Abstract
Free full text

OREX-1038: a potential new treatment for pain with low abuse liability and limited adverse effects
Associated Data
Abstract
Drugs targeting mu opioid receptors are the mainstay of clinical practice for treating moderate to severe pain. While they can offer excellent analgesia, their use can be limited by adverse effects, including constipation, respiratory depression, tolerance, and abuse liability. Multifunctional ligands acting at mu opioid and nociceptin/orphanin FQ peptide receptors might provide antinociception with substantially improved adverse-effect profiles. This study explored one of these ligands, OREX-1038 (BU10038), in several assays in rodents and nonhuman primates. Binding and functional studies confirmed OREX-1038 to be a low efficacy agonist at mu opioid and nociceptin/orphanin FQ peptide receptors and an antagonist at delta and kappa opioid receptors with selectivity for opioid receptors over other proteins. OREX-1038 had long-acting antinociceptive effects in post-surgical and complete Freund’s adjuvant (CFA)-induced thermal hyperalgesia assays in rats and a warm water tail withdrawal assay in monkeys. OREX-1038 was active for ≥24 hours in each antinociception assay, and its effects in monkeys did not diminish over 22 days of daily administration. This activity was coupled with limited effects on physiological signs (arterial pressure, heart rate and body temperature) and no evidence of withdrawal after administration of naltrexone or discontinuation of treatment in monkeys receiving OREX-1038 daily. Over a range of doses, OREX-1038 was only transiently self-administered, which diminished rapidly to non-significant levels; overall both OREX-1038 and buprenorphine maintained less responding than remifentanil. These results support the concept of dual mu and nociceptin/orphanin FQ peptide receptor partial agonists having improved pharmacological profiles compared with opioids currently used to treat pain.
Introduction
Pain remains a significant public health burden worldwide. Current estimates suggest acute and chronic pain affects 125 million adults in the US (Skolnick and Volkow, 2016) and 1 in 5 adults globally, with 1 in 10 diagnosed with chronic pain (Goldberg and McGee, 2011). While there are a variety of pharmacological and non-pharmacological approaches to treat pain, opioid receptor agonists remain the most widely used, with more than 142 million prescriptions in the US in 2020 (https://www.cdc.gov/drugoverdose/rxrate-maps/). Despite their effectiveness for relieving pain, opioids have a number of limitations, including the development of tolerance and physical dependence, and the ability to induce respiratory depression and constipation. Moreover, the current opioid epidemic is due, at least in part, to the abuse-related effects of these drugs, which have contributed to high rates of overdose deaths (Compton et al., 2016) and have increased research efforts aimed at identifying and advancing alternative safe and effective treatments for pain (Skolnick and Volkow, 2016; Grosser et al., 2017; Collins et al., 2018).
Opioid receptor ligands can be developed with reduced adverse effects (Chang et al., 2015; Yekkirala et al., 2017). In addition to abuse-deterrent formulations of current opioids, there is substantial effort towards the discovery and development of novel opioids, such as ligands with actions that are restricted to peripheral (Walker, 2018) or inflamed tissues (Spahn et al., 2017), bivalent ligands (Yekkirala et al., 2013), ligands for putative heteromeric opioid receptors (Yekkirala et al., 2011), and biased ligands, particularly those that favor the G-protein signaling pathway over the β-arrestin signaling pathway (Smith et al., 2018). A number of structurally diverse biased ligands have been developed (Soergel et al., 2014; Manglik et al., 2016; Viscusi et al., 2016; Schmid et al., 2017). While initial studies suggested that these biased ligands could provide pain relief with fewer adverse effects, including limited respiratory depression, further characterization of these compounds have revealed only trends towards an improved profile relative to morphine (Singla et al., 2017; Hill et al., 2018).
Another strategy that might improve the treatment of pain is to develop multifunctional ligands that target more than one receptor (Gunther et al., 2017). Often this approach involves activation of mu opioid receptors along with, for example, activation of kappa opioid receptors (Khroyan et al., 2017), blockade of delta opioid receptors (Healy et al., 2013), or activation of nociceptin/orphanin FQ peptide receptors (Ding et al., 2016). One example of this approach is OREX-1038 (also called BU10038) that arose from a program to develop compounds with binding profiles similar to buprenorphine, which has some advantages over other mu opioid receptor agonists. In a recent study, OREX-1038 was effective in an assay of antinociception with fewer adverse effects than other opioids after systemic and intrathecal administration in nonhuman primates (Kiguchi et al., 2019). The current studies extended the evaluation of OREX-1038, demonstrating antinociceptive activity in models of postoperative and inflammatory pain, coupled with low abuse liability and no evidence for the development of tolerance or physical dependence.
Materials and Methods
Drug Synthesis
OREX-1038 was synthesized as part of a series of molecules that are analogues of naltrexone and, like buprenorphine, designed to have activity at mu opioid and nociceptin/orphanin FQ peptide receptors (Figure 1). OREX-1038 used for these studies was provided by Orexigen.
In vitro characterization of OREX-1038
Cell lines and cell culture.
Chinese Hamster Ovary (CHO) cell lines stably expressing human delta opioid receptors or human nociceptin/orphanin FQ peptide receptors were generous gifts from Lawrence Toll at the Torrey Pines Institute for Molecular Studies (Port St Lucie, FL, USA), and CHO cell lines stably expressing human mu opioid receptors or human kappa opioid receptors were generous gifts from John M. Streicher at the University of Arizona (Tucson, AZ, USA). Cells were cultured in 50:50 DMEM/F12 media with 10% heat inactivated FBS and 1X penicillin/streptomycin supplement (Gibco Life Sciences, Grand Island, NY, USA) in a 37°C humidified incubator with 5% CO2 atmosphere. Propagation cultures were further maintained with 500 μg/ml G418 (Geneticin reagent; Gibco Life Sciences, Grand Island, NY, USA). Cultures were propagated for no more than 30 passages before discarding. Cell pellets for experiments were prepared by growth in 15 cm2 plates and harvested with 5 mM EDTA in 50 mM Tris HCl, pH 7.4. They were resuspended in 50 mM Tris HCl, pH 7.4, and homogenized with a tissue grinder for 15–30 seconds on ice. Crude membranes were spun down at 15,000 g for 30 minutes. This process was repeated and membrane preparations were stored at −80°C prior to use.
Competition radioligand binding.
Affinity of OREX-1038 for each type of opioid receptor as well as for nociceptin/orphanin FQ peptide receptors was determined by conducting competition binding studies. Membrane preparations from CHO cells expressing human mu, kappa, or delta opioid receptors were diluted to 10–20 μg/reaction with a fixed concentration of 3H-diprenorphine (0.2 nM) and varying concentrations of competitor ligand; Kd values for diprenorphine are 0.38 nM at mu opioid receptors, 0.14 nM at kappa opioid receptors, and 0.33 nM at delta opioid receptors. For membrane preparations from CHO cells expressing human nociceptin/orphanin FQ peptide receptors, 3H-nociceptin (0.1 nM) was used in an analogous manner; the Kd value for nociception is 0.05 nM at these receptors. Competitor ligands were OREX-1038, buprenorphine, and naltrexone for all 4 receptor types along with naloxone for the 3 opioid receptors and nociceptin for nociceptin/orphanin FQ peptide receptors. These reactions were miniaturized to a 400-μl volume in 96-well plates. Membrane preparations containing opioid receptors were incubated at 37°C for 3 hours and membrane preparations containing nociceptin/orphanin FQ peptide receptors were maintained at room temperature for 60 minutes. The reactions were terminated by rapid filtration through 24-well format GF/B filter mat (PerkinElmer, Inc., Waltham, MA, USA), washed with cold 25 mM Trish HCl, pH 7.4, buffer, dried, and then combined with ECOLUME™ scintillation cocktail (MP Biomedicals, Santa Cruz, CA, USA). The plates were read in a MicroBeta2 96-well format 6 detector scintillation counter (PerkinElmer, Inc., Waltham, MA, USA). KI values were calculated using the IC50 of each competitor ligand and the previously established KD of 3H-diprenorphine in each cell line using a competition binding model and GraphPad Prism version 7.0 for Windows (GraphPad Software, San Diego, CA, USA).
[35S]GTPγS Coupling.
Stimulation of [35S]GTPγS binding was used to determine whether OREX-1038 has pharmacological efficacy at opioid and nociceptin/orphanin FQ peptide receptors. As described previously (Traynor and Nahorski, 1995), membranes from CHO cells expressing human mu, kappa, or delta opioid receptors or human nociceptin/orphanin FQ peptide receptors were combined with various concentrations of drugs or their respective controls along with approximately 50 pM [35S]GTPγS (PerkinElmer Inc. Waltham, MA, USA) and 10–15 μg/reaction GTPγS assay buffer (50 mM Tris HCl, pH 7.4, 125 mM NaCl, 5 mM MgCl2, 1 mM EDTA and 30 μM GDP). For each reaction, 200 μl was incubated at 30°C for 50 minutes in 96-well plates then collected and measured as described above for the binding experiments. Competitor ligands were OREX-1038, DAMGO, buprenorphine and naltrexone for mu opioid receptors, and OREX-1038, nociceptin, buprenorphine and naltrexone for nociceptin/orphanin FQ peptide receptors. EC50 and EMAX values were calculated using the three-variable log(agonist) versus response curve (GraphPad Prism). Antagonist experiments were conducted with membrane preparations expressing human kappa and delta opioid receptors, which were preincubated with OREX-1038, buprenorphine or naltrexone for 2 minutes followed by: 1) addition of 1 μM U69,593, an agonist selective for kappa opioid receptors, to preparations expressing kappa opioid receptors, or 2) addition of 500 nM deltorphin-II, an agonist selective for delta opioid receptors, to preparations expressing delta opioid receptors. IC50 and IMAX values were calculated using the three-variable log(inhibitor) versus response curve (GraphPad Prism).
Off-target screening.
A Tier 2 in vitro Safety Pharmacology Profiling panel was used to determine potential off-target liabilities of 10 mM OREX-1038 (Eurofins Panlabs Taiwan Ltd, Taipai, Taiwan). A total of 87 enzymatic and binding assays were conducted and are listed in Supplemental Table 1.
Materials.
Reagents and solvents were purchased from commercial sources and used without further purification. With the exception of OREX-1038, chemicals and biochemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Hudson, NH, USA), unless otherwise noted. Radioactive compounds were purchased from PerkinElmer (Waltham, MA, USA).
Pharmacokinetics of OREX-1038
Subjects.
Pharmacokinetic studies were conducted in 4 different species using various routes of administration. In separate groups of 3 male CD-1 mice, OREX-1038 was given i.v., s.c., and p.o. In Sprague-Dawley rats, groups of 3 males and 3 females received OREX-1038 i.v., s.c., or p.o. Groups of 5 male New Zealand white rabbits were given OREX-1038 i.v., s.c., or s.l. Finally, in cynomolgus monkeys, groups of 2 males and 2 females received OREX-1038 i.v., s.c., or p.o. Rodents and monkeys were selected because they were used for antinociceptive studies and rabbits provided an extra species that allowed for assessment of sublingual bioavailability.
Procedure.
Blood samples were collected at times ranging from 5 min to 24 hour after OREX-1038 administration. Within 1 hour of collection, blood samples were centrifuged at 3000 × g at 4°C and plasma was collected. Plasma OREX-1038 concentrations were quantified by protein precipitation followed by HPLC-MS/MS analysis. Drug administration, sample collection and plasma analyses were performed at Aptuit (Verona, Italy; mouse and rat), Absorption Systems (Exton, PA, USA; rabbit) and Covance Laboratories Inc. (Madison, WI, USA; monkey).
Data analyses.
Pharmacokinetic parameters were calculated from the time course of the plasma concentrations using a non-compartmental model (Phoenix WinNonlin software, version 7.0, Certara Companies, Princeton, NJ, USA). For i.v. dosing, the maximum plasma concentrations (C0) were estimated by extrapolation of the first two time points back to t=0. For other routes of administration, the observed maximum plasma concentration (Cmax) and time to reach maximum plasma concentration (Tmax) were reported. Areas under the concentration-time curves (AUC) were calculated using the linear trapezoidal rule with calculation to the last quantifiable data point and with extrapolation to infinity if applicable. Plasma half-life (t½) was obtained by dividing 0.693 by the slope of the terminal elimination phase. Mean residence time (MRT) was calculated by dividing the area under the moment curve (AUMC) by the AUC. Clearance was obtained by dividing dose by AUC. Steady-state volume of distribution (Vss) was calculated by multiplying clearance by MRT. Bioavailability was estimated by dividing the individual dose-normalized AUC value following s.c., s.l., or p.o. administration by the average dose-normalized AUC value following i.v. administration. Dose-normalized AUC values were AUC values obtained from concentration-time curves (individual curves following s.c., s.l., or p.o. administration and group average curves following i.v. administration) divided by OREX-1038 dose. Samples below the limit of quantitation (0.5 ng/ml) were treated as zero for pharmacokinetic data analysis.
