Abstract
Free full text
Sex-related differences in violence exposure, neural reactivity to threat, and mental health
Abstract
The prefrontal cortex (PFC), hippocampus, and amygdala play an important role in emotional health. However, adverse life events (e.g., violence exposure) affect the function of these brain regions, which may lead to disorders such as depression and anxiety. Depression and anxiety disproportionately affect women compared to men, and this disparity may reflect sex differences in the neural processes that underlie emotion expression and regulation. The present study investigated sex differences in the relationship between violence exposure and the neural processes that underlie emotion regulation. In the present study, 200 participants completed a Pavlovian fear conditioning procedure in which cued and non-cued threats (i.e., unconditioned stimuli) were presented during functional magnetic resonance imaging. Violence exposure was previously assessed at four separate time points when participants were 11–19 years of age. Significant threat type (cued versus non-cued) ×
× sex and sex
sex and sex ×
× violence exposure interactions were observed. Specifically, women and men differed in amygdala and parahippocampal gyrus reactivity to cued versus non-cued threat. Further, dorsolateral PFC (dlPFC) and inferior parietal lobule (IPL) reactivity to threat varied positively with violence exposure among women, but not men. Similarly, threat-elicited skin conductance responses varied positively with violence exposure among women. Finally, women reported greater depression and anxiety symptoms than men. These findings suggest that sex differences in threat-related brain and psychophysiological activity may have implications for mental health.
violence exposure interactions were observed. Specifically, women and men differed in amygdala and parahippocampal gyrus reactivity to cued versus non-cued threat. Further, dorsolateral PFC (dlPFC) and inferior parietal lobule (IPL) reactivity to threat varied positively with violence exposure among women, but not men. Similarly, threat-elicited skin conductance responses varied positively with violence exposure among women. Finally, women reported greater depression and anxiety symptoms than men. These findings suggest that sex differences in threat-related brain and psychophysiological activity may have implications for mental health.
Introduction
Healthy emotional function depends upon emotion expression and regulation processes mediated by the prefrontal cortex (PFC), hippocampus, and amygdala. Disruption of these emotion processes leads to the development of emotion-related disorders (e.g., anxiety, depression) [1–3]. Women have higher prevalence of emotion-related disorders [4] and differ from men in emotional processes that are supported by the PFC, hippocampus, and amygdala [5]. The PFC, hippocampus, and amygdala support emotion learning, expression, and regulation processes that are important for healthy emotional function [6–8]. However, negative life events (e.g., violence exposure) may lead to changes among these brain regions resulting in emotional dysfunction [9, 10]. Therefore, determining how neural function varies with negative life events is important for understanding the relationship between these events and emotional function.
Women in the United States are almost twice as likely as men to meet diagnostic criteria for a mood or anxiety disorder during their lifetime [11, 12]. Sex-related differences in emotion-function may underlie this disparity in the rates of mood and anxiety disorders [11–16]. Further, prior work has demonstrated that there are sex-related differences in the brain function that underlies emotional expression and regulation [14, 17–19]. Taken together, this prior work suggests that differences in brain function may underlie sex differences in emotion regulation and the subsequent development of depression and anxiety. Therefore, understanding the mechanisms that underlie sex differences in emotional function may offer novel insight into sex disparities among these disorders.
Sex differences in emotional function may be mediated by sex differences in brain function [20]. For example, prior work has found greater PFC and amygdala activity in women than men during negatively-valenced emotion tasks, including the evaluation of threats [19, 21, 22]. Additionally, neural activation elicited by sad facial expressions was lateralized to the left hemisphere in men, but right hemisphere in women, suggesting laterality differences between women and men [23]. Further, women show greater PFC activation and less amygdala deactivation than men during the cognitive reappraisal of emotion [18]. Additionally, prior work has demonstrated greater amygdala and orbitofrontal activity in women, and greater PFC and superior parietal cortex activity in men during the cognitive control of emotion [24]. Although sex differences in neural activity vary across studies, women and men often show differences in the brain regions recruited during emotion-related tasks [25–27]. Thus, sex-related differences in mood and anxiety symptoms may reflect sex differences in emotion regulation and expression [18, 19]. Finally, these differences may underlie the higher rates of anxiety and depression observed among women in prior studies [11, 28, 29].
Violence exposure can affect emotional function [30, 31]. Moreover, several studies have demonstrated that neural activity during emotion-related tasks varies with violence exposure [32–35]. Specifically, violence exposure during childhood and adolescence is associated with alterations in the PFC, hippocampus, and amygdala response to emotion-related stimuli during childhood and adolescence [33–35]. Given that healthy emotional function relies upon the PFC, hippocampus, and amygdala, violence exposure may disrupt the function of these brain regions [9]. For example, those that experience violence are more likely to develop depression and anxiety than those who have not been exposed to violence [36]. The effects of violence exposure may be especially prominent during adolescence as the brain regions underlying emotion regulation continue to develop [37]. Further, sex differences in emotional processes may contribute to differences in the way men and women respond to violence. For example, women who have experienced an assault are twice as likely to develop depression, anxiety, and posttraumatic stress disorder than men [38, 39], and experience more severe post-assault symptoms [40]. Taken together, these prior findings suggest there are sex differences in the effects of violence on emotion regulation processes.
Pavlovian fear conditioning is commonly used to investigate the expression and regulation of emotion. Pavlovian fear conditioning is a procedure in which a neutral stimulus is repeatedly paired with an aversive threat (unconditioned stimulus (UCS)). As the relationship between the neutral stimulus and the threat is learned, the neutral stimulus becomes a warning signal (conditioned stimulus (CS)) and anticipatory responses (conditioned responses (CR)) elicited by the warning signal are expressed. Typically, the expression of an anticipatory CR to the warning signal is taken as evidence that learning has occurred. In contrast, the emotional response to the threat (UCS) is typically considered an automatic, reflexive response that does not require learning. However, learning can also be measured by changes in the threat-elicited response (unconditioned response (UCR)). Specifically, behavioral and neural responses to predictable threats are typically smaller than responses to unpredictable threats [32, 41–46]. The reduced response amplitude to predictable compared to unpredictable threat is called conditioned UCR diminution, which is mediated by a PFC-hippocampus-amygdala neural circuit [41–45, 47]. Investigating the relationship between conditioned UCR diminution and violence exposure may further elucidate the relationship between violence and changes in brain regions that support threat-related emotional processes.
