Abstract
Free full text

Biology of lung macrophages in health and disease
SUMMARY
Tissue-resident alveolar and interstitial macrophages and recruited macrophages are critical players in innate immunity and maintenance of lung homeostasis. Until recently, assessing the differential functional contributions of tissue-resident versus recruited macrophages has been challenging because they share overlapping cell surface markers, making it difficult to separate them using conventional methods. This review describes how scRNA-seq and spatial transcriptomics can separate these subpopulations and help unravel the complexity of macrophage biology in homeostasis and disease. First, we provide a guide to identifying and distinguishing lung macrophages from other mononuclear phagocytes in humans and mice. Second, we outline emerging concepts related to the development and function of the various lung macrophages in the alveolar, perivascular, and interstitial niches. Finally, we describe how different tissue states profoundly alter their functions, including acute and chronic lung disease, cancer, and aging.
INTRODUCTION
Despite the saying “fresh air will do you good”, the 10,000 liters of air we breathe every day is potentially contaminated with pathogens, allergens, and man-made pollutants that are the root cause of infectious, allergic, environmental, and neoplastic disease. The lungs have evolved to maximize on the vital processes of gas exchange, and, as such, mammals carry many alveoli that facilitate the rapid diffusion of oxygen and carbon dioxide. The alveolocapillary membrane is a delicate 0.3-μm-thick interface between the inhaled air and the bloodstream; when spread out, its surface spans the size of a tennis court. Thus, this vast and thin barrier provides an ample portal through which inhaled pathogens can rapidly enter the bloodstream (Willart et al., 2009). To protect the lung from external pathogens entering via air or blood, a rapid surveillance and defense system is needed, but inflammation needs to be kept to a minimum, so that the microanatomy of the alveolocapillary membranes is not damaged. Defense of the lung is therefore a unique and delicate balancing act.
Alveolar macrophages (AMs) are the first-line defenders of the alveoli and airways, while lung interstitial macrophages (IM) act as gatekeepers of the vasculature and lung interstitium (Figure 1) (Bain and MacDonald, 2022; Hume et al., 2020). AMs and IMs are examples of tissue resident macrophages (TRMs); fulfilling the homeostatic, metabolic, and repair functions required for the organ of residence, while simultaneously acting as a sentinel phagocytic immune cell (Bleriot et al., 2020; Guilliams et al., 2022; Mulder et al., 2021). Yet, considerable differences exist between these lung TRMs in their transcriptional profile, ontogeny, phenotype, location, and function. Moreover, during inflammation, recruited monocytes become part of the lung macrophage pool, referred to as recruited macrophages (recMacs), displaying their own unique transcriptional signature and function. In this review, we will discuss the development and function of the various lung macrophage subsets as they live in their alveolar, perivascular, and interstitial niches in homeostasis and how this function can be profoundly altered by disease.
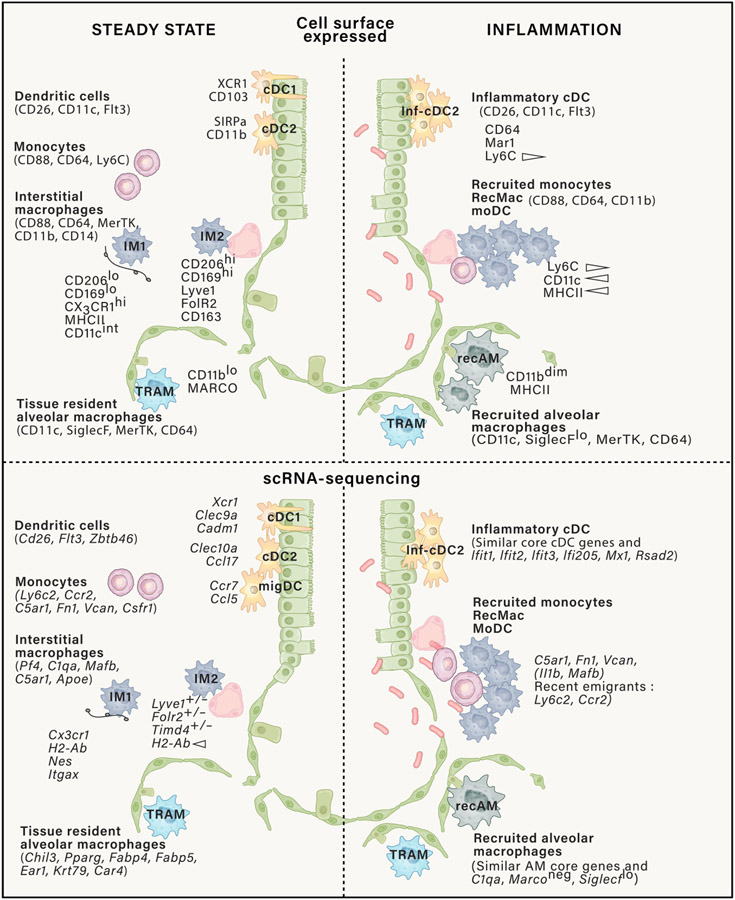
Top panel: CD45+ mononuclear phagocytes represented in the figure have varying surface expression of CD11c and CD11b. Prior to defining mononuclear phagocyte cell types using protein-based approaches such as flow cytometry, to avoid contamination, other immune cell types need to be excluded using lineage markers expressed on lymphoid cells (NK1.1, CD19, and CD3), neutrophils (Ly6G), and eosinophils (SiglecF+, SSChi, CD11c−CD64−). CD88 versus CD26 distinguishes monocytes and macrophages from cDCs. Note that monocytes do express a little CD26, but cDCs are CD88 negative. Activated monocytes and macrophages demonstrate high CD88 expression. AMs and IMs highly express MerTK (R&D: Polyclonal Goat IgG #AF591) and CD64 compared with recently recruited monocytes, which have low expression of MerTK, CD64, and F4/80 and varying surface expression of MHCII, CD11c, and Ly6C. Ly6C expression on recruited monocytes is lost over time. CD206 expression on in vivo macrophage does not define M2 macrophages since all AMs highly express CD206 and are not all M2-like. IMs express CD206 to a high and intermediate degree, as do cDC2s, recruited monocytes and macrophages. The two extreme profiles of IMs are represented here, although IMs, currently, based on the markers represented, are more a gradient of cell types than two clearly defined cell types. Similarly, cDC2 has multiple subtypes such as CD301+ and CD301− cDC2 (not represented here). Bottom panel: some commonly used genes to identify murine mononuclear phagocytes in scRNA-seq datasets. Like protein cell surface expression, CD88 versus CD26 gene expression help distinguish monocytes and macrophages from DCs. cDC, conventional dendritic cells; TRAM, tissue resident alveolar macrophage; IM, interstitial macrophage; moDC, monocyte-derived DC; recAM, recruited alveolar macrophages; recMac, recruited macrophages; Inf-cDC, inflammatory conventional DCs.
Lung macrophage heterogeneity reflects anatomical niche occupancy and function
As tissue-specific macrophages, AMs display a unique phenotype and transcriptional signature, which is dictated by the lung environment (Gautier et al., 2012). AMs live on the luminal side of the alveolar niche, surrounded by type I and type II alveolar epithelial cells, capillary endothelial cells, and alveolar interstitial fibroblasts, that provide the cytokine-rich nurturing scaffold for AMs to thrive (Westphalen et al., 2014) (Figure 1). They are abundant in the lung as just the upper lobe of the human lung already contains 1.5 × 109 AMs, the majority of which are localized to the diffusing area of the lung and a minority in the conducting small airways (Hume et al., 2020). Lung IMs mainly reside in the interstitial space around the bronchovascular bundle, though a few have been found within the airspace and alveolar interstitium (Hume et al., 2020). The IMs locate adjacent to many nonhematopoietic cell types such as neurons, lymphatic vessels, blood vessels, and hematopoietic cells (Chakarov et al., 2019; Gibbings et al., 2017; Keerthivasan et al., 2021; Schyns et al., 2019; Ural et al., 2020). In contrast to AMs, IMs are present throughout the body and display a similar transcriptional profile across multiple organs, suggesting they have a conserved, specialized function regardless of the organ they reside in (Dick et al., 2022; Gibbings et al., 2017).
Precise identification of lung macrophages by flow cytometry is complex. At steady state, distinguishing IMs from AMs, dendritic cells (DCs), and extravascular monocytes is still relatively straight-forward with sufficient markers (Gibbings and Jakubzick, 2018a, b; Gibbings et al., 2017; Leach et al., 2020) (Figure 1; Table 1). A reliable discrimination method to distinguish intravascular myeloid cells from tissue residents is by intravenous injection of CD45 antibodies 5 min prior to the sacrifice of the animal, which is a more pervasive method than perfusion alone (Desch et al., 2016; Gibbings et al., 2017). The differential expression of CD88 and CD26 helps distinguish monocytes and macrophages (both are CD88+) from CD26+ conventional DCs (Dutertre et al., 2019; Gibbings and Jakubzick, 2018a; Guilliams et al., 2016; Li et al., 2022; Nakano et al., 2015). C5AR1 (the gene encoding CD88) and DPP4 (CD26) may also be used at the transcriptional level. Exclusion markers are equally important to clean up the dataset within the myeloid gate, such as Ly6G for neutrophils in mice and CD15 in humans (Table 1).
In humans, AMs can be identified by flow cytometry and CITE-seq as large auto-fluorescent cells, expressing surface HLA-DR, CD43, CD88, CD169, CD206, CD10 (MME), CD11c, CD36, CD141, CD64, the scavenging antigen uptake receptors MARCO, and low amounts of CD14 (Bharat et al., 2015; Bosteels et al., 2020a; Desch et al., 2016; Leach et al., 2020; Misharin et al., 2013; Snelgrove et al., 2008; Vermaelen and Pauwels, 2004; Yu et al., 2015). In mice, CD11c, SiglecF, CD64, F4/80, and MerTK are useful phenotyping markers. In humans and mice, key AM genes include FABP4, MARCO, and PPARγ (Leach et al., 2020; Serezani et al., 2022). In mice, other robustly expressed genes are type 4 carbonic anhydrase (Car4), chitinase-like protein Ym1 (Chil3), CD2, several of the ribonucleases (Ear1), and keratins (Krt79) (Sajti et al., 2020; Schneider et al., 2014; van de Laar et al., 2016) (Figure 2; Table 1).
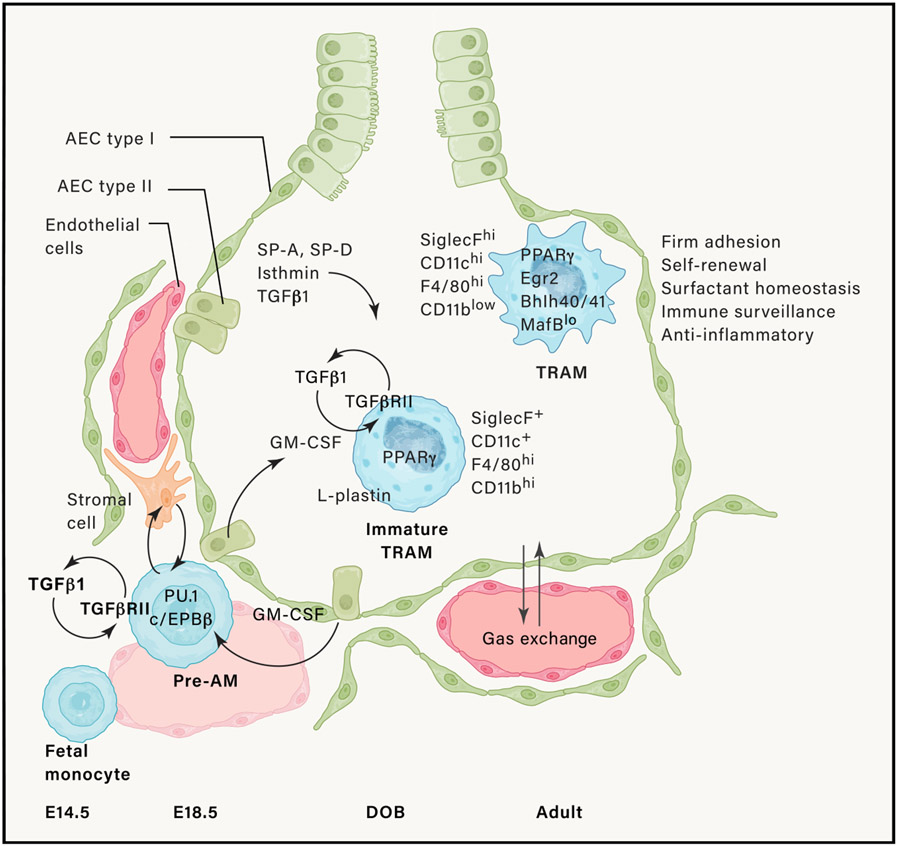
Around embryonic (E) day 18.5, fetal monocytes exit the lung vasculature and under the influence of autocrine TGF-β turn on a macrophage differentiation program characterized by expression of macrophage lineage-determining transcription factors. These cells next migrate to the alveolar niche where around the day of birth (DOB), they develop into immature TRAM, under the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) produced by type II alveolar epithelial cells and helped by macrophage-intrinsic transforming growth factor beta (TGF-β). This induces the expression of the transcription factor PPARγ that controls a large part of the AM-specific transcriptome. Mature TRAMs also contain transcription factors that allow for AM self-renewal. AM identity is instructed by other epithelial-cell-derived molecules like surfactant proteins A and D (SP-A and SP-D), TGF-β, and isthmin.