In vivo characterization of OREX-1038 in rats
Subjects.
Male Sprague-Dawley rats (Charles River Laboratories, Calco, Italy) were divided into groups containing 10–11 rats and housed in cages with solid bottoms in rooms maintained on a 12-hour light/dark cycle. Sawdust litter was used except that it was replaced by Alfa-dry litter after surgery for rats participating in the Brennan model. Food (Altromin R, A. Rieper SpA, Bolzano, Italy) and water were available ad libitum. Animals were housed, and experiments performed, in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International).
Brennan model of incisional pain.
The antinociceptive effects of OREX-1038 were determined using two different hyperalgesia procedures. The first procedure was the Brennan model of incisional pain (Brennan et al., 1996), which measured latencies for rats to remove their hind paws from a thermal stimulus (Hargreaves et al., 1988) before and after surgery during which an incision was created. Rats (250–300 g) were anesthetized with isoflurane and a paw incision was made in the plantar aspect of the right hind paw. On the day after surgery, rats received s.c. injections of vehicle (1:9 mixture of 10% solutol:10% 2-hydroxypropyl-β-cyclodextrin), morphine (5 mg/kg), or OREX-1038 (3, 10, or 30 mg/kg) in a volume of 5 ml/kg, and latencies were redetermined 2, 6 and 24 hours later. Because tolerance can develop to the effects of opioid receptor agonists, the development of tolerance was examined in rats that received 5 mg/kg morphine (s.c.) or 10 mg/kg OREX-1038 (s.c.) once a day for 7 consecutive days with paw withdrawal latencies measured 2, 6 and 24 hours after administration of the last dose of drug. Data were expressed as mean latency (seconds) ± 1 SEM, plotted as a function of time since injection of test compound, and analysed using repeated-measures ANOVA followed by Fisher’s test to determine whether latencies were changed after administration of test compound compared with administration of vehicle. Statistical significance was set at P<0.05.
Complete Freund’s Adjuvant (CFA) inflammation model.
Hyperalgesia was also examined by administering CFA in a hind paw to induce inflammation and measuring latencies for rats to remove hind paws from a thermal stimulus before and after CFA injection (Hargreaves et al., 1988; Takayama et al., 2011). Rats (225–275 g) received an intraplantar (i.pl.) injection of CFA (1 mg/ml of Mycobacterium tuberculosis, heat killed and dried, in 0.85 ml paraffin oil and 0.15 ml mannide monooleate). On the day after CFA administration, rats received s.c. injections of vehicle (1:9 mixture of 10% solutol:10% 2-hydroxypropyl-β-cyclodextrin), diclofenac (30 mg/kg in 0.5% methylcellulose in water), or OREX-1038 (3, 10, or 30 mg/kg) in a volume of 5 ml/kg, and latencies were redetermined 2, 6 and 24 hours later. Diclofenac is a non-steroidal anti-inflammatory drug that is widely used clinically for relieving pain and inflammation in conditions such as arthritis. The development of edema induced by CFA injection was also quantified by measuring the volume of the hind paw using a plethysmometer immediately before injection of CFA, immediately before treatment with a test compound or vehicle (24 hours after CFA), and 24 hours post-treatment (48 hours after CFA administration). Hyperalgesia data were expressed as mean latency (seconds) ± 1 SEM, plotted as a function of time since injection of test compound, and analysed using repeated-measures ANOVA followed by Fisher’s test to determine whether latencies were changed after administration of test compound compared with administration of vehicle. Edema data were expressed as mean paw volume (ml) ± 1 SEM; in addition, the edema rate (%) was calculated according to the following expression: 100% X [(test paw volume − control paw volume)/(control paw volume] and plotted as a function of dose. Statistical significance was set at P<0.05.
Gastrointestinal transit.
Because constipation can limit the clinical use of opioids for pain, gastrointestinal transit was examined. Food, but not water, was withheld from each rat the night before the experiment. Rats received an injection of vehicle, morphine (30 mg/kg), or OREX-1038 (3, 30, or 100 mg/kg) and were returned to the home cage. Two hours after the injection, they received 2 ml of charcoal suspension by gavage, and 30 minutes later, rats were anesthetized with isoflurane and terminated by cervical dislocation. After exposing the intestine, the distance that the charcoal travelled from the pyloric sphincter as well as the total intestinal length were measured. Relative distance was the ratio of distance travelled by the charcoal divided by intestinal length; these ratios were expressed as mean relative distance ± 1 SEM, plotted as a function of dose, and analysed using repeated-measures ANOVA to determine whether ratios were different after administration of test compound compared with ratios after administration of vehicle.
Rotarod.
To examine the effects of OREX-1038 on motor coordination, rats (272–318 g) were placed on a rotarod apparatus (Model 47700, Ugo Basile SRL, Gemonio, Italy). The study was performed over 3 days. On the first day, rats were placed on the apparatus that turned at a fixed speed (4 rpm) for at least 120 seconds. Four hours later, they were again placed on the apparatus turning at a fixed speed (4 rpm) for 1 minute and then at an accelerating speed (4–40 rpm) for 100–120 seconds. On the second day, rats were tested on the rotarod apparatus turning at a fixed speed (4 rpm) for 1 minute and then at an accelerating speed (4–40 rpm) until they fell off or 5 minutes elapsed, whichever occurred first, and latency (seconds) to fall was recorded. The third day was the test day when rats received vehicle, diazepam (10 mg/kg, i.p., 30 minutes before test), or OREX-1038 (5, 10, or 20 mg/kg, s.c., 120 minutes before test). During the test, rats were placed on the rotarod turning at a fixed speed (4 rpm) for 1 minute and then at an accelerating speed (4–40 rpm). Latency to fall was recorded for each rat (up to a cut-off of 5 minutes). Data were expressed as mean latency (seconds) ± 1 SEM, plotted as a function of dose, and analysed using ANOVA followed by Dunnett’s test to determine whether latencies were different after administration of a test compound compared with administration of vehicle. Statistical significance was set at P<0.05.
In vivo characterization of OREX-1038 in rhesus monkeys
Subjects.
Four adult male (subjects IP, AC, FI, GU; 8.6–11.4 kg) and six adult female (LI, SA, LU, ME, PR, RU; 4.6–10.6 kg) rhesus monkeys participated in these studies. Monkeys were individually housed with unlimited access to water in their home cages and received primate chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI, USA), fresh fruit, and peanuts daily. They were maintained on a 14-hour light and 10-hour dark cycle. Monkeys received a variety of drugs in previous experiments and were maintained, and all experiments were performed, in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals, 8th edition (2011).
Surgery.
Indwelling venous catheters were implanted using methods that have been described previously (e.g. Gerak et al., 2019). Monkeys were sedated with 10 mg/kg ketamine (s.c.; Henry Schein, Dublin, OH, USA), and then intubated and maintained on 2 l/minute oxygen and isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX, USA). A polyurethane catheter (SIMS Deltec Inc., St. Paul, MN, USA) was placed in a vein, such as a femoral or jugular, and tunneled s.c. to the back where it was connected to a vascular access port (Access Technologies, Skokie, IL, USA). Telemetry devices (model L11, Data Sciences International, Arden Hills, MN, USA) were placed in the right flank with positive ECG leads tunneled to the lower left quadrant of the thorax and negative ECG leads tunneled to the upper right quadrant. The systemic blood pressure catheter was placed in a femoral artery. Absorbable suture (Ethicon Inc., Somerville, NJ, USA) was used to close muscle, tissue, and skin incision sites. Penicillin B&G (40,000 IU/kg) and meloxicam (0.1–0.2 mg/kg) were given to animals postoperatively.
Warm water tail withdrawal.
Monkeys (IP, LU, LI, and SA) were seated in primate chairs (Primate Products, Miami, FL, USA). Tails were placed in mugs containing water at 40 or 50°C and the latency to remove them from the water was measured with a stopwatch. The maximum time that tails remained in water was 20 sec. The acute effects of drug or vehicle were examined for up to 72 hours by conducting several sessions. Each session lasted at least 30 minutes and began with measurements of latencies for monkeys to remove their tails from water at 40 and 50°C. For all sessions, latencies were obtained from 40°C water 15 minutes after the first measurement, and latencies were obtained from water at both temperatures 30 minutes after the first measurement. During the first session, these control measurements were followed immediately by a single injection of vehicle, morphine (1.8 mg/kg, s.c.), or OREX-1038 (0.001, 0.003, 0.01, 0.03, 0.1, and 0.3 mg/kg, s.c.). Thereafter, tail-withdrawal latencies from water at both temperatures was determined every 30 minutes for 3 hours with an additional measurement of latency from 40°C water obtained midway between those assessments. Monkeys were returned to the home cage upon completion of the 3-hour session. A second session began 5 hours and 30 minutes after the injection; this 30-minute session was identical to the first 30 minutes of the first session with the latencies obtained the end of the session used for the 6-hour time point. Additional 30-minute sessions were conducted using the same strategy as that described for the 6-hour time point to obtain latencies 24, 30, 48 and 72 hours after injections.
Antinociception data are presented as a percentage of the maximum possible effect (20 seconds) according to the following expression: 100% X [(test latency - control latency)/(20 seconds - control latency)] and are plotted as a function of time since injection. A dose-effect curve for OREX-1038, administered acutely, was obtained by calculating the area under the curve (AUC) for antinociception data collected 0.5–3 hours after injection with areas plotted as a function of dose.
Drug self-administration.
Monkeys were seated in primate chairs in ventilated, sound-attenuating chambers. Custom-built response panels, equipped with stimulus lights and two response levers, were mounted on the front panel of each chamber. Infusions were delivered by activation of syringe pumps (Razel Scientific Instruments Inc., Stamford, CT, USA) located outside of chambers. Syringes in pumps were connected to vascular access ports using 20-g Huber-point needles (Access Technologies, Skokie, IL, USA) with a 185-cm extension set (Abbott Laboratories, Stone Mountain, GA, USA). Experimental events were controlled and data were recorded by a computer using Med-PC IV software (Med Associates Inc., St. Albans, VT, USA).
During self-administration sessions, monkeys could respond to receive an infusion of drug or vehicle under a fixed ratio 30:timeout 180-second schedule of reinforcement. Sessions lasted 90 minutes and began with activation of the syringe pump to fill the catheter with the solution that was available for self-administration. Beginning one minute after the start of the loading infusion, the red stimulus light above the active lever was illuminated for 5 seconds and a noncontingent priming infusion was delivered. When delivery of the noncontingent infusion was complete, the green stimulus light above the active lever was illuminated and 30 responses on the active lever turned off the green light, illuminated the red light for 5 seconds, and initiated the drug infusion and 180-second timeout. Responses on the inactive lever during the response period and responses on both levers during the timeout were recorded but had no programmed consequence. The end of the timeout period and beginning of the next response period was signaled by illumination of the green light above the active lever. After sessions, catheters and ports were flushed and locked with 3 ml of saline containing 100 U/ml of heparin.
Reinforcing effects of buprenorphine were evaluated in 4 monkeys (ME, GU, FI, and AC) who initially responded for 0.00032 mg/kg/infusion remifentanil; for this study, buprenorphine and remifentanil were dissolved in saline. Monkeys responded for remifentanil for at least 5 sessions and until responding was stable, as defined by 3 consecutive sessions in which at least 20 infusions were delivered and the average number of infusions obtained in each of those 3 sessions varied by not more than ± 20% of the 3-session mean. Then, saline was substituted for remifentanil for at least 4 sessions and until 8 or fewer infusions were obtained for 3 consecutive sessions. Following the first substitution with saline, 0.1 mg/kg/infusion buprenorphine was available for a minimum of 5 sessions and until responding met the stability criteria with either remifentanil or saline as indicated above, or for 10 sessions, whichever occurred first. Thereafter, saline was substituted for buprenorphine, followed by a test with 0.03 mg/kg/infusion buprenorphine. Tests with saline and progressively smaller doses of buprenorphine alternated until the smallest unit dose of buprenorphine (0.0001 mg/kg/infusion) was tested, at which point, saline was substituted once again followed by a final test with remifentanil. This approach was chosen for buprenorphine based on published data on self-administration of buprenorphine in rhesus monkeys (e.g. Mello et al. 1981).
Reinforcing effects of OREX-1038 were evaluated in 4 monkeys (PR, FI, AC, RU), two of which (AC and FI) also participated in the buprenorphine experiment. Monkeys initially responded for 0.00032 mg/kg/infusion remifentanil, with remifentanil dissolved in 10% w/v 2-hydroxypropyl-β-cyclodextrin vehicle (i.e., the same vehicle used for OREX-1038). After responding was stable, as defined above, vehicle was substituted for remifentanil followed by tests with different unit doses of OREX-1038. The method of testing with OREX-1038 was similar to that used with buprenorphine, but not identical: the three smallest unit doses of OREX-1038 (0.0003, 0.001, and 0.003 mg/kg/infusion) were tested first, in a mixed order across monkeys, after which, responding for remifentanil was re-established. Following the intervening test with remifentanil, the three largest unit doses of OREX-1038 (0.01, 0.03, and 0.1 mg/kg/infusion) were tested in an ascending order before a final test with remifentanil. As with buprenorphine, all tests with drug were separated by vehicle extinction sessions. Two sets of OREX-1038 doses were studied because it was unclear what the effective dose range would be, and it was safer to start with smaller doses and increase dose systematically. It should be noted that this study tested a 333-fold range of doses of OREX-1038.
