Abstract
Background
Understanding the natural history of anal high-risk human papillomavirus (hrHPV) infection is key for designing anal cancer prevention programs but has not been systematically characterized.Methods
We reanalyzed data from 34 studies including 16 164 individuals in 6 risk groups defined by human immunodeficiency virus (HIV) status, sex, and male sexuality: men who have sex with men (MSM) and people with HIV (MSMWH), HIV-negative MSM, women with HIV (WWH), HIV-negative women, men who have sex with women (MSW) with HIV (MSWWH), and HIV-negative MSW. We used Markov models to estimate incidence and clearance of 13 hrHPV types and their determinants.Results
Human papillomavirus (HPV) 16 had the highest incidence-clearance ratio of the hrHPV types. MSMWH had the highest hrHPV incidence (eg, 15.5% newly HPV-16 infected within 2 years), followed by HIV-negative MSM (7.5%), WWH (6.6%), HIV-negative women (2.9%), MSWWH (1.7%), and HIV-negative MSW (0.7%). Determinants of HPV-16 incidence included HIV status and number of sexual partners for MSM, women, and MSW, and anal sex behavior for MSM only. HPV-16 clearance was lower for people with HIV (PWH) and lower for prevalent than incident infection. Among MSM, increasing age was associated with lower clearance of prevalent, but not incident, HPV-16 infection.Conclusions
This robust and unifying analysis of anal hrHPV natural history is essential to designing and predicting the impact of HPV vaccination and HPV-based screening programs on anal cancer prevention, particularly in MSM and PWH. Importantly, it demonstrates the higher carcinogenic potential of longstanding anal prevalent hrHPV infection than more recent incident infection.Free full text

Incidence and Clearance of Anal Human Papillomavirus Infection in 16 164 Individuals, According to Human Immunodeficiency Virus Status, Sex, and Male Sexuality: An International Pooled Analysis of 34 Longitudinal Studies
Abstract
Background
Understanding the natural history of anal high-risk human papillomavirus (hrHPV) infection is key for designing anal cancer prevention programs but has not been systematically characterized.
Methods
We reanalyzed data from 34 studies including 16 164 individuals in 6 risk groups defined by human immunodeficiency virus (HIV) status, sex, and male sexuality: men who have sex with men (MSM) and people with HIV (MSMWH), HIV-negative MSM, women with HIV (WWH), HIV-negative women, men who have sex with women (MSW) with HIV (MSWWH), and HIV-negative MSW. We used Markov models to estimate incidence and clearance of 13 hrHPV types and their determinants.
Results
Human papillomavirus (HPV) 16 had the highest incidence-clearance ratio of the hrHPV types. MSMWH had the highest hrHPV incidence (eg, 15.5% newly HPV-16 infected within 2 years), followed by HIV-negative MSM (7.5%), WWH (6.6%), HIV-negative women (2.9%), MSWWH (1.7%), and HIV-negative MSW (0.7%). Determinants of HPV-16 incidence included HIV status and number of sexual partners for MSM, women, and MSW, and anal sex behavior for MSM only. HPV-16 clearance was lower for people with HIV (PWH) and lower for prevalent than incident infection. Among MSM, increasing age was associated with lower clearance of prevalent, but not incident, HPV-16 infection.
Conclusions
This robust and unifying analysis of anal hrHPV natural history is essential to designing and predicting the impact of HPV vaccination and HPV-based screening programs on anal cancer prevention, particularly in MSM and PWH. Importantly, it demonstrates the higher carcinogenic potential of longstanding anal prevalent hrHPV infection than more recent incident infection.
Of 29 000 high-risk human papillomavirus (hrHPV)–attributable anal squamous cell carcinomas (ASCCs) estimated to be diagnosed worldwide in 2018, 10 000 occur in men and 19 000 in women [1]. ASCC risk is low in the general population (1–2 cases per 100 000 person-years) but is known to be elevated in certain subgroups, such as human immunodeficiency virus (HIV)–negative men who have sex with men (MSM; approximately 20 cases per 100 000 person-years), women with HIV (WWH; approximately 30 cases), men who have sex with women (MSW) with HIV (MSWWH; approximately 30 cases), and especially MSM with HIV (MSMWH; approximately 100 cases) [2].
Anal hrHPV infection is the etiologic agent for ASCC and human papillomavirus (HPV)-16 is the most carcinogenic type among both HIV-negative people and people with HIV (PWH) [3]. Differences in ASCC incidence by risk group are likely driven by variations in the underlying natural history of anal hrHPV (HPV-16) infection, through increased hrHPV (HPV-16) incidence, known to be heavily influenced by sexual behavior [4], or decreased hrHPV (HPV-16) clearance, known to be worsened by immune dysfunction related to HIV infection [5].
Robust estimates of type-specific anal hrHPV incidence and clearance by risk group are essential to designing and predicting the population-wide impact of HPV-based ASCC prevention programs. These programs include primary prevention through HPV vaccination, as well as secondary prevention through, for example, HPV–based screening algorithms, somewhat analogous to cervical cancer screening.
Many longitudinal studies have reported the natural history of anal HPV infection, focusing on specific risk groups (Supplementary Table 1). However, heterogeneity in study design, such as duration and follow-up intervals, differences in analytic definitions of incidence and clearance [6], as well as relatively small sample sizes and number of events, hamper comparisons across risk groups, limiting their applicability to inform risk-targeted ASCC prevention interventions. Thus, we performed a collaborative-pooled reanalysis of individual-level data, underpinned by a statistical method allowing the pooling of data across studies of different duration and follow-up intervals, to provide a unified estimate of anal hrHPV incidence and clearance across risk groups stratified by HIV status, sex, and male sexuality.