AMs have been previously considered as a relatively homogenous population of cells. Single-cell sequencing studies of healthy human AMs are quickly revealing their heterogeneity (Mould et al., 2021). At least 4 superclusters of AMs containing 14 subclusters can be discriminated across several individuals, with IFI27 and APOC2 as defining differentially expressed genes. One of the major determinants of heterogeneity appears to be the highly regulated production of certain combinations of chemokines, metallothioneins, interferon (IFN)-inducible genes, cholesterol-biosynthesis-related genes, and IGF1 by AM subsets. Moreover, analysis across several diseases including cystic fibrosis, chronic obstructive pulmonary disease (COPD), and COVID-19 demonstrated that these superclusters and their subclusters are universally observed (Li et al., 2022).
| MOUSE* | ||||||
|---|---|---|---|---|---|---|
| scRNA-seq | ||||||
| AMs | IMs | recMacs | cDC1 | cDC2 | InfcDC2 | pDCs |
| Chil3 | C1qa/b/c | early rec Ly6c2 | Cd24a | Dpp4 | Ifit1 | Siglech |
| Fabp5 | Pf4 | early rec Ccr2 | Itgae | Zbtb46 | Ifit1bl1 | Ly6d |
| Flt1 | C5ar1 | Cd14 | Xcr1 | Clec10a | Ifit2 | Ccr9 |
| Pparg | Apoe | Fn1 | Batf3 | Ccl17 | Ifit | Cox6a2 |
| Fabp4 | Cd14 | Vcan | Cadm1 | Ccl22 | Ifit3b | Plac8 |
| Siglecf | Csf1r | IL1b | Dpp4 | Fabp5 | Ifi205 | Ly6c2 |
| Car4 | Mafb | S100a6 | Zbtb46 | S100a6 | Ifi206 | Tcf4 |
| Ear1 | Mrc1 int/hi | S100a4 | Id2 | S100a4 | Rsad2 | Bst2 |
| Krt79 | Folr2 −/+ | Apoe | Flt3 | Flt3 | Phf11d | |
| Lyve1 −/+ | Mafb | Irf8 | Irf4 | Mx1 | ||
| Timd4 −/+ | C5ar1 | Tlr3 | H2-Ab1 | Cmpk2 | ||
| H2-Ab1 +/− | Ms4a4c | Clec9a | CD209a | Helz2 | ||
| Cx3cr1 hi/lo | Cst3 | Ccr7 (migDC) | ||||
| CD163 lo/hi | Wdfy4 | Ccl5 (migDC) | ||||
| Shared with other MPs | Activted IMs | Activted recMacs | Cxcr3 | |||
| Lpl | Lpl | Lpl | Rnase6 | |||
| Mpeg1 | Mpeg1 | Mpeg1 | Ccr7 (migDC) | |||
| Clec4n | Clec4n | Clec4n | Ccl5 (migDC) | |||
| Calr | Calr | Calr | ||||
| Siglec1 | Siglec1 | Siglec1 | ||||
| HUMAN* | ||||||
|---|---|---|---|---|---|---|
| scRNA-seq | ||||||
| AMs | IMs | recMacs | cDC1 | cDC2 | scRNA | pDCs |
| FABP4 | LGMN (pan) | S100A8 | CLEC9A | CD1C | FCER1A | GZMB |
| SERPING1 | MARCKS (pan) | FN1 | XCR1 | CD1E | CD1C | JCHAIN |
| APOC1 | FOLR2 (subtype 1) | VCAN | CADM1 | CLEC10A | CLEC10A | MZB1 |
| CD52 | SELENOP (subtype 1) | RNASE1 | BTLA | FCER1A | CD300E | CLEC4C |
| PCOLCE2 | F13A1 (subtype 1) | FCN1 | IDO1 | FCGR2B | FCER1G | CLIC3 |
| INHBA | SLC40A1 (subtype 1) | EMP1 | DNASE1L3 | CCL17 | FCGR2A | SPIB |
| MME (CD10) | SPP1 (subtype 2) | CD14 | CCND1 | PKIB | F13A1 | TCF4 |
| CES1 | PLA2G7 (subtype 2) | S100B | LGALS2 (panDC) | CD36 | ||
| GPD1 | MMP9 (subtype 2) | LGALS2 (panDC) | BIRC3 (migDCs) | CD163 | ||
| CFD | HAMP (subtype 2) | CD14 | ||||
| RBP4 | ||||||
| ITIH5 | ||||||
| SCD | ||||||
Unlike AMs, which can easily be isolated from the lung environment by bronchoalveolar lavage, IMs require a digestion and enrichment protocol from whole mouse or tissue excised human lungs to obtain high enough cell counts for transcriptional and functional analyses (Atif et al., 2018; Gibbings and Jakubzick, 2018a). IMs also rapidly die upon removal from their tissue niche. Therefore, to observe heterogeneity of IMs using single-cell RNA sequencing (scRNA-seq), in addition to rapid processing, it is important to exclude contaminating cell types, including AMs and recMacs, since both cell types are highly abundant and will dilute the granularity of an IM dataset. In mice, IMs universally express Pf4, C1q, and CD88. In humans, IMs selectively express LGMN and MARCKS. However, contrary to the mouse, human AMs also express C1Q, and therefore C1Q cannot be used as a distinguishing AM versus IM gene (Leach et al., 2020; Li et al., 2022). In addition to differentially expressed genes (DEGs) (Table 1), key reference genes help support the identification of a given scRNA-seq cluster. These genes may not be included in the top DEGs and might be shared with other cell types but are essential to validate the given classification of a cluster. For instance, macrophage identity genes include transcription factors, growth factors, and cell surface molecules such as Mafb, Csf1r, and Cd14. In a recent scRNA-seq study, three IM subtypes have been identified across multiple organs, including the lung, and based on the expression of Timd4, Lyve1, Folr2, Ccr2, and H2-Ab (Dick et al., 2022; Gibbings et al., 2017). Others have identified only two IM subtypes: CD206hi and CD206int IMs (Chakarov et al., 2019; Lim et al., 2018; Schyns et al., 2019; Ural et al., 2020) (Figure 1; Table 1). Even though these populations encompass a simplistic view, their identification in mouse tissue by flow and immunohistochemistry (IHC) can be performed by staining for C1q, in addition to MerTK, CD64, F4/80, or the CX3CR1 reporter mouse (Hume et al., 2020; McCubbrey et al., 2017; Meghraoui-Kheddar et al., 2020; Ogawa et al., 2022).
Added complexity in identifying lung macrophages arises during the perturbation of homeostasis, such as under inflammation, sterile injury, or aging. Under these conditions, recMacs, which derive from circulating classical Ly6C+ monocytes, migrate into the lung and can be easily confused with TRMs. RecMacs upregulate all the surface molecules used to distinguish IMs from other mononuclear phagocytes in steady state (Bosteels et al., 2020b; Gibbings et al., 2015; Nakano et al., 2015) (Figure 1). RecMacs initially display a remarkably altered phenotype and responsiveness. Some recMacs can acquire overlapping features with TRMs, such as recruited alveolar macrophages (recAMs) (Aegerter et al., 2020; Arafa et al., 2022; Guillon et al., 2020; Misharin et al., 2017), while other recMacs are phenotypically distinct, providing functional roles in epithelial repair, induction of fibrosis, or enhanced inflammation (Grant et al., 2021; McCubbrey et al., 2018; Misharin et al., 2017; Mould et al., 2019; Ogawa et al., 2022). The phenotypic overlap between recMacs and TRMs, combined with the promiscuity of many myeloid markers in inflammatory contexts such as those expressed on inflammatory DCs, has resulted in descriptions in the literature of an overarching and generic functional response by “lung macrophages” that might be more accurately annotated to individual cell types in future research. Sequencing either at the transcriptomic or epigenetic level, or making use of a traceable monocyte-descent marker in adoptive transfer models, has been shown to provide a reliable way to distinguish recMacs from TRMs.
Lung macrophages develop from multiple precursors in homeostasis and inflammation
Over 50 years ago, van Furth proposed the mononuclear phagocyte system, in which the central theme was that all TRM are derived or replenished from adult circulating monocytes (van oud Alblas and van Furth, 1979). From the start, this model was contested, as radiation chimera studies in the rat, parabiotic studies in the mouse and monocyte depletion experiments demonstrated that resident lung macrophages can self-replenish without input from circulating monocytic progenitors (Sawyer et al., 1982; Tarling and Coggle, 1982; Volkman and Gowans, 1965). More recent research from several labs has shown that mouse and human AMs develop prenatally from circulating fetal progenitors, that occupy the alveolar niche during perinatal lung growth, and self-maintain later in life through local low-grade proliferation in the absence of inflammation, without input from circulating adult monocytes (Figure 2) (Evren et al., 2022; Gomez Perdiguero et al., 2015; Guilliams et al., 2013; Hoeffel et al., 2015; Jakubzick et al., 2013; Mass et al., 2016; Schulz et al., 2012; van de Laar et al., 2016; Yona et al., 2013). scRNA-seq analysis and Brdu pulse-chase experiments indeed show that at any given time, up to 5%–10% of AMs have Ki67 expression or active BrdU incorporation (Coggle and Tarling, 1984; Hashimoto et al., 2013; Li et al., 2022; Mould et al., 2021). With increasing age of mice, there seems to be a larger component of resident AM replaced from circulating monocytes (Liu et al., 2019); however, parabiotic mice suggest that replacement is minimal if the mouse experiences no major injury or insult (Hashimoto et al., 2013; Jakubzick et al., 2013). In human patients undergoing lung transplantation, macrophage origin, and replacement can also be followed, and reports supporting donor replacement by host recMacs (Byrne et al., 2020), as well as persistence of donor TRMs have been shown (Eguiluz-Gracia et al., 2016; Nayak et al., 2016). More research in humans and mice is warranted to determine what signals control TRM replacement.
Within the first week of murine life, alveolar epithelial-cell-derived granulocyte-macrophage colony-stimulating factor (GM-CSF, encoded by Csf2) provides the instructive cytokine signal that programs fetal monocytes to become AMs in the alveolar niche, along a gradual differentiation scheme (Figure 2) (Gschwend et al., 2021; Guilliams et al., 2013; Schneider et al., 2014; Suzuki et al., 2008; Tazawa et al., 2019). While M-CSF (Csf1) deficient mice have AMs, GM-CSF (Csf2)- or GM-CSFR (Csf2r)-deficient mice lack AMs from birth. This instructive process is enforced by autocrine transforming growth factor-β (TGF-β) signaling and lung epithelial ανβ6 integrin-mediated presentation of TGF-β to AMs (Branchett et al., 2021; Koth et al., 2007; Meliopoulos et al., 2016; Yu et al., 2017). Triggering of these cytokine receptors leads to stable expression of the transcription factor (TF) PPARγ. To be able to respond to GM-CSF, the progenitors need to reach the alveolar space, and the actin binding protein L-Plastin (encoded by Lpl) is crucial for reaching the alveolar niche and upregulation of PPARγ (Todd et al., 2016). PPARγ is well known for regulating several lipid handling transporters and enzymes involved in surfactant turnover, and in the absence of this TF, AMs are absent and replaced by pro-inflammatory macrophages as pulmonary alveolar proteinosis develops (Schneider et al., 2014). AMs have also been shown to rely on Egr2, Bhlhe40, Bhlhe41, Bach2, and Klf4 for development and self-maintenance. These TFs instruct and maintain the unique identity and function of AMs by binding to enhancer regions of AM specific genes, on top of the generic macrophage transcriptional program that is controlled by pioneer or lineage-determining TFs such as C/EBPβ and PU.1 that appear earlier during AM development (Figure 2) (Daniel et al., 2020; Gorki et al., 2021; Mass et al., 2016; Rauschmeier et al., 2019; Sajti et al., 2020; Schneider et al., 2014; Yu et al., 2017). Mouse AM also have a great capacity for proliferation and stem cell-like replenishment, partially imparted by the low expression of the transcription factors MafB and cMaf, and high expression of Bhlhe40 and Bhlhe41, which control self-renewal genes (Busch et al., 2019; Rauschmeier et al., 2019; Soucie et al., 2016; Subramanian et al., 2022). Strikingly, VitD3 is required for restricting proliferation because accumulation of AMs is seen in Vdr−/− mice (Hu et al., 2019).
Steady-state IMs also originate from the yolk sac and are then replaced by fetal liver monocytes. IMs then either self-replenish without further input from circulating monocytes or are gradually replaced by monocytes at different rates and to a different extent according to the subtype of IM (Chakarov et al., 2019; Dick et al., 2022; Gibbings et al., 2017). Whereas AMs require GM-CSF, IMs are dependent on M-CSF for development and maintenance in the lung, as exemplified by the fact that Op/Op mice lack IMs due to deficiency in M-CSF and inhibitors of the kinase activity of CSF-1R or blocking CSF-1R antibodies deplete IMs (Lim et al., 2018; Ural et al., 2020). More research is needed on additional niche-instructive signals and precise TFs that govern the differentiation program of IMs, as this could also open possibilities for selective targeting of these cells. Using epigenetics analysis and TF binding motif enrichment on gene expression differences between mouse strains, important TFs have already been predicted, but remain to be validated (Sajti et al., 2020).
During lung infection or other inflammatory insults, the diversity of macrophage populations expands, and populations of recruited macrophages, recAMs and recMacs, replace or join the pool of resident AMs and IMs (Figure 1). These cells develop from CCR2-positive Ly6Chi monocytic precursors, express CD115, and depend on the cytokine M-CSF for their development in lung tissues and alveolar space. This pathway of lung inflammatory macrophage development is the reflection of the original mononuclear phagocyte system concept in which the circulating monocyte is the precursor for tissue macrophages (Volkman et al., 1983).