Infusions obtained during the last 3 sessions of each phase were averaged for individual monkeys and used to indicate levels of intake under stable responding. Self-administration dose-effect data for stable responding were analyzed using a repeated-measures ANOVA on the number of infusions obtained for the first and last remifentanil and vehicle conditions of each experiment as well as each unit dose of buprenorphine or OREX-1038. Data for the buprenorphine and OREX-1038 experiments were analyzed separately. If there was a significant effect of condition, a Dunnett’s post-hoc test was conducted, comparing each condition to the first vehicle phase. A second analysis was conducted including the number of infusions obtained during the last session of each vehicle phase and the first 3 sessions of each test phase using a two-way, repeated-measures ANOVA with day and dose as within-subject factors; significant effects were analyzed further using a Dunnett’s post-hoc test, which compared the number of infusions obtained on each test day to the corresponding vehicle session.
Daily treatment with OREX-1038.
Effects of chronic treatment with OREX-1038 were examined by administering a single dose (0.03 mg/kg) once daily for 22 days with injections given at 0800 hours. Several dependent variables were measured before, during and after chronic treatment to characterize fully the development of tolerance and dependence. To determine whether tolerance developed, tail withdrawal latencies were obtained periodically using 30-minute sessions as described above. Latencies were determined at 1030 hours, which was 2.5 hours after daily administration of OREX-1038 or vehicle and a time when the peak effects of acutely administered OREX-1038 were observed. Tests for the development of tolerance were conducted the day before chronic treatment began, on treatment days 1, 8, 15 and 22 as well as 1, 2 and 7 days after discontinuation of OREX-1038 treatment (an acute injection of 0.03 mg/kg OREX-1038 was administered at 0800 hours 7 days after discontinuation of daily treatment with OREX-1038).
To determine whether opioid physical dependence developed during daily treatment with OREX-1038, the emergence of withdrawal signs was assessed following administration of the opioid receptor antagonist naltrexone, which would be expected to precipitate withdrawal in opioid-dependent monkeys, and following discontinuation of OREX-1038 treatment. Physiological parameters, including mean arterial pressure, heart rate, and body temperature, were monitored using implanted telemetry devices, which collected data continuously when activated. Telemetry devices sent signals to TRX-1 transceivers located in front of home cages. Mean arterial pressure (mmHg), heart rate (beats per minute), body temperature (°C), and activity (counts per minute) were collected using Ponemah® software (Data Sciences International, Arden Hills, MN, USA). In addition, directly observable signs were monitored by two individuals experienced with the behavior of these monkeys and blind to treatment conditions. Monkeys were observed in their home cage before, during, and after OREX-1038 treatment. The presence or absence of signs were recorded for 15 seconds of every minute for 8 consecutive minutes; these signs have been scored previously during opioid withdrawal in rhesus monkeys (Becker et al., 2008; Gerak et al., 2015; Gerak and France, 2016).
Tests for precipitated withdrawal occurred on days 18 and 19 of daily OREX-1038 treatment. The normal daily dose of OREX-1038 was given at 0800 hours. The transmitters were activated at 0845 hours on day 18 and turned off at 1615 hours on day 19. Behavioral observations were recorded at 1045 hours followed by an injection that was administered at 1100 hours; on day 18, the injection was saline, and on day 19, the injection was 0.1 mg/kg naltrexone, which precipitates withdrawal in monkeys treated daily with morphine (Maguire et al., 2020). Behavioral observations were also recorded at 1130 hours.
Transmitters were activated for at least 24 consecutive hours before and on days 1, 2, and 22 of daily OREX-1038 treatment. Telemetry data were not collected while monkeys were out of their home cage for antinociception tests (1000–1200 hours). On those days, behavioral observations were recorded at 0930 hours. After 22 days of treatment, vehicle replaced OREX-1038 at 0800 hours. For the next 3 days, transmitters were activated and behavioral observations were recorded at 0930 hours.
Mean arterial pressure, heart rate, and body temperature were recorded every minute and averaged across a 30-minute period to obtain one value per monkey for each dependent variable. When saline or naltrexone was given to assess precipitated withdrawal, the 30-minute period began at 1115 hours, which was 15 minutes after administration of saline or naltrexone. To monitor for the emergence of signs following discontinuation of OREX-1038 treatment, the 30-minute period began at 0900 hours, which was 1 hour after the daily injection of OREX-1038 or saline. Directly observable signs were analyzed individually, and for each sign, the frequency with which it occurred was averaged among monkeys (± 1 SEM); any signs that occurred during more than one observation period on at least one day of withdrawal were analyzed using a one-factor (treatment condition) repeated-measures ANOVA.
Drugs.
Morphine sulfate, remifentanil hydrochloride, buprenorphine hydrochloride, and naltrexone hydrochloride were generously provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD, USA) and dissolved in saline. Doses of OREX-1038 were calculated using the base with a correction factor of 1.08 (salt weight / base weight) and solutions of OREX-1038 were prepared fresh daily. 2-Hydroxypropyl-β-cyclodextrin (Accela ChemBio, Inc., San Diego, CA, USA) was dissolved in sterile saline at a concentration of 0.1 g per ml. All self-administered drug was delivered i.v. in a volume of 1 ml per 10 kg of body weight; infusion durations ranged from 13 to 21 seconds. For all other experiments, injections were given s.c.
Results
In vitro characterization of OREX-1038
Competition radioligand binding.
Like naltrexone, naloxone, and buprenorphine, OREX-1038 had affinity for mu, delta, and kappa opioid receptors, although its affinity at each of these receptors (Table 1) was higher than the affinities of the other opioid receptor ligands. Unlike naltrexone, OREX-1038 and buprenorphine could also bind to nociceptin/orphanin FQ peptide receptors, although their affinities at those receptors was much lower than their affinities for opioid receptors. Compared with buprenorphine, OREX-1038 had 7 to 8-fold higher affinity for both mu opioid and nociceptin/orphanin FQ peptide receptors (Table 1).
Table 1
Binding Affinity of Compounds at Opioid Receptors
| Compound | Binding Ki (nM) | |||
|---|---|---|---|---|
| mu receptor | delta receptor | kappa receptor | NOP receptor | |
| OREX-1038 | 0.16±0.08 | 0.42±0.04 | 0.79±0.40 | 177±40 |
| Buprenorphine | 1.3±0.17 | 10±2.6 | 0.87±0.43 | 1300±321 |
| Naltrexone | 1.2±0.15 | 53±12 | 3.7±1.10 | NB |
| Naloxone | 10±1.4 | 46±9.9a | 25.7±6.1 | |
| Nociceptin | 0.03±0.006a | |||
Competition binding at mu, delta, and kappa opioid receptors performed against [3H]-diprenorphine and at nociceptin/orphanin FQ peptide (NOP) receptors against [3H]-nociceptin in 50 mM Tris HCl pH 7.4. N = 3; NB = No binding at 10 mM
[35S]GTPγS Coupling.
Agonist activity of OREX-1038 at mu opioid receptors was compared to that of the high efficacy agonist DAMGO. While the EC50 of OREX-1038 was lower than that of DAMGO, its EMAX was only 28 ± 4.4%, indicating that OREX-1038 had low pharmacological efficacy at mu opioid receptors (Table 2). Similarly, OREX-1038 had lower pharmacological efficacy than nociceptin at nociceptin/orphanin FQ peptide receptors, with an EC50 of 160 ± 51 nM and an EMAX of 47 ± 9.0 (Table 2). Potency and pharmacological efficacy of buprenorphine was similar to that of OREX-1038 at the three opioid receptors, although potency of buprenorphine was substantially lower at nociceptin/orphanin FQ peptide receptors. OREX-1038 antagonized the effects of deltorphin-II, an agonist selective for delta opioid receptors, and U69,593, an agonist selective for kappa opioid receptors. The IC50 of OREX-1038 at each of these receptors was comparable to that of buprenorphine (Table 2).
Table 2
[35S]GTPγS Agonist and Antagonist Activity of OREX-1038 at opioid receptors
| Compound | mu receptor | NOP receptor | kappa receptor | delta receptor | ||||
|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | EMAXa | EC50 (nM) | EMAX | IC50 (nM) | IMAXb | IC50 (nM) | IMAX | |
| OREX-1038 | 1.5±0.67 | 28±4.4 | 160±51 | 47±9.0 | 9.0±1.4 | 90±4 | 25±9 | 87±2 |
| DAMGO | 33±3.1 | 98±5 | ||||||
| Nociceptin | 0.52±0.061 | 98±3.7 | ||||||
| Buprenorphine c | 0.79±0.31d | 34±6.2d | >1000 | 35±3.8 | 3.0±0.40 | 92±5.2 | 46±23 | 84±2.2 |
| Naltrexone | 5.5±3.8 | −18±3.0 | NS | NS | 42±1.7 | 83±9 | 490±200 | 90±7.1 |
Membrane preparations were incubated with ~50 pM [35S]GTPγS, 30 mM GDP in 50 mM Tris HCl, 125 mM NaCl, 5 mM MgCl2, 1 mM EDTA, as in methods section. N = 3; Data presented as average±SEM; NS = No Stimulation.
Off-target screening.
OREX-1038 exhibited no agonist activity and minimal antagonist activity against a panel of 87 non-opioid receptors (See Supplemental Table 1), supporting the selectivity of OREX-1038 for opioid and nociceptin/orphanin FQ peptide receptors. Perhaps the most notable activity of OREX-1038 at other sites involves its partial inhibition of L-type calcium channels. Initial examination of the safety profile of OREX-1038 also showed that it did not inhibit drug transporters, have cardiac liability or genotoxicity, or induce micronuclei in human peripheral blood lymphocytes (See Supplemental Table 2 for safety pharmacology).
Pharmacokinetics of OREX-1038
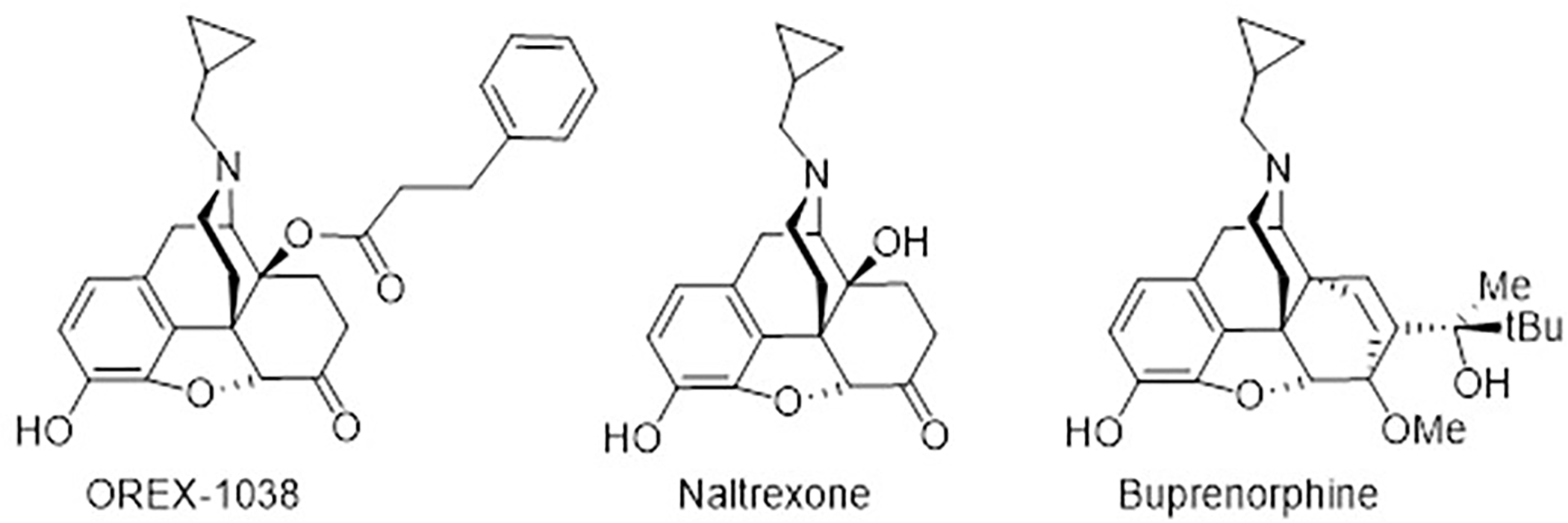
The pharmacokinetic profile of OREX-1038 was evaluated in mice, rats, rabbits, and cynomolgus monkeys using various routes of administration. While oral bioavailability was poor, bioavailability after s.c. administration was high, ranging from 321% in mouse to 63.7% in rabbit (See Supplemental Table 3 for pharmacokinetic parameters). Following s.c. administration, peak concentrations of OREX-1038 were achieved at 2, 2, 0.7 and 3 hours for mice, rats, rabbits, and monkeys, respectively (Figure 2 and Supplemental Table 3). In rabbits, Cmax and Tmax following s.l. administration were comparable to those of s.c. administration, but the t½ was shorter, leading to a decrease in exposure (AUClast and AUCinf) relative to s.c. and a bioavailability of 34.7% (Figure 2 and Supplemental Table 3). In rats, peak concentration (873 ng/ml) following s.c. administration was observed at 2 hours, the earliest time point measured. The brain-to-plasma concentration ranged from 1.5 to 2.9 (See Supplemental Figure 1 for brain and plasma concentrations at various times after administration).