METHODS
Data Collection
We conducted a systematic review on studies reporting on anal HPV incidence or clearance, by searching MEDLINE and Embase for studies published between 1 January 1986 and 31 January 2022, using the search strategy detailed in Supplementary Data 1. Studies were eligible if they used polymerase chain reaction to detect type-specific anal HPV DNA (at least for HPV-16) at multiple time points. Authors of eligible studies were contacted and invited to share individual-level data on the following variables: (1) HIV status, (2) sex, (3) male sexuality (MSW or MSM), (4) age, and, for each eligible study visit, (5) date and (6) type-specific anal HPV result. Additional variables (cytopathological diagnosis, sexual behavior, smoking, etc) were also shared, if available.
Data Analysis
A 2-state (HPV-negative and HPV-positive) continuous-time Markov model (CTMM) was used to estimate HPV incidence rate (from HPV negative to HPV positive) and clearance rate (from HPV positive to HPV negative) (Supplementary Data 2) [7]. This model was well suited for the pooled analysis, since it allows individuals to switch between the 2 states at any point in time, not only at the time of the visit when HPV was tested. Estimating the constant transmission rate addresses 3 issues: (1) the nature of interval-censored data on HPV status, namely the time of the visit does not capture the exact time of incidence or clearance of an infection; (2) the variability in HPV visit intervals for individuals within studies; and (3) the variability in visit interval protocols across studies. Importantly, clearance rates were estimated separately for infection that was detected at baseline (prevalent infection) or at follow-up visit (incident infection), by generating a covariable “infection type.”
We ran separate CTMMs for 13 individual hrHPV types (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) (group 1/2A; International Agency for Research on Cancer) [8], by using HIV status (negative and positive), population (MSM, women, and MSW), age group (<25, 25–34, 35–44, 45–54, or ≥55 years) and infection type (prevalent and incident) as covariables. This allowed postestimation of type-specific incidence and clearance, expressed as events per 1000 person-months, in 6 risk groups (MSMWH, HIV-negative MSM, WWH, HIV-negative women, MSWWH, and HIV-negative MSW), as well as incidence-clearance ratio and mean duration of infection for the 13 hrHPV types.
Other outputs, with a focus on HPV-16, were also estimated. First, hazard ratios of risk factors of HPV-16 incidence and clearance were evaluated. Second, the cumulative probability of HPV-16 incidence and clearance up to 2 years was estimated by 6 risk groups and, for MSM and women, stratified by HIV status and age group. Last, we estimated HPV-16 prevalence among MSM and women at 2 years after baseline, according to HIV status, infection type (as a surrogate for infection duration: prevalent infection equated to infection duration ≥2 years and incident infection to duration <2 years) and, for MSM only, age group. R software (version 4.0.3, http://www.r-project.org, R Core Team 2021) was used for all analyses.
RESULTS
The systematic review identified 48 eligible longitudinal studies with 25 238 individuals, of which 34 studies with 21 069 individuals (83.5%) contributed data (Supplementary Figure 1). Among the 21 069 individuals, 16 164 (76.7%) had ≥2 valid anal HPV results (contributing a total of 56 048 eligible visits) and were included in analyses, of whom 29.4% were MSMWH (n = 4745), 24.0% HIV-negative women (n = 3877), 21.4% HIV-negative MSM (n = 3459), 16.6% HIV-negative MSW (n = 2691), 6.6% WWH (n = 1062), and 2.0% MSWWH (n = 330) (Supplementary Tables 1 and 2).
The HPV-16 incidence was highest in MSMWH (14.8 per 1000 person-months [95% confidence interval (CI), 13.5–16.1]), followed by HIV-negative MSM (9.1 [8.2–10.1]), WWH (7.9 [6.9–9.1]), HIV-negative women (4.9 [4.3–5.6]), MSWWH (3.2 [2.1–5.0]), and HIV-negative MSW (2.0 [1.3–3.1]) (Figure 1AA). For HPV-16 clearance, the same ranking by risk group was observed, being lowest in MSMWH (61.5 per 1000 person-months [95% CI, 55.6–67.9]), then HIV-negative MSM (95.5 [86.0–106.0]), WWH (96.2 [84.7–109.3]), HIV-negative women (149.5 [130.7–170.9]), MSWWH (170.4 [125.2–231.8]), and HIV-negative MSW (264.6 [193.9–361.0]) (Figure 1BB). Similar patterns by risk group tended to be observed for incidence and clearance of all other hrHPV types. Of all hrHPV types, HPV-16 tended to have the highest incidence-clearance ratio of all hrHPV types, a pattern that was most evident among MSMWH, HIV-negative MSM, WWH, and HIV-negative women (Figure 2).
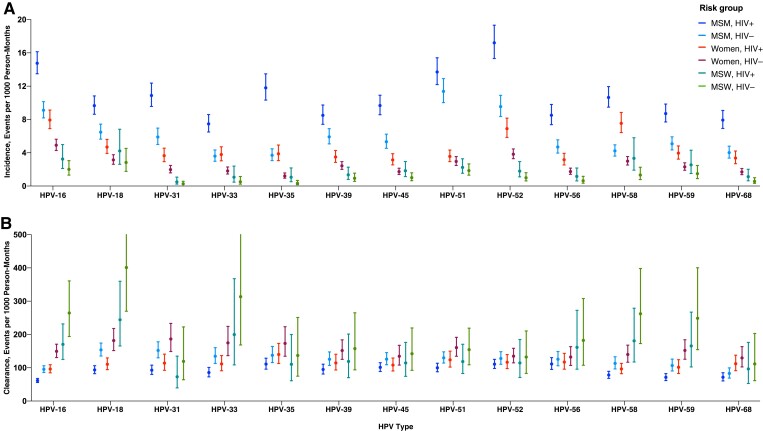
Incidence and clearance of anal type-specific high-risk human papillomavirus (HPV) infection in 6 risk groups. Error bars represent 95% confidence intervals. Abbreviations: HIV+, with human immunodeficiency virus (HIV); HIV-, HIV negative; MSM, men who have sex with men; MSW, men who have sex with women.
