Abstract
Background
Malaria in pregnancy has been associated with worse cognitive outcomes in children, but its association with behavioral outcomes and the effectiveness of malaria chemoprevention on child neurodevelopment are not well characterized.Methods
To determine if more effective malaria chemoprevention in mothers and their children results in better neurodevelopment, 305 pregnant women were randomly assigned to 3 doses of sulfadoxine-pyrimethamine, 3 doses of dihydroartemisinin-piperaquine (DP), or monthly DP during pregnancy, and their 293 children were assigned to DP every 3 months or monthly DP from 2 to 24 months of age. Cognition, language, and motor function were assessed at 12, 24. and 36 months of age, and attention, memory, behavior, and executive function were assessed at 24 and 36 months of age.Results
Children of mothers with versus without malaria in pregnancy had worse scores on cognitive, behavioral, and executive function outcomes at 24 months. Clinical malaria in children within the first 12 months was similarly associated with poorer scores in behavior and executive function at 24 months, language at 24 and 36 months, and motor function scores at 36 months. However, more effective malaria chemoprevention in the mothers and children was not associated with better outcomes.Conclusions
Malaria in pregnancy was associated with worse cognitive, behavioral, and executive function scores in affected children, but more effective malaria chemoprevention measures did not result in better outcomes. Malaria chemoprevention prior to and early in gestation and with even higher efficacy in mothers and children may be required to prevent neurodevelopmental impairment in children. Clinical Trials Registration. NCT02557425.Free full text

Effect of Malaria and Malaria Chemoprevention Regimens in Pregnancy and Childhood on Neurodevelopmental and Behavioral Outcomes in Children at 12, 24, and 36 Months: A Randomized Clinical Trial
Abstract
Background
Malaria in pregnancy has been associated with worse cognitive outcomes in children, but its association with behavioral outcomes and the effectiveness of malaria chemoprevention on child neurodevelopment are not well characterized.
Methods
To determine if more effective malaria chemoprevention in mothers and their children results in better neurodevelopment, 305 pregnant women were randomly assigned to 3 doses of sulfadoxine-pyrimethamine, 3 doses of dihydroartemisinin-piperaquine (DP), or monthly DP during pregnancy, and their 293 children were assigned to DP every 3 months or monthly DP from 2 to 24 months of age. Cognition, language, and motor function were assessed at 12, 24. and 36 months of age, and attention, memory, behavior, and executive function were assessed at 24 and 36 months of age.
Results
Children of mothers with versus without malaria in pregnancy had worse scores on cognitive, behavioral, and executive function outcomes at 24 months. Clinical malaria in children within the first 12 months was similarly associated with poorer scores in behavior and executive function at 24 months, language at 24 and 36 months, and motor function scores at 36 months. However, more effective malaria chemoprevention in the mothers and children was not associated with better outcomes.
Conclusions
Malaria in pregnancy was associated with worse cognitive, behavioral, and executive function scores in affected children, but more effective malaria chemoprevention measures did not result in better outcomes. Malaria chemoprevention prior to and early in gestation and with even higher efficacy in mothers and children may be required to prevent neurodevelopmental impairment in children.
Clinical Trials Registration. NCT02557425.
In malaria-endemic regions, malaria may hinder children's ability to achieve their full developmental potential [1, 2]. Cerebral malaria and severe malarial anemia in childhood are associated with long-term neurocognitive impairment [1], while uncomplicated malaria is associated with worse academic outcomes in schoolchildren. However, malaria chemoprevention programs in schoolchildren have led to modest or no improvement in child cognition and educational achievement [3–6].
In animal malaria studies, mice pups exposed to malaria in pregnancy (MIP) have impaired learning and memory scores that persist in adulthood [7]. In humans, MIP is associated with disruptions in the uterine environment, altering the regulation of inflammation, angiogenesis, and metabolism in ways that could predispose the child to neurodevelopmental disorders [8]. Recent human studies have shown that MIP is associated with impaired function in different cognitive domains at 1, 2, and 6 years [9, 10].
Artemisinin combination therapy (ACT) is the primary treatment for malaria and is increasingly being used for malaria prevention in children [11, 12] and pregnant women [11]. Early animal studies showed neurotoxicity with artemisinin derivative treatment [13, 14], but artemisinin neurotoxicity has not been shown in humans [15]. Adverse outcomes are not increased in mothers who received ACT in pregnancy or in their children [16, 17], but the effect of ACT in mothers on neurodevelopmental outcomes in their children has not been evaluated. In the present study, we evaluated cognition, behavior, and executive function in a cohort of children who, along with their mothers, were enrolled in a randomized clinical trial of malaria chemoprevention regimens, including ACT-containing regimens. We hypothesized that chemoprevention regimens that led to a greater reduction of malaria episodes in mothers and children would result in better neurodevelopmental outcomes in children.
METHODS
Trial Design
PROMOTE (Prevention of malaria and HIV disease in Tororo) was a double-blind randomized clinical trial in which pregnant women and their offspring were allocated to 5 malaria chemoprevention regimens in a 2:1:1:1:1 ratio. In the present study, PROTECT (PROphylaxis against malaria To Enhance Child Development), these children were evaluated for neurodevelopmental outcomes at 12, 24, and 36 months of age (±2 months).
Participants
The studies were conducted at Tororo District Hