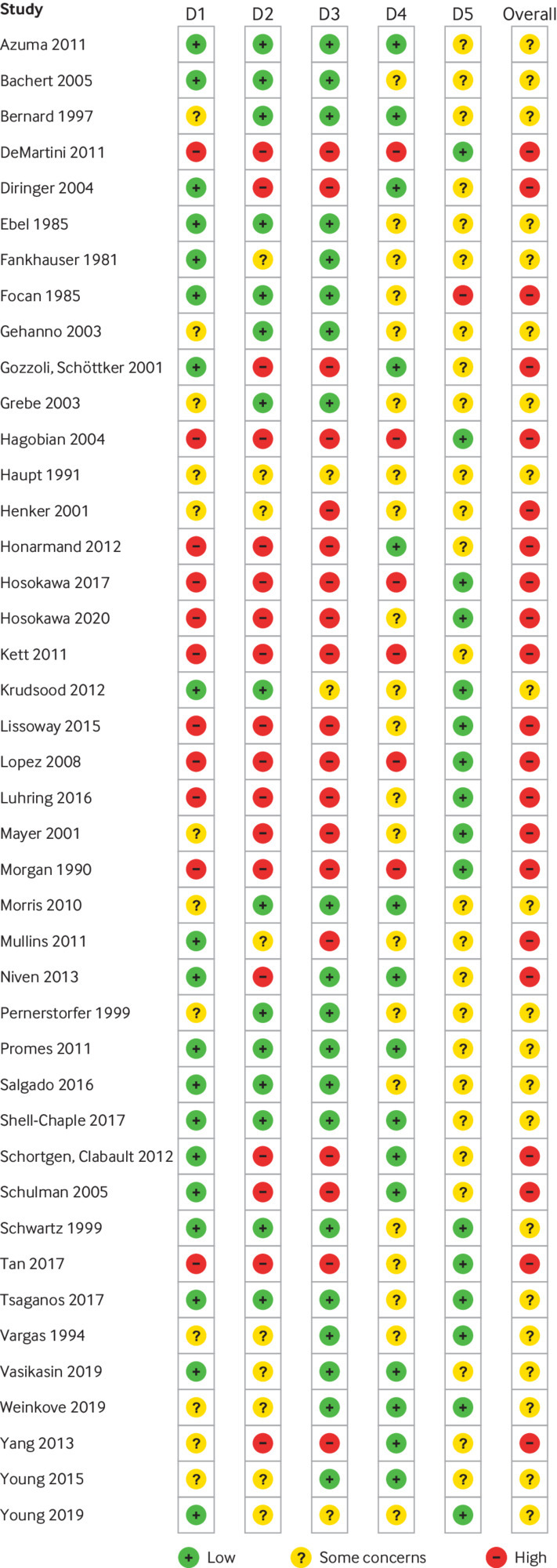
Risk of bias summary for randomised controlled trials included in evidence synthesis. Risk of bias assessment used Cochrane risk of bias tool 2 (RoB2). D1=bias arising from randomisation process; D2=bias due to deviations from intended intervention; D3=bias due to missing outcome data; D4=bias in measurement of outcome; D5=bias in selection of reported result
All cause mortality
Sixteen trials (19 comparisons) with a total of 2415 participants reported on all cause mortality. The included trials assessed the effects of five different fever therapy interventions: ibuprofen versus placebo (three trials),47 48 49 paracetamol versus placebo (six trials),50 51 52 53 54 55 physical cooling versus no intervention (three trials),56 57 58 antipyretics plus physical cooling versus no intervention (two trials),18 59 and physical cooling plus antipyretics versus antipyretics (two trials).60 61 All 2415 participants were admitted to hospital; 2050 were critically ill, and 251 were non-critically ill (supplementary table S1). One trial included both critically ill and non-critically ill patients (120/2415) (supplementary table S1). Of the 2415 participants, 1658 had infectious fever and 477 had non-infectious fever (supplementary table S1). For 286/2415 participants, the origin of fever was unknown (supplementary table S1). A total of 294 (23.0%) of 1281 fever therapy participants died compared with 258 (22.6%) of 1140 control participants.