The present study investigated sex differences in the relationship between violence exposure and threat-related neural activity. Functional magnetic resonance imaging (fMRI) was used to study brain activity to cued versus non-cued threat in participants whose violence exposure was previously assessed. We hypothesized that (1) non-cued threats would elicit greater skin conductance responses (SCR) and fMRI signal reactivity within the amygdala, hippocampus, PFC, insula, and inferior parietal lobule (IPL) in accordance with prior work [41–45], and that (2) greater violence exposure would be associated with greater differential (i.e., cued versus non-cued threat) activity within these brain regions, and that these effects would be more pronounced in women than men. The present study extends our understanding of the role of sex differences and violence exposure in threat-related emotional function.
Materials and methods
Participants
Two hundred twenty three right-handed volunteers were recruited from the Birmingham, Alabama cohort of the Healthy Passages Study (details on this cohort are provided in the Supplement and Supplementary Tables 1 and 3). Twenty three participants were excluded from the current analyses due to excessive motion or missing data. Therefore, 200 adults (Mean age ±
± SD
SD =
= 20.78
20.78 ±
± 1.37, range
1.37, range =
= 18–23 years, 102 women, 98 men) were included in the present study. Exclusion criteria for this study included standard magnetic resonance imaging (MRI) contraindications (e.g., metallic devices, metallic foreign body, pacemaker), prior head injury, blood/circulation disorders, pregnancy, spinal cord abnormalities, history of seizures, history of psychotic symptoms, and left-handedness. Participants were not excluded for use of prescription medication. All participants provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
18–23 years, 102 women, 98 men) were included in the present study. Exclusion criteria for this study included standard magnetic resonance imaging (MRI) contraindications (e.g., metallic devices, metallic foreign body, pacemaker), prior head injury, blood/circulation disorders, pregnancy, spinal cord abnormalities, history of seizures, history of psychotic symptoms, and left-handedness. Participants were not excluded for use of prescription medication. All participants provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Stimuli
Participants completed a Pavlovian fear conditioning task in which two pure tones (700 and 1300 Hz; 10
Hz; 10 s duration) served as warning (CS+) and safety (CS−) signals. The warning signal (CS+) coterminated with a loud (100-dB) white noise (0.5
s duration) served as warning (CS+) and safety (CS−) signals. The warning signal (CS+) coterminated with a loud (100-dB) white noise (0.5 s duration, 100% pairing rate) that served as the threat (i.e., UCS), while the safety signal (CS−) was presented alone. The threat (UCS) was also presented alone (UCS alone) on some trials. Each stimulus was presented 12 times (18
s duration, 100% pairing rate) that served as the threat (i.e., UCS), while the safety signal (CS−) was presented alone. The threat (UCS) was also presented alone (UCS alone) on some trials. Each stimulus was presented 12 times (18 s inter-trial interval) during each of two fMRI scans (total of 24 CS+, 24 CS−, 24 UCS alone). The tones that served as warning and safety signals were counterbalanced across participants and all stimuli were presented in a pseudo-random order such that no stimulus was presented more than twice consecutively. Analyses assessed differential threat-elicited responses (i.e., UCRs) to the cued threat (i.e., UCS) that coterminated with the CS+ (i.e., CS
s inter-trial interval) during each of two fMRI scans (total of 24 CS+, 24 CS−, 24 UCS alone). The tones that served as warning and safety signals were counterbalanced across participants and all stimuli were presented in a pseudo-random order such that no stimulus was presented more than twice consecutively. Analyses assessed differential threat-elicited responses (i.e., UCRs) to the cued threat (i.e., UCS) that coterminated with the CS+ (i.e., CS +
+ UCS) versus the non-cued threat (i.e., UCS alone). The present study focused on differences in the response to cued (CS
UCS) versus the non-cued threat (i.e., UCS alone). The present study focused on differences in the response to cued (CS +
+ UCS) versus non-cued (UCS alone) threat.
UCS) versus non-cued (UCS alone) threat.
SCR
SCR data were sampled (10 kHz) with two radio-translucent electrodes attached to the thenar and hypothenar eminences of the non-dominant hand using a Biopac MP150 MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) using methods described in prior work [48]. Additional details of SCR analyses are described in the Supplement.
kHz) with two radio-translucent electrodes attached to the thenar and hypothenar eminences of the non-dominant hand using a Biopac MP150 MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) using methods described in prior work [48]. Additional details of SCR analyses are described in the Supplement.
Threat expectancy
Threat expectancy was measured continuously (40 Hz sampling rate) throughout the conditioning procedure using methods described previously [48]. Participants rated their expectancy of threat on a 0 to 100 scale (0
Hz sampling rate) throughout the conditioning procedure using methods described previously [48]. Participants rated their expectancy of threat on a 0 to 100 scale (0 =
= certain the threat would not be presented; 50
certain the threat would not be presented; 50 =
= uncertain the threat would be presented; 100
uncertain the threat would be presented; 100 =
= certain the threat would be presented). Additional details of the threat expectancy measure are provided in the Supplement.
certain the threat would be presented). Additional details of the threat expectancy measure are provided in the Supplement.
Violence exposure
Violence exposure was assessed for each participant at four time points, ages 11, 13, 16, and 19 (see Supplement for further information) using the Healthy Passages violence exposure measure [49–51]. At each time point, participants reported their exposure to violent incidents in the previous 12 months. Each participant reported how frequently they had been a victim of a threat of violence, physical violence, violence with injury requiring medical treatment, or violence involving a gun or knife. A four-point frequency scale ranging from 0 (Never) to 3 (Many times) was used to score responses. Ratings were averaged within each time point, and then times 1–4 were averaged to reflect cumulative violence exposure.
Depression symptoms
Depression symptoms were assessed at Time 4 using the Diagnostic Interview Schedule for Children Predictive Scales (Major Depression Subscale) [52]. Participants answered 6 dichotomous questions regarding depression symptoms experienced in the past 12 months. Responses were coded 0 (No) or 1 (Yes) and summed to create an index of depression symptoms. The scale had good internal consistency (Cronbach’s α =
= 0.72).