Bioavailability of OREX-1038 after i.v. p.o., s.c., or s.l. administration in CD1 mice, Sprague-Dawley rats, New Zealand white rabbits and cynomolgus monkeys. Blood samples were collected up to 24 hours after administration of OREX-1038. Ordinate: plasma concentrations of OREX-1038 (ng/ml). Abscissa: time (hours) after administration of OREX-1038.
In vivo characterization of OREX-1038 in rodents
Brennan model of incisional pain.
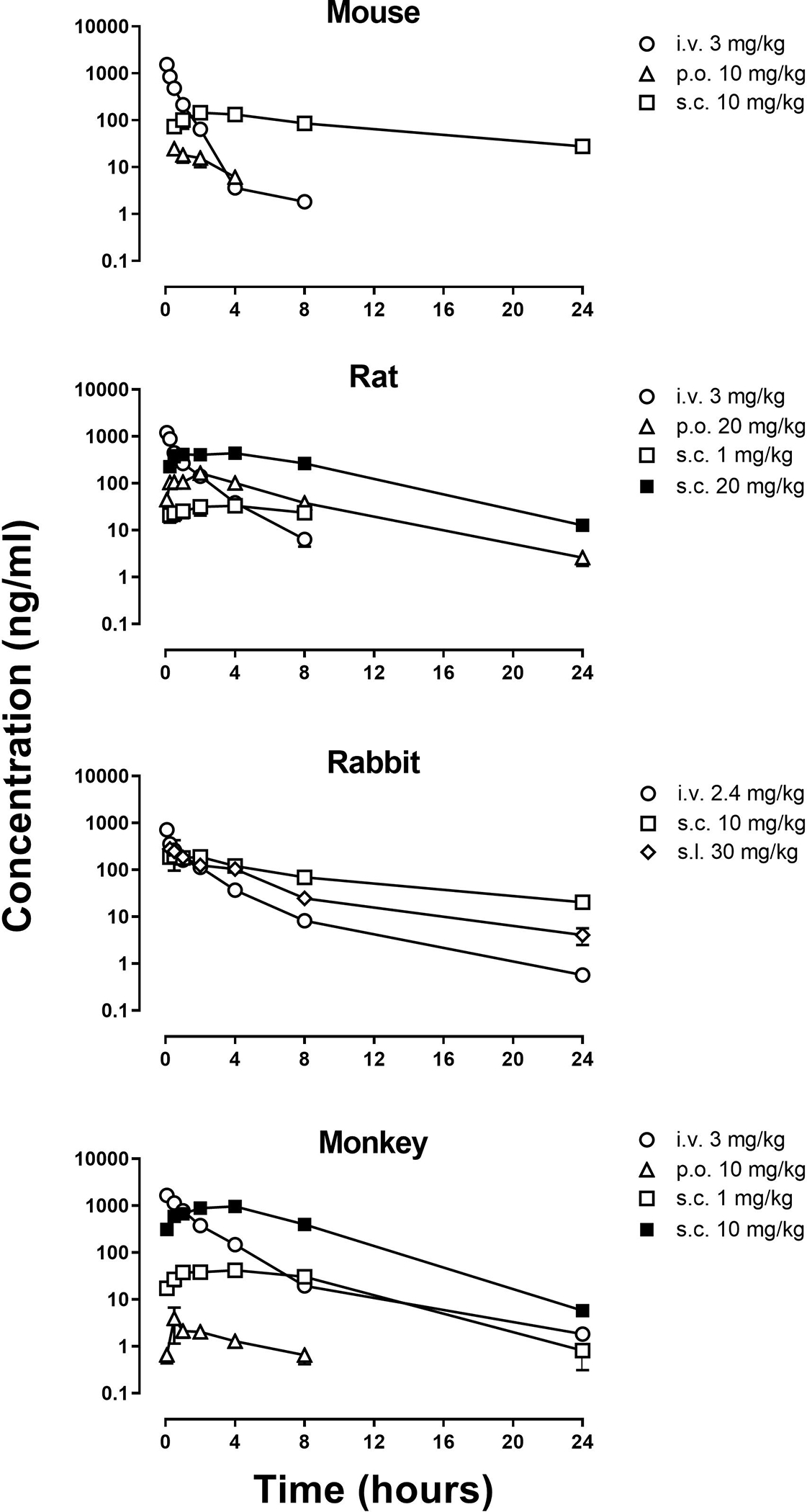
Incision of the hind paw significantly reduced latencies for vehicle-treated rats to remove their hind paw from a thermal stimulus, decreasing latencies from 13.6 ± 1.1 seconds before surgery to 5.43 ± 0.89 seconds after surgery (squares, Figures 3A and and3B);3B); latencies were similar 2, 6, and 24 hours after vehicle administration. In contrast, paw-withdrawal latencies were not decreased 2 hours after administration of morphine or the larger 2 doses of OREX-1038 compared with latencies obtained before surgery. For example, average latency was 13.1 ± 2.5 seconds after 5 mg/kg morphine and 15.6 ± 2.8 seconds after 10 mg/kg OREX-1038, compared with pre-surgery latencies of 13.3 ± 1.1 and 13.4 ± 1.2 seconds, respectively (points above 2, Figures 3A and and3B).3B). Blockade of the hyperalgesic effects by OREX-1038 was dose-related with a latency of 8.8 ± 0.9 seconds obtained 2 hours after the smallest dose of OREX-1038 (3 mg/kg). The anti-hyperalgesic effects of morphine were no longer evident 6 hours after administration; however, OREX-1038 retained the anti-hyperalgesic effect for up to 24 hours after administration (Figures 3A and and3B).3B). Daily administration of 5 mg/kg morphine blunted its effects on hyperalgesia, whereas daily administration of 10 mg/kg OREX-1038 did not alter its effects, with similar responses obtained in groups treated acutely and chronically (See Supplemental Figure 2 for withdrawal latencies). Supporting the findings from the Brennan procedure, OREX-1038 also had robust antinociceptive effects in the mouse tail flick assay (See Supplemental Figure 3 for % of maximum possible effect).
Antinociceptive effects of OREX-1038 and morphine in male Sprague-Dawley rats (n=10 or 11 per treatment group). In tests of post-surgical thermal hyperalgesia (Brennan model, Panels A and B), paw-withdrawal latencies are shown for one time point before surgery (pre-surgery baseline; surgery was 1 day before hyperalgesia assessment) and 2, 6 and 24 hours after administration of vehicle (squares), 5 mg/kg morphine (triangles, Panel A) and 3, 10 and 30 mg/kg OREX-1038 (circles, Panel B). Antinociceptive effects of diclofenac and OREX-1038 were evaluated in Sprague-Dawley rats (n=10 or 11 per treatment group) using CFA-induced thermal hyperalgesia (Panels C and D). Paw-withdrawal latencies are shown for one time point before CFA (pre-CFA baseline; CFA-injection was 1 day before hyperalgesia assessment) and 2, 6 and 24 hours after administration of vehicle (squares), 30 mg/kg diclofenac (triangles, Panel C) and 3, 10 and 30 mg/kg OREX-1038 (circles, Panel D). Edema rate (% increase over baseline) for diclofenac (Panel E) and OREX-1038 (Panel F). Right hind-paw volume was determined immediately before injection of CFA, immediately before treatment with vehicle or test compound (24 hours after CFA), and 24 hour later (48 hours after CFA).
Complete Freund’s Adjuvant (CFA) inflammation model.
In rats, injection of CFA into a hind paw significantly decreased paw-withdrawal latency from 13.5 ± 3.6 seconds before CFA administration to 4.5 ± 3.1 seconds after CFA and vehicle administration (squares, Figures 2C and and2D).2D). Diclofenac (30 mg/kg) and OREX-1038 (30 mg/kg) significantly blocked the hyperalgesic effects of inflammation, producing latencies of 7.3 ± 2.1 seconds and 8.6 ± 2.5 seconds, respectively, 2 hours after administration (points above 2, Figures 3C and and3D).3D). Each dose of OREX-1038, across a 10-fold dose range, was partially effective 2 hours after administration, although the larger 2 doses (10 and 30 mg/kg) retained this anti-hyperalgesic effect for 24 hours whereas the effect of 3 mg/kg waned over 24 hours. Diclofenac, but not OREX-1038, decreased edema 48 hours after CFA injection (Figures 3E and and3F3F).
Gastrointestinal transit.
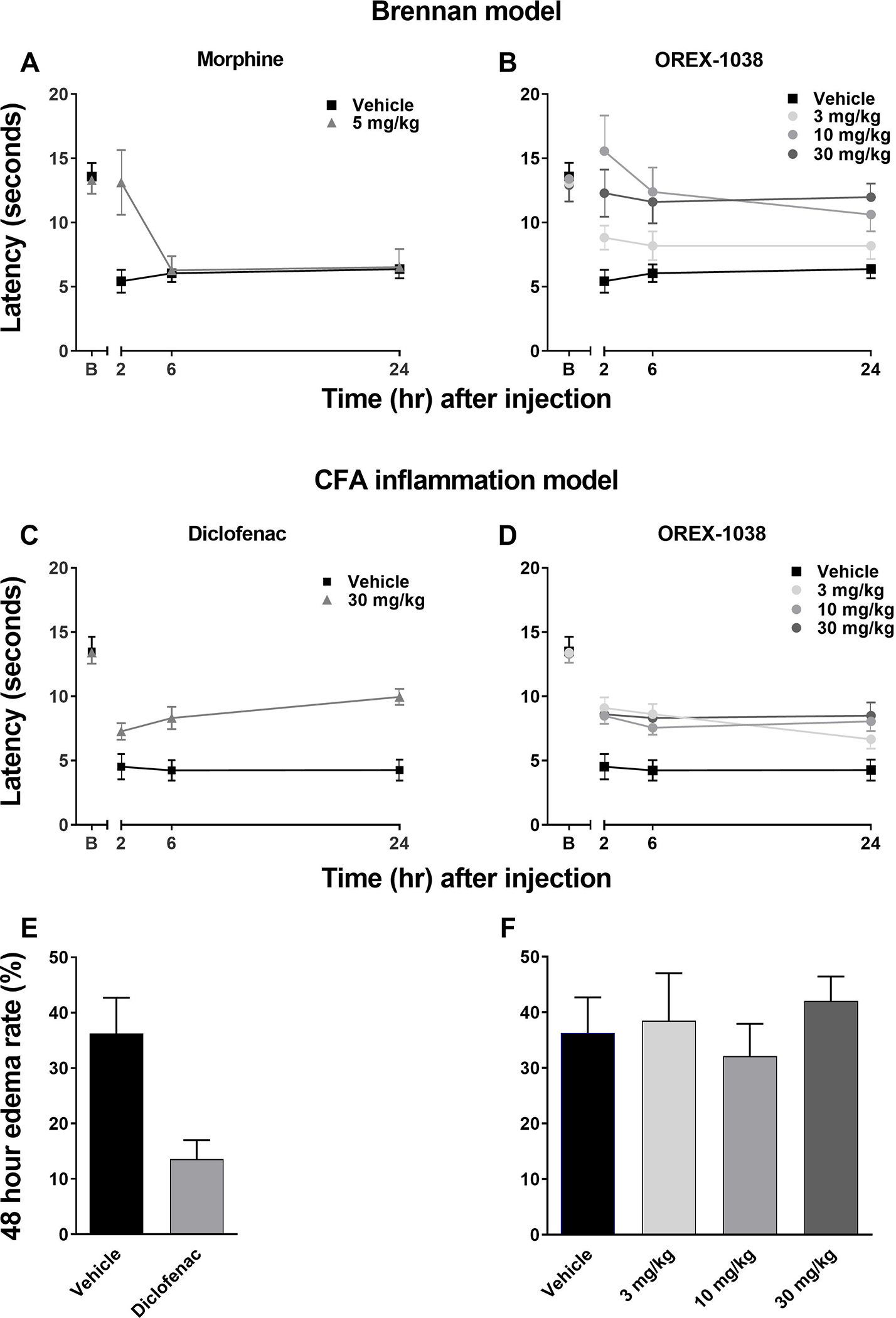
In rats, a dose of morphine (30 mg/kg) that was 6-fold larger than the dose that produced anti-hyperalgesic effects in the incisional model significantly reduced gastrointestinal transit distance, decreasing the relative distance traveled from 0.62 ± 0.02 after vehicle administration to 0.22 ± 0.02 after morphine administration (Figure 4A). In contrast, OREX-1038 did not substantially alter gastrointestinal transit; relative distance traveled was 0.53 ± 0.06 following administration of a dose that was 10-fold larger than the smallest effective dose of OREX-1038 in the incisional model of hyperalgesia (Figure 4A).