Meta-analysis of all cause mortality did not show evidence of a difference (risk ratio 1.04, 95% confidence interval 0.90 to 1.19; I2=0%; P=0.62; 16 trials; high certainty evidence) (fig 2; supplementary table S3). Quantitative measures of heterogeneity (I2=0%) combined with visual inspection of the forest plot did not show signs of significant heterogeneity (fig 2). Trial sequential analysis showed that we could reject the hypothesis that fever therapy reduces the risk of all cause mortality by 22% (fig 3). We assessed this outcome result as being at high risk of bias and the certainty of the evidence as being high (supplementary table S3). None of the subgroup analyses showed evidence of a difference (supplementary figures S3-S10). The assessment time points varied between trials, ranging from one day after randomisation to 90 days after randomisation.54 56
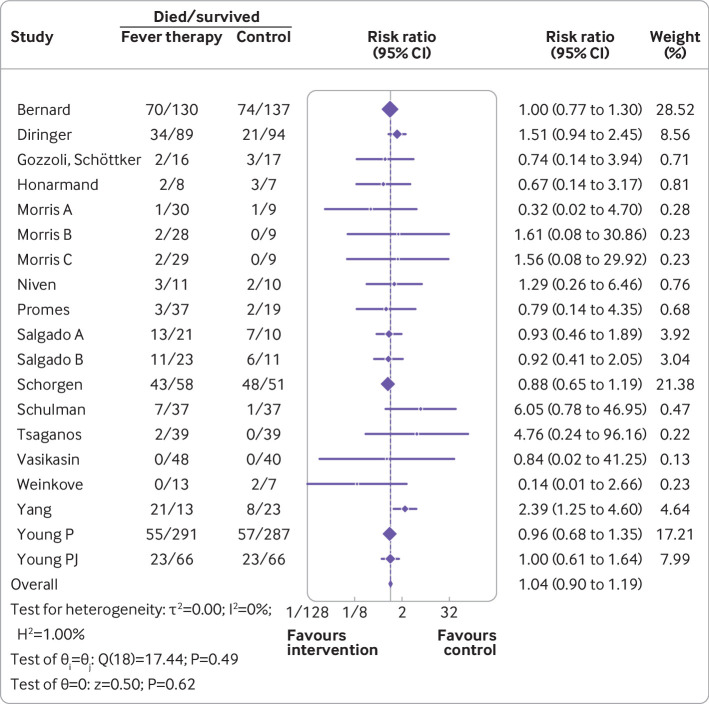
Random effects meta-analysis comparing fever therapy versus control interventions for all cause mortality (risk ratio 1.04, 95% confidence interval 0.90 to 1.19; P=0.62; I2=0%; 16 trials)
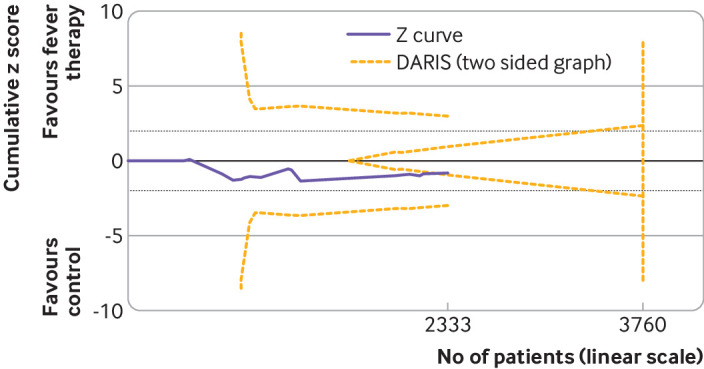
Trial sequential analysis of fever therapy versus control interventions for all cause mortality. Two sided trial sequential analysis graph of fever therapy versus control interventions for all cause mortality in 16 trials. Diversity adjusted required information size (DARIS) was calculated on basis of all cause mortality proportion in control group of 22.6%, relative risk reduction of 22% in experimental group, type I error (α) of 2%, and type II error (β) of 10% (90% power). Diversity was 10%. Required information size was calculated to be 3760 participants. Cumulative z curve (purple line) did not cross trial sequential monitoring boundaries for either benefit or harm. Cumulative z curve did cross inner wedge futility line (yellow outward sloping lines). Black dashed lines show conventional boundary (α=5%)
Serious adverse events
Sixteen trials (19 comparisons) with a total of 2415 participants reported on serious adverse events. The included trials assessed the effects of five different fever therapy interventions: ibuprofen versus placebo (three trials),47 48 49 paracetamol versus placebo (six trials),50 51 52 53 54 55 physical cooling versus no intervention (three trials),56 57 58 antipyretics plus physical cooling versus no intervention (two trials),18 59 and physical cooling plus antipyretics versus antipyretics (two trials).60 61 All 2415 participants were admitted to hospital; 2050 were critically ill, and 251 were non-critically ill (supplementary table S1). One trial included both critically ill and non-critically ill patients (120/2415). Of the 2415 participants, 1658 had infectious fever and 477 had non-infectious fever (supplementary table S1). For 286/2415 participants the origin of fever was unknown (supplementary table S1). A total of 307 (24.0%) of 1281 trial participants had a serious adverse event in the fever therapy group compared with 276 (24.2%) of 1140 in the control group.