0.72).
Anxiety symptoms
Anxiety symptoms were assessed at Time 4 using the Revised Children’s Manifest Anxiety Scale (Physiological Anxiety subscale) [53]. Participants answered 10 dichotomous questions regarding anxiety symptoms experienced in the past 12 months. Responses were coded 0 (No) or 1 (Yes) and summed to create an index of anxiety symptoms. The scale had good internal consistency (Cronbach’s α =
= 0.65).
0.65).
Posttraumatic stress disorder (PTSD) symptoms
PTSD symptoms were assessed at Time 4 using the Child PTSD Symptom Scale [54]. Participants answered 17 questions regarding PTSD symptoms experienced within the past 2 weeks. Responses ranged from 0 (not at all or only 1 time) to 3 (five or more times per week/almost always). The scale had good internal consistency (Cronbach’s α =
= 0.94). Five participants did not complete the questionnaire; therefore, all PTSD analyses include 195 participants.
0.94). Five participants did not complete the questionnaire; therefore, all PTSD analyses include 195 participants.
Functional imaging
FMRI was completed using 3T Siemens Allegra and Prisma scanners. Acquisition parameters and preprocessing methods are described in the Supplement. All analyses were performed using the Analysis of Functional NeuroImages (AFNI) [version: AFNI_18.2.05] software package [55]. A multiple regression analysis was completed to create functional activation maps for each participant using a gamma-variate hemodynamic response function with reference waveforms to model each stimulus (i.e., warning cue, CS+; safety cue, CS−; cued threat, CS +
+ UCS; non-cued threat, UCS alone). Separate regressors were used to account for head motion in six directions and motor activity associated with movement of the joystick. Volumes with excessively high motion (≥3% of voxels greater than five times the median absolute deviation of the timeseries) were censored from the first-level analysis. Percent signal change was used as a measure of the BOLD response amplitude to each stimulus type. The data were then converted to the Talairach and Tournoux stereotaxic coordinate system [56] and resampled to a 1
UCS; non-cued threat, UCS alone). Separate regressors were used to account for head motion in six directions and motor activity associated with movement of the joystick. Volumes with excessively high motion (≥3% of voxels greater than five times the median absolute deviation of the timeseries) were censored from the first-level analysis. Percent signal change was used as a measure of the BOLD response amplitude to each stimulus type. The data were then converted to the Talairach and Tournoux stereotaxic coordinate system [56] and resampled to a 1 mm3 resolution.
mm3 resolution.
Statistical analyses
Statistical analyses were completed using IBM SPSS Statistics Version 25 and AFNI [55]. Independent samples t-tests assessed sex differences in violence exposure. Linear mixed effects (LME) models were completed to test the effects of cued versus non-cued threat, sex, violence exposure, and interactions between these terms on SCR, threat expectancy, and fMRI signal responses. A nominal significance threshold was set at p <
< 0.05 for SCR and threat expectancy analyses. Analyses of covariance were used to determine differences in depression and anxiety between women and men (covariates: family income, neighborhood disadvantage, race (described in the Supplement)). Voxelwise analyses of fMRI data (AFNI’s 3dLME [57]) were completed to assess main effects of sex, violence exposure, and threat type (cued versus non-cued), as well as interaction effects between sex, violence exposure, and threat type. To reduce the number of voxelwise comparisons, group level analyses were performed using a mask to restrict analyses to anatomical regions of interest (PFC, cingulate gyrus, insula, IPL, parahippocampal gyrus (PHG), hippocampus, amygdala) based on prior work [41, 44, 45, 47]. Monte Carlo simulations (AFNI’s 3dClustSim) were performed with the autocorrelation function option to correct for multiple comparisons. A voxelwise threshold of p
0.05 for SCR and threat expectancy analyses. Analyses of covariance were used to determine differences in depression and anxiety between women and men (covariates: family income, neighborhood disadvantage, race (described in the Supplement)). Voxelwise analyses of fMRI data (AFNI’s 3dLME [57]) were completed to assess main effects of sex, violence exposure, and threat type (cued versus non-cued), as well as interaction effects between sex, violence exposure, and threat type. To reduce the number of voxelwise comparisons, group level analyses were performed using a mask to restrict analyses to anatomical regions of interest (PFC, cingulate gyrus, insula, IPL, parahippocampal gyrus (PHG), hippocampus, amygdala) based on prior work [41, 44, 45, 47]. Monte Carlo simulations (AFNI’s 3dClustSim) were performed with the autocorrelation function option to correct for multiple comparisons. A voxelwise threshold of p <
< 0.005 and a cluster volume threshold of 540
0.005 and a cluster volume threshold of 540 mm3 were used to achieve a corrected significance threshold of p
mm3 were used to achieve a corrected significance threshold of p <
< 0.05. Additional simulations were performed for small volume correction using masks of the parahippocmapal gyrus, hippocampus, and amygdala. Simulations used a voxelwise threshold of p
0.05. Additional simulations were performed for small volume correction using masks of the parahippocmapal gyrus, hippocampus, and amygdala. Simulations used a voxelwise threshold of p <
< 0.005 and a cluster threshold of 169
0.005 and a cluster threshold of 169 mm3 to achieve a corrected significance threshold of p
mm3 to achieve a corrected significance threshold of p <
< 0.05. Scanner, race, family income, and neighborhood disadvantage were used as covariates in all analyses, as these covariates have been found to vary with brain function and violence exposure [32]. Although the abovementioned regions of interest were the focus of the present study, a voxelwise whole brain analysis was also completed (see Supplementary Table 2). AFNI’s 3dClustSim was used as described above to determine the cluster volume threshold (i.e., 608
0.05. Scanner, race, family income, and neighborhood disadvantage were used as covariates in all analyses, as these covariates have been found to vary with brain function and violence exposure [32]. Although the abovementioned regions of interest were the focus of the present study, a voxelwise whole brain analysis was also completed (see Supplementary Table 2). AFNI’s 3dClustSim was used as described above to determine the cluster volume threshold (i.e., 608 mm3 with p
mm3 with p <
< 0.005uncorrected) for a corrected significance threshold of p
0.005uncorrected) for a corrected significance threshold of p <
< 0.05.