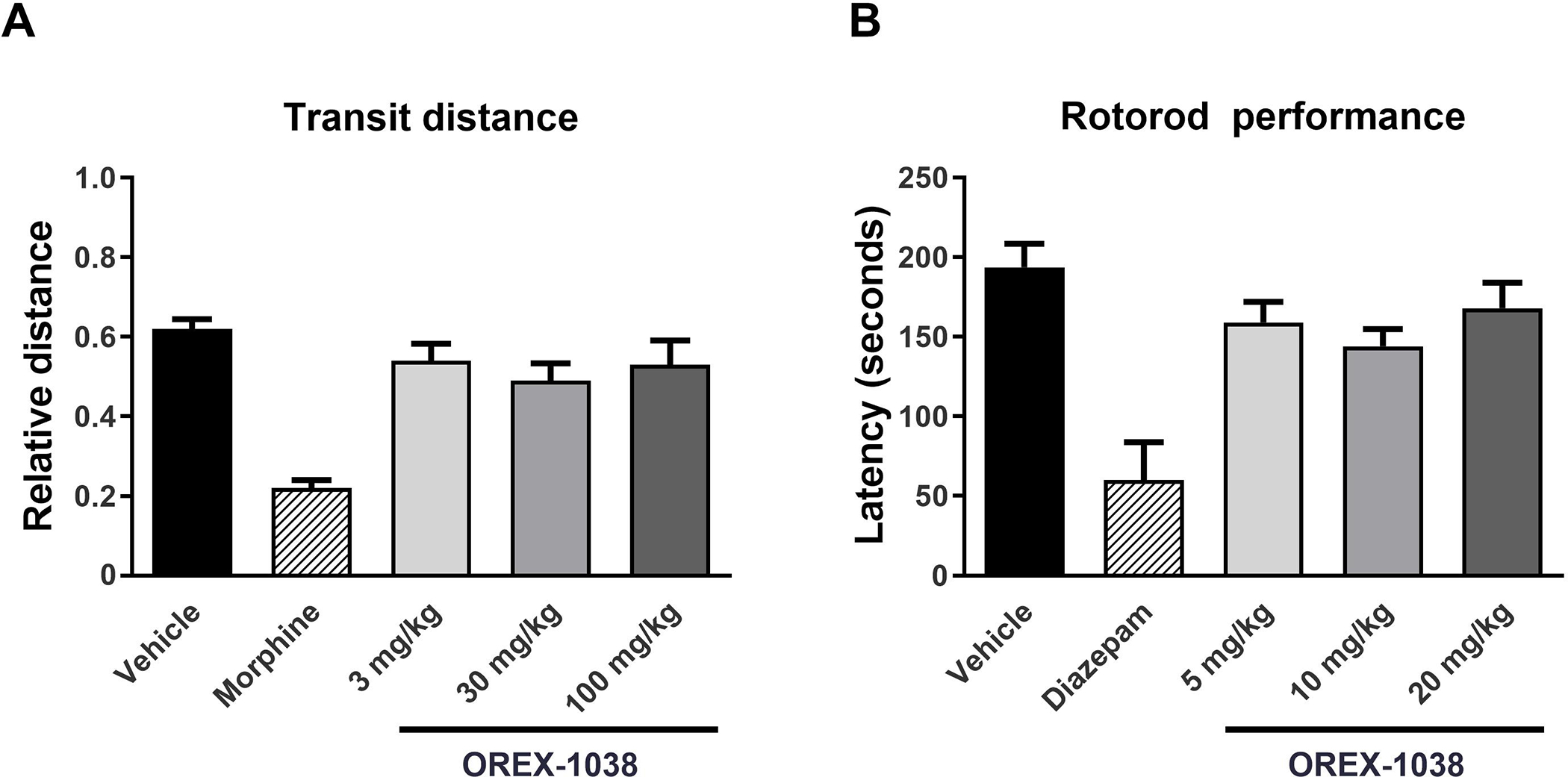
Gastrointestinal transit of a charcoal meal in male rats (Panel A). The distance that a charcoal meal travelled along the intestine from the pyloric sphincter was measured, as was the total intestinal length after vehicle, 30 mg/kg morphine, or 3, 30 and 100 mg/kg OREX-1038 (10 rats per treatment group). Motor coordination as measured (time spent on rotarod in seconds) using in the rotarod test (Panel B). OREX-1038 was administered at doses of 5, 10 and 20 mg/kg (s.c.) 120 minutes before the test. The benzodiazepine diazepam was included as reference compound, at the dose of 10 mg/kg (i.p.), 30 minutes before initiation of the test (10 rats per treatment group).
Rotarod.
Diazepam robustly decreased time spent on the rotarod, reducing the time from 193.6 ± 14.9 seconds after vehicle administration to 60.1 ± 23.7 seconds after diazepam administration (Figure 4B). In contrast, OREX-1038 did not substantially alter rotarod performance with rats remaining on the apparatus for an average of 168.1 ± 15.9 seconds following administration of 20 mg/kg OREX-1038 (Figure 4B).
In vivo characterization of OREX-1038 in rhesus monkeys
Warm water tail withdrawal.
Under control (no drug) conditions, tail withdrawal latencies, averaged across four monkeys, were 20 (maximum possible) and 3.7 ± 1.1 seconds from 40 and 50°C water, respectively. After administration of saline, latency for monkeys to remove their tails from 50°C water did not increase during the initial 3-hour session or when monkeys were tested again 6 hours after saline (triangles, Figure 5A). In contrast, morphine (1.8 mg/kg) increased tail withdrawal latency with an increase to at least 80% of the maximum possible effect within 1 hour of injection (circles, Figure 5A). This antinociceptive effect of morphine lasted up to 6 hours and was no longer evident 24 hours after injection. A dose of 0.03 mg/kg OREX-1038 produced an intermediate but sustained effect; latencies were between 30 and 40% of the maximum possible effect for 48 hours after injection and the effect was no longer evident 72 hours after injection (squares, Figure 5A). A dose-effect curve for OREX-1038 plotting the area under the time-effect curve obtained between 0.5 and 3 hours after drug administration is shown in Figure 5B. OREX-1038 significantly increased tail withdrawal latency across a wide range of doses spanning 0.001 to 0.3 mg/kg; however, the maximum effect produced by OREX-1038 was less than 50% of the maximum effect of morphine.
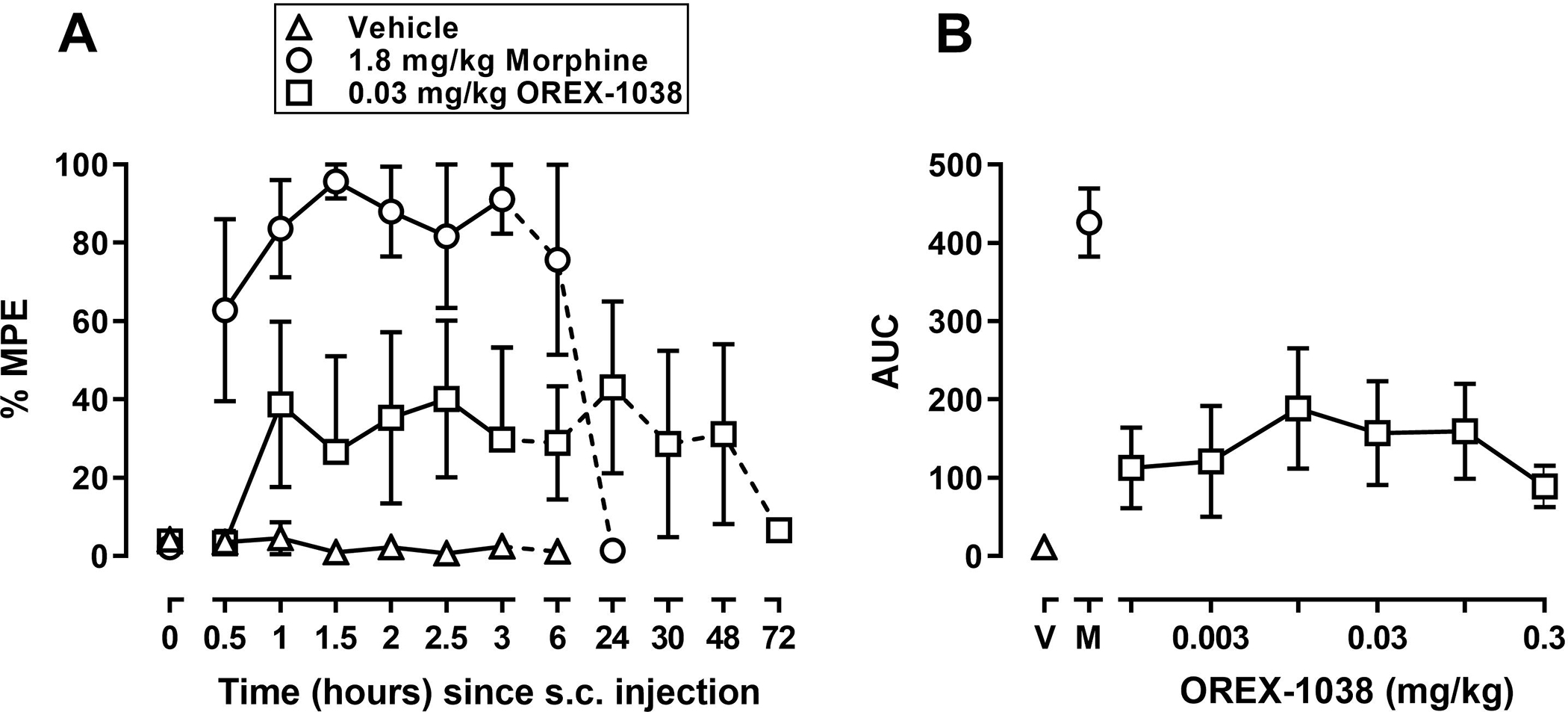
Antinociceptive effects of OREX-1038 in four monkeys. Tail-withdrawal latencies, expressed as a percentage of the maximum possible effect, are shown in the left panel at various times after administration of saline (triangles), 1.8 mg/kg morphine (circles), and 0.03 mg/kg OREX-1038 (squares). The effects of multiple doses of OREX-1038 were examined over time; area under the curve (AUC) was determined from latencies obtained between 0.5 and 3 hours after drug administration and are plotted as a function of dose in the right panels. Left ordinate: latency to remove tails from water maintained at 50°C, expressed as a percentage of the maximum possible latency, which was 20 seconds. Left abscissa: time since injection (hours). Right ordinate: AUC. Right abscissa: dose of OREX-1038 (mg/kg); the point above V indicates the AUC after vehicle and the point above M indicates the AUC after 1.8 mg/kg morphine.
Drug self-administration.
The number of infusions obtained, averaged across the last 3 sessions of each condition when responding was considered stable, is plotted for each condition in Figure 6. When remifentanil was available in saline or β-cyclodextrin vehicle at the beginning of the experiment, monkeys obtained on average (± 1 SEM) of 27.8 ± 0.29 and 25.5 ± 1.1 infusions, respectively; at the end of the experiment, responding for remifentanil was not significantly different, with monkeys obtaining 27.6 ± 0.5 and 26.5 ± 1.1 infusions (symbols above “Rem”, Figure 6). When saline or β-cyclodextrin vehicle alone was substituted for drug, the number of infusions obtained decreased to 2.4 ± 0.8 and 1.9 ± 0.7 infusions, respectively, at the beginning of the experiment and to 1.5 ± 0.9 and 0.5 ± 0.3 infusions, respectively, at the end of the experiment (symbols above “V”, Figure 6). For buprenorphine, the mean number of infusions increased then decreased as a function of dose (circles, Figure 6), with the maximum number of infusions (12.4 ± 4.8), on average, occurring when 0.003 mg/kg/infusion was available for self-administration, which was less than half of the number of infusions maintained by remifentanil. According to a repeated-measures ANOVA, there was a significant effect of condition for the buprenorphine experiment [F(10,30)=8.04, p<.0001]; however, only remifentanil was significantly different from the first saline phase according to the Dunnett’s post-hoc test (filled symbols). For OREX-1038, the mean number of infusions obtained also tended to increase and then decrease as a function of dose (squares, Figure 6). However, across the range of doses tested, fewer infusions were obtained compared with remifentanil and buprenorphine. The maximum number of infusions (6.3 ± 4.2 at a dose of 0.01 mg/kg/infusion) was approximately ¼ of the maximum maintained by remifentanil and ½ of the maximum maintained by buprenorphine. According to a repeated-measures ANOVA, there was a significant effect of condition for the OREX-1038 experiment [F(9,27)=30.1, p<.0001]; however, only remifentanil was significantly different from vehicle according to the Dunnett’s post-hoc test.