AM function is instructed by homeostatic and inflammatory environmental conditions
AMs adhere to and often crawl tightly attached to the luminal side of alveolar epithelial cells. This important surveillance function is mediated by the actin cytoskeleton and dysregulated in the absence of surfactant-protein A (Meliopoulos et al., 2016; Phelps et al., 2011). Only one in every three alveoli has an AM at any given time point (Westphalen et al., 2014), but migration of AMs from one alveolus to another by squeezing through the connecting pores of Kohn can occur, under the integrin-dependent guidance of the complement system component C5a (Neupane et al., 2020). In their exposed position, AMs continuously capture, phagocytose, conceal, and neutralize a large cargo of inhaled pathogens and particles (Westphalen et al., 2014). This is done in an immunologically “silent” way, i.e., without triggering neutrophil influx and causing excessive inflammation, which could damage the delicate alveoli. It has been estimated that the pool of AMs of the rat can even conceal up to 109 inhaled heat killed bacteria before the adaptive immune system is set in motion (MacLean et al., 1996). By expressing scavenging and apoptotic cell receptors like MARCO and Axl, AMs also avidly efferocytose apoptotic granulocytes and epithelial cells and phagocytose cellular and extracellular matrix debris that deposits on the alveolar wall (Fujimori et al., 2015; Roberts et al., 2017). During efferocytosis, AMs produce epithelial repair cytokines, like trefoil factor 2 (Hung et al., 2019). In addition to their phagocytic function in the innate immune system, AMs handle and recycle surfactant, a phospholipid detergent that keeps alveoli in an open inflated state, crucial for gas exchange. Genetic or acquired deficiency of AMs causes surfactant accumulation and leads to pulmonary alveolar proteinosis in humans and mice (Dranoff et al., 1994).
When their phagocytic function is surpassed, AMs initiate inflammation via the production of chemokines and cytokines (such as type I IFNs, TNF-α, and IL-1β) that recruit and activate neutrophils, monocytes, and DCs (Goritzka et al., 2015; Mould et al., 2019). The inflammatory response is not evenly distributed among all AMs recovered from the lungs, and chemokine production appears in a highly coordinated manner (Li et al., 2022; Xu-Vanpala et al., 2020), suggesting the existence of a specific transcriptional program that dictates a specialized function, tailored to the need of the stimulus, or the specialized location in the lung. As well as their ability to initiate inflammation, AMs are also responsible for resolving inflammation through the secretion of immunoregulatory cytokines like TGF-β, IL-1RA, and prostaglandins (Branchett et al., 2021; Jardine et al., 2019; Thepen et al., 1991).
The unique environment occupied by AMs and signals provided by the surrounding stroma and epithelium has been shown to confer many functional properties of these cells, which is well revealed following their removal from the lung (Bleriot et al., 2020; Janssen et al., 2020; Roquilly et al., 2020; Subramanian et al., 2022; Svedberg et al., 2019). Following ex vivo culture, AMs lose expression of genes involved in lipid metabolism, oxygen response, TGF-β signaling, and adhesion molecules. They also respond differently in the absence of lung-derived restraints, becoming more responsive to the cytokine IL-4 ex vivo (Svedberg et al., 2019) and more phagocytic and efferocytic (Janssen et al., 2008). Importantly, the transfer to plastic is in itself a plastic phenotype, as a bona fide in vivo AM profile is restored once ex vivo cultured cells are re-established in the lung (Subramanian et al., 2022). The environmental signals that constrain AM efferocytosis include surfactant proteins A and D that signal through SIRPα (Janssen et al., 2008). The epithelium and stroma in general are likely responsible for a multitude of AM phenotypes, but this has not yet been dissected in full detail. However, with acute and chronic inflammation and aging, the alteration of the AM niche is reflected in their functional outcomes (McQuattie-Pimentel et al., 2021).
Cellular metabolism dictates unique AM functions
The environment of the lung has a direct influence on cell metabolism that is tightly linked to function, particularly with respect to differentiation and inflammation (Mould et al., 2017; Svedberg et al., 2019). The environment of the lung is well supplied with oxygen and lipids, yet actively depleted of glucose to prevent bacterial outgrowth (Baker and Baines, 2018). This is reflected in a low ability of luminal AMs to use glucose (Mould et al., 2017; Svedberg et al., 2019), a constraint imposed by the lung environment, as AMs become more glucose responsive outside of the lung environment; upregulating the glycolysis as well as PI3K-Akt, mTOR, and C/EBPβ pathways (Subramanian et al., 2022; Svedberg et al., 2019). Interestingly, AMs derived from different regions of the human lung appear to utilize different metabolic pathways, again reflecting tissue dictated heterogeneity (Lavrich et al., 2018). Macrophages with an “alternatively activated” phenotype use the tricarboxylic acid (TCA) cycle to provide ATP via oxidative phosphorylation, whereas a “classically activated” macrophage will divert citrate from the TCA cycle for fatty acid oxidation, and preferentially utilize glycolysis (O’Neill et al., 2016). The nuclear receptor PPARγ directly regulates genes involved in lipid metabolism and fatty acid β-oxidation to enable macrophage function (Schneider et al., 2014) (Figure 3). However, just as the M1/M2 dichotomy of macrophages has proved reductive, the metabolism of a cell within a tissue cannot be easily simplified. The many metabolic pathways that supplement and regulate each other within a cell are interconnected to allow rapid environmental adaptation and response. Upon infection, the lung is liable to fast and extreme changes in glucose concentration (Baker and Baines, 2018). Increased glucose provides a benefit for cellular growth and can enable proliferation. Additionally, the generation of NADPH by the pentose phosphate pathway during glycolysis is required to produce reactive oxygen species and anti-bacterial defense. The metabolic profile of recAMs has been shown to differ, and even one-month post-influenza infection recAMs have not yet upregulated key AM genes involved in lipid- or fatty acid metabolism (Aegerter et al., 2020) Figure 3), and may have a reliance on creatine metabolism (Guillon et al., 2020).
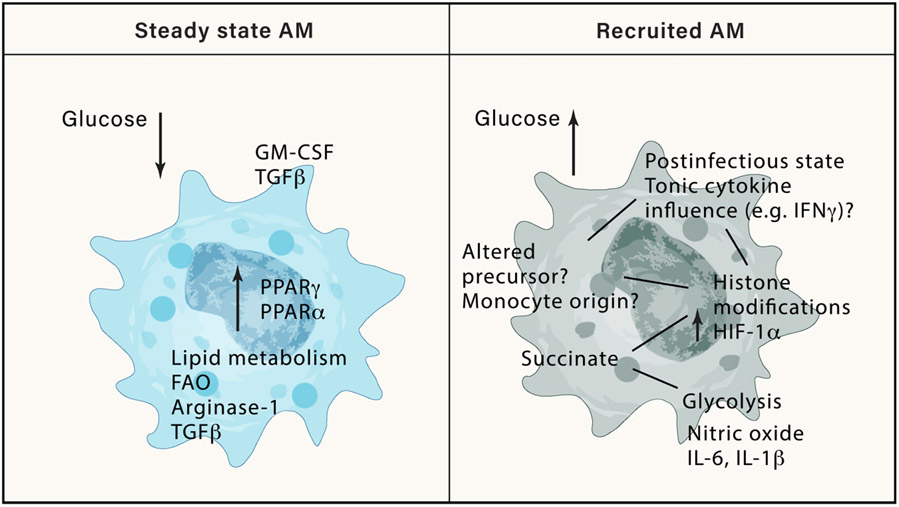
Steady-state AMs exist in an environment of low glucose and other environmental signals that instruct their metabolism and inflammatory output. PPARγ is a key AM transcription factor induced by GM-CSF, which regulates lipid metabolism, a key feature of AM functionality. In contrast, recAMs develop in an environment of higher glucose accessibility, and due to an altered origin or altered environmental signals, remain in an altered metabolic state characterized by high HIF1a that results in increased of inflammatory cytokines and nitric oxide.
Inflammation and infection results in a mixed population of AMs: Recruited monocytes contribute to an altered responsiveness of the lung
During acute lung infection, or inflammation, a reduction in the resident AM population is often seen and referred to as the “macrophage disappearance reaction”, and this is certainly the case in SARS-CoV2 infection (Grant et al., 2021; Liao et al., 2020). It is still unclear whether tissue resident alveolar macrophages (TRAMs) are directly infected and die, or whether TRAMs disappear by loss of a survival signal due to the infection of airway or alveolar epithelial cells. The efferocytic role of AMs promotes a pro-repair, anti-inflammatory phenotype, which may be counterproductive during acute inflammation, and therefore their temporary disappearance may prove beneficial to license effective inflammation (Herold et al., 2011; Miki et al., 2021). The disappearance of AMs could also be caused by their detachment from epithelial cells and subsequent disposal by mucociliary transport. Whether AMs die or migrate during inflammation, the lungs must urgently fill the alveolar niche to preserve lung function and gas exchange. The signals that govern this are still unknown. Repopulation may occur either by local proliferation of the remaining resident, terminally differentiated AMs (Jenkins et al., 2011; Minutti et al., 2017; Mould et al., 2019), or differentiation of recruited Ly6Chi monocyte precursors (recAMs) (Aegerter et al., 2020; Gschwend et al., 2021; Machiels et al., 2017; Mould et al., 2017). RecAMs have a different transcriptional, epigenetic, and functional phenotype than embryonically derived AMs, despite a similar expression of CD11c and CD64 and slightly lower SiglecF. However, recAMs do not comprise the entire AM population. TRMs are capable of simultaneous expansion and often seen as impervious to the viral insult, retaining a naive AM phenotype (Aegerter et al., 2020). In contrast, bone marrow-derived monocytes, as precursors of multiple populations, have considerable potential for education. As monocytes differentiate into recAMs during acute or chronic inflammation, the local signals that instruct AMs during homeostatic development may be lost or altered (Larsen et al., 2021). This may result in an interrupted or alternative differentiation of recAMs, who retain open chromatin regions relative to their monocyte origin for an extended period. This epigenetic legacy, as a natural result of differentiation (Aegerter et al., 2020; Chen et al., 2022), is distinct from “trained immunity". Trained immunity is defined operationally as a process by which an initial pathogenic insult leads to a lasting epigenetic imprint of innate immune cells, such that cellular responses become faster and greater upon repeated contacts with homologous or heterologous stimuli (Divangahi et al., 2021). While the “imprinting” of resident AMs has been reported, this is always in the apparent absence of recAMs, outlining two possibilities. Either monocytes are recruited but not sufficiently detected and recAMs are responsible for altered population reactivity, as previously shown (Aegerter et al., 2020; Chen et al., 2022; Machiels et al., 2017; Misharin et al., 2017). Or, in the absence of monocyte recruitment, the AMs gain plasticity. It has been demonstrated that AM phenotype is not always a result of intrinsic alterations to the cell but rather of extrinsic signals provided by the niche (Roquilly et al., 2020).
The altered reactivity of infection-derived recAMs have beneficial roles in some contexts. Compared with TRMs, they produce higher amounts of IL-6 to protect against Streptococcus pneumoniae and Mtb infection (Aegerter et al., 2020; Mata et al., 2021), express higher Arginase-1 which results in resistance to helminth infection (Chen et al., 2022), produce amphiregulin in response to cell injury signals to repair vascular injury in helminth infection (Minutti et al., 2019), and restrict type-2 inflammation in house-dust mite induced allergic asthma (Machiels et al., 2017). These phenotypes are surprisingly long term and have persisted over a month following recruitment. In other instances, like in severe COVID-19, the acute replacement of homeostatic AMs by pro-inflammatory and profibrotic monocyte-derived cells is detrimental. The considerable cytokine (IL-1, IL-6, TNF-α) and chemokine production by RecAMs can contribute to lung damage and induce a systemic syndrome resembling cytokine release syndrome (Grant et al., 2021; Liao et al., 2020). .
AMs are generally described as having a less mature and more inflammatory phenotype during chronic inflammation or aging. Multiple causes can induce such a profile; altered instructions from the niche, accumulation of epigenetic changes in resident cells, or replacement of embryonic-derived AMs by recAMs. Equally, all three factors may play a role. AMs from older mice are more prone to replacement by monocyte-derived cells, which have down-regulated cell cycle genes, less Ki67 staining, and increased production of the transcription factors MafB and Mafc, as well as increased CD11b (Angelidis et al., 2019; McQuattie-Pimentel et al., 2021; Wong et al., 2017). Bulk sequencing and proteomics of the aged mouse lung demonstrate an inflammatory signature of increased IL-6, IL-1β, and TNF-α coinciding with the downregulation of PPARγ (Angelidis et al., 2019). These classic indications of monocyte origin have been confirmed by fate-mapping studies in the Ms4a3 mouse (Liu et al., 2019). Functionally, AMs from older mice have a lower phagocytic and efferoctyic capacity (Wong et al., 2017). However, these macrophages do not age in isolation. Deposition of matrix, and changes in the ECM have been identified in the aging lung. Old mice downregulate collagen XIV that may disrupt the ability of decorin to regulate TGF-β, a key signaling factor for AM maturation (Angelidis et al., 2019). Conversely, collagen IV is upregulated, and increased expression of matrix genes and growth factors (Areg, Ntf3, Vegfa, Igf1) are seen. Indeed, the cellular niche has been shown to impose an aged phenotype on AMs because old AMs transferred into young mice were able to restore their “young phenotype” and vice versa (McQuattie-Pimentel et al., 2021). The transfer of young macrophages to old mice is able to provide protection during influenza (Wong et al., 2017). While conducting these studies in humans is technically difficult, parallels can be drawn, as a more inflammatory profile is also seen in AMs from older humans, with a lower expression of cell adhesion and proliferation markers (McQuattie-Pimentel et al., 2021). However, a caveat to many murine aging and inflammation studies is the relatively sterile environment in which they are conducted. This contrasts with humans, who experience considerably more exposure to ambient pathogens, pollutants, and infections throughout their life. Thus, the situation in the human lung may be considerably different to that of the mouse, given the repetitive infections and insults which come with advancing age.
How exactly an altered lung environment changes AM polarization and function is highly complex and multifactorial. MicroRNAs (miRNAs) can regulate the expression of many genes simultaneously, and exosome-bound miRNAs could be important in the communication between niche cells and AMs in health and disease states, such communication heavily influenced by disease risk factors like smoking, alcohol consumption, and prior microbial exposure (Sturrock et al., 2014). For instance, miR-155 has been found to be induced in AMs of humans and mice exposed to lipopolysaccharide (LPS)-induced lung injury and cigarette smoke, and promote the expression of several pro-inflammatory genes, such that miR-155 deficient mice resist cigarette smoke-induced lung injury (De Smet et al., 2020); (Wang et al., 2016). MiR-155 is also involved in controlling the function of post-viral recAM, and in the absence of miR-155, post-influenza recAMs exposed to bacteria produced more IL-17 and IL-23 and mice are better protected against secondary bacterial infection (Podsiad et al., 2016). MiR-146a is an important miRNA induced not only by microbial exposure, but also by mechanostretch in AMs, e.g., induced by injurious mechanical ventilation. This miRNA dampens the activation of AMs and miR-146a overexpression reduces mechanical ventilation induced lung injury (Bobba et al., 2021).
Interstitial macrophages perform homeostatic and inflammatory functions
There are still few data on the functional role of IM subtypes apart from common macrophage features. Due to the proximity of IMs to non-hematopoietic cells, it is logical to assume that IMs communicate with adjacent cell types and that this crosstalk promotes the survival of both macrophages and adjacent cell types during the development and regeneration of the lung, although these mediators are still largely unknown. Lyve-1+ macrophages in the aorta communicate with hyaluronic acid on vascular smooth muscle cells to regulate collagen synthesis in the latter and maintain vascular tone by preventing arterial stiffness (Lim et al., 2018). In the lung, loss of Lyve-1+ IMs using a genetic targeting strategy leads to increased vascular leakage (Chakarov et al., 2019). However, in the absence of IMs in Csf1op/op mice, lung function is not affected (Ural et al., 2020). Perhaps tissue-resident IMs are therefore not solely required for normal maintenance of lung nonhematopoietic cells, and instead, the crosstalk between a tissue-specific macrophage and its adjacent cell may have other functions. It has indeed been shown that CCR2-dependent cells and IL-4Rα signaling are required for lung regeneration following partial pneumonectomy (Lechner et al., 2017). Monocytes enter tissue continuously, surveying the environment during steady-state conditions and abundantly during inflammation (Jakubzick et al., 2013; Rodero et al., 2015). Recruited monocytes have a naive M0-like phenotype; a plastic cell capable of differentiation into a spectrum of phenotypes, including into an “alternatively activated” macrophage, which can secrete numerous growth factors required for structural cell development and survival (Mould et al., 2019). Thus, perhaps a combination of surveying recruited monocytes and TRMs will provide the cellular crosstalk with nonhematopoietic cells currently theorized.
Some IM functions have been suggested based on their transcriptional profile and anatomical location but have not yet been adequately investigated. IMs might have a role as neuroimmune communicators, leukocyte chemoattractants, and secrete immune modulators (Fu et al., 2022b; Ural et al., 2020). The production of immunoregulatory cytokines like IL-10, TGF-β, IGF-1, and reactive oxygen scavengers such as SOD2 suggest that IMs might have an important immunoregulatory role (Bedoret et al., 2009; Kawano et al., 2016; Martinez et al., 1997; Ural et al., 2020). In the induced absence of IMs (in Cd169Cre × Cx3CR1LStopL–DTR mice), sublethal influenza virus infection runs a more severe course, with heightened production of chemokines and cytokines (Ural et al., 2020). In a model of systemic LPS-induced endothelial injury, lung endothelium-derived Rspondin3 profoundly expands and alters the IM metabolism and epigenome to suppress lung inflammation (Zhou et al., 2020). In a model of hypoxia-induced acute respiratory distress syndrome, M-CSF is able to promote the differentiation of monocytes into tissue injury restoring macrophages (Mirchandani et al., 2022). The precise functional role of major histocompatibility complex (MHC) class II on lung IMs is unclear but could be important for providing a long-term residence niche for tissue resident CD4+ T cells, that derive tonic survival signals from this interaction (Snyder et al., 2021; Tang et al., 2022). This subset of MHCII+CX3CR1+ IM has also been shown to be recruited to sites of emboli that clog up lung capillaries in the lung vascular filter, which contribute to the formation of T-cell rich inflammatory lesions around intravascular embolic antigens (Willart et al., 2009). All in all, there is much to explore of IMs function and tools to selectively investigate their functional role in health and disease are just beginning to unfold.
Macrophages demonstrate an altered phenotype and function in chronic lung diseases
In the previous sections, we discussed the many alterations in resident AMs and IMs, particularly the confounding effects of recruited macrophages that dominate the macrophage population in acute inflammatory and infectious settings. However, certain chronic lung diseases are also prone to altering lung macrophage biology. In some, these cells are even seen as important culprits in the disease process.
A major disease in which lung macrophages are of principal importance is tuberculosis, caused by the bacterium Mycobacterium tuberculosis (Mtb), responsible for over 1.5 million annual deaths. AMs are the primary cells to be infected by Mtb and ultimately act as a bacterial reservoir. AMs have a gene profile associated with easy access to iron (Pisu et al., 2020), and their slow cytokine and chemokine production in response to infection permits bacterial replication (Papp et al., 2018; Rothchild et al., 2019), which is even more prominent in AMs from infants (Goenka et al., 2020). Experimental infection of AMs in vivo but not in vitro caused upregulation of NRF-2 associated genes, which prevent host cell death and therefore permits bacterial replication (Rothchild et al., 2019), demonstrating niche-derived signals that promote infection. Elegant intravital imaging, combined with intravascular labeling, and monocyte-deficient mice demonstrated how resident luminal AMs actively migrate into the interstitium to transfer bacteria to IMs (Cohen et al., 2018; Huang et al., 2018; Mata et al., 2021). This is likely an anti-bacterial host response, as IMs exert high stress, low iron condition on Mtb (Huang et al., 2018; Pisu et al., 2020). RecAMs also represent an ample host for infection (Antonelli et al., 2010; Cohen et al., 2018), and monocyte-derived AMs derived from old mice harbor more bacteria (Lafuse et al., 2019). Strikingly, pulmonary-delivered Bacille Calmette-Guérin (BCG) has been shown to be contained within AMs for up to five months post-vaccination, demonstrating not only their longevity, but profound ability to act as self-sacrificing protectants of the lung (Mata et al., 2021).
Lung fibrosis can result from defective lung repair following acute or chronic lung injury, a manifestation of autoimmune collagen vascular disorders, or an idiopathic process, often with a very poor patient clinical outcome (Figure 4). In a vast body of literature, it was mainly proposed that lung fibrosis is associated with the differentiation of macrophages to a profibrotic “alternatively activated” phenotype expressing the mannose receptor CD206, producing profibrotic chemokines (like CCL17, CCL18, and CCL23) and several matrix remodeling enzymes and components like fibronectin. Indeed, macrophage-mediated fibrosis can be blocked by interfering with IL-4Rα signaling (Jakubzick et al., 2003; Kropski and Blackwell, 2019; McCubbrey et al., 2018; Ogawa et al., 2022; Prasse et al., 2006). In animal models of bleomycin-induced lung fibrosis, recAMs are the preeminent lung macrophage driving the profibrotic process (Gibbons et al., 2011; McCubbrey et al., 2018). Some recruited bone marrow-derived monocytes are already poised for an alternatively activated signature because they express the marker Chil3 or contain a segregated nucleus and atypical morphology (SAT-M monocytes), but it is also possible that pro-repair genes are instructed by environmental cues of the fibrotic niche (Fukushima et al., 2020; Ikeda et al., 2018; Satoh et al., 2017). Macrophage-driven fibrosis results most likely from an initially appropriate response triggered by the ingestion of dead cells or damage-associated molecular patterns (DAMPS) and acts to stimulate epithelial and vascular repair, through the release of growth factors, like VEGF-A, amphiregulin, platelet-derived growth factor (PDGF-A and B), and TGF-β (Minutti et al., 2019). However, when out of balance or prolonged, an epithelial-endothelial-fibroblast-macrophage fibrotic niche can occur around an injured area. Monocyte-derived recAMs recruited by CXCL12 initially depend on M-CSF rather than GM-CSF and produce excessive amounts of growth factors that stimulate the differentiation of myofibroblasts. These myofibroblasts, in turn, synthesize collagen, a process that can be maintained because recAMs have a long-life span (Aran et al., 2019; Fukushima et al., 2020; Greiffo et al., 2020; Joshi et al., 2020; Misharin et al., 2017; Satoh et al., 2017). Alternatively, in a silica-induced fibrosis model Lyve1neg MHCII+ IMs that locally divide or recMacs produce many profibrotic cytokines like PDGF-A and PDGF-B, TGF-β, IGF-1, TGF-β receptors, and matrix remodeling enzymes. These macrophages express the complement component C1q, and it has been elegantly shown that C1qa-DTR mediated depletion of these cells abrogates fibrosis but enhances inflammation (Ogawa et al., 2022). In mice, IMs are also a source of IL-10, and triggering of IL-10R on recMacs stimulates a PDGF-A-dependent profibrotic program in a bleomycin model, suggesting that anti-inflammatory pathways might be intrinsically associated with fibrosis when unabated (Bhattacharyya et al., 2022).
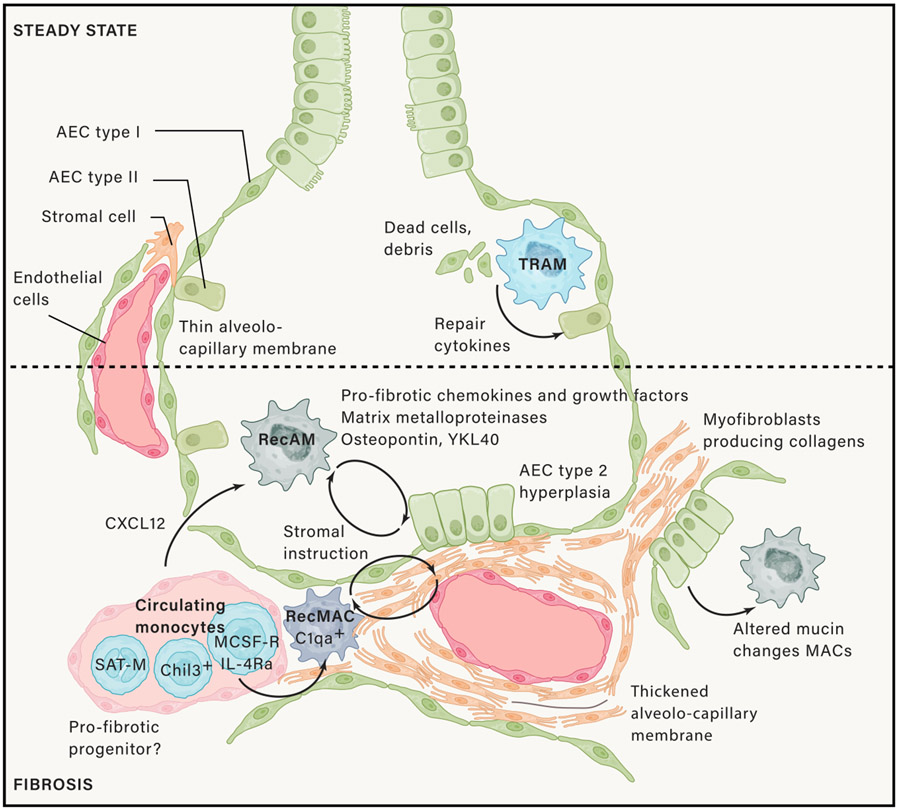
In homeostasis, TRAMs clear out apoptotic cells and other debris and, in this process, produce pro-repair genes like amphiregulin and TGF-β that is necessary for normal tissue homeostasis and repair. In fibrosis developing in response to injury, or due to an idiopathic epithelial injury, however monocytes are recruited and as these develop into recMacs or recAMs, they become programmed to contribute to fibrosis by producing profibrotic growth factors and chemokines, matrix metalloproteinases, matrix components, and other mediators. These cause activation of type 2 epithelial cells, which become hyperplastic, and activation of myofibroblasts that produce too much collagens. However, both epithelial cells and myofibroblasts then produce cytokines that can activate the recMacs to contribute further to fibrosis and can activate other niche cells to produce more collagens. This becomes a self-sustaining functional unit in progressive fibrosis.
In humans with various profibrotic lung diseases, a subset of AMs expressing SPP1 (osteopontin), CHI3L1 (YKL-40), MARCKS, IL1RN, PLA2G7, and MMP9 has been described (Reyfman et al., 2019). YKL-40 is a known severity marker in idiopathic lung fibrosis but is also able to promote fibrosis by further programming of recruited monocytes to become M2-like profibrotic fibroblasts by triggering the CRTH2 chemokine receptor (Cao et al., 2022). The chemokine CCL18 is strongly associated with lung fibrosis and is produced by an AM subset that clusters separately from other AMs, suggesting that only some AMs might be profibrotic (Li et al., 2022; Prasse et al., 2006; Serezani et al., 2022). A profibrotic population of macrophages has recently been found in the BAL fluid of COVID-19 patients with severe acute lung injury, yet co-expressed high amounts of IM-markers CD163, Lyve-1, and C1q and fibrosis promoting genes like SPP1 and legumain, suggesting that profibrotic AMs might differentiate from monocytes over a phenotype resembling IMs, or IMs enter the airspace and contribute to fibrosis (Bosteels et al., 2020a; Wendisch et al., 2021; Li et al., 2022). Specifically, in idiopathic pulmonary fibrosis, scRNA-seq on AMs identified genes upregulated by IFN-γ, cautioning that the binary M1/M2 macrophage view of fibrosis being associated with M2 has been an oversimplification (Serezani et al., 2022).
These new insights in macrophage-associated lung fibrosis are slowly leading to new therapeutic avenues, such as targeting TGF-β (Singh et al., 2022; Xu et al., 2022) and M-CSF (Joshi et al., 2020). With increasing age, a known risk factor for lung fibrosis, recMacs occupy the AM niche and contribute to fibrosis, and this has been linked to aging-associated loss of GM-CSF responsiveness (McQuattie-Pimentel et al., 2021). Therefore, an alternative therapeutic option might be to promote differentiation of homeostatic AMs, through inhalation of GM-CSF, a strategy that has been tested in several profibrotic lung diseases like pulmonary alveolar proteinosis, and even COVID-19-associated acute lung injury (Bosteels et al., 2020a; Tazawa et al., 2019). Also, the complement protein and chitinase-like protein family are largely unexplored yet highly interesting targets for immune intervention in fibrotic diseases.