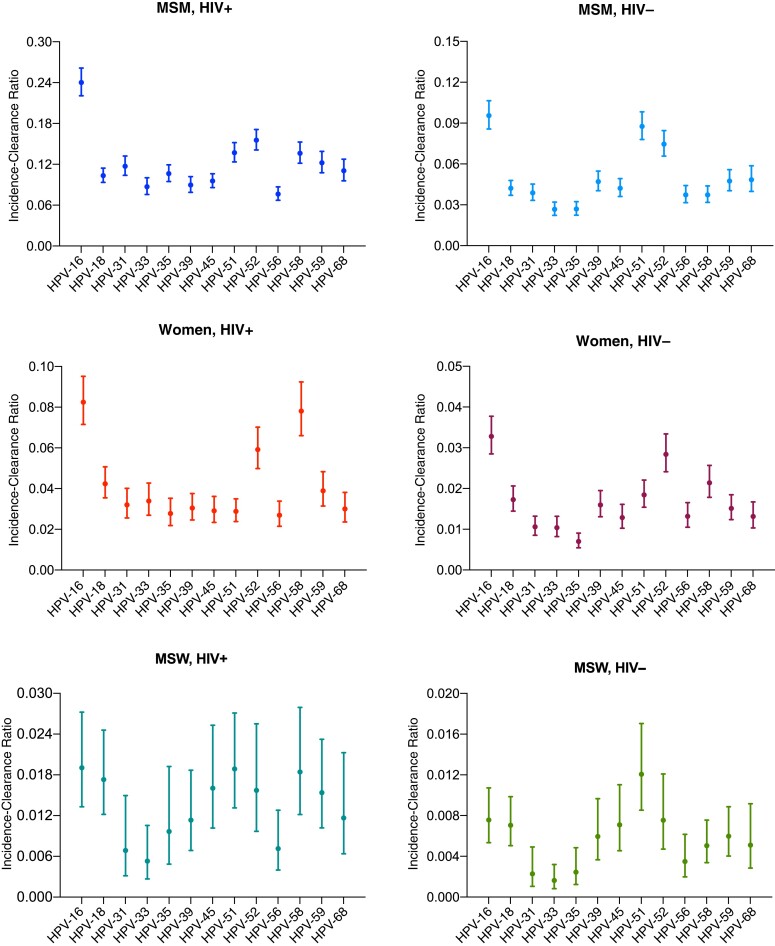
Incidence-clearance ratios for anal type-specific high-risk human papillomavirus (HPV) infection in 6 risk groups. Error bars represent 95% confidence intervals. Abbreviations: HIV+, with human immunodeficiency virus (HIV); HIV-, HIV negative; MSM, men who have sex with men; MSW, men who have sex with women.
Of the 16 750 hrHPV infections observed, a greater proportion were prevalent (n = 11 356) than incident (n = 5394) infection, both overall and in each risk group (Supplementary Figure 2). The mean duration of prevalent infections was longer than that of incident infections (Figure 3). For HPV-16, for example, the mean durations for prevalent versus incident infection were 3.8 (95% CI, 3.5–4.1) versus 1.2 (1.1–1.4) years in MSMWH, 2.2 (1.9–2.4) versus 0.8 (.7–1.0) in HIV-negative MSM, 2.5 (2.0–3.1) versus 0.7 (.6–.8) in WWH, and 0.8 (.6–.9) versus 0.5 (.4–.6) in HIV-negative women. Infections were too few to allow this type of analysis for MSW (Supplementary Figure 2).
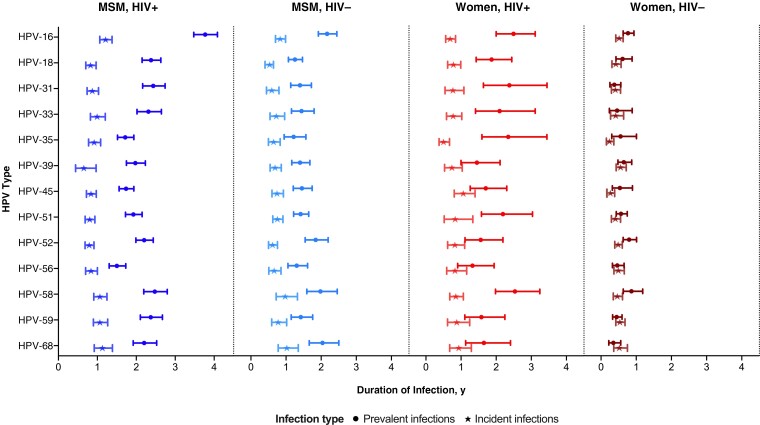
Mean duration of anal high-risk human papillomavirus (HPV) prevalent and incident infection in 4 risk groups. Error bars represent 95% confidence intervals. Abbreviations: HIV+, with human immunodeficiency virus (HIV); HIV-, HIV negative; MSM, men who have sex with men.
Among MSM, women, and MSW, the HPV-16 incidence was higher in younger people, with HIV, and those with more lifetime and recent sexual partners, and among MSM and women, it was higher in current smokers (Table 1). Ever having receptive anal sex and high (lifetime and recent) number of anal sexual partners were associated with higher HPV-16 incidence in MSM but not in women. Lower CD4 cell counts and higher HIV viral load were associated with HPV-16 incidence among MSMWH and WWH, although differences were not always statistically significant.