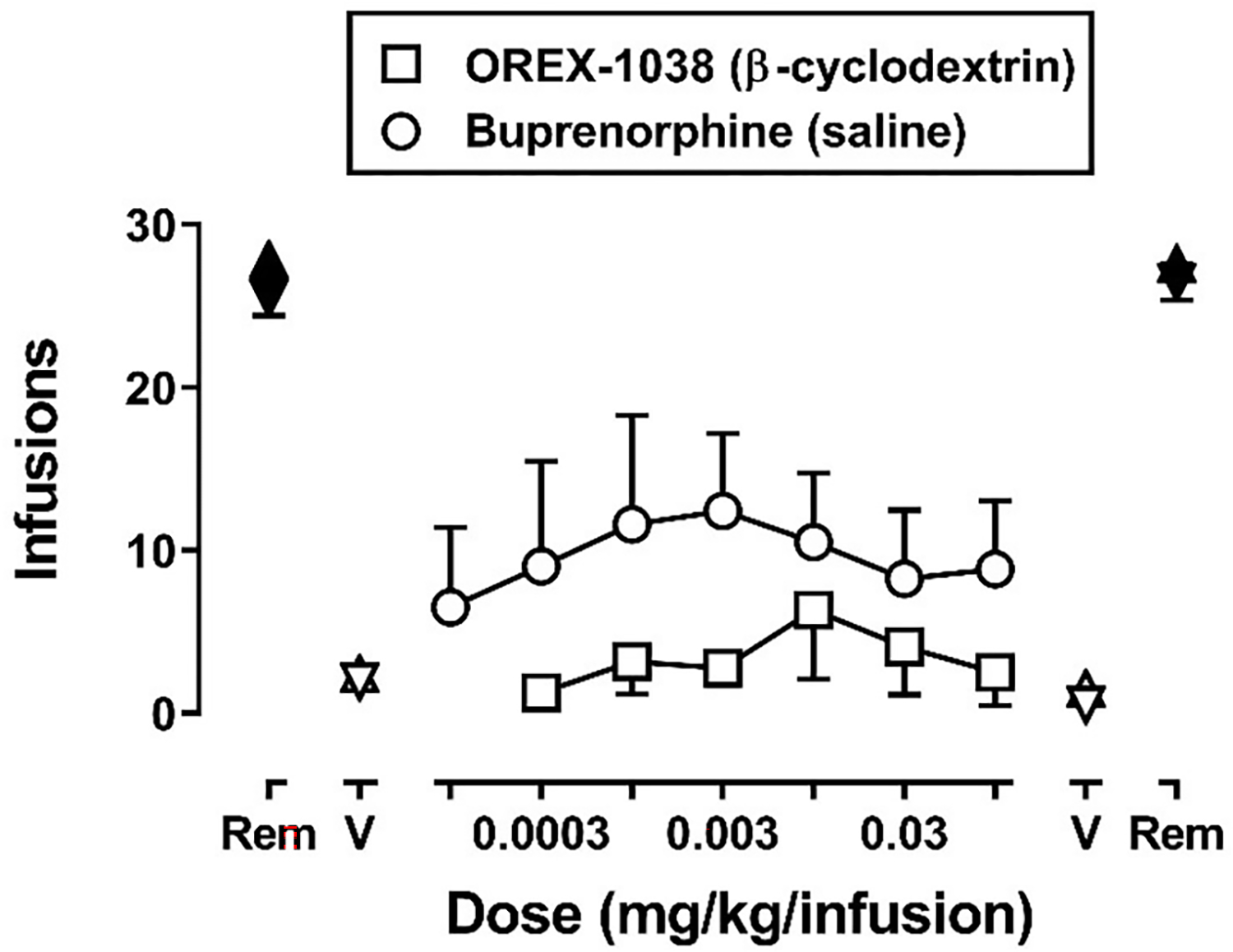
Number of infusions obtained of remifentanil (0.00032 mg/kg/infusion), vehicle, and varying unit doses of OREX-1038 or buprenorphine in monkeys (n=4 per experiment) responding under a fixed-ratio 30 schedule of i.v. self-administration during 90-minute sessions. Data points indicate the mean (± 1 SEM) of the number of infusions obtained during the last 3 sessions of each condition. Symbols above “Rem” and “V” indicate the number of infusions obtained during tests with remifentanil and vehicle, respectively, conducted at the beginning (left side) and end (right side) of the experiment. Squares and circles indicate the number of infusions obtained with increasing doses of OREX-1038 and buprenorphine, respectively. The drug vehicles used for each experiment are indicated in the parentheses; see “Drugs” for further details. Data for the buprenorphine and OREX-1038 experiments were analyzed separately using repeated-measures ANOVA. Filled symbols indicate data that are significantly different from the first vehicle condition of each experiment according to a Dunnett’s post-hoc test (p<.05).
Figure 7 shows the number of infusions obtained during the last vehicle session and the first three drug sessions for tests with each unit dose of OREX-1038 and buprenorphine as well as for the last tests with remifentanil in each experiment. For tests with OREX-1038 (Figure 7A), the number of vehicle infusions obtained during the last vehicle session of each test (diamonds) was low, with less than 3 infusions obtained per session. The number of infusions obtained with each unit dose of OREX-1038 and with remifentanil increased significantly during the first session when the drug was available for self-administration (filled squares and filled triangles). According to a two-way, repeated measures ANOVA, there were significant main effects of dose [F(6,54)=12.3, p<.0001] and day [F(3,54)=24.4, p=.0001] as well as a significant dose by day interaction [F(18,54)=6.82, p<.0001]. For OREX-1038 the number of infusions obtained decreased progressively across sessions, whereas the number of remifentanil infusions obtained remained stable across sessions. For all unit doses of OREX-1038, except 0.01 mg/kg/infusion, the number of infusions obtained did not differ from vehicle by the second or third session of the test.
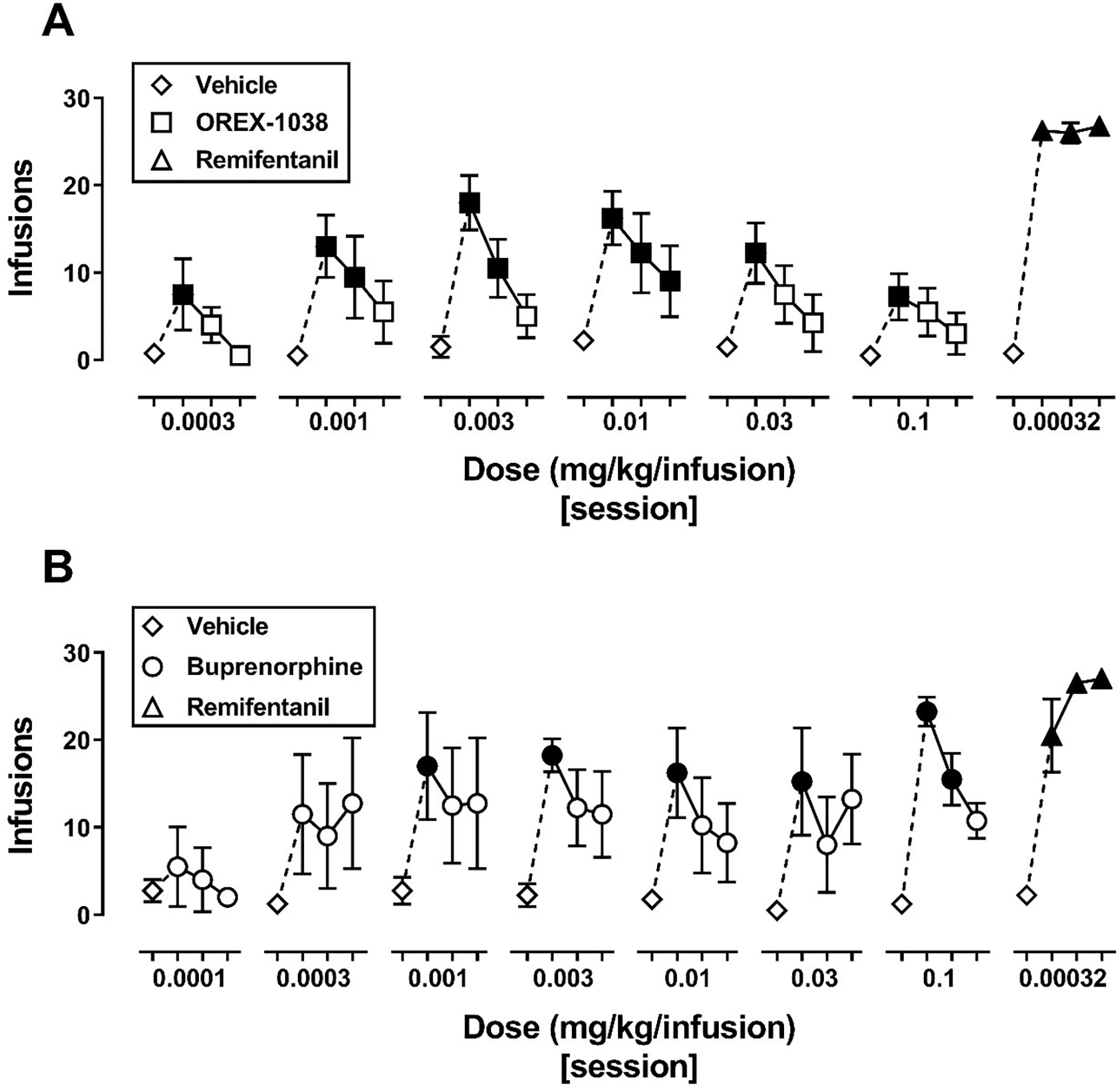
Number of infusions obtained across sessions for 4-session blocks during the OREX-1038 (panel A) and buprenorphine (panel B) self-administration experiments. Data points indicate the mean (± 1 SEM) number of infusions obtained (n=4 monkeys). In both panels, diamonds indicate data from the last session of vehicle preceding each test, and triangles indicate data from the final test with remifentanil. Squares (panel A) and circles (panel B) indicate the number of infusions obtained with increasing doses of OREX-1038 and buprenorphine, respectively. Breaks in the abscissa indicate a different condition. Data were analyzed using a two-way repeated-measures ANOVA with dose and day as within-subject factors. Filled symbols indicate data that are significantly different from the respective vehicle session for each condition according to a Dunnett’s post-hoc test (p<.05).
For tests with buprenorphine (Figure 7B), the number of vehicle infusions obtained during the last vehicle session of each test (diamonds) remained low. The number of infusions obtained with unit doses of buprenorphine of 0.001 mg/kg/infusion and larger and with remifentanil increased significantly during the first session when the drug was available for self-administration (filled circles). According to a two-way, repeated measures ANOVA, there were significant main effects of dose [F(7,63)=2.7, p=.04] and day [F(3,63)=20.1, p=.0003] but no dose by day interaction (p=.053). As with OREX-1038, the number of buprenorphine infusions decreased progressively across sessions whereas the number of remifentanil infusions remained significantly higher than vehicle.
Daily treatment with OREX-1038.
Tail-withdrawal latency did not change substantially during daily treatment with 0.03 mg/kg OREX-1038. Before treatment with OREX-1038 began and 3 hours after an injection of vehicle, monkeys removed their tails from 50°C water quickly with a latency of 2.7 ± 1.1 seconds (1.7% of the maximum possible effect; triangle above V, Figure 8). On the first day of treatment and 3 hours after administration of 0.03 mg/kg OREX-1038, tail-withdrawal latency increased to 61.7% of the maximum possible effect and the effect of that dose of OREX-1038 was only modestly decreased on day 22 of repeated treatment (47.1 ± 10.2% of the maximum possible effect; Figure 8). When treatment was discontinued, latencies returned to control values within 1 day of the last dose of OREX-1038. The effect of an acute injection of 0.03 mg/kg OREX-1038, administered 7 days after discontinuation of daily treatment, was similar to effects obtained during daily treatment with OREX-1038 (compare squares, Figure 8).