Meta-analysis of serious adverse events did not show evidence of a difference (risk ratio 1.02, 0.89 to 1.17; I2=0%; P=0.78; 16 trials; high certainty evidence) (fig 4; supplementary table S3). Quantitative measures of heterogeneity (I2=0%) combined with visual inspection of the forest plot did not show signs of significant heterogeneity (fig 4). Trial sequential analysis showed that we could reject the hypothesis that fever therapy reduces the relative risk of serious adverse events by 23% (fig 5). We assessed this outcome result as being at high risk of bias and the certainty of the evidence as being high (supplementary table S3). None of the subgroup analyses showed evidence of a difference (supplementary figures S12-S19). The assessment time points varied between trials, ranging from one day after randomisation to 90 days after randomisation.54 56
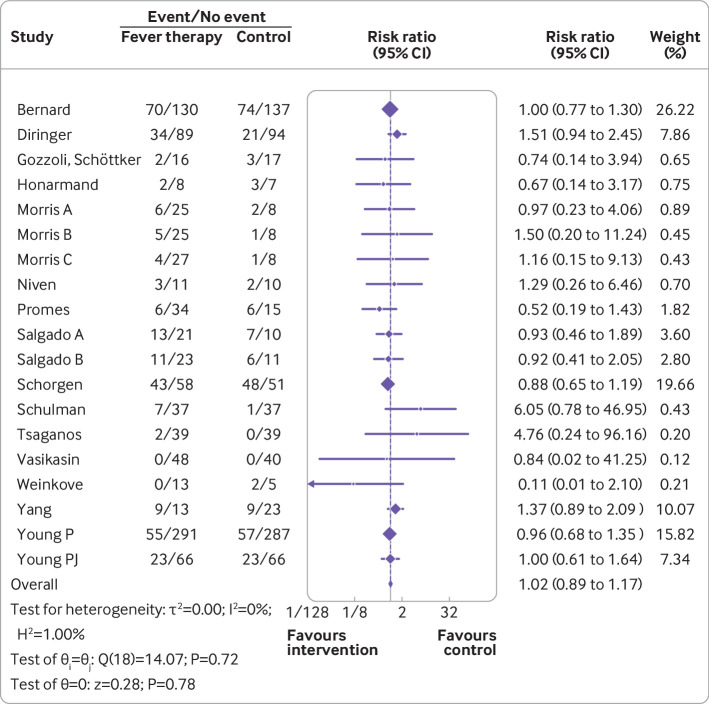
Random effects meta-analysis comparing fever therapy versus control interventions for serious adverse events (risk ratio 1.02, 95% confidence interval 0.89 to 1.17; P=0.78; I2=0%; 16 trials)
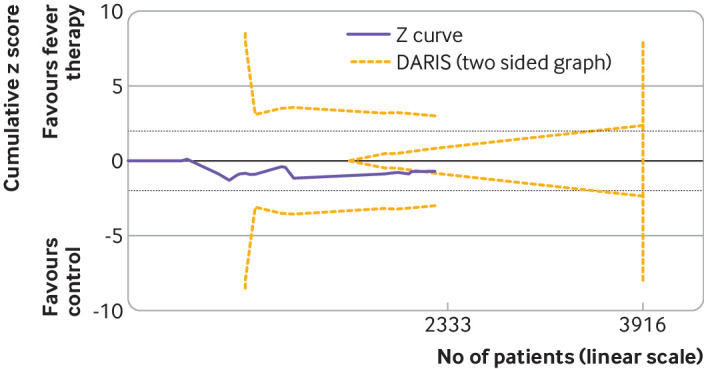
Trial sequential analysis of fever therapy versus control interventions for serious adverse events. Two sided trial sequential analysis graph of fever control interventions versus control interventions for serious adverse events in 16 trials. Diversity adjusted required information size (DARIS) was calculated on basis of all cause mortality proportion in control group of 22.6%, relative risk reduction of 23% in experimental group, type I error (α) of 2%, and type II error (β) of 10% (90% power). Diversity was 21%. Required information size was calculated to be 3916 participants. Cumulative Z curve (purple line) did not cross trial sequential monitoring boundaries for either benefit or harm. Cumulative Z curve did cross inner wedge futility line (yellow outward sloping lines). Black dashed lines show conventional boundary (α=5%).
Secondary outcomes
One trial (37 participants), assessed quality of life using the EQ-5D-5L descriptive system at 24, 48, and 72 hours and found no difference between fever therapy and control intervention.53 Meta-analysis and trial sequential analysis showed that the hypothesis that fever therapy reduces the risk of non-serious adverse events could be neither confirmed nor rejected (risk ratio 0.92, 0.67 to 1.25; I2=66.5%; P=0.58; four trials; very low certainty evidence). Findings for resolution of fever and reduction of fever are provided in the supplementary results.
Discussion