Table 1.
Risk Factors for Incidence and Clearance of Anal Human Papillomavirus 16 Infection in Men Who Have Sex With Men, Women, and Men Who Have Sex With Women
| Risk Factor | Incidence, aHRa (95% CI) | Clearance, aHRa (95% CI) | ||||
|---|---|---|---|---|---|---|
| MSM | Women | MSW | MSM | Women | MSW | |
| Age group, y | ||||||
| ȃ<25 | Reference | Reference | Reference | Reference | Reference | Reference |
| ȃ25–34 | .84 (.65–1.09) | .83 (.61–1.13) | .49 (.15–1.61) | .74 (.58–.94)b | .90 (.68–1.20) | .68 (.22–2.05) |
| ȃ35–44 | .73 (.56–.94)b | .96 (.69–1.34) | .50 (.17–1.50) | .63 (.50–.80)b | 1.14 (.80–1.61) | .79 (.31–2.01) |
| ȃ45–54 | .55 (.42–.72)b | .38 (.24–.61)b | .32 (.10–1.05) | .51 (.40–.65)b | 1.15 (.76–1.73) | .64 (.22–1.85) |
| ȃ≥55 | .31 (.23–.42)b | .79 (.41–1.52) | .25 (.03–2.21) | .42 (.32–.55)b | 1.29 (.72–2.31) | .66 (.20–2.16) |
| ȃAge (per 10 y) | .77 (.72–.81)b | .85 (.76–.94)b | .72 (.52–.99)b | .81 (.77–.85)b | 1.07 (.95–1.19) | .91 (.71–1.17) |
| HIV status | ||||||
| ȃNegative | Reference | Reference | Reference | Reference | Reference | Reference |
| ȃPositive | 1.42 (1.22–1.64)b | 1.90 (1.47–2.46)b | 3.33 (1.46–7.64)b | .68 (.60–.77)b | .47 (.36–.62)b | .37 (.18–.75)b |
| Lifetime no. of sexual partnersc | ||||||
| ȃLow | Reference | Reference | Reference | Reference | Reference | Reference |
| ȃHigh | 1.22 (1.01–1.47)b | 2.58 (1.86–3.57)b | 4.79 (1.38–16.7)b | .90 (.76–1.06) | 1.00 (.75–1.35) | .61 (.21–1.75) |
| No. of recent sexual partnersc | ||||||
| ȃLow | Reference | Reference | Reference | Reference | Reference | Reference |
| ȃHigh | 1.76 (1.38–2.23)b | 1.67 (1.12–2.47)b | 1.90 (.52–6.91) | 1.14 (.91–1.44) | 1.09 (.76–1.57) | 1.70 (.44–6.56) |
| Ever having receptive anal sex | ||||||
| ȃNo | Reference | Reference | … | Reference | Reference | … |
| ȃYes | 1.43 (1.11–1.85)b | .79 (.60–1.04) | … | 1.18 (92–1.51) | .78 (.61–1.01) | … |
| Lifetime no. of anal sexual partnersc | ||||||
| ȃLow | Reference | Reference | … | Reference | Reference | … |
| ȃHigh | 1.58 (1.18–2.13)b | .79 (.60–1.04) | … | 1.24 (.95–1.61) | .78 (.61–1.01) | … |
| No. of recent anal sexual partnersc | ||||||
| ȃLow | Reference | Reference | … | Reference | Reference | … |
| ȃHigh | 1.45 (1.17–1.80)b | .80 (.48–1.34) | … | 1.04 (.86–1.26) | .97 (.63–1.47) | … |
| Current smoking | ||||||
| ȃNo | Reference | Reference | … | Reference | Reference | … |
| ȃYes | 1.21 (1.03–1.43)b | 1.58 (1.14–2.18)b | … | .94 (.81–1.09) | .98 (.73–1.31) | … |
| Presence of anal HSILs at baseline | ||||||
| ȃNo | Reference | … | … | Reference | … | … |
| ȃYes | 1.13 (.89–1.42) | … | … | .66 (.54–.79)b | … | … |
| Infection type | ||||||
| ȃPrevalent | … | … | … | Reference | Reference | … |
| ȃIncident | … | … | … | 2.44 (2.16–2.74)b | 2.24 (1.81–2.78)b | … |
| Among individuals with HIV only | ||||||
| Current CD4 cell count | ||||||
| ȃ>500/µL | Reference | Reference | … | Reference | Reference | … |
| ȃ350–500/µL | 1.26 (1.02–1.55)b | .99 (.59–1.66) | … | 1.16 (.97–1.39) | .94 (.59–1.49) | … |
| ȃ<350/µL | 1.12 (.89–1.42) | 1.65 (1.09–2.48)b | … | 1.00 (.82–1.23) | .91 (.63–1.32) | … |
| Current HIV viral load, copies/mL | ||||||
| ȃ<50 | Reference | Reference | … | Reference | Reference | … |
| ȃ50–10 000 | 1.06 (.81–1.39) | 2.47 (1.55–3.92)b | … | .91 (.72–1.14) | .97 (.66–1.43) | … |
| ȃ>10 000 | 1.14 (.92–1.40) | 1.94 (1.18–3.19)b | … | .76 (.63–.92)b | .68 (.44–1.05) | … |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSILs, high-grade squamous intraepithelial lesions; MSM, men who have sex with men; MSW, men who have sex with women.
HRs were estimated from separate models for each variable and adjusted by age group and HIV status for incidence and clearance analyses, and also by infection type for clearance analyses, as appropriate.
Significant aHRs relative to the reference group.