0.05.
Secondary analyses
Functional MRI, anxiety, depression, and PTSD
Moderation analyses: secondary analyses were completed to determine whether sex moderated the association between the fMRI signal response to threat type and anxiety, depression, and PTSD symptoms. Data from the main effect of threat type contrast (Table 1) were used as predictor variables. Using PROCESS [58, 59], multiple regression analyses were completed to assess whether sex moderated the association between the neural response to threat and anxiety, depression, and PTSD symptoms. Conditional effects of the neural response to threat and sex on anxiety, depression, and PTSD symptoms were assessed. Race, family income, neighborhood disadvantage, and scanner were included as covariates.
Table 1
Brain regions that show threat-elicited activity.
| Main effect of threat type | Hemisphere | F-statistic | Talairach coordinates (x,y,z) | Cluster size (mm3) |
|---|---|---|---|---|
| dmPFC | Right | 33.16 | 7, 31, 36 | 40,575 |
| dlPFC | Left | 14.97 | −29, 4, 56 | 1789 |
| Left(2) | 18.63 | −45, 18, 42 | 1316 | |
| Right | 14.26 | 35, 3, 38 | 1361 | |
| Insula | Right | 36.20 | 31, 20, −5 | 4582 |
| Right(2) | 26.08 | 46, −16, 7 | 783 | |
| Left | 42.22 | −26, 20, −4 | 5584 | |
| PCC | Right | 16.82 | 7, −29, 24 | 3064 |
| IPL | Right | 27.03 | 53, −46, 25 | 881 |
| Left | 24.01 | −56, −55, 38 | 686 | |
| Main effect of sex | ||||
| dmPFC | Right | 22.59 | 2, 18, 53 | 4075 |
| dlPFC | Right | 14.87 | 22, 47, 28 | 869 |
| PCC | Right | 15.83 | 5, −41, 25 | 1438 |
| IPL | Left | 13.32 | −40, −50, 49 | 688 |
Sex × × Threat type Threat type | ||||
| dlPFC | Left | 14.18 | −20, 41, 36 | 1017 |
| Precentral gyrus | Left | 17.08 | −45, 12, 7 | 2464 |
| Insula | Left | 17.32 | −37, −11, 0 | 1172 |
| Left(2) | 21.96 | −45, 14, 15 | 610 | |
| Right | 18.90 | 42, −8, 0 | 5911 | |
| Mid Cingulate | Right | 19.28 | 14, −16, 42 | 1489 |
| PCC | Right | 16.76 | 8, −42, 9 | 2466 |
| IPL | Left | 14.76 | −48, −34, 26 | 1505 |
| Amygdala | Right | 22.16 | 21, −6, −22 | 374 |
| Parahippocampal gyrus | Right | 16.71 | 23, −23, −25 | 301 |
Sex × × Violence exposure Violence exposure | ||||
| dlPFC | Right | 16.75 | 31, 23, 28 | 1073 |
| IPL | Left | 15.99 | −42, −48, 47 | 688 |
Coordinates refer to the location of the peak voxel within each volume of activation. F-statistic values refer to the F value at the peak voxel within each volume of activation. Significance threshold pcorrected <
< 0.05. Numbers within parentheses in the Hemisphere column reflect a distinct ROI within a particular brain region [e.g., dlPFC Left(2) is the 2nd of two ROIs within the left dlPFC].
0.05. Numbers within parentheses in the Hemisphere column reflect a distinct ROI within a particular brain region [e.g., dlPFC Left(2) is the 2nd of two ROIs within the left dlPFC].
dlPFC dorsolateral prefrontal cortex, dmPFC dorsomedial prefrontal cortex, PCC posterior cingulate cortex, IPL inferior parietal lobule.
Results
Violence exposure
No differences were observed in the amount of violence exposure between women and men (women: M =
= 0.13
0.13 ±
± 0.02, range
0.02, range =
= 0.00–3.00; men: M
0.00–3.00; men: M =
= 0.14
0.14 ±
± 0.02, range
0.02, range =
= 0.00–3.00, t[198]
0.00–3.00, t[198] =
= −0.64, p
−0.64, p =
= 0.52; two-tailed; 95% CI: −0.06–0.03). No sex differences were observed for violence exposure at any time point (Supplementary Table 3).
0.52; two-tailed; 95% CI: −0.06–0.03). No sex differences were observed for violence exposure at any time point (Supplementary Table 3).
Depression symptoms
Women (M =
= 2.38, SEM
2.38, SEM =
= 0.16, range
0.16, range =
= 0.00–6.00) reported more depression symptoms than men (M
0.00–6.00) reported more depression symptoms than men (M =
= 1.73, SEM
1.73, SEM =
= 0.16, range
0.16, range =
= 0.00–5.00), F[1,195]
0.00–5.00), F[1,195] =
= 9.62, p
9.62, p =
= 0.002. Among women, violence exposure varied positively with depression symptoms (r
0.002. Among women, violence exposure varied positively with depression symptoms (r =
= 0.26, p
0.26, p =
= 0.01), while there was no relationship for men (r
0.01), while there was no relationship for men (r =
= 0.15, p
0.15, p =
= 0.16); however, these correlations were not different (z
0.16); however, these correlations were not different (z =
= 0.84, p
0.84, p =
= 0.40). Full results are presented in the Supplement (Supplementary Table 4).
0.40). Full results are presented in the Supplement (Supplementary Table 4).
Anxiety symptoms
Women (M =
= 3.12, SEM
3.12, SEM =
= 0.21, range
0.21, range =
= 0.00–9.00) reported more anxiety symptoms than men (M
0.00–9.00) reported more anxiety symptoms than men (M =
= 2.51, SEM
2.51, SEM =
= 0.21, range
0.21, range =
= 0.00–9.00), F[1,195]
0.00–9.00), F[1,195] =
= 4.91, p
4.91, p =
= 0.03. Among women and men, violence exposure varied positively with anxiety symptoms (women: r
0.03. Among women and men, violence exposure varied positively with anxiety symptoms (women: r =
= 0.24; p
0.24; p =
= 0.02, men: r
0.02, men: r =
= 0.23, p
0.23, p =
= 0.02). Full results are presented in the Supplement (Supplementary Table 5).