In this systematic review with meta-analyses and trial sequential analyses, we showed that fever therapy does not seem to affect the risk of death or serious adverse events in febrile adults. We found almost no signs of statistical heterogeneity, and none of the predefined subgroup analyses showed evidence of a difference in all cause mortality or serious adverse events, which supports the validity of our meta-analysis results. We found insufficient evidence to confirm or reject the hypothesis that fever therapy influences quality of life or non-serious adverse events.
Type of fever therapy
In this systematic review, we chose to include both antipyretic drugs and physical cooling when assessing the effects of fever therapy. The physiological effects and underlying mechanisms of action of antipyretic drugs and physical cooling are different. Antipyretic drugs mainly affect body temperature by lowering the thermal setpoint and are reliant on a functioning thermoregulatory centre to have an effect.62 Physical cooling does not affect the thermoregulatory centre and acts by removing heat from the body without affecting the hypothalamic setpoint; the cooling is thus forced on the body, which may cause adverse effects. Decreasing the core body temperature without affecting the hypothalamic setpoint induces cold defensive responses mediated via the sympathetic nervous system such as shivering, tachycardia, and peripheral vasoconstriction, which lead to discomfort for the patient and increased metabolic stress.63 The compensatory responses to the discrepancy between the body temperature and the thermal setpoint generate and retain heat, counteracting the cooling and increasing the metabolic demand. These cold defensive responses may be reduced pharmacologically with muscle paralysing drugs, analgesics, and sedatives, which in turn may lead to additional adverse effects. Antipyretics decrease the setpoint and therefore do not induce cold defensive responses or affect the metabolic demand.64
The different mechanisms of action and adverse effects have spawned theories that specific approaches may be tailored to patients with different origins of fever to adapt a treatment regimen to achieve superior results. Physical cooling is theorised to be more appropriate than antipyretics for patients with acute brain injury, as the thermoregulatory centre could be compromised,65 and antipyretics could be more appropriate in patients with infectious fever.66 Clinically, however, antipyretic refractory infectious fever is commonly observed, raising questions about the validity of these theories. Most trials analysed in the meta-analyses and trial sequential analyses investigated antipyretic drugs (9/16; 56%) rather than physical cooling (5/16; 31%), and only two trials investigated a combination of antipyretics and physical cooling (2/16; 13%). Hence, even though we did not identify signs of heterogeneity, our results primarily show the effects of antipyretic drugs, which needs to be considered when interpreting our results.