Obstructive airway diseases like asthma and COPD have also been studied extensively. In allergic asthma, many rodent studies have suggested that AMs have an important dampening role in disease inception by capturing allergens in an immunologically silent way while suppressing the function of DCs and antigen-specific T cells (Lauzon-Joset et al., 2013; Soroosh et al., 2013; Strickland et al., 1993; Thepen et al., 1989, 1992). Other studies have suggested that AMs are more pro-inflammatory, an observation that may be confounded because of the accumulation of recAMs (Kurowska-Stolarska et al., 2009; Lee et al., 2015; Naessens et al., 2012). In humans with asthma, AMs show increased antigen-presenting capacity, costimulatory molecule, and adhesion molecule expression (Balbo et al., 2001; Chanez et al., 1993; Tang et al., 1998; Vieira Braga et al., 2019; Viksman et al., 2002). The function of IMs in asthma is emerging. Interleukin-10 derived from IMs has a dampening effect on the activation of lung DCs and may prevent overt immune reactions to allergens (Bedoret et al., 2009), although the IL-10 production is not extensive (Sabatel et al., 2017), and recMacs may be a more prominent source of this cytokine (Tewari et al., 2021). One subset of lung IMs are phenotypically similar to monocyte-derived DCs (moDCs), which are known to be a copious source of chemokines that recruit effector and regulatory T cells, as well as innate effector cells like eosinophils and neutrophils to the lung (Fu et al., 2022b; Medoff et al., 2009; Plantinga et al., 2013). Recently, IL-9, mainly produced by ILC2 and T helper 9 (Th9) cells in asthma, has been shown to promote activation of IL-9R-positive lung IMs, leading to the acquisition of Arg1 expression and production of chemokines that regulate allergic airway inflammation, suggesting that IMs, or recMacs resembling IMs, could also be proinflammatory (Fu et al., 2022b). Following Sendai virus infection, IMs produce increased amounts of triggering receptor expressed on myeloid cells (TREM-2). Strikingly, TREM-2 is cleaved into sTREM-2 and prevents apoptosis of IMs, leading to a prolonged M2-like state, characterized by overproduction of IL-13 and expression of Chil3 and Arg1, and intense collaboration with ILC2 (Wu et al., 2015, 2020). Clearly, more work and better tools are necessary before we can fully understand the role of macrophages in asthma.
In COPD, AMs have long been known to be in an activated state, acting even as orchestrators of the disease process, producing increased amounts of cytokines, chemokines, ROS, and matrix-degrading enzymes that could contribute to the recruitment of CD8+ T cells and neutrophils and to the process of alveolar destruction that characterizes lung emphysema (Barnes, 2004). The metabolic alterations associated with chronic smoke inhalation in AMs profoundly affect the phagocytic capacity of AMs, explaining how COPD patients might be at risk for bacterial and viral infections, often the cause of exacerbation of disease (Belchamber et al., 2019; Kono et al., 2021; Wrench et al., 2018). New scRNA-seq of COPD patients has revealed the accumulation of AMs expressing a broad range of metal-binding metallothioneins (Sauler et al., 2022). Deficiency in VitD3 or genetic polymorphisms in VitD pathway genes is a risk factor for COPD development. In Vdr−/− mice, a GM-CSF mediated expansion of ROS-producing AMs leads to progressive emphysema of the lung (Hu et al., 2019). Other pathways that control AM numbers can also lead to emphysema when disrupted. Isthmin-1 is produced by lung structural cells and promotes AM apoptosis by triggering the cell surface Grp78 receptor. Ism1−/− mice develop progressive emphysema, characterized by increased MMP12 production from AMs (Lam et al., 2022). These studies illustrate how AM activation needs to be tightly balanced to avoid lung structural damage to the alveolus. In this regard, it is well-known that interrupting endothelial or epithelial function alters AM activation and vice versa and can lead to emphysema, suggesting equilibrium in the alveolar niche is crucial (Jakkula et al., 2000).
Specific macrophage subsets participate in the tumor microenvironment
Lung cancer is very prominent particularly in smokers, although non-small-cell lung cancer (NSCLC) is also increasingly seen in non-smokers. Due to the highly vascularized nature of the gas-exchange apparatus and blood-filtering capacity, the lung is also a very frequent site of metastasis of other primary cancers. A detailed description of the biology of lung macrophages in cancer is beyond the scope of this paper, and the reader is referred to other detailed review articles (Hegde et al., 2021; Ma et al., 2022). Several scRNA-seq studies have unraveled the extraordinary complexity of macrophages in the lung tumor microenvironment (TME), and the dynamic nature of macrophage behavior as the tumor or the metastatic lesion sets up symbiosis in the host lung (Casanova-Acebes et al., 2021; Huggins et al., 2021; Lavin et al., 2017). These studies revealed there to be at least 7 different types of lung macrophages, including lipid-associated macrophages and specific tumor-associated macrophages, some of which are pro-angiogenic and immunosuppressive. Macrophages shape the TME, tumor immunity, and response to immunotherapy, which makes them an important target for cancer treatment. Using lineage tracing, it has been shown that developing NSCLC lesions in the mouse initially accumulate TRMs close to tumor cells to promote epithelial-mesenchymal transition and invasiveness in tumor cells, and these TRMs also induced a potent regulatory T cell response that protects tumor cells from adaptive immunity (Casanova-Acebes et al., 2021; Loyher et al., 2018). Depleting TRMs diminishes and alters the phenotype of regulatory T cells, promoting the accumulation of CD8+ T cells and decreasing tumor invasiveness and growth. During tumor growth, TRMs become redistributed at the periphery of the TME, which gradually become dominated by recMac in both mouse and human NSCLC (Casanova-Acebes et al., 2021). The precise signals that alter lung macrophages in the TME are still unclear. CD4+ T cell-derived IL-9 promotes the expansion of both IMs in lung tumor models, and IL-9 is overexpressed in NSCLC lesions. Mechanistically, the IL-9 macrophage axis required arginase 1 to mediate tumor growth, and adoptive transfer of Arg1+ lung macrophage to Il9r−/− mice promoted tumor growth (Fu et al., 2022a). Modulating macrophages in cancer is still difficult, as we still lack a complete understanding of the molecular and functional diversity of the tumor macrophage compartment before this can lead to effective new therapies.
Outstanding questions in the lung macrophage field
Despite the now well-recognized heterogeneity of lung macrophages, it is presently unclear if heterogeneity would reflect the different origins of AMs, micro-anatomical specializations of AMs, or historical remnants of various insults that might have altered the epigenetic and phenotypic landscape of the cells. Likewise, the precise molecular and transcriptional signals that control the development, self-replenishment, and demise of TRMs versus recruited macrophages during inflammation are currently unknown. While it has been shown that both embryonic, as well as adult, precursors give rise to transcriptionally nearly identical macrophage when they occupy an empty niche at birth, this situation could be completely altered in the aged, diseased, and injured lung, when peripheral instruction or training would be permanently changed. Recruited macrophage could have equally powerful homeostatic functions when lung homeostasis returns, or alternatively, continue to promote inflammation. The latter scenario would initially benefit host defense but could eventually become detrimental in chronic lung disease. Moreover, how recruited macrophage and TRM can co-exist is unknown, and whether co-existence has beneficial functions for lung immunity. Very relevant to lung disease, we poorly understand how chronic environmental airborne exposure like air pollution, cigarette smoke, allergen exposure, farm dust exposure, and infection history affects macrophage imprinting in the lung.
To solve these questions, we urgently need better genetic tools to study lung macrophage biology. Current fate-mapping or chimeric studies, while useful in delineating many features of differentiation, can also under-represent the contribution of bone marrow-derived cells to the macrophage population if reconstitution of the circulating precursors is incomplete. Permanent replacement of AMs by monocyte-derived cells carrying a defective gene or carrying a congenic marker to follow AM differentiation can be forced experimentally when adult mice are irradiated to empty the alveolar niche, a process inevitably causing lung inflammation, alveolocapillary leak, and genotoxic stress to self-maintaining AMs, cautioning experimental design. Many studies that describe alterations in infection ascribed AM depletions before or during infections, or in the context of respiratory allergy have used clodronate liposomes to deplete lung macrophages. This method, as well as being non-specific, can act as an adjuvant to many innate and adaptive immune cells. Indeed, any method of AM depletion will be inflammatory, given the cardinal role of these cells in maintaining homeostasis. Exquisite care must be taken to effectively identify recAMs from TRMs, so as not to ascribe a mistaken function to an otherwise innocent population.
Finally, lung macrophages are primarily phagocytes, but have many additional functions that are still largely unexplored. Both AMs and IMs can capture antigens, express MHC class I and II molecules and, in disease states, also costimulatory molecules, but it is still insufficiently understood if and how they interact with T and B cells. The field of lung macrophage biology has been obscured by the inherent complexity of myeloid cells that express many overlapping markers and metastable activation states. In the lungs, many of the functions attributed to resident AMs and IMs might therefore have been functions of recruited monocytes or macrophages that only have a short lifespan. The advent of scRNA-seq technologies and spatial transcriptomics will hopefully soon unravel this complexity in homeostasis and disease, lead to better tools to study these cells in vivo, and identify how we can best exploit this knowledge to understand, prevent, and treat lung disease.
ACKNOWLEDGMENTS
B.N.L. is supported by a FWO Methusalem grant, and by an FWO Excellence of Science grant. He has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 789384). H.A. is supported by an FWO-VLAIO Fellowship. C.V.J. is supported by National Institutes of Health grant R35 HL155458.
REFERENCES
- Aegerter H, Kulikauskaite J, Crotta S, Patel H, Kelly G, Hessel EM, Mack M, Beinke S, and Wack A (2020). Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol 21, 145–157. [Europe PMC free article] [Abstract] [Google Scholar]
- Angelidis I, Simon LM, Fernandez IE, Strunz M, Mayr CH, Greiffo FR, Tsitsiridis G, Ansari M, Graf E, Strom TM, et al. (2019). An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun 10, 963. [Europe PMC free article] [Abstract] [Google Scholar]
- Antonelli LRV, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, and Sher A (2010). Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest 120, 1674–1682. [Europe PMC free article] [Abstract] [Google Scholar]
- Arafa EI, Shenoy AT, Barker KA, Etesami NS, Martin IM, Lyon De Ana C, Na E, Odom CV, Goltry WN, Korkmaz FT, et al. (2022). Recruitment and training of alveolar macrophages after pneumococcal pneumonia. JCI Insight 7, e150239. [Europe PMC free article] [Abstract] [Google Scholar]
- Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, Chak S, Naikawadi RP, Wolters PJ, Abate AR, et al. (2019). Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol 20, 163–172. [Europe PMC free article] [Abstract] [Google Scholar]
- Atif SM, Gibbings SL, and Jakubzick CV (2018). Isolation and identification of interstitial macrophages from the lungs using different digestion enzymes and staining strategies. Methods Mol. Biol 1784, 69–76. [Europe PMC free article] [Abstract] [Google Scholar]
- Bain CC, and MacDonald AS (2022). The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity. Mucosal Immunol. 15, 223–234. [Europe PMC free article] [Abstract] [Google Scholar]
- Baker EH, and Baines DL (2018). Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest 153, 507–514. [Abstract] [Google Scholar]
- Balbo P, Silvestri M, Rossi GA, Crimi E, and Burastero SE (2001). Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin. Exp. Allergy 31, 625–636. [Abstract] [Google Scholar]
- Barnes PJ (2004). Alveolar macrophages as orchestrators of COPD. COPD 1, 59–70. [Abstract] [Google Scholar]
- Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, et al. (2009). Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest 119, 3723–3738. [Europe PMC free article] [Abstract] [Google Scholar]
- Belchamber KBR, Singh R, Batista CM, Whyte MK, Dockrell DH, Kilty I, Robinson MJ, Wedzicha JA, Barnes PJ, and Donnelly LE; COPD-MAP consortium (2019). Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur. Respir. J 54, 1802244. [Abstract] [Google Scholar]
- Bharat A, Bhorade SM, Morales-Nebreda L, Mc Quattie-Pimentel AC, Soberanes S, Ridge K, DeCamp MM, Mestan KK, Perlman H, Budinger GRS, and Misharin AV (2015). Flow cytometry reveals similarities between lung macrophages in humans and mice. Am. J. Respir. Cell Mol. Biol 54, 147–149. [Europe PMC free article] [Abstract] [Google Scholar]
- Bhattacharyya A, Boostanpour K, Bouzidi M, Magee L, Chen TY, Wolters R, Torre P, Pillai SK, and Bhattacharya M (2022). IL10 trains macrophage profibrotic function after lung injury. Am. J. Physiol. Lung Cell Mol. Physiol 322, L495–L502. [Europe PMC free article] [Abstract] [Google Scholar]
- Blériot C, Chakarov S, and Ginhoux F (2020). Determinants of resident tissue macrophage identity and function. Immunity 52, 957–970. [Abstract] [Google Scholar]