The categories for numbers of sexual partners (lifetime or recent; overall or anal) were defined by the combination of median value for each population and the availability of categorical variables from contributed studies. For lifetime number of sexual partners, low was defined as ≤200 for MSM and ≤3 for women and MSW; high, as >200 for MSM and >3 for women and MSW. For recent sexual partners, low was defined as ≤5 for MSM and ≤1 for women and MSW; high, as >5 for MSM and >1 for women and MSW. For lifetime number of anal sexual partners, low was defined as ≤50 for MSM and 0 for women; high, as >50 for MSM and >0 for women. For recent anal sexual partners, low was defined as ≤3 for MSM and 0 for women; high, as >3 for MSM and >0 for women.
Among the 3 populations, HPV-16 clearance was significantly lower in PWH than in HIV-negative people (Table 1). For both MSM and women, clearance of HPV-16 incident infection was higher than that of prevalent infection. Older age and presence of anal high-grade squamous intraepithelial lesions (HSILs) at baseline were associated with lower clearance in MSM. HIV viral load was significantly associated with lower clearance in MSMWH, and a consistent nonsignificant association was observed in WWH.
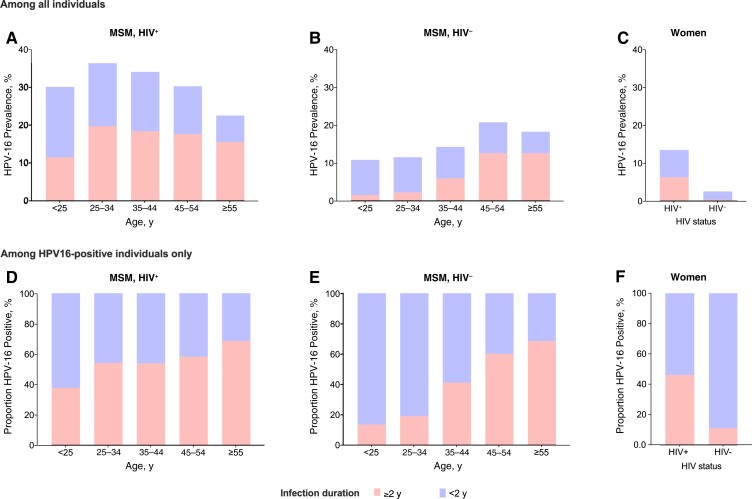
The 2-year cumulative HPV-16 incidence was 15.5% (95% CI, 14.6%–16.6%) in MSMWH, 7.5% (6.9%–8.3%) in HIV-negative MSM, 6.6% (5.8%–7.4%) in WWH, 2.9% (2.5%–3.3%) in HIV-negative women, 1.7% (1.2%–2.4%) in MSWWH, and 0.7% (.5%–1.0%) in HIV-negative MSW (Figure 4AA). The cumulative HPV-16 incidence was higher in youngest MSMWH (Figure 4DD) and HIV-negative women (Supplementary Figure 3), but the age effect was less clear for HIV-negative MSM (Figure 4GG) and WWH (Supplementary Figure 3).
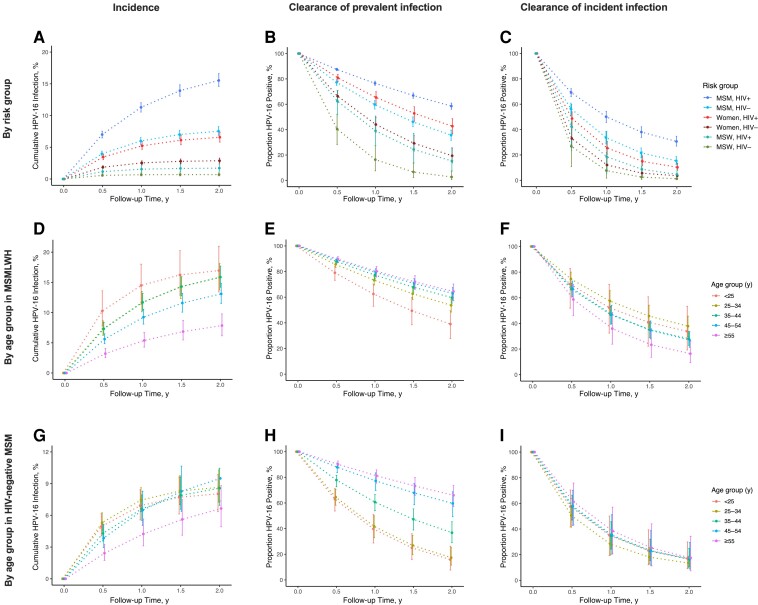
Cumulative incidence and clearance of anal human papillomavirus (HPV) 16 infection by risk group, as well as by age group in men who have sex with men (MSM) with human immunodeficiency virus (HIV) (MSMWH) and HIV-negative (HIV-) MSM. Error bars represent 95% confidence intervals. Abbreviations: HIV-, with HIV; MSW, men who have sex with women.
After 2 years, HPV-16 prevalent infection persisted in 58.6% of MSMWH, 42.7% of WWH, 35.7% of HIV-negative MSM, 19.4% of HIV-negative women, 15.3% of MSWWH, and 2.7% of HIV-negative MSW (Figure 4BB). For each of these risk groups, the respective 2-year persistence for incident infection was lower, namely, 30.6%, 10.2%, 15.4%, 3.6%, 4.6%, and 1.2% (Figure 4CC). There was a strong relationship between age and persistence of HPV-16 prevalent infection, both for MSMWH and HIV-negative MSM (Figures 4EE and and44HH), but not for incident infection (Figures 4FF and and44II). There were no differences in clearance of prevalent or incident HPV-16 infection by age in WWH or HIV-negative women (Supplementary Figure 3).