Strengths and limitations in relation to other studies
Previous studies have primarily focused on specific patient groups, such as critically ill patients and those with sepsis, or specific fever control interventions.20 21 22 23 24 Our review differs from previous research in that we have included a wider variety of both participants and fever therapies, allowing us, unlike previous studies, to obtain sufficient power by reaching required information sizes. The implications of including a variety of different interventions and a diverse patient group may entail results that are difficult to interpret if significant statistical heterogeneity is present. We therefore stated in the protocol that meta-analyses would be done only if the trials showed low levels of heterogeneity. With our analyses indicating low levels of heterogeneity and our subgroup analyses showing no subgroup differences, we deemed meta-analyses to be valid and did them including all identified trials. The relative effect, regardless of intervention type and patient group, being similar confirms and verifies our decision to pool these trials. We do, however, acknowledge that tests of heterogeneity most likely are underpowered.
The scope of this systematic review was wider in the population studied compared with previous research because our ambition was to assess the effects of fever therapy with regard to the outcomes most important to patients—that is, mortality and serious adverse events. Trials designed to investigate and report on these outcomes will inevitably focus on sicker patient populations; the participants included in the important analyses were all admitted to hospital, and most were critically ill. Our results therefore say little about fever therapy for milder disease states in the wider non-admitted population.
Biological rationale
Whether aiming for a benefit in terms of mortality and morbidity with fever therapy is biologically and physiologically plausible is questionable. From a phylogenetic perspective, with the conserved fever response, one might expect fever to be beneficial and that fever therapy should be avoided. However, that the physiological stress caused by fever shifts the balance in a vulnerable and critically ill patient towards worse outcomes is equally plausible.67 Nevertheless, different types of fever therapy have been part of usual care across medical specialties for decades. Results of non-randomised studies are conflicting, and the results from our analyses do not indicate any effects of the interventions studied. One important finding from this study is that the total sample size of participants included in randomised trials was relatively small and that less than half of the trials included reported on hard outcomes. We investigated the smallest intervention effects we could reject using trial sequential analyses: 22% for mortality and 23% for serious adverse events. We recognise that smaller intervention effects may be beneficial for the affected patients and thus clinically relevant. Our results indicate that further trials should therefore be powered for detecting smaller and more realistic intervention effects.
Strengths and limitations of study
Our review has several strengths. Our method was predefined in detail and published before we did our literature search. We searched all relevant databases, we used an eight step assessment suggested by Jakobsen and colleagues to assess the clinical significance of our results, and we used trial sequential analysis to reduce the risks of type I and type II errors. Furthermore, we did meta-analyses with both fixed effects and random effects meta-analysis, we investigated subgroup differences, and we assessed the certainty of the evidence through GRADE. The main limitation of our review was the low methodological quality of the included trials, with half of the included trials assessed as being of some concerns and half assessed as being at high risk of bias. The inclusion of active comparator trials (that is, physical cooling plus antipyretics versus antipyretics, antipyretics plus physical cooling versus physical cooling, or aggressive treatment versus standard treatment) along with experimental versus control trials is a potential complicating factor with regard to the interpretation of the results. However, in the absence of heterogeneity between trials, as in our study, this should not be considered limiting to our results. Aiming to be inclusive, we accepted a wide variety of patients and interventions. Despite no heterogeneity being seen, specific cases may exist in which fever therapy is beneficial or harmful.
Meaning of study
Antipyretics are routinely administered in care both in and out of hospital, and physical cooling is applied in severe fever not responding to antipyretic drugs. Our results suggest little to no difference in effect between fever therapy and no fever therapy; realistic, while still clinically relevant, intervention effects of fever therapy have so far not been properly studied, however. Fever therapy may be investigated in further adequately powered high quality randomised trials also including a health economics perspective, to define implications for patients and society.