0.02). Full results are presented in the Supplement (Supplementary Table 5).
Posttraumatic stress disorder (PTSD) symptoms
There were no differences in PTSD symptoms between women (M =
= 9.69, SEM
9.69, SEM =
= 1.15, range
1.15, range =
= 0.00–51.00) and men (M
0.00–51.00) and men (M =
= 7.72, SEM
7.72, SEM =
= 0.90, range
0.90, range =
= 0.00–37.00), F[1,190]
0.00–37.00), F[1,190] =
= 1.10, p
1.10, p =
= 0.30. Among men, violence exposure varied positively with PTSD symptoms (r
0.30. Among men, violence exposure varied positively with PTSD symptoms (r =
= 0.27, p
0.27, p =
= 0.01), while there was no relationship for women (r
0.01), while there was no relationship for women (r =
= 0.11, p
0.11, p =
= 0.30); however, these correlations were not significantly different (z
0.30); however, these correlations were not significantly different (z =
= 1.14, p
1.14, p =
= 0.25). Full results are presented in the Supplement (Supplementary Table 6).
0.25). Full results are presented in the Supplement (Supplementary Table 6).
SCR
SCR data from 85 participants were excluded from analyses due to poor signal quality/equipment malfunction (n =
= 13) or non-responsiveness (n
13) or non-responsiveness (n =
= 72). Poor signal quality was defined as excessive noise in the signal that prevented identification of stimulus-evoked SCRs. Equipment malfunction was defined as no signal observed during scanning due to a problem with equipment set up or function. Participants exhibiting no responses greater than 0.05
72). Poor signal quality was defined as excessive noise in the signal that prevented identification of stimulus-evoked SCRs. Equipment malfunction was defined as no signal observed during scanning due to a problem with equipment set up or function. Participants exhibiting no responses greater than 0.05 µS were considered non-responders. The LME analysis revealed a significant main effect of violence exposure (F[1,109]
µS were considered non-responders. The LME analysis revealed a significant main effect of violence exposure (F[1,109] =
= 11.37, p
11.37, p <
< 0.001). Specifically, SCR varied positively with violence exposure (r
0.001). Specifically, SCR varied positively with violence exposure (r =
= 0.19, p
0.19, p =
= 0.04). There was also a significant sex
0.04). There was also a significant sex ×
× violence exposure interaction (F[1,109]
violence exposure interaction (F[1,109] =
= 18.95, p
18.95, p <
< 0.001). Specifically, SCR to threat varied positively with violence exposure among women (r
0.001). Specifically, SCR to threat varied positively with violence exposure among women (r =
= 0.52, p
0.52, p <
< 0.001) but not for men (r
0.001) but not for men (r =
= −0.07, p
−0.07, p =
= 0.60). Since the LME analysis did not reveal a significant difference in threat type, a follow-up paired samples t-test was conducted to determine whether there were differences in SCR by threat type. SCR to non-cued threat (M
0.60). Since the LME analysis did not reveal a significant difference in threat type, a follow-up paired samples t-test was conducted to determine whether there were differences in SCR by threat type. SCR to non-cued threat (M =
= 0.43, SEM
0.43, SEM =
= 0.03) was larger than to cued threat (M
0.03) was larger than to cued threat (M =
= 0.39, SEM
0.39, SEM =
= 0.03; Mean difference: 0.03, t[1,114]
0.03; Mean difference: 0.03, t[1,114] =
= −2.56, p
−2.56, p =
= 0.01; two-tailed; 95% CI: 0.01–0.06), consistent with prior work [43, 44, 46, 47].
0.01; two-tailed; 95% CI: 0.01–0.06), consistent with prior work [43, 44, 46, 47].
Threat expectancy
The LME revealed a significant main effect for threat type (F[1,193] =
= 90.57, p
90.57, p <
< 0.001) such that expectancy was greater for cued (M
0.001) such that expectancy was greater for cued (M =
= 70.46, SEM
70.46, SEM =
= 1.26) than non-cued (M
1.26) than non-cued (M =
= 55.27, SEM
55.27, SEM =
= 0.98) threat. No other significant effects were observed.
0.98) threat. No other significant effects were observed.
Functional MRI
The LME analysis of fMRI data revealed main effects of threat type and sex, as well as threat type ×
× sex and violence exposure
sex and violence exposure ×
× sex interactions. The fMRI results showed a main effect of threat type, such that the fMRI response was greater to non-cued than to cued threat within the dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC), insula, and IPL consistent with prior research (Table 1) [41, 43–45, 60]. We also observed a main effect of sex within the dlPFC, dmPFC, IPL, and PCC (Table 1). Specifically, threat-elicited activity within these regions was greater in women than men.
sex interactions. The fMRI results showed a main effect of threat type, such that the fMRI response was greater to non-cued than to cued threat within the dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC), insula, and IPL consistent with prior research (Table 1) [41, 43–45, 60]. We also observed a main effect of sex within the dlPFC, dmPFC, IPL, and PCC (Table 1). Specifically, threat-elicited activity within these regions was greater in women than men.