At 2 years after baseline, HPV-16 prevalence peaked at age 25–34 years and decreased with age in MSMWH, whereas in HIV-negative MSM, the prevalence increased with age (Figures 5AA and and55BB). Notably, the proportion of HPV-16 infections with a duration ≥2 years (ie, already prevalent at baseline) increased from 38% at age <25 years to 69% at age ≥55 years in MSMWH (Figure 5DD) and from 13% to 68% in HIV-negative MSM (Figure 5EE). Among women (for whom we observed no apparent age effect), HPV-16 prevalence was higher in WWH than in HIV-negative women (Figure 5CC), and the proportion of HPV-16 infections with a duration ≥2 years was higher in WWH (46%) than in HIV-negative women (11%) (Figure 5FF).
DISCUSSION
This pooled reanalysis of individual-level longitudinal data from 34 studies built a robust picture of the natural history of anal hrHPV infection across 6 ASCC risk groups, underpinned by statistical methods (CTMM) that allowed us to overcome the inconsistencies in design and analytical approaches of previous individual studies. We confirmed that HIV status, sex, and male sexuality are important population-level determinants of both anal hrHPV incidence and clearance. In all risk groups, clearance of incident hrHPV infection was higher than that of prevalent infection. Age-specific differences in hrHPV clearance in MSM were predominantly observed for prevalent but not incident infection, suggesting that age-specific differences are more likely linked to duration of HPV infection rather than age of the infected individual.
Compared with other hrHPV types, HPV-16 had the highest incidence-clearance ratio and longest infection duration, as previously reported [4, 9, 10]. This finding could explain why HPV-16 was the most prevalent type, both at baseline in our pooled data set (Supplementary Figure 4), as well as in other studies [11, 12], and it supports the uniquely high potential of anal HPV-16 infection to progress to ASCC [3]. Notably, the incidence-clearance ratio for HPV-18 was no different from that many other hrHPV types, corroborating evidence that the carcinogenicity of HPV-18 in the anus is not as apparent as in the cervix [1].
Most previous individual reports on anal hrHPV natural history have not been powered to separate clearance of prevalent infection from incident infection. Furthermore, prevalent hrHPV infection is not homogeneous but is rather a mixture of antecedent natural histories. Prevalent infection may be recently acquired, like incident infection, or be longstanding and likely to have greater oncogenic potential. Indeed, using a similar CTMM approach, Plummer et al [13] found that the longer a cervical hrHPV infection had persisted, the greater the probability that it would further persist. In the current study, we were able to provide robust evidence of the same tendency in the anus. First, incident anal HPV infection was shown to clear significantly faster than prevalent infection, consistently for all hrHPV types and across different risk groups, as previously reported [9, 14]. Moreover, prevalent HPV-16 infection was more likely to clear in young than in older MSM, irrespective of HIV status. This finding is consistent with the higher cervical HPV clearance observed in young women [15] and is expected to be related to differences in prior duration of infection by age. Indeed, our CTMM approach demonstrated that prevalent infection in older MSM is disproportionately represented by longstanding infection. The absence of an age effect on clearance of incident HPV-16 infection also suggests that differences are driven by the duration of the infection rather than by age. On a similar note, the observation of a lower clearance of prevalent HPV-16 infection in MSM than in women or MSW (even after adjustment for HIV status and age; Supplementary Table 3) suggests that prevalent infection in MSM is more likely to represent longstanding persistent infection, and hence to have greater oncogenic potential, than that detected in women and MSW of a similar age.
HIV positivity was confirmed as an important risk factor for HPV-16 incidence and clearance among all 3 populations, consistent with the evidence that PWH have higher HPV prevalence and ASCC risk than their HIV-uninfected counterparts [2, 11]. Additional evidence for the role of HIV-related immunosuppression on the natural history of HPV infection in the current study comes from the observation that CD4 cell count and HIV viral load were determinants of HPV-16 incidence and clearance in PWH.
Having anal HSILs was a strong risk factor for lower HPV-16 clearance in MSM, highlighting the strong causal link between HPV-16 and anal HSILs [3, 16]. Indeed, the presence of underlying HSILs may partially contribute to the lower HPV-16 clearance observed in older MSM. Current smoking was associated with higher anal HPV-16 incidence, likely reflecting confounding by sexual behavior but corroborating evidence that anal cancer is elevated among smokers [17].
The current study highlighted important differences in anal HPV-16 acquisition by sex. Number of sexual partners, whether lifetime or recent, was confirmed as a strong determinant of HPV-16 incidence in both MSM and women. Number of anal sexual partners, on the other hand, was strongly associated with HPV-16 incidence in MSM, but not in women, despite adequate sample sizes. This suggests that anal HPV-16 incidence is similar in women whether or not they have anal sexual intercourse, and it supports other lines of evidence that anal HPV is frequently acquired from the cervix/external genital region. These include strong correlations in type-specific HPV detection between the genitals and anus among women [18] (and MSW [19]), as well as associations between front-to-back post-toilet wiping or anal touching and female anal HPV infection [20, 21].
HPV clearance, defined as a single positive result followed by a single negative result, offers a real-life picture of repeat anal HPV testing. Nevertheless, HPV contamination, intravisit clearance, or reinfection, driven by recent sexual activity, can mimic lack of clearance. Thus, HPV clearance rate may remain underestimated, particularly in highly exposed groups, and this may partly explain why incident infection is observed to clear less often in MSM than in women and MSW, even after adjustment for HIV status and age (Supplementary Table 3). Of note, the CTMM developed for this analysis allows for individuals to switch HPV status between visits and so better addresses this issue than the standard person-time approach based only on observed events. Indeed, estimates of incidence and clearance, as well as differences in these measures between risk groups, were much lower from a standard person-time approach (Supplementary Figure 5) than from our CTMM approach, as shown previously [9]. However, our estimates were generally consistent with previous individual studies using CTMM for MSM [9, 10, 22].