- Bobba CM, Fei Q, Shukla V, Lee H, Patel P, Putman RK, Spitzer C, Tsai M, Wewers MD, Lee RJ, et al. (2021). Nanoparticle delivery of micro-RNA-146a regulates mechanotransduction in lung macrophages and mitigates injury during mechanical ventilation. Nat. Commun 12, 289. [Europe PMC free article] [Abstract] [Google Scholar]
- Bosteels C, Maes B, Van Damme K, De Leeuw E, Declercq J, Delporte A, Demeyere B, Vermeersch S, Vuylsteke M, Willaert J, et al. (2020a). Sargramostim to treat patients with acute hypoxic respiratory failure due to COVID-19 (SARPAC): A structured summary of a study protocol for a randomised controlled trial. Trials 21, 491. [Europe PMC free article] [Abstract] [Google Scholar]
- Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N, De Prijck S, Bosteels V, Vandamme N, Martens L, et al. (2020b). Inflammatory type 2 cDCs acquire features of cdc1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52, 1039–1056.e9. [Europe PMC free article] [Abstract] [Google Scholar]
- Branchett WJ, Cook J, Oliver RA, Bruno N, Walker SA, Stölting H, Mack M, O’Garra A, Saglani S, and Lloyd CM (2021). Airway macrophage-intrinsic TGF-beta1 regulates pulmonary immunity during early-life allergen exposure. J. Allergy Clin. Immunol 147, 1892–1906. [Europe PMC free article] [Abstract] [Google Scholar]
- Busch CJL, Favret J, Geirsdóttir L, Molawi K, and Sieweke MH (2019). Isolation and long-term cultivation of mouse alveolar macrophages. Bio. Protoc 9, e3302. [Europe PMC free article] [Abstract] [Google Scholar]
- Byrne AJ, Powell JE, O’Sullivan BJ, Ogger PP, Hoffland A, Cook J, Bonner KL, Hewitt RJ, Wolf S, Ghai P, et al. (2020). Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J. Exp. Med 217, e20191236. [Europe PMC free article] [Abstract] [Google Scholar]
- Cao Y, Rudrakshala J, Williams R, Rodriguez S, Sorkhdini P, Yang AX, Mundy M, Yang D, Palmisciano A, Walsh T, et al. (2022). CRTH2 mediates pro-fibrotic macrophage differentiation and promotes lung fibrosis. Am. J. Respir. Cell Mol. Biol 67, 201–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, Brown M, Chang C, Troncoso L, Chen ST, et al. (2021). Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 595, 578–584. [Europe PMC free article] [Abstract] [Google Scholar]
- Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, Zhang XM, Foo S, Nakamizo S, Duan K, et al. (2019). Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363,eaau0964. [Abstract] [Google Scholar]
- Chanez P, Vignola AM, Lacoste P, Michel FB, Godard P, and Bousquet J (1993). Increased expression of adhesion molecules (ICAM-1 and LFA-1) on alveolar macrophages from asthmatic patients. Allergy 48, 576–580. [Abstract] [Google Scholar]
- Chen F, El-Naccache DW, Ponessa JJ, Lemenze A, Espinosa V, Wu W, Lothstein K, Jin L, Antao O, Weinstein JS, et al. (2022). Helminth resistance is mediated by differential activation of recruited monocyte-derived alveolar macrophages and arginine depletion. Cell Rep. 38, 110215. [Europe PMC free article] [Abstract] [Google Scholar]
- Coggle JE, and Tarling JD (1984). The proliferation kinetics of pulmonary alveolar macrophages. J. Leukoc. Biol 35, 317–327. [Abstract] [Google Scholar]
- Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, Sherman DR, Gerner MY, and Urdahl KB (2018). Alveolar macrophages provide an early mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24, 439–446.e4. [Europe PMC free article] [Abstract] [Google Scholar]
- Daniel B, Czimmerer Z, Halasz L, Boto P, Kolostyak Z, Poliska S, Berger WK, Tzerpos P, Nagy G, Horvath A, et al. (2020). The transcription factor EGR2 is the molecular linchpin connecting STAT6 activation to the late, stable epigenomic program of alternative macrophage polarization. Genes Dev. 34, 1474–1492. [Europe PMC free article] [Abstract] [Google Scholar]
- De Smet EG, Van Eeckhoutte HP, Avila Cobos F, Blomme E, Verhamme FM, Provoost S, Verleden SE, Venken K, Maes T, Joos GF, et al. (2020). The role of miR-155 in cigarette smoke-induced pulmonary inflammation and COPD. Mucosal Immunol. 13, 423–436. [Abstract] [Google Scholar]
- Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, Slansky JE, Jacobelli J, Mason R, Ito Y, et al. (2016). Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am. J. Respir. Crit. Care Med 193, 614–626. [Europe PMC free article] [Abstract] [Google Scholar]
- Dick SA, Wong A, Hamidzada H, Nejat S, Nechanitzky R, Vohra S, Mueller B, Zaman R, Kantores C, Aronoff L, et al. (2022). Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol 7, eabf7777. [Abstract] [Google Scholar]
- Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR, Dominguez-Andres J, et al. (2021). Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat. Immunol 22, 2–6. [Europe PMC free article] [Abstract] [Google Scholar]
- Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. (1994). Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264, 713–716. [Abstract] [Google Scholar]
- Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, et al. (2019). Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity 51, 573–589.e8. [Abstract] [Google Scholar]
- Eguíluz-Gracia I, Schultz HHL, Sikkeland LIB, Danilova E, Holm AM, Pronk CJH, Agace WW, Iversen M, Andersen C, Jahnsen FL, and Baekkevold ES (2016). Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax 71, 1006–1011. [Abstract] [Google Scholar]
- Evren E, Ringqvist E, Doisne JM, Thaller A, Sleiers N, Flavell RA, Di Santo JP, and Willinger T (2022). CD116+ fetal precursors migrate to the perinatal lung and give rise to human alveolar macrophages. J. Exp. Med 219,e20210987. [Europe PMC free article] [Abstract] [Google Scholar]
- Fu Y, Pajulas A, Wang J, Zhou B, Cannon A, Cheung CCL, Zhang J, Zhou H, Fisher AJ, Omstead DT, et al. (2022a). Mouse pulmonary interstitial macrophages mediate the pro-tumorigenic effects of IL-9. Nat. Commun 13, 3811. [Europe PMC free article] [Abstract] [Google Scholar]
- Fu Y, Wang J, Zhou B, Pajulas A, Gao H, Ramdas B, Koh B, Ulrich BJ, Yang S, Kapur R, et al. (2022b). An IL-9-pulmonary macrophage axis defines the allergic lung inflammatory environment. Sci. Immunol 7, eabi9768. [Europe PMC free article] [Abstract] [Google Scholar]
- Fujimori T, Grabiec AM, Kaur M, Bell TJ, Fujino N, Cook PC, Svedberg FR, MacDonald AS, Maciewicz RA, Singh D, and Hussell T (2015). The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol. 8, 1021–1030. [Europe PMC free article] [Abstract] [Google Scholar]
- Fukushima K, Satoh T, Sugihara F, Sato Y, Okamoto T, Mitsui Y, Yoshio S, Li S, Nojima S, Motooka D, et al. (2020). Dysregulated expression of the nuclear exosome targeting complex component Rbm7 in nonhematopoietic cells licenses the development of fibrosis. Immunity 52, 542–556.e13. [Abstract] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. (2012). Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol 13, 1118–1128. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbings SL, and Jakubzick CV (2018a). A consistent method to identify and isolate mononuclear phagocytes from human lung and lymph nodes. Methods Mol. Biol 1799, 381–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbings SL, and Jakubzick CV (2018b). Isolation and characterization of mononuclear phagocytes in the mouse lung and lymph nodes. Methods Mol. Biol 1809, 33–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, Bratton DL, Janssen W, and Jakubzick CV (2015). Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126, 1357–1366. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, Leach SM, Bratton DL, Henson PM, Janssen WJ, and Jakubzick CV (2017). Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cell Mol. Biol 57, 66–76. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. (2011). Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med 184, 569–581. [Abstract] [Google Scholar]
- Goenka A, Prise IE, Connolly E, Fernandez-Soto P, Morgan D, Cavet JS, Grainger JR, Nichani J, Arkwright PD, and Hussell T (2020). Infant alveolar macrophages are unable to effectively contain mycobacterium tuberculosis. Front. Immunol 11, 486. [Europe PMC free article] [Abstract] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, and Rodewald HR (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. [Europe PMC free article] [Abstract] [Google Scholar]
- Goritzka M, Makris S, Kausar F, Durant LR, Pereira C, Kumagai Y, Culley FJ, Mack M, Akira S, and Johansson C (2015). Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med 212, 699–714. [Europe PMC free article] [Abstract] [Google Scholar]
- Gorki AD, Symmank D, Zahalka S, Lakovits K, Hladik A, Langer B, Maurer B, Sexl V, Kain R, and Knapp S (2021). Murine ex vivo cultured alveolar macrophages provide a novel tool to study tissue-resident macrophage behavior and function. Am. J. Respir. Cell Mol. Biol 66, 64–75. [Europe PMC free article] [Abstract] [Google Scholar]
- Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, Abbott DA, Donnelly HK, Donayre A, Goldberg IA, et al. (2021). Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 590, 635–641. [Europe PMC free article] [Abstract] [Google Scholar]
- Greiffo FR, Viteri-Alvarez V, Frankenberger M, Dietel D, Ortega-Gomez A, Lee JS, Hilgendorff A, Behr J, Soehnlein O, Eickelberg O, and Fernandez IE (2020). CX3CR1-fractalkine axis drives kinetic changes of monocytes in fibrotic interstitial lung diseases. Eur. Respir. J 55, 1900460. [Abstract] [Google Scholar]
- Gschwend J, Sherman SPM, Ridder F, Feng X, Liang HE, Locksley RM, Becher B, and Schneider C (2021). Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J. Exp. Med 218, e20210745. [Europe PMC free article] [Abstract] [Google Scholar]
- Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, Bujko A, Martens L, Thoné T, Browaeys R, et al. (2022). Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 185, 379–396.e38. [Europe PMC free article] [Abstract] [Google Scholar]
- Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, and Lambrecht BN (2013). Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med 210, 1977–1992. [Europe PMC free article] [Abstract] [Google Scholar]
- Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger M, De Prijck S, et al. (2016). Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45, 669–684. [Europe PMC free article] [Abstract] [Google Scholar]
- Guillon A, Arafa EI, Barker KA, Belkina AC, Martin I, Shenoy AT, Wooten AK, Lyon De Ana C, Dai A, Labadorf A, et al. (2020). Pneumonia recovery reprograms the alveolar macrophage pool. JCI Insight 5, 133042. [Europe PMC free article] [Abstract] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. (2013). Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804. [Europe PMC free article] [Abstract] [Google Scholar]
- Hegde S, Leader AM, and Merad M (2021). MDSC: Markers, development, states, and unaddressed complexity. Immunity 54, 875–884. [Europe PMC free article] [Abstract] [Google Scholar]
- Herold S, Mayer K, and Lohmeyer J (2011). Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol 2, 65. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, et al. (2015). C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678. [Europe PMC free article] [Abstract] [Google Scholar]
- Hu G, Dong T, Wang S, Jing H, and Chen J (2019). Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicine 45, 563–577. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang L, Nazarova EV, Tan S, Liu Y, and Russell DG (2018). Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med 215, 1135–1152. [Europe PMC free article] [Abstract] [Google Scholar]
- Huggins DN, LaRue RS, Wang Y, Knutson TP, Xu Y, Williams JW, and Schwertfeger KL (2021). Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 81, 5284–5295. [Europe PMC free article] [Abstract] [Google Scholar]
- Hume PS, Gibbings SL, Jakubzick CV, Tuder RM, Curran-Everett D, Henson PM, Smith BJ, and Janssen WJ (2020). Localization of macrophages in the human lung via design-based stereology. Am. J. Respir. Crit. Care Med 201, 1209–1217. [Europe PMC free article] [Abstract] [Google Scholar]
- Hung LY, Sen D, Oniskey TK, Katzen J, Cohen NA, Vaughan AE, Nieves W, Urisman A, Beers MF, Krummel MF, and Herbert DR (2019). Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol. 12, 64–76. [Europe PMC free article] [Abstract] [Google Scholar]
- Ikeda N, Asano K, Kikuchi K, Uchida Y, Ikegami H, Takagi R, Yotsumoto S, Shibuya T, Makino-Okamura C, Fukuyama H, et al. (2018). Emergence of immunoregulatory Ym1(+)Ly6C(hi) monocytes during recovery phase of tissue injury. Sci. Immunol 3, eaat0207. [Abstract] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, and Abman SH (2000). Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol 279, L600–L607. [Abstract] [Google Scholar]
- Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, and Hogaboam CM (2003). Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J. Immunol 171, 2684–2693. [Abstract] [Google Scholar]
- Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, et al. (2013). Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 39, 599–610. [Europe PMC free article] [Abstract] [Google Scholar]
- Janssen WJ, Danhorn T, Harris C, Mould KJ, Lee FFY, Hedin BR, D’Alessandro A, Leach SM, and Alper S (2020). Inflammation-induced alternative pre-mRNA splicing in mouse alveolar macrophages. G3 (Bethesda) 10, 555–567. [Europe PMC free article] [Abstract] [Google Scholar]
- Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson PM, and Gardai SJ (2008). Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am. J. Respir. Crit. Care Med 178, 158–167. [Europe PMC free article] [Abstract] [Google Scholar]
- Jardine L, Wiscombe S, Reynolds G, McDonald D, Fuller A, Green K, Filby A, Forrest I, Ruchaud-Sparagano MH, Scott J, et al. (2019). Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat. Commun 10, 1999. [Europe PMC free article] [Abstract] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, and Allen JE (2011). Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288. [Europe PMC free article] [Abstract] [Google Scholar]