There was a significant threat type ×
× sex interaction within the right amygdala (Supplementary Table 7 and Fig. 1a). Specifically, a greater fMRI signal response was observed within the amygdala to non-cued threat in women (M
sex interaction within the right amygdala (Supplementary Table 7 and Fig. 1a). Specifically, a greater fMRI signal response was observed within the amygdala to non-cued threat in women (M =
= 0.08, SEM
0.08, SEM =
= 0.02) than men (M
0.02) than men (M =
= 0.03, SEM
0.03, SEM =
= 0.02; t[1,193]
0.02; t[1,193] =
= 2.19, p
2.19, p =
= 0.03). Further, the response to cued threat was smaller for women (M
0.03). Further, the response to cued threat was smaller for women (M =
= 0.02, SEM
0.02, SEM =
= 0.02) than men (M
0.02) than men (M =
= 0.09, SEM
0.09, SEM =
= 0.02) within the amygdala (t[1,193]
0.02) within the amygdala (t[1,193] =
= −3.03, p
−3.03, p =
= 0.003; Fig. 1a). There was also a significant threat type
0.003; Fig. 1a). There was also a significant threat type ×
× sex interaction within the right PHG (Supplementary Table 7 and Fig. 1b). PHG activity to non-cued threat was larger in women (M
sex interaction within the right PHG (Supplementary Table 7 and Fig. 1b). PHG activity to non-cued threat was larger in women (M =
= 0.11, SEM
0.11, SEM =
= 0.02) than men (M
0.02) than men (M =
= 0.05, SEM
0.05, SEM =
= 0.02; t[1,193]
0.02; t[1,193] =
= 2.42, p
2.42, p =
= 0.02). Further, PHG activity to cued threat was smaller in women (M
0.02). Further, PHG activity to cued threat was smaller in women (M =
= 0.07, SEM
0.07, SEM =
= 0.02) than men (M
0.02) than men (M =
= 0.14, SEM
0.14, SEM =
= 0.02; t[1,193]
0.02; t[1,193] =
= −2.13, p
−2.13, p =
= 0.04, Fig. 1b). Finally, there was a significant threat type
0.04, Fig. 1b). Finally, there was a significant threat type ×
× sex interaction within the left dlPFC, left IPL, left precentral gyrus, bilateral insula, right mid cingulate gyrus, and right PCC (Supplementary Table 7).
sex interaction within the left dlPFC, left IPL, left precentral gyrus, bilateral insula, right mid cingulate gyrus, and right PCC (Supplementary Table 7).
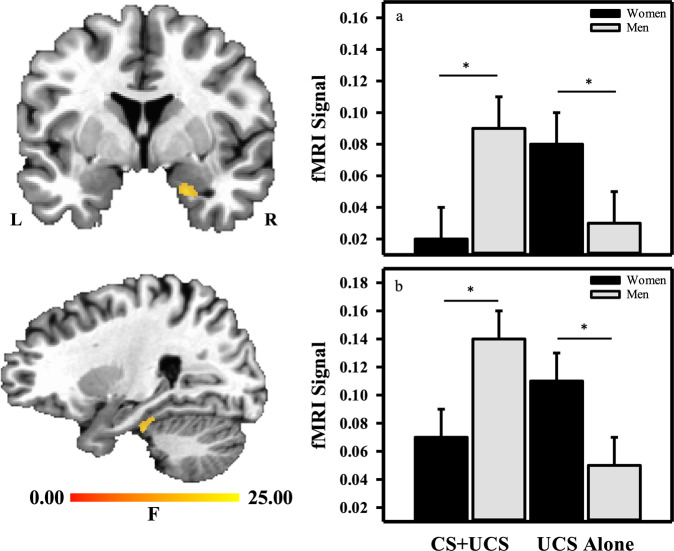
a A threat type ×
× sex interaction was observed within the right (a) amygdala and (b) parahippocampal gyrus. Women (black bars) exhibited a smaller response to cued threat (CS
sex interaction was observed within the right (a) amygdala and (b) parahippocampal gyrus. Women (black bars) exhibited a smaller response to cued threat (CS +
+ UCS) than men (gray bars). In contrast, women exhibited a larger response to non-cued (UCS Alone) threat than men. *p
UCS) than men (gray bars). In contrast, women exhibited a larger response to non-cued (UCS Alone) threat than men. *p <
< 0.05.
0.05.
A significant sex ×
× violence exposure interaction was observed within the right dlPFC and left IPL (Table 1). Among women, violence exposure varied positively with the fMRI response within the dlPFC and IPL. In men, violence exposure varied negatively with the fMRI response within the dlPFC, while no relationship was observed within the IPL (Fig. 2).
violence exposure interaction was observed within the right dlPFC and left IPL (Table 1). Among women, violence exposure varied positively with the fMRI response within the dlPFC and IPL. In men, violence exposure varied negatively with the fMRI response within the dlPFC, while no relationship was observed within the IPL (Fig. 2).
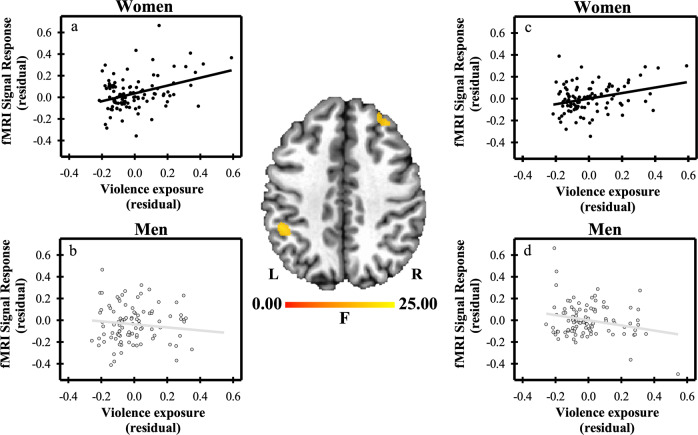
A sex ×
× violence exposure interaction was observed within the left IPL. a Women (black scatterplot) demonstrated a positive relationship (r
violence exposure interaction was observed within the left IPL. a Women (black scatterplot) demonstrated a positive relationship (r =
= 0.37, p
0.37, p <
< 0.01) between violence exposure and the IPL response to threat, while b men (gray scatterplot) demonstrated no relationship (r
0.01) between violence exposure and the IPL response to threat, while b men (gray scatterplot) demonstrated no relationship (r =
= −0.12, p
−0.12, p =
= 0.24). A similar sex
0.24). A similar sex ×
× violence exposure interaction was also observed within the right dlPFC. c Women (black scatterplot) demonstrated a positive relationship (r
violence exposure interaction was also observed within the right dlPFC. c Women (black scatterplot) demonstrated a positive relationship (r =
= 0.31, p
0.31, p <
< 0.01) between violence exposure and the dlPFC response to threat, while d men (gray scatterplot) demonstrated a negative relationship (r
0.01) between violence exposure and the dlPFC response to threat, while d men (gray scatterplot) demonstrated a negative relationship (r =
= −0.22, p
−0.22, p =
= 0.04).
0.04).