Other strengths of CTMM, in addition to those mentioned above, include the avoidance of separating follow-up into 2 discrete datasets for incidence and clearance. However, combining studies with different visit intervals precluded us from investigating stricter criteria for incidence or clearance (eg, a requirement for 2 subsequent positive, or negative, visits, respectively) or analyzing groups of HPV types (eg, any hrHPV). Other limitations should also be noted. First, our CTMM approach cannot overcome any lack of representativeness in the original study population. Most notably, HIV-negative MSM recruited into HPV studies are at higher-than-average HPV infection risk compared with their general population [11, 12]. Second, small numbers of MSWWH and few HPV infections in HIV-negative MSW hampered the robustness of estimates in MSW. Furthermore, most individuals were from the United States, Europe, or Asia, with underrepresentation of Africa, even for PWH.
Gender-neutral HPV vaccination of children is expected to be the long-term solution for ASCC prevention in all risk groups, including MSM [23]. However, MSM will not receive protection from female-only vaccination [24]. This, together with evidence of vaccine effectiveness against anal HPV infection [25, 26], has led certain countries to recommend targeted vaccination for MSM and other high-risk populations, such as PWH [27]. When predicting the relative public health benefits of age-targeted MSM versus gender-neutral vaccination, however, it is important to consider that incident infection tends to be shorter lived and that the fraction of prevalent infection that represents longstanding persistent infection increases with age. Thus, in cohorts of increasing age, an increasingly important proportion of future ASCC can be expected to arise from existing prevalent HPV infection and will not be prevented by HPV vaccination. Of relevance, clinical trials in PWH aged >26 years old were unable to demonstrate efficacy against anal HPV infection [28, 29].
Populations at highest risk of ASCC, which were not eligible for childhood HPV vaccination, could benefit from anal cancer screening [30], analogous to HPV-based cervical cancer screening, but with a particular focus on HPV-16, given its unique anal carcinogenicity [3]. However, up to 30% of MSMWH, and 15% of HIV-negative MSM and WWH are HPV-16 positive at any given time [12, 31] and may not all be at sufficient risk to warrant referral for high-resolution anoscopy, an invasive technique for which there is limited available clinical expertise [12]. Referral only of patients with persistent HPV infection, a necessary requirement for ASCC development, may thus improve risk stratification. The current study provides the indicators to make predictions in screening programs based on persistent infection. For example, retesting MSM and PWH found to be HPV-16 positive at the first visit after 1 year would avoid referral of about a quarter of patients, and retesting those found to be positive at 2 years would avoid referral of about half.
In conclusion, robust estimates of anal HPV incidence and clearance inform anal HPV natural history by risk group. They are key to designing and predicting the impact of ASCC prevention algorithms, including primary (HPV vaccination) and secondary (HPV-based screening) prevention modalities for targeted high-risk populations. Most notably, our study demonstrates that the carcinogenic potential of longstanding prevalent anal hrHPV infection is higher than that of more recent incident infection.
Contributor Information
Feixue Wei, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer (IARC/WHO), Lyon, France.
Marc T Goodman, Cancer Prevention and Control Program, Cedars Cancer, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Ningshao Xia, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, Fujian, China.
Jun Zhang, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, Fujian, China.
Anna R Giuliano, Center for Immunization and Infection Research in Cancer (CIIRC), Moffitt Cancer Center, Tampa, Florida, USA.
Gypsyamber D’Souza, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Nancy A Hessol, Department of Clinical Pharmacy, University of California, San Francisco, California, USA.
Maarten F Schim van der Loeff, Department of Infectious Diseases, GGD Amsterdam, Amsterdam, Netherlands.
Jianghong Dai, School of Public Health, Xinjiang Medical University, Urumqi, Xinjiang, China.
Karin Neukam, Unidad Clínica de Enfermedades Infecciosas y Medicina Preventiva, UCEIMP, Instituto de Biomedicina de Sevilla, CSIC, Universidad de Sevilla, Hospital Universitario Virgen del Rocío, Seville, Spain.
Alexandra de Pokomandy, Chronic Viral Illness Service, McGill University Health Centre and Department of Family Medicine, McGill University, Montreal, Quebec, Canada.
I Mary Poynten, The Kirby Institute, University of New South Wales, Kensington, Sydney, New South Wales, Australia.
Ronald B Geskus, Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam. Centre for Tropical Medicine and Global Health, University of Oxford, Oxford, UK.
Joaquin Burgos, Infectious Diseases Department, Hospital Universitari Vall d’Hebron, Barcelona, Spain. Vall d’Hebron Institut de Recerca (VHIR), Universitat Autónoma de Barcelona, Barcelona, Spain.
Isabelle Etienney, Proctology, Diaconesses-Croix Saint Simon Hospital, Paris, France.
Anna-Barbara Moscicki, Department of Pediatrics, University of California, Los Angeles, California, USA.
Maria Gabriella Donà, Sexually Transmitted Infections (STI)/HIV Unit, San Gallicano Dermatological Institute IRCCS, Rome, Italy.
Maura L Gillison, Thoracic Head and Neck Medical Oncology Department, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Alan G Nyitray, Center for AIDS Intervention Research and Clinical Cancer Center, Medical College of Wisconsin, Milwaukee, Wisconsin, USA.
Rebecca G Nowak, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Evy Yunihastuti, Faculty of Medicine Universitas Indonesia/Cipto Mangunkusumo Hospital, Jakarta, Indonesia.
Huachun Zou, School of Public Health (Shenzhen), Sun Yat-sen University, Shenzhen, China. Kirby Institute, University of New South Wales, Sydney, Australia.