- Joshi N, Watanabe S, Verma R, Jablonski RP, Chen CI, Cheresh P, Markov NS, Reyfman PA, McQuattie-Pimentel AC, Sichizya L, et al. (2020). A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J 55, 1900646. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawano H, Kayama H, Nakama T, Hashimoto T, Umemoto E, and Takeda K (2016). IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int. Immunol 28, 489–501. [Abstract] [Google Scholar]
- Keerthivasan S, Şenbabaoğlu Y, Martinez-Martin N, Husain B, Verschueren E, Wong A, Yang YA, Sun Y, Pham V, Hinkle T, et al. (2021). Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity 54, 1511–1526.e8. [Abstract] [Google Scholar]
- Kono Y, Colley T, To M, Papaioannou AI, Mercado N, Baker JR, To Y, Abe S, Haruki K, Ito K, and Barnes PJ (2021). Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Sci. Rep 11, 335. [Europe PMC free article] [Abstract] [Google Scholar]
- Koth LL, Alex B, Hawgood S, Nead MA, Sheppard D, Erle DJ, and Morris DG (2007). Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1. Am. J. Respir. Cell Mol. Biol 37, 651–659. [Europe PMC free article] [Abstract] [Google Scholar]
- Kropski JA, and Blackwell TS (2019). Progress in understanding and treating idiopathic pulmonary fibrosis. Annu. Rev. Med 70, 211–224. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. (2009). IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol 183, 6469–6477. [Abstract] [Google Scholar]
- Lafuse WP, Rajaram MVS, Wu Q, Moliva JI, Torrelles JB, Turner J, and Schlesinger LS (2019). Identification of an increased alveolar macrophage subpopulation in old mice that displays unique inflammatory characteristics and is permissive to mycobacterium tuberculosis infection. J. Immunol 203, 2252–2264. [Europe PMC free article] [Abstract] [Google Scholar]
- Lam TYW, Nguyen N, Peh HY, Shanmugasundaram M, Chandna R, Tee JH, Ong CB, Hossain MZ, Venugopal S, Zhang T, et al. (2022). ISM1 protects lung homeostasis via cell-surface GRP78-mediated alveolar macrophage apoptosis. Proc. Natl. Acad. Sci. USA 119. e2019161119. [Europe PMC free article] [Abstract] [Google Scholar]
- Larsen SB, Cowley CJ, Sajjath SM, Barrows D, Yang Y, Carroll TS, and Fuchs E (2021). Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell 28, 1758–1774.e8. [Europe PMC free article] [Abstract] [Google Scholar]
- Lauzon-Joset JF, Marsolais D, Langlois A, and Bissonnette EY (2013). Dysregulation of alveolar macrophages unleashes dendritic cell-mediated mechanisms of allergic airway inflammation. Mucosal Immunol. 7, 155–164. [Abstract] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir EAD, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. (2017). Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765.e17. [Europe PMC free article] [Abstract] [Google Scholar]
- Lavrich KS, Speen AM, Ghio AJ, Bromberg PA, Samet JM, and Alexis NE (2018). Macrophages from the upper and lower human respiratory tract are metabolically distinct. Am. J. Physiol. Lung Cell Mol. Physiol 315, L752–L764. [Europe PMC free article] [Abstract] [Google Scholar]
- Leach SM, Gibbings SL, Tewari AD, Atif SM, Vestal B, Danhorn T, Janssen WJ, Wager TD, and Jakubzick CV (2020). Human and mouse transcriptome profiling identifies cross-species homology in pulmonary and lymph node mononuclear phagocytes. Cell Rep. 33, 108337. [Europe PMC free article] [Abstract] [Google Scholar]
- Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, and Rock JR (2017). Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21, 120–134.e7. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly EAB, et al. (2015). Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol 52, 772–784. [Europe PMC free article] [Abstract] [Google Scholar]
- Li X, Kolling FW, Aridgides D, Mellinger D, Ashare A, and Jakubzick CV (2022). ScRNA-seq expression of IFI27 and APOC2 identifies four alveolar macrophage superclusters in healthy BALF. Life Sci. Alliance 5. e202201458. [Europe PMC free article] [Abstract] [Google Scholar]
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, et al. (2020). Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med 26, 842–844. [Abstract] [Google Scholar]
- Lim HY, Lim SY, Tan CK, Thiam CH, Goh CC, Carbajo D, Chew SHS, See P, Chakarov S, Wang XN, et al. (2018). Hyaluronan receptor LYVE-1-expressing macrophages maintain arterial tone through hyaluronan-mediated regulation of smooth muscle cell collagen. Immunity 49, 1191–1341. e327. [Abstract] [Google Scholar]
- Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, Shin A, Huang W, Dress RJ, Dutertre CA, et al. (2019). Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell 178, 1509–1525.e19. [Abstract] [Google Scholar]
- Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadière B, et al. (2018). Macrophages of distinct origins contribute to tumor development in the lung. J. Exp. Med 215, 2536–2553. [Europe PMC free article] [Abstract] [Google Scholar]
- Ma RY, Black A, and Qian BZ (2022). Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563. [Abstract] [Google Scholar]
- Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, Desmecht D, Lallemand F, Martinive P, Hammad H, et al. (2017). A gamma-herpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol 18, 1310–1320. [Abstract] [Google Scholar]
- MacLean JA, Xia W, Pinto CE, Zhao L, Liu HW, and Kradin RL (1996). Sequestration of inhaled particulate antigens by lung phagocytes: A mechanism for the effective inhibition of pulmonary cell- mediated immunity. Am. J. Pathol 148, 657–666. [Europe PMC free article] [Abstract] [Google Scholar]
- Martinez JA, King TE Jr., Brown K, Jennings CA, Borish L, Mortenson RL, Khan TZ, Bost TW, and Riches DW (1997). Increased expression of the interleukin-10 gene by alveolar macrophages in interstitial lung disease. Am. J. Physiol 273, L676–L683. [Abstract] [Google Scholar]
- Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, et al. (2016). Specification of tissue-resident macrophages during organogenesis. Science 353, aaf4238. [Europe PMC free article] [Abstract] [Google Scholar]
- Mata E, Tarancon R, Guerrero C, Moreo E, Moreau F, Uranga S, Gomez AB, Marinova D, Domenech M, Gonzalez-Camacho F, et al. (2021). Pulmonary BCG induces lung-resident macrophage activation and confers long-term protection against tuberculosis. Sci. Immunol 6, eabc2934. [Abstract] [Google Scholar]
- McCubbrey AL, Allison KC, Lee-Sherick AB, Jakubzick CV, and Janssen WJ (2017). Promoter specificity and efficacy in conditional and inducible transgenic targeting of lung macrophages. Front. Immunol 8, 1618. [Europe PMC free article] [Abstract] [Google Scholar]
- McCubbrey AL, Barthel L, Mohning MP, Redente EF, Mould KJ, Thomas SM, Leach SM, Danhorn T, Gibbings SL, Jakubzick CV, et al. (2018). Deletion of c-FLIP from CD11b(hi) macrophages prevents development of bleomycin-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol 58, 66–78. [Europe PMC free article] [Abstract] [Google Scholar]
- McQuattie-Pimentel AC, Ren Z, Joshi N, Watanabe S, Stoeger T, Chi M, Lu Z, Sichizya L, Aillon RP, Chen CI, et al. (2021). The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Invest 131, 140299. [Europe PMC free article] [Abstract] [Google Scholar]
- Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, and Luster AD (2009). CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. J. Immunol 182, 623–635. [Europe PMC free article] [Abstract] [Google Scholar]
- Meghraoui-Kheddar A, Barthelemy S, Boissonnas A, and Combadière C (2020). Revising CX3CR1 expression on murine classical and non-classical monocytes. Front. Immunol 11, 1117. [Europe PMC free article] [Abstract] [Google Scholar]
- Meliopoulos VA, Van de Velde LA, Van de Velde NC, Karlsson EA, Neale G, Vogel P, Guy C, Sharma S, Duan S, Surman SL, et al. (2016). An epithelial integrin regulates the amplitude of protective lung interferon responses against multiple respiratory pathogens. PLoS Pathog. 12, e1005804. [Europe PMC free article] [Abstract] [Google Scholar]
- Miki H, Pei H, Gracias DT, Linden J, and Croft M (2021). Clearance of apoptotic cells by lung alveolar macrophages prevents development of house dust mite-induced asthmatic lung inflammation. J. Allergy Clin. Immunol 147, 1087–1092.e3. [Europe PMC free article] [Abstract] [Google Scholar]
- Minutti CM, Jackson-Jones LH, García-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, et al. (2017). Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080. [Europe PMC free article] [Abstract] [Google Scholar]
- Minutti CM, Modak RV, Macdonald F, Li F, Smyth DJ, Dorward DA, Blair N, Husovsky C, Muir A, Giampazolias E, et al. (2019).A macrophagepericyte axis directs tissue restoration via amphiregulin-induced transforming growth factor beta activation. Immunity 50, 645–654.e6. [Europe PMC free article] [Abstract] [Google Scholar]
- Mirchandani AS, Jenkins SJ, Bain CC, Sanchez-Garcia MA, Lawson H, Coelho P, Murphy F, Griffith DM, Zhang A, Morrison T, et al. (2022). Hypoxia shapes the immune landscape in lung injury and promotes the persistence of inflammation. Nat. Immunol 23, 927–939. [Europe PMC free article] [Abstract] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GRS, and Perlman H (2013). Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol 49, 503–510. [Europe PMC free article] [Abstract] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, et al. (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med 214, 2387–2404. [Europe PMC free article] [Abstract] [Google Scholar]
- Mould KJ, Barthel L, Mohning MP, Thomas SM, McCubbrey AL, Danhorn T, Leach SM, Fingerlin TE, O’Connor BP, Reisz JA, et al. (2017). Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am. J. Respir. Cell Mol. Biol 57, 294–306. [Europe PMC free article] [Abstract] [Google Scholar]
- Mould KJ, Jackson ND, Henson PM, Seibold M, and Janssen WJ (2019). Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI Insight 4, 126556. [Europe PMC free article] [Abstract] [Google Scholar]
- Mould KJ, Moore CM, McManus SA, McCubbrey AL, McClendon JD, Griesmer CL, Henson PM, and Janssen WJ (2021). Airspace macrophages and monocytes exist in transcriptionally distinct subsets in healthy adults. Am. J. Respir. Crit. Care Med 203, 946–956. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulder K, Patel AA, Kong WT, Piot C, Halitzki E, Dunsmore G, Khalilnezhad S, Irac SE, Dubuisson A, Chevrier M, et al. (2021). Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883–1900.e5. e1885. [Abstract] [Google Scholar]
- Naessens T, Vander Beken S, Bogaert P, Van Rooijen N, Lienenklaus S, Weiss S, De Koker S, and Grooten J (2012). Innate imprinting of murine resident alveolar macrophages by allergic bronchial inflammation causes a switch from hypoinflammatory to hyperinflammatory reactivity. Am. J. Pathol 181, 174–184. [Abstract] [Google Scholar]
- Nakano H, Moran TP, Nakano K, Gerrish KE, Bortner CD, and Cook DN (2015). Complement receptor C5aR1/CD88 and dipeptidyl peptidase-4/CD26 define distinct hematopoietic lineages of dendritic cells. J. Immunol 194, 3808–3819. [Europe PMC free article] [Abstract] [Google Scholar]
- Nayak DK, Zhou F, Xu M, Huang J, Tsuji M, Hachem R, and Mohanakumar T (2016). Long-term persistence ofdonor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses. Am. J. Transplant 16, 2300–2311. [Europe PMC free article] [Abstract] [Google Scholar]
- Neupane AS, Willson M, Chojnacki AK, Vargas E Silva Castanheira F, Morehouse C, Carestia A, Keller AE, Peiseler M, DiGiandomenico A, Kelly MM, et al. (2020). Patrolling alveolar macrophages conceal bacteria from the immune system to maintain homeostasis. Cell 183, 110–125.e11. [Abstract] [Google Scholar]
- Ogawa T, Shichino S, Ueha S, Bando K, and Matsushima K (2022). Profibrotic properties of C1q(+) interstitial macrophages in silica-induced pulmonary fibrosis in mice. Biochem. Biophys. Res. Commun 599, 113–119. [Abstract] [Google Scholar]
- O’Neill LAJ, Kishton RJ, and Rathmell J (2016). A guide to immunometabolism for immunologists. Nat. Rev. Immunol 16, 553–565. [Europe PMC free article] [Abstract] [Google Scholar]
- Papp AC, Azad AK, Pietrzak M, Williams A, Handelman SK, Igo RP Jr., Stein CM, Hartmann K, Schlesinger LS, and Sadee W (2018). Ampli-Seq transcriptome analysis of human alveolar and monocyte-derived macrophages over time in response to Mycobacterium tuberculosis infection. PLoS One 13, e0198221. [Europe PMC free article] [Abstract] [Google Scholar]
- Phelps DS, Umstead TM, Quintero OA, Yengo CM, and Floros J (2011). In vivo rescue of alveolar macrophages from SP-A knockout mice with exogenous SP-A nearly restores a wild type intracellular proteome; actin involvement. Proteome Sci. 9, 67. [Europe PMC free article] [Abstract] [Google Scholar]
- Pisu D, Huang L, Grenier JK, and Russell DG (2020). Dual RNA-seq of Mtb-infected macrophages in vivo reveals ontologically distinct host-pathogen interactions. Cell Rep. 30, 335–350.e4. [Europe PMC free article] [Abstract] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, et al. (2013). Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335. [Abstract] [Google Scholar]
- Podsiad A, Standiford TJ, Ballinger MN, Eakin R, Park P, Kunkel SL, Moore BB, and Bhan U (2016). MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway. Am. J. Physiol. Lung Cell Mol. Physiol 310, L465–L475. [Europe PMC free article] [Abstract] [Google Scholar]
- Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Müller-Quernheim J, and Zissel G (2006). A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Respir. Crit. Care Med 173, 781–792. [Abstract] [Google Scholar]
- Rauschmeier R, Gustafsson C, Reinhardt A, A-Gonzalez N, Tortola L, Cansever D, Subramanian S, Taneja R, Rossner MJ, Sieweke MH, et al. (2019). Bhlhe40 and Bhlhe41 transcription factors regulate alveolar macrophage self-renewal and identity. EMBO J. 38, e101233. [Europe PMC free article] [Abstract] [Google Scholar]
- Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, et al. (2019). Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med 199, 1517–1536. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, and Barton GM (2017). Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 47, 913–927.e6. [Europe PMC free article] [Abstract] [Google Scholar]