Functional MRI, anxiety, depression, and PTSD
Secondary analyses were completed to determine whether sex moderated the association between the fMRI response to threat type and anxiety, depression, and PTSD symptoms. Moderation analyses were not corrected for multiple comparisons; therefore, results should be interpreted with caution. Anxiety: sex did not moderate the association between the neural response to threat (cued or non-cued) and anxiety (Supplementary Table 8). Depression: sex moderated the association between the right insula response to non-cued threat and depression. Specifically, there was a positive association between the insula response and depression symptoms in men, but not women (Supplementary Table 9). Although the interaction between the left IPL response to non-cued threat and depression symptoms was significant, the slopes for men and women were not significant (Supplementary Table 9). Sex did not moderate the association between cued threat and depression (Supplementary Table 9). PTSD: sex moderated the association between the right IPL response to cued threat and PTSD symptoms. Specifically, the IPL response varied positively with PTSD symptoms in women, but not men (Supplementary Table 10). Sex also moderated the association between left IPL response to cued threat and PTSD symptoms. Specifically, the left IPL response varied positively with PTSD symptoms in women, but not men (Supplementary Table 10). Sex did not moderate the association between non-cued threat and PTSD symptoms (Supplementary Table 10).
Discussion
The present study investigated sex differences in the relationship between violence exposure and the neural response to threat. We found that women showed greater threat-elicited activity within the dlPFC, dmPFC, IPL, and PCC than men. Further, we found that women demonstrated greater amygdala and PHG responses to non-cued threat than men, while the response to cued threat was greater in men than women. We also found that dlPFC and IPL activity varied positively with violence exposure in women. In contrast, activity within these regions showed a negative (dlPFC) or no (IPL) relationship with violence exposure in men. Further, women reported greater depression and anxiety symptoms than men, while no sex differences were observed in PTSD symptoms. Additionally, anxiety symptoms varied positively with violence exposure in women and men; however, depression symptoms and violence exposure varied positively in women only. Lastly, PTSD symptoms varied positively with violence exposure in men only. These results suggest that adolescent violence exposure may differentially impact the neural function that supports threat-related emotion processes in women and men, and varies with depression, anxiety, and PTSD symptoms in both women and men. Findings from the present study provide novel insight into the neural circuitry underlying sex differences in emotional function.
The present study demonstrated sex differences in neural reactivity to cued and non-cued threat. Specifically, we found greater dlPFC, cingulate, IPL, and precentral gyrus responses to non-cued threat in women than men, while no differences were observed in responses to cued threat. Further, insula reactivity to cued threat was greater in men than women, while no differences were observed to non-cued threat. The dlPFC, cingulate, and IPL are important for threat-related learning and the cognitive control of emotion [41, 44, 61, 62]. Further, prior work has demonstrated greater dlPFC and IPL activity under uncertain task conditions [63–65] and linked activity within these brain regions to anxiety [66, 67]. Additionally, amygdala and PHG activation to non-cued threat was greater in women than men. In contrast, amygdala and PHG activation to cued threat was greater in men than in women (Fig. 1). Differences observed in these responses to threat may also be linked to threat predictability. For example, prior work indicates that unpredictable threats are perceived as more aversive than predictable threats [68, 69], which is consistent with other research that has demonstrated larger autonomic responses to unpredictable than predictable threats [41–43, 45, 47, 70]. Similar findings have been observed within neuroimaging data. Specifically, greater brain activity to unpredictable threats has been linked to anxiety about the uncertainty of the threat [68]. In the present study, women showed greater amygdala and PHG activation to non-cued threat, which may reflect greater affective responding when threats are uncertain. Prior work indicates that affective responses to uncertainty are larger in those with anxiety disorders [71], which occur more frequently in women [11]. In fact, women in the present study reported greater anxiety symptoms than men. Overall, the findings from the present study suggest there is greater recruitment of brain regions important for both cognitive control (e.g., dlPFC, IPL) and affective responding (e.g., amygdala and PHG) in women when threats are unpredictable. Thus, the current findings suggest that the dlPFC, IPL, amygdala, and PHG response to unpredictable threat is greater in women which may be linked to symptoms of anxiety.
The present study also demonstrated sex differences in the relationship between violence exposure and the neural response to threat (Fig. 2). Specifically, women who experienced greater violence exposure showed a greater response to threat within the dlPFC and IPL. In contrast, men demonstrated a negative relationship between violence exposure and dlPFC activity and no relationship between violence exposure and IPL activity. The dlPFC and IPL are important components of the fronto-parietal network and modulate threat-elicited brain activity [42, 45, 72, 73]. Further, greater dlPFC and IPL activation is associated with increased emotion regulation and cognitive control [62, 72, 74]. Thus, the present results suggest that women who have been exposed to higher levels of violence exposure may recruit brain regions that are important for the cognitive control of emotion during threat compared to men who show the opposite pattern. Furthermore, women demonstrated a positive relationship between violence exposure and SCR to threat, while men demonstrated no relationship. Taken together, these findings suggest that the brain and psychophysiological response to threat in women, but not in men, may be linked to their exposure to violence.
Results from the present study demonstrate sex-related differences in neural reactivity to threat. These findings suggest that neural reactivity to threat varies with violence exposure. Prior studies demonstrate that as the brain continues to develop during adolescence (i.e., puberty), trauma (e.g., violence exposure) may alter the neural response to threat due to increased sensitivity to violence and other forms of trauma [75–79]. Consistent with the suggestion that adolescents may show greater sensitivity to adverse events, prior work demonstrates greater psychophysiological reactivity to laboratory stressors as adolescents age and reach pubertal maturation [80–82]. Thus, violence exposure during this period of development may result in greater physiological reactivity. Further, prior research has found a greater risk of anxiety, depression, and PTSD throughout both adolescence and adulthood in those exposed to greater prepubertal and pubertal maltreatment and violence [83, 84]. Taken together, findings from previous studies suggest that violence exposure during adolescence may enhance the response to threat which may influence future mental health symptoms.