Conclusion
Fever therapy does not seem to affect the risk of death or serious adverse events in febrile adults. We found insufficient evidence to confirm or reject the hypothesis that fever therapy influences quality of life or the risk of non-serious adverse events.
What is already known on this topic
The effects of fever therapy in febrile patients are unclear
Previous trials have focused on either specific patient groups or specific fever therapies, limiting the statistical power
Aggregated evidence is needed to evaluate the overall effect of fever therapy interventions to identify the beneficial and harmful effects of fever therapy in adults
What this study adds
A systematic review with meta-analyses and trial sequential analyses found that fever therapy does not seem to affect the risk of death and serious adverse events
Insufficient evidence was found to confirm or reject the hypothesis that fever therapy influences quality of life or the risk of non-serious adverse events
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Notes
Contributors: The authors of the published protocol (NS, NN, and JCJ) developed the design of this study. JH and AC did the literature search, independently screened and selected the trials, and independently extracted the data from selected trials. JH and JCJ were responsible for statistical analyses. JH wrote the first draft of the manuscript. AC and NS provided advice at different stages. NN and JCJ provided critical advice and support throughout the process of writing and analysing this study. NN obtained funding. NN and JCJ contributed equally to this paper and share co-senior authorship. All authors approved the final version of the manuscript. JH is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by grants from the Swedish Research Council, grant number 2017-02627. The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Swedish Research Council for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: A plain language summary of this work will be distributed to the wider community via press and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not needed.
Data availability statement
Data and analyses will be available on request from the corresponding author (JH).
References
Articles from The BMJ are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/bmj-2021-069620
Read article for free, from open access legal sources, via Unpaywall:
https://www.bmj.com/content/bmj/378/bmj-2021-069620.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/131552990
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1136/bmj-2021-069620
Article citations
Postoperative fever and clinical outcomes after endoscopic surgery for spontaneous intracerebral hemorrhage: a retrospective database study.
BMC Neurol, 24(1):392, 15 Oct 2024
Cited by: 0 articles | PMID: 39407147 | PMCID: PMC11476804
Largely ignored-but pathogenetically significant: ambient temperature in rodent sepsis models.
Intensive Care Med Exp, 12(1):104, 14 Nov 2024
Cited by: 0 articles | PMID: 39542953 | PMCID: PMC11564610
Comparison of Wireless Continuous Axillary and Core Temperature Measurement after Major Surgery.
Sensors (Basel), 24(14):4469, 10 Jul 2024
Cited by: 0 articles | PMID: 39065867 | PMCID: PMC11281130
From fever to action: diagnosis, treatment, and prevention of acute undifferentiated febrile illnesses.
Pathog Dis, 82:ftae006, 01 Feb 2024
Cited by: 1 article | PMID: 38614961 | PMCID: PMC11067964
Review Free full text in Europe PMC
Beneficial and harmful effects of tricyclic antidepressants for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis.
BMJ Ment Health, 27(1):e300730, 22 Jan 2024
Cited by: 1 article | PMID: 39093721
Review
Go to all (10) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of level of sedation on outcomes in critically ill adult patients: a systematic review of clinical trials with meta-analysis and trial sequential analysis.
EClinicalMedicine, 71:102569, 28 Mar 2024
Cited by: 0 articles | PMID: 38572080 | PMCID: PMC10990717
Prophylactic drug management for febrile seizures in children.
Cochrane Database Syst Rev, 6:CD003031, 16 Jun 2021
Cited by: 8 articles | PMID: 34131913 | PMCID: PMC8207248
Review Free full text in Europe PMC
Beta-blockers for suspected or diagnosed acute myocardial infarction.
Cochrane Database Syst Rev, 12:CD012484, 17 Dec 2019
Cited by: 19 articles | PMID: 31845756 | PMCID: PMC6915833
Review Free full text in Europe PMC
L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis.
Cochrane Database Syst Rev, 5:CD012410, 15 May 2018
Cited by: 38 articles | PMID: 29762873 | PMCID: PMC6494563
Review Free full text in Europe PMC