- Rodero MP, Poupel L, Loyher PL, Hamon P, Licata F, Pessel C, Hume DA, Combadière C, and Boissonnas A (2015). Immune surveillance of the lung by migrating tissue monocytes. Elife 4, e07847. [Europe PMC free article] [Abstract] [Google Scholar]
- Roquilly A, Jacqueline C, Davieau M, Mollé A, Sadek A, Fourgeux C, Rooze P, Broquet A, Misme-Aucouturier B, Chaumette T, et al. (2020). Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol 21, 636–648. [Abstract] [Google Scholar]
- Rothchild AC, Olson GS, Nemeth J, Amon LM, Mai D, Gold ES, Diercks AH, and Aderem A (2019). Alveolar macrophages generate a noncanonical NRF2-driven transcriptional response to Mycobacterium tuberculosis in vivo. Sci. Immunol 4, eaaw6693. [Europe PMC free article] [Abstract] [Google Scholar]
- Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, Olivier S, Toussaint M, Pirottin D, Xiao X, et al. (2017). Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 46, 457–473. [Abstract] [Google Scholar]
- Sajti E, Link VM, Ouyang Z, Spann NJ, Westin E, Romanoski CE, Fonseca GJ, Prince LS, and Glass CK (2020). Transcriptomic and epigenetic mechanisms underlying myeloid diversity in the lung. Nat. Immunol 21, 221–231. [Europe PMC free article] [Abstract] [Google Scholar]
- Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, et al. (2017). Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 541, 96–101. [Abstract] [Google Scholar]
- Sauler M, McDonough JE, Adams TS, Kothapalli N, Barnthaler T, Werder RB, Schupp JC, Nouws J, Robertson MJ, Coarfa C, et al. (2022). Characterization of the COPD alveolar niche using single-cell RNA sequencing. Nat. Commun 13, 494. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawyer RT, Strausbauch PH, and Volkman A (1982). Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab. Invest 46, 165–170. [Abstract] [Google Scholar]
- Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, and Kopf M (2014). Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol 15, 1026–1037. [Abstract] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, et al. (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [Abstract] [Google Scholar]
- Schyns J, Bai Q, Ruscitti C, Radermecker C, De Schepper S, Chakarov S, Farnir F, Pirottin D, Ginhoux F, Boeckxstaens G, et al. (2019). Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun 10, 3964. [Europe PMC free article] [Abstract] [Google Scholar]
- Serezani APM, Pascoalino BD, Bazzano JMR, Vowell KN, Tanjore H, Taylor CJ, Calvi CL, McCall AS, Bacchetta MD, Shaver CM, et al. (2022). Multi-platform single-cell analysis identifies immune cell types enhanced in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 67, 50–60. [Europe PMC free article] [Abstract] [Google Scholar]
- Singh A, Chakraborty S, Wong SW, Hefner NA, Stuart A, Qadir AS, Mukhopadhyay A, Bachmaier K, Shin JW, Rehman J, and Malik AB (2022). Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis. Proc. Natl. Acad. Sci. USA 119. e2121098119. [Europe PMC free article] [Abstract] [Google Scholar]
- Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, and Hussell T (2008). A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol 9, 1074–1083. [Abstract] [Google Scholar]
- Snyder ME, Sembrat J, Noda K, Myerburg MM, Craig A, Mitash N, Harano T, Furukawa M, Pilewski J, McDyer J, et al. (2021). Human lung-resident macrophages colocalize with and provide costimulation to PD1(hi) tissue-resident memory T cells. Am. J. Respir. Crit. Care Med 203, 1230–1244. [Europe PMC free article] [Abstract] [Google Scholar]
- Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, and Croft M (2013). Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med 210, 775–788. [Europe PMC free article] [Abstract] [Google Scholar]
- Soucie EL, Weng Z, Geirsdóttir L, Molawi K, Maurizio J, Fenouil R, Mossadegh-Keller N, Gimenez G, VanHille L, Beniazza M, et al. (2016). Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science 351, aad5510. [Europe PMC free article] [Abstract] [Google Scholar]
- Strickland DH, Thepen T, Kees UR, Kraal G, and Holt PG (1993). Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology 80, 266–272. [Abstract] [Google Scholar]
- Sturrock A, Mir-Kasimov M, Baker J, Rowley J, and Paine R 3rd (2014). Key role of microRNA in the regulation of granulocyte macrophage colony-stimulating factor expression in murine alveolar epithelial cells during oxidative stress. J. Biol. Chem 289, 4095–4105. [Europe PMC free article] [Abstract] [Google Scholar]
- Subramanian S, Busch CJL, Molawi K, Geirsdottir L, Maurizio J, Vargas Aguilar S, Belahbib H, Gimenez G, Yuda RAA, Burkon M, et al. (2022). Long-term culture-expanded alveolar macrophages restore their full epigenetic identity after transfer in vivo. Nat. Immunol 23, 458–468. [Abstract] [Google Scholar]
- Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, et al. (2008). Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med 205, 2703–2710. [Europe PMC free article] [Abstract] [Google Scholar]
- Svedberg FR, Brown SL, Krauss MZ, Campbell L, Sharpe C, Clausen M, Howell GJ, Clark H, Madsen J, Evans CM, et al. (2019). The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat. Immunol 20, 571–580. [Europe PMC free article] [Abstract] [Google Scholar]
- Tang C, Rolland JM, Li X, Ward C, Bish R, and Walters EH (1998). Alveolar macrophages from atopic asthmatics, but not atopic nonasthmatics, enhance interleukin-5 production by CD4+ T cells. Am. J. Respir. Crit. Care Med 157, 1120–1126. [Abstract] [Google Scholar]
- Tang X-Z, Kreuk LSM, Cho C, Metzger RJ, and Allen CDC (2022). Bronchus-associated macrophages are positioned for soluble antigen capture from the airway lumen and for local Th2 cell activation. Preprint at bioRxiv, 10.1016/2020.2009.2018.247742. [CrossRef] [Google Scholar]
- Tarling JD, and Coggle JE (1982). Evidence for the pulmonary origin of alveolar macrophages. Cell Tissue Kinet. 15, 577–584. [Abstract] [Google Scholar]
- Tazawa R, Ueda T, Abe M, Tatsumi K, Eda R, Kondoh S, Morimoto K, Tanaka T, Yamaguchi E, Takahashi A, et al. (2019). Inhaled GM-CSF for Pulmonary Alveolar Proteinosis. N. Engl. J. Med 381, 923–932. [Abstract] [Google Scholar]
- Tewari A, Prabagar MG, Gibbings SL, Rawat K, and Jakubzick CV (2021). LN Monocytes Limit DC-Poly I:C Induced Cytotoxic T Cell Response via IL-10 and Induction of Suppressor CD4 T Cells. Front. Immunol 12, 763379. [Europe PMC free article] [Abstract] [Google Scholar]
- Thepen T, McMenamin C, Girn B, Kraal G, and Holt PG (1992). Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin. Exp. Allergy 22, 1107–1114. [Abstract] [Google Scholar]
- Thepen T, McMenamin C, Oliver J, Kraal G, and Holt PG (1991). Regulation of immune responses to inhaled antigen by alveolar macrophages (AM) : differential effects of AM elimination in vivo on the induction of tolerance versus immunity. Eur. J. Immunol 21, 2845–2850. [Abstract] [Google Scholar]
- Thepen T, Van Rooijen N, and Kraal G (1989). Alveolar macrophage elimination in vivo is associated in vivo with an increase in pulmonary immune responses in mice. J. Exp. Med 170, 499–509. [Europe PMC free article] [Abstract] [Google Scholar]
- Todd EM, Zhou JY, Szasz TP, Deady LE, D’Angelo JA, Cheung MD, Kim AHJ, and Morley SC (2016). Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli. Blood 128, 2785–2796. [Europe PMC free article] [Abstract] [Google Scholar]
- Ural BB, Yeung ST, Damani-Yokota P, Devlin JC, de Vries M, Vera-Licona P, Samji T, Sawai CM, Jang G, Perez OA, et al. (2020). Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol 5, eaax8756. [Europe PMC free article] [Abstract] [Google Scholar]
- van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, and Guilliams M (2016). Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity 44, 755–768. [Abstract] [Google Scholar]
- van oud Alblas AB, and van Furth R (1979). Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J. Exp. Med 149, 1504–1518. [Europe PMC free article] [Abstract] [Google Scholar]
- Vermaelen K, and Pauwels R (2004). Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 61, 170–177. [Abstract] [Google Scholar]
- Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, Brouwer S, Gomes T, Hesse L, Jiang J, et al. (2019). A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med 25, 1153–1163. [Abstract] [Google Scholar]
- Viksman MY, Bochner BS, Peebles RS, Schleimer RP, and Liu MC (2002). Expression of activation markers on alveolar macrophages in allergic asthmatics after endobronchial or whole-lung allergen challenge. Clin. Immunol. 104, 77–85. [Abstract] [Google Scholar]
- Volkman A, and Gowans JL (1965). The Origin of Macrophages from Bone Marrow in the Rat. Br. J. Exp. Pathol 46, 62–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Volkman A, Chang NC, Strausbauch PH, and Morahan PS (1983). Differential effects of chronic monocyte depletion on macrophage populations. Lab. Invest 49, 291–298. [Abstract] [Google Scholar]
- Wang W, Liu Z, Su J, Chen WS, Wang XW, Bai SX, Zhang JZ, and Yu SQ (2016). Macrophage micro-RNA-155 promotes lipopolysaccharide-induced acute lung injury in mice and rats. Am. J. Physiol. Lung Cell Mol. Physiol 311, L494–L506. [Abstract] [Google Scholar]
- Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, Mache C, Chua RL, Knoll R, Timm S, et al. (2021). SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 184, 6243–6261.e27. [Europe PMC free article] [Abstract] [Google Scholar]
- Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, and Bhattacharya J (2014). Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506, 503–506. [Europe PMC free article] [Abstract] [Google Scholar]
- Willart MAM, Jan de Heer H, Hammad H, Soullié T, Deswarte K, Clausen BE, Boon L, Hoogsteden HC, and Lambrecht BN (2009). The lung vascular filter as a site of immune induction for T cell responses to large embolic antigen. J. Exp. Med 206, 2823–2835. [Europe PMC free article] [Abstract] [Google Scholar]
- Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, and Goldstein DR (2017). Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J. Immunol 199, 1060–1068. [Europe PMC free article] [Abstract] [Google Scholar]
- Wrench C, Belchamber KBR, Bercusson A, Shah A, Barnes PJ, Armstrong-James D, and Donnelly LE (2018). Reduced clearance of fungal spores by chronic obstructive pulmonary disease GM-CSF- and M-CSF-derived macrophages. Am. J. Respir. Cell Mol. Biol 58, 271–273. [Abstract] [Google Scholar]
- Wu K, Byers DE, Jin X, Agapov E, Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober DL, et al. (2015). TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med 212, 681–697. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu K, Wang X, Keeler SP, Gerovac BJ, Agapov EV, Byers DE, Gilfillan S, Colonna M, Zhang Y, and Holtzman MJ (2020). Group 2 innate lymphoid cells must partner with the myeloid-macrophage lineage for long-term postviral lung disease. J. Immunol 205, 1084–1101. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu D, Bhattacharyya S, Wang W, Ifergan I, Wong MYAC, Procissi D, Yeldandi A, Bale S, Marangoni RG, Horbinski C, et al. (2022). PLG nanoparticles target fibroblasts and MARCO+ monocytes to reverse multiorgan fibrosis. JCI Insight 7, e151037. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu-Vanpala S, Deerhake ME, Wheaton JD, Parker ME, Juvvadi PR, MacIver N, Ciofani M, and Shinohara ML (2020). Functional heterogeneity of alveolar macrophage population based on expression of CXCL2. Sci. Immunol 5, eaba7350. [Europe PMC free article] [Abstract] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. (2013). Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu X, Buttgereit A, Lelios I, Cansever D, Becher B, and Greter M (2017). TGF-beta controls the development and homeostatis of alveolar macrophages. Immunity. [Abstract] [Google Scholar]
- Yu YRA, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, Kraft M, Hollingsworth JW, Gunn MD, and Tighe RM (2015). Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am. J. Respir. Cell Mol. Biol 54, 13–24. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou B, Magana L, Hong Z, Huang LS, Chakraborty S, Tsukasaki Y, Huang C, Wang L, Di A, Ganesh B, et al. (2020). The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic-epigenetic reprogramming and resolves inflammatory injury. Nat. Immunol 21, 1430–1443. [Europe PMC free article] [Abstract] [Google Scholar]
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/135865354
Article citations
Periodontal conditions and salivary microbiota are potential indicators to distinguish silicosis: an exploratory study.
BMC Microbiol, 24(1):438, 28 Oct 2024
Cited by: 0 articles | PMID: 39465426 | PMCID: PMC11514746
The deadly dance of alveolar macrophages and influenza virus.
Eur Respir Rev, 33(174):240132, 30 Oct 2024
Cited by: 0 articles | PMID: 39477353 | PMCID: PMC11522969
Review Free full text in Europe PMC
Progesterone promotes CXCl2-dependent vaginal neutrophil killing by activating cervical resident macrophage-neutrophil crosstalk.
JCI Insight, 9(20):e177899, 22 Oct 2024
Cited by: 0 articles | PMID: 39298265 | PMCID: PMC11529979
Cancer-induced systemic pre-conditioning of distant organs: building a niche for metastatic cells.
Nat Rev Cancer, 10 Oct 2024
Cited by: 0 articles | PMID: 39390247
Review
Inhaled GM-CSF administered during ongoing pneumovirus infection alters myeloid and CD8 T cell immunity without affecting disease outcome.
Front Immunol, 15:1439789, 08 Oct 2024
Cited by: 0 articles | PMID: 39439800 | PMCID: PMC11493702
Go to all (135) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Advances on physiology and pathology of subpopulations of macrophages in the lung tissue].
Zhejiang Da Xue Xue Bao Yi Xue Ban, 53(5):650-658, 01 Oct 2024
Cited by: 0 articles | PMID: 39343742 | PMCID: PMC11528147
Review Free full text in Europe PMC
Proteomics: An advanced tool to unravel the role of alveolar macrophages in respiratory diseases.
Int J Biochem Cell Biol, 134:105966, 05 Mar 2021
Cited by: 2 articles | PMID: 33677070
Review
Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury.
J Immunol Methods, 403(1-2):7-16, 23 Nov 2013
Cited by: 37 articles | PMID: 24280595
Alveolar Macrophages.
Cell Immunol, 330:86-90, 20 Jan 2018
Cited by: 139 articles | PMID: 29370889
Review