Carmen Hidalgo-Tenorio, Early Clinical Trial Unit. Biosanitary Institute (IBS.Granada). Infectious Diseases Unit. University Hospital Virgen de las Nieves, Granada, Spain.
Nittaya Phanuphak, Institute of HIV Research and Innovation, Bangkok, Thailand.
Jean-Michel Molina, Department of Infectious diseases, University of Paris Cité, St-Louis Hospital, Paris, France.
Alice M Schofield, Institute of Cancer Sciences, The University of Manchester, Manchester, UK.
Stephen Kerr, HIV-NAT, Thai Red Cross AIDS Research Centre, and Research Affairs, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Song Fan, School of Public Health, Southwest Medical University, Luzhou, China.
Yong Lu, School of Public Health, the Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guiyang, China.
Jason J Ong, Central Clinical School, Monash University, Melbourne, Australia.
Admire T Chikandiwa, Wits RHI, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Sirinya Teeraananchai, Department of Statistics, Faculty of Science, Kasetsart University, Bangkok, Thailand.
Nicola Squillace, Infectious Diseases Unit ASST-Monza, San Gerardo Hospital-University of Milano-Bicocca, Monza, Italy.
Dorothy J Wiley, School of Nursing, University of California, Los Angeles, California, USA.
Joel M Palefsky, Department of Medicine, University of California, San Francisco, California, USA.
Damien Georges, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer (IARC/WHO), Lyon, France.
Catharina J Alberts, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer (IARC/WHO), Lyon, France.
Gary M Clifford, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer (IARC/WHO), Lyon, France.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Ashish A Deshmukh for his comments on the manuscript, Susan Gamon for her help on text editing, and the other colleagues involved in original studies: Ting Wu, Shoujie Huang, and Yingying Su (reference 27 in the Supplementary Data); Luis F López Cortés, César Sotomayor de la Piedra, and Pompeyo Viciana (reference 22 in the Supplementary Data); Cristina González, Montserrat Torres, Jorge Del Romero, Pompeyo Viciana, Mar Masiá, José R Blanco, Mauricio Iribarren, Silvia De Sanjosé, Beatriz Hernández-Novoa, Marta Ortiz, and Julia Del Amo (reference 12 in the Supplementary Data); Alessandra Latini, Francesca Rollo, Maria Benevolo, Massimo Giuliani, and Amalia Giglio (references 11 and 33 in the Supplementary Data); Nipat Teeratakulpisarn, Tuti Parwati Merati, I. Ketut Agus Somia, Iskandar Azwa, Ilias A Yee, Wifanto Saditya Jeo, and Hanny Nilasari (reference 28 in the Supplementary Data); Isabelle Charrreau, Laurence Meyer, David Veyer, Laurent Cotte, Constance Delaugerre, and Catherine Capitant (reference 29 in the Supplementary Data); and Davide Bernasconi (reference 32 in the Supplementary Data).
Disclaimer. The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
References
Articles from Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cid/ciac581
Read article for free, from open access legal sources, via Unpaywall:
https://escholarship.org/content/qt8445x6cw/qt8445x6cw.pdf?t=rx89lv
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/133042234
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/cid/ciac581
Article citations
Clearance of anal and penile HPV 6, 11, 16, and 18 DNA and antibodies among adolescent men who have sex with men (HYPER): An observational cohort study.
Vaccine X, 20:100551, 01 Sep 2024
Cited by: 0 articles | PMID: 39290530 | PMCID: PMC11405912
Clinical Predictors and Outcomes of Invasive Anal Cancer for People With HIV in an Inception Cohort.
Clin Infect Dis, 79(3):709-716, 01 Sep 2024
Cited by: 0 articles | PMID: 38573010 | PMCID: PMC11426273
Prevalence and concordance of penile, anal, and oral human papillomavirus infections among sexually active heterosexual men in Ibadan, Nigeria.
Cancer Causes Control, 03 Oct 2024
Cited by: 0 articles | PMID: 39361165
Longitudinal Screening for Oral High-Risk Non-HPV16 and Non-HPV18 Strains of Human Papillomavirus Reveals Increasing Prevalence among Adult and Pediatric Biorepository Samples: A Pilot Study.
Vaccines (Basel), 12(8):895, 08 Aug 2024
Cited by: 0 articles | PMID: 39204021 | PMCID: PMC11360083
Safety and Immunogenicity of the Nonavalent Human Papillomavirus Vaccine in Women Living with HIV.
Vaccines (Basel), 12(8):838, 25 Jul 2024
Cited by: 1 article | PMID: 39203964 | PMCID: PMC11359547
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Incidence and Clearance of Anal High-risk Human Papillomavirus Infections and Their Determinants Over 5 Years Among Human Immunodeficiency Virus-negative Men Who Have Sex With Men.
Clin Infect Dis, 68(9):1556-1565, 01 Apr 2019
Cited by: 11 articles | PMID: 30169621
Human Papillomavirus-Associated Anal Cancer Incidence and Burden Among US Men, According to Sexual Orientation, Human Immunodeficiency Virus Status, and Age.
Clin Infect Dis, 77(3):419-424, 01 Aug 2023
Cited by: 5 articles | PMID: 37017078 | PMCID: PMC10681657
Prevalence of and factors associated with anal high-risk human papillomavirus in urban Tanzanian men who have sex with men, 2011-2012.
Int J STD AIDS, 33(7):672-679, 07 May 2022
Cited by: 2 articles | PMID: 35531601 | PMCID: PMC9189599
Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis.
Lancet Oncol, 13(5):487-500, 23 Mar 2012
Cited by: 519 articles | PMID: 22445259
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: K07 CA225403
NIH
World Health Organization (1)
WHO generic grant number for open-access policy
World Organization
Grant ID: 001