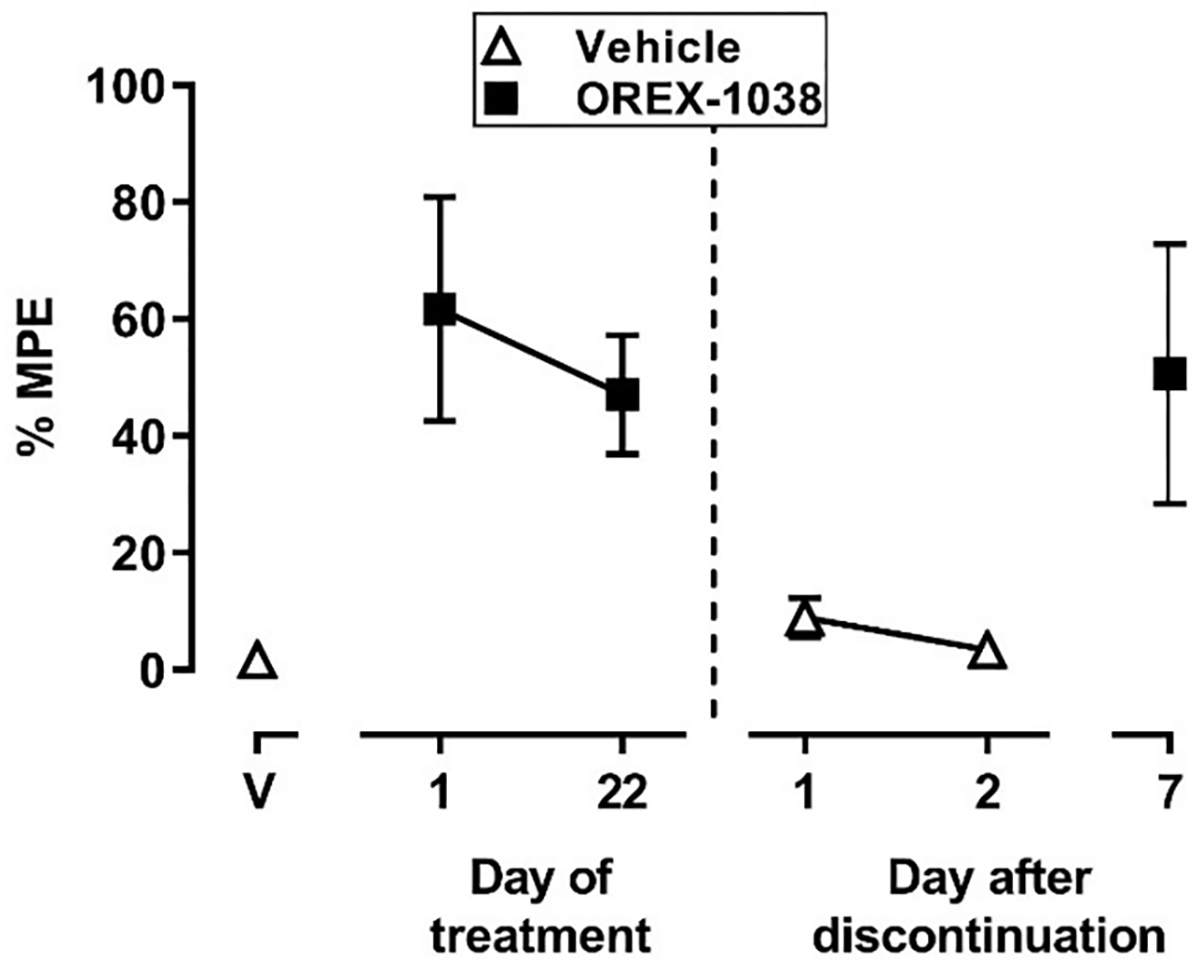
Antinociceptive effects before, during and after daily treatment with OREX-1038. The triangle above V shows the tail-withdrawal latency, expressed as a percentage of the maximum possible effect, that was obtained 3 hours after administration of vehicle on the day before daily treatment began. The squares above 1 and 22 days of treatment show latencies obtained 3 hours after administration of the 0.03 mg/kg OREX-1038 on the first and last day of daily treatment. Points after the vertical dashed line show data that were obtained on different days after discontinuation of treatment either 3 hours after administration of vehicle (triangles) or after a supplemental injection of 0.03 mg/kg OREX-1038 (square). Ordinate: latency to remove tails from water maintained at 50°C, expressed as a percentage of the maximum possible latency, which was 20 seconds. Abscissa: day of treatment or after discontinuation of treatment.
Changes in physiological and behavioral signs were monitored before, during and after repeated treatment with OREX-1038. Before treatment began, average values over a 30-minute period (0900–0930) were 116.9 ± 3.8 mmHg, 137.7 ± 13.1 beats/minute, and 37.4 ± 0.4 °C for mean arterial pressure, heart rate and body temperature, respectively. The cardiovascular parameters were decreased over the same 30-minute period during the first few days of treatment with 0.03 mg/kg OREX-1038, such that on the second day of treatment mean arterial pressure was decreased to 101.8 ± 4.3 mmHg and heart rate was decreased to 104 ± 11.0 beats/minute; these values remained decreased throughout the treatment period (Figures 9A and and9B).9B). In contrast, body temperature did not change initially, although it was decreased by the end of treatment (Figure 9C). Administration of 0.1 mg/kg naltrexone did not alter mean arterial pressure, heart rate, or body temperature. All three physiological parameters increased after discontinuation of treatment with the cardiovascular parameters similar to those obtained before treatment began within 3 days of the last dose of OREX-1038. Directly observable signs of opioid withdrawal, such as unusual tongue movement, tongue protrusion, twitch, vocalization, and wet-dog shakes, were not changed after OREX-1038 treatment or after administration of naltrexone during OREX-1038 treatment (data not shown).
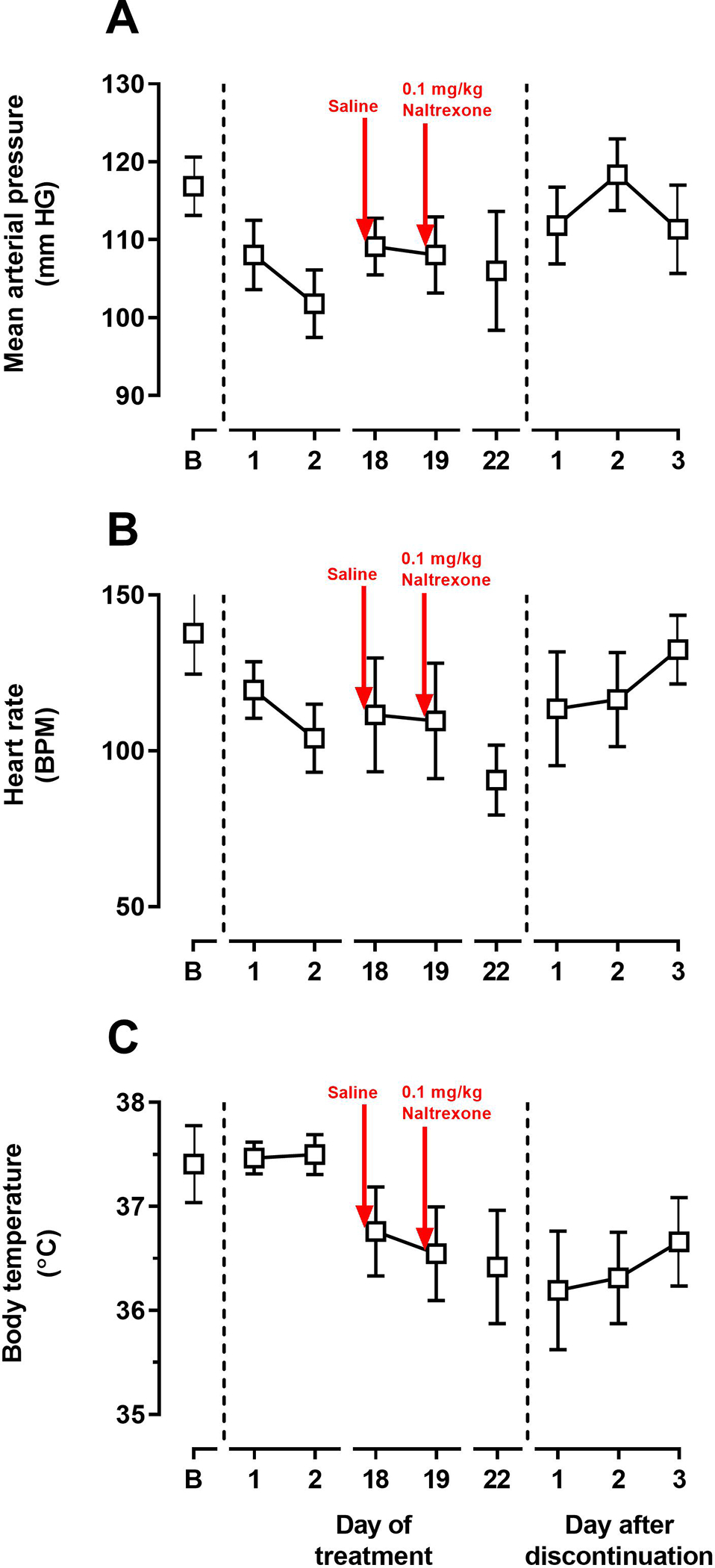
Mean arterial pressure (top panel), heart rate (middle panel) and body temperature (bottom panel) before, during and after daily treatment with OREX-1038. The vertical dashed lines indicate the beginning and end of daily treatment. Values were collected every minute and data shown in the figure were averaged across a 30-minute period. Before and after daily treatment, vehicle was administered at 0800 and during daily treatment with 0.03 mg/kg was administered at 0800. On days 18 and 19 of daily treatment (points immediately after the red arrows in the figure), a second injection of saline or 0.1 mg/kg naltrexone, respectively, was given and physiological data are shown for the 30-minute period from 1115–1145 hours; for all other days, physiological data are shown for the 30-period from 0900–0930 hours. Ordinates: upper panel, mean arterial pressure (mmHg); middle panel, heart rate (beats per minute); bottom panel, body temperature (°C). Abscissa: day of treatment or after discontinuation of treatment.
Discussion
The current opioid epidemic, which is partly due to chronic pain sufferers transitioning from therapeutic use of prescription opioids to misuse and abuse of prescription and other opioids, underscores the need to develop new, effective analgesics with fewer adverse effects. OREX-1038 was developed to be a partial agonist at mu opioid and nociceptin/orphanin FQ peptide receptors and a competitive antagonist at delta and kappa opioid receptors. A previous report showed it to have potent and long lasting antinociceptive and anti-allodynic effects (Kiguchi et al., 2019). In this earlier report, OREX-1038 (there called BU10038) was evaluated in the warm water tail withdrawal and capsaicin-induced thermal allodynia procedures in rhesus monkeys and had equivalent effectiveness but extended duration of action and greater potency compared with morphine, a prototypic mu opioid receptor agonist and commonly used analgesic. Importantly, OREX-1038 also displayed a significantly improved adverse effect profile (e.g., little or no self-administration, respiratory depression, itch, physical dependence, or tolerance) compared with morphine. The current study builds upon earlier work by evaluating effects of OREX-1038 under an extended range of antinociception procedures, along with additional testing for potential adverse effects (gastric motility, sedation, abuse liability, tolerance and withdrawal after daily dosing, and physiological signs) and preliminary adsorption, distribution, metabolism, excretion and toxicology (ADME-tox) assessments. These effects were compared to those of morphine to determine whether OREX-1038 might offer advantages over commonly used opioid analgesics.
Binding and functional studies confirm that OREX-1038, an oxymorphone, is a partial agonist at mu opioid and nociceptin/orphanin FQ peptide receptors and a competitive antagonist at delta and kappa opioid receptors with excellent selectivity for opioid receptors over other proteins. Its affinity at nociceptin/orphanin FQ peptide receptors is greater than that of buprenorphine, and this translates into greater potency at nociceptin/orphanin FQ peptide receptors in the [35S]GTPγS assay. OREX-1038 shares similar chemical (e.g. N-cyclopropylmethyl and C3-phenolic groups) and physicochemical properties with buprenorphine, including clogP (2.87 for OREX-1038, compared to 3.57 for buprenorphine) and molecular weight (473.6 g/mol for OREX-1038 compared to 467.6 g/mol for buprenorphine). Buprenorphine displays poor oral bioavailability, although it can be delivered successfully sublingually, which implies that delivery of OREX-1038 would also be best by the subcutaneous or sublingual routes. This is supported by the data showing improved bioavailability by these routes. That brain concentrations are 1.5- to 2.9-fold higher than plasma concentration following subcutaneous administration further supports the use of this route of administration. Assessment of potentially active metabolites of OREX-1038 is warranted, given that active metabolites of buprenorphine have been well documented.
Opioids, such as morphine, are commonly used to treat pain during post-surgical care. For this reason, effects of OREX-1038 were evaluated using the Brennan model of incisional pain (Brennan et al., 1996). Anti-hyperalgesic activity was evaluated in rats with pain induced by an incision into the plantar surface of the hind paw. OREX-1038 was as effective as morphine 2 hours after administration, and while the effects of morphine dissipated within 6 hours, OREX-1038 was still active after 24 hours. OREX-1038 was also effective in the CFA inflammation model, again displaying an extended duration of action of at least 24 hours. Activity in the CFA inflammation model was shown to be due to antinociceptive rather than anti-inflammatory effects, because unlike the anti-inflammatory drug diclofenac, OREX-1038 did not reduce edema produced by CFA injection. Because OREX-1038 had no effect in the rotarod test at doses as large as or larger than doses used in the hyperalgesia models, it is unlikely that sedation contributed to activity in these assays.