Results from secondary analyses demonstrate that sex moderated the association between the neural response to threat and depression and PTSD symptoms. First, among men, the right insula response to non-cued threat varied positively with depression symptoms, while there was no association among women, and no associations for cued threat. The insula is important for integrating external stimuli with internal bodily states, and supports the processing of emotion-related stimuli [85–87]. Prior studies have shown a positive association between the insula response to negative stimuli and depression symptoms [88]. Results from the present study suggest that greater depression symptoms in men are associated with greater activity in brain regions important for emotion processing when threat is uncertain. Additionally, we found that among women, the association between PTSD symptoms and bilateral IPL responses to cued threat varied positively while there was no association for men, and no associations for non-cued threat. Prior studies have demonstrated a positive association between PTSD symptom severity and IPL responses to emotional stimuli [89]. Given that the IPL is important for cognitive control of emotion, women with higher PTSD symptoms may recruit the IPL to a greater extent than women with lower PTSD symptoms, to modulate emotional responses when threats are predictable. Overall, these findings suggest there are sex-related differences in brain regions that underlie emotion expression. Further, the present findings align with prior studies that suggest that men and women may use different brain regions to regulate emotions [25–27]. While these findings provide novel information regarding differences in brain regions that underlie emotion expression in response to threat in women and men, these results were not corrected for multiple comparisons, and thus, should be interpreted with caution.
Strengths and limitations
The present study has several strengths. Specifically, the present study includes a large community sample with approximately equal numbers of men and women, increasing statistical power and generalizability. However, the present study is not without limitation. For example, the present study is cross sectional; thus, we cannot determine whether violence exposure influences neural activity or whether the relationships observed were due to preexisting differences. Thus, future studies should implement longitudinal neuroimaging designs to determine whether violence exposure impacts the neural response to threat over time. Additionally, prior work suggests that reproductive hormones and menstrual cycle phase may impact the brain function that supports fear learning processes [17, 90]. Follow-up analyses in the present study suggest that the neural response to threat did not vary as a function of hormonal medication (Supplementary Tables 11 and 12). However, future studies should assess the presence of reproductive hormones and menstrual cycle phase when examining sex differences in neural function. Finally, cultural factors (e.g., socialization, coping style) were not assessed in the present study, but may modulate sex differences in emotion expression [91, 92]. Thus, future studies should assess cultural factors when examining sex differences in the emotional response to threat.
Conclusion
The current study found sex differences in the neural response to threat, as well as sex differences in the relationship between violence exposure and threat-elicited neural activity. Although prior studies have investigated sex differences in neural function during Pavlovian fear conditioning [93–95] this study is the first to investigate sex differences in the neural and psychophysiological response to threat that are linked to violence exposure. Additionally, results suggest that there are sex differences in the neural response to threat uncertainty which may be linked to sex-related differences in symptoms of anxiety. Overall, findings from the present study suggest that sex differences in the neural response to threat may underlie sex differences in emotional function.
Acknowledgements
The authors would like to thank Peyton Thetford for assistance with data collection.
Author contributions
SM, MAS, MNE, STE, and DCK conceptualized and designed the project. HED, NGH, AMG, KHW, MDW, DRH, DCK, and SM have full access to the data. HED, NGH, AMG, KHW, and MDW collected data for the project. HED, DRH, and NGH analyzed the data. HED, DRH, NGH, DCK, and SM interpreted the data. HED, DRH, NGH, and DCK revised the work critically for important intellectual content. SM, MAS, MNE, STE, DCK, HED, NGH, AMG, KHW, and MDW had final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was supported by the National Institutes of Health (Grant number: R01 MH098348). The original Healthy Passages study, from which the participants in the present study were recruited, was approved by the Centers for Disease Control and Prevention and the institutional review boards of the study sites and supported by cooperative agreements (CCU409679, CCU609653, CCU915773, U48DP000046, U48DP000057, U48DP000056, U19DP002663, U19DP002664, and U19DP002665). The funding sources had no role in the analysis or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01430-1.
References
Articles from Neuropsychopharmacology are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41386-022-01430-1
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9630543
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/135053743
Article citations
Sex Differences in Response Inhibition-Related Neural Predictors of Posttraumatic Stress Disorder in Civilians With Recent Trauma.
Biol Psychiatry Cogn Neurosci Neuroimaging, 9(7):668-680, 22 Mar 2024
Cited by: 1 article | PMID: 38522649
Impact of handgun ownership and biological sex on startle reactivity to predictable and unpredictable threats.
Int J Psychophysiol, 197:112297, 05 Jan 2024
Cited by: 0 articles | PMID: 38185419
Sex Differences in the Effects of Cognitive Reappraisal Training on Conditioned Fear Responses.
Int J Environ Res Public Health, 19(23):15837, 28 Nov 2022
Cited by: 0 articles | PMID: 36497911 | PMCID: PMC9739676
Mitigating the impact of adolescence isolation on the development of social anxiety: A potential role for oxytocin.
Front Behav Neurosci, 16:1038236, 13 Oct 2022
Cited by: 0 articles | PMID: 36311867 | PMCID: PMC9608628
Review Free full text in Europe PMC
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Negative life experiences contribute to racial differences in the neural response to threat.
Neuroimage, 202:116086, 08 Aug 2019
Cited by: 48 articles | PMID: 31401241 | PMCID: PMC6819267
Anticipatory prefrontal cortex activity underlies stress-induced changes in Pavlovian fear conditioning.
Neuroimage, 174:237-247, 16 Mar 2018
Cited by: 10 articles | PMID: 29555429 | PMCID: PMC5949265
Stress-Induced Changes in Effective Connectivity During Regulation of the Emotional Response to Threat.
Brain Connect, 12(7):629-638, 31 Dec 2021
Cited by: 1 article | PMID: 34541896 | PMCID: PMC9634990
Neural mechanisms of human temporal fear conditioning.
Neurobiol Learn Mem, 136:97-104, 28 Sep 2016
Cited by: 10 articles | PMID: 27693343 | PMCID: PMC5124389
Funding
Funders who supported this work.
NIA NIH HHS (1)
Grant ID: P30 AG066444
NIMH NIH HHS (1)
Grant ID: R01 MH098348
U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (1)
Grant ID: MH098348
U.S. Department of Health & Human Services | National Institutes of Health (1)
Grant ID: MH098348
 1
1