In the Brennan model of incisional pain, OREX-1038 reversed hyperalgesia such that latencies were similar to those obtained before surgery and equivalent to those of morphine. This finding is consistent with the earlier work (Kiguchi et al., 2019) where OREX-1038 was fully effective in models of acute noxious stimulus (50°C warm water tail withdrawal assay) and capsaicin-induced allodynia in rhesus monkeys. In contrast, in the current study, OREX-1038 produced approximately half the maximal response of morphine in the warm water tail withdrawal procedure. While maximal antinociceptive effects of OREX-1038 in the current study were less than those observed with morphine in the earlier study (Kiguchi et al., 2019), the duration of action was greatly extended for OREX-1038 with antinociceptive effects still apparent after 48 hours.
In addition to antinociceptive effects observed under a variety of conditions, OREX-1038 appears to have fewer adverse effects compared with other mu opioid receptor agonists. For example, repeated administration did not change the effectiveness of OREX-1038. Given the extended duration of action of OREX-1038 in the various antinociception assays, Kiguchi et al. (2019) employed a once every two days dosing strategy in rhesus monkeys to monitor for the development of tolerance. In the current study, a more frequent (once daily) dosing schedule was used in both rats and monkeys to assess the development of tolerance. Consistent with earlier studies, and despite more frequent (daily) administration of OREX-1038 in the current study, the effects of OREX-1038 did not diminish during 22 days of once-daily dosing in rhesus monkeys, suggesting that tolerance did not develop. However, the antinociceptive effects of OREX-1038 dissipated within 24 hours of the last dose following 22 days of treatment, whereas those effects were evident for longer than 24 hours when the same dose was administered acutely; this apparent decrease in duration of action with repeated administration might be an indicator of the development of mild tolerance and suggests that more substantial tolerance might occur when doses larger than those needed to produce antinociception are administered for longer periods.
Another adverse effect that has limited the clinical use of opioids is abuse liability, which was assessed in the current study using a self-administration procedure in rhesus monkeys. Over a range of doses, OREX-1038 transiently increased self-administration with responding for OREX-1038 diminishing rapidly over days to non-significant levels. The dose-response curve for OREX-1038 had a flattened bell shape. Compared with remifentanil, OREX-1038 and buprenorphine maintained, on average, fewer infusions obtained across a broad range of unit doses, perhaps suggesting limited reinforcing effects compared to higher efficacy opioids such as remifentanil. On the other hand, low levels of drug intake might be the result of the long duration of action of buprenorphine and OREX-1038. Determining whether differences in intake are due to pharmacodynamic (e.g., intrinsic efficacy) or pharmacokinetic (e.g., duration of action) properties of these drugs requires additional studies under a broader range of experimental conditions, for example, using longer timeouts after each infusion. Based upon these data, the abuse potential of OREX-1038 appears to be less than that of remifentanil and not different from that of buprenorphine.
OREX-1038 appears to produce significant antinociception with little evidence of sedation or tolerance development after chronic dosing, low abuse liability, and no effect on gastric motility. There were some effects of OREX-1038 on physiological signs (in contrast to the lack of effect reported by Kiguchi et al., 2019) but these were limited and rapidly reversed upon discontinuation of chronic treatment. Of note, neither cessation of OREX-1038 nor administration of naltrexone resulted in the emergence of withdrawal signs. A selective low efficacy agonist at nociceptin/orphanin FQ peptide receptors, SER100, has been evaluated in a small group of patients with isolated systolic hypertension and had a small, but significant, effect lowering systolic blood pressure (Kantola et al., 2017).
The current study further supports the strategy of developing of multifunctional ligands targeting more than one receptor (Gunther et al., 2017). For the treatment of pain, this approach typically involves activation of mu opioid receptors along with, for example, activation of kappa opioid receptors (PPL-101 and PPL-103; Khroyan et al., 2017) or blockade of delta opioid receptors (UMB 425; Healy et al., 2013). In addition, compounds from three separate chemical series have been shown to have very similar agonist activity at mu opioid and nociceptin/orphanin FQ peptide receptors, including BU08028 (Ding et al., 2016), AT-121 (Ding et al., 2018), and OREX-1038 (current study; Kiguchi et al., 2019). In monkeys, these compounds have significant antinociceptive effects and fewer adverse effects, as compared to opioids commonly used for pain. Taken together, results of these studies support the notion that dual acting mu opioid/nociceptin/orphanin FQ peptide receptor partial agonists might provide a unique pharmacological profile and substantiate the notion that opioids can be developed that have an improved therapeutic profile compared to opioids currently used for pain.
Supplementary Material
Supplemental Figures
Supplemental Tables
Acknowledgements:
The authors thank Eli Desarno, Steven Garza, Sarah Howard, and Jade Juarez for their expert technical assistance.
Conflicts of Interest and Sources of Funding:
Industrial funding; the majority of this work was funded by Orexigen Therapeutics Inc. LA, BB, and PF were employed by Orexigen Therapeutics while engaged in this research project. SMH is an inventor on the patent that describes OREX-1038/BU10038 and was a consultant to Orexigen Therapeutics during this project. Other sources of support include the National Institutes of Health [Grant R37DA039997 (JRT) and T32DA007268 (KMO; PI:JRT)] and the Welch Foundation [Grant AQ-0039 (CPF)].
References
- Becker GL, Gerak LR, Koek W, France CP (2008). Antagonist-precipitated and discontinuation-induced withdrawal in morphine-dependent rhesus monkeys. Psychopharmacology 201:373–382. [Europe PMC free article] [Abstract] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF (1996). Characterization of a rat model of incisional pain. Pain 64:493–501. [Abstract] [Google Scholar]
- Chang DS, Raghavan R, Christiansen S, Cohen SP (2015). Emerging targets in treating pain. Curr Opin Anaesthesiol 28:379–397. [Abstract] [Google Scholar]
- Collins FS, Koroshetz WJ, Volkow ND (2018). Helping to end addiction over the long-term. JAMA 320:129–130. [Europe PMC free article] [Abstract] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT (2016). Nonmedical prescription-opioid use and heroin use. N Engl J Med 374:1269. [Abstract] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, et al. (2016). A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci USA 113:E5511–5518. [Europe PMC free article] [Abstract] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, et al. (2018). A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10(456):eaar3483. 10.1126/scitranslmed.aar3483. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gerak LR, France CP (2016). Combined treatment with morphine and Δ9-tetrahydrocannabinol in rhesus monkeys: antinociceptive tolerance and withdrawal. J Pharmacol Exp Ther 357:357–366. [Europe PMC free article] [Abstract] [Google Scholar]
- Gerak LR, Zanettini C, Koek W, France CP (2015). Cross-tolerance to cannabinoids in morphine-tolerant rhesus monkeys. Psychopharmacology 232:3637–3647. [Abstract] [Google Scholar]
- Gerak LR, Collins GT, Maguire DR, France CP (2019). Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp Clin Psychopharmacology 27:78–86. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldberg DS, McGee SJ (2011). Pain as a global public health priority. BMC Public Health 11:770–775. [Europe PMC free article] [Abstract] [Google Scholar]
- Grosser T, Woolf CJ, FitzGerald GA (2017). Time for nonaddictive relief of pain. Science 355:1026–1027. [Abstract] [Google Scholar]
- Günther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, et al. (2017). Targeting multiple opioid receptors—improved analgesics with reduced side effects? Br J Pharmacol 175:2857–2868. [Europe PMC free article] [Abstract] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [Abstract] [Google Scholar]
- Healy JR, Bezawada P, Shim J, Jones JW, Kane MA, MacKerell AD Jr, et al. (2013). Synthesis, modeling, and pharmacological evaluation of UMB 425, a mixed mu agonist/delta antagonist opioid analgesic with reduced tolerance liabilities. ACS Chem Neurosci 4:1256–1266. [Europe PMC free article] [Abstract] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, et al. (2018). The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661. [Europe PMC free article] [Abstract] [Google Scholar]
- Hillhouse TM, Olson K, Hallahan JE, Rysztak LG, Sears BF, Meurice C, et al. (2021). The buprenorphine analogue BU10119 attenuates drug-primed and stress-induced cocaine reinstatement in mice. J Pharmacol Exp Ther 378:287–299. [Europe PMC free article] [Abstract] [Google Scholar]
- Kantola I, Scheinin M, Gulbrandsen T, Meland N, Smerud KT (2017). Safety, tolerability, and antihypertensive effect of SER100, an opiate receptor-like 1 (ORL-1) partial agonist in patients with isolated systolic hypertension. Clin Pharmacol Drug Dev 6:584–591. [Abstract] [Google Scholar]
- Khroyan TV, Cippitelli A, Toll N, Lawson JA, Crossman W, Polgar WE, Toll L (2017). In vitro and in vivo profile of PPL-101 and PPL-103: mixed opioid partial agonist analgesics with low abuse potential. Front Psychiatry 8:article 52. [Europe PMC free article] [Abstract] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, et al. (2019). BU10038 as a safe opioid analgesic with few side-effects after systemic and intrathecal administration in primates. Br J Anaesth 122:e146–e156. [Europe PMC free article] [Abstract] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. (2016). Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [Europe PMC free article] [Abstract] [Google Scholar]
- Maguire DR, Gerak LR, Cami-Kobeci G, Husbands SM, France CP, Belli B, Flynn P (2020). OREX-1019: a novel treatment of opioid use disorder and relapse prevention. J Pharmacol Exp Ther 372:205–215. [Europe PMC free article] [Abstract] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH (1981). Buprenorphine self-administration by rhesus monkey. Pharmacology Biochemistry and Behavior 15:215–225. [Abstract] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, et al. (2017). Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171:1165–1175. [Europe PMC free article] [Abstract] [Google Scholar]
- Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, et al. (2017). A randomized, Phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 10:2413–2424. [Europe PMC free article] [Abstract] [Google Scholar]
- Skolnick P, Volkow ND (2016). Re-energizing the development of pain therapeutics in light of the opioid epidemic. Neuron 92:294–297. [Abstract] [Google Scholar]
- Smith JS, Lefkowitz RJ, Rajagopal S (2018). Biased signaling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 17:243–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. (2014). Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155:1829–1835. [Abstract] [Google Scholar]
- Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, et al. (2017). A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355:966–969. [Abstract] [Google Scholar]
- Takayuma K, Hirose A, Suda I, Miyazaki A, Oguchi M, Onotogi M, Fotopoulos G (2011). Comparison of the anti-inflammatory and analgesic effects in rats of diclofenac-sodium, felbinac and indomethacin patches. Int J Biomed Sci 7:222–229. [Europe PMC free article] [Abstract] [Google Scholar]
- Traynor JR, Nahorski SR (1995). Modulation by the mu-opioid agonists of guanosine-5’-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47:848–854. [Abstract] [Google Scholar]
- Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, et al. (2016). A randomized, Phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 157:264–272. [Abstract] [Google Scholar]
- Walker G (2018). The opioid crisis: a 21st century pain. Drugs Today 54:283–286. [Abstract] [Google Scholar]
- Yekkirala AS, Kalyuzhny AE, Portoghese PS (2013). An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol 8:1412–1416. [Europe PMC free article] [Abstract] [Google Scholar]
- Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS (2011). N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc Natl Acad Sci USA 108:5098–5103. [Europe PMC free article] [Abstract] [Google Scholar]
- Yekkirala AS, Roberson DP, Bean BP, Woolf CJ (2017). Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16:810. [Europe PMC free article] [Abstract] [Google Scholar]
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/134100376
Article citations
Nociceptin Receptor-Related Agonists as Safe and Non-addictive Analgesics.
Drugs, 83(9):771-793, 20 May 2023
Cited by: 2 articles | PMID: 37209211
Review
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates.
Br J Anaesth, 122(6):e146-e156, 01 Mar 2019
Cited by: 29 articles | PMID: 30916003 | PMCID: PMC6676776
OREX-1019: A Novel Treatment of Opioid Use Disorder and Relapse Prevention.
J Pharmacol Exp Ther, 372(2):205-215, 20 Nov 2019
Cited by: 6 articles | PMID: 31748404 | PMCID: PMC6978692
Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists.
J Pharmacol Exp Ther, 331(3):946-953, 27 Aug 2009
Cited by: 51 articles | PMID: 19713488 | PMCID: PMC2784721
The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability.
ACS Chem Neurosci, 4(2):214-224, 06 Nov 2012
Cited by: 48 articles | PMID: 23421672 | PMCID: PMC3582300
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDA NIH HHS (2)
Grant ID: R37 DA039997
Grant ID: T32 DA007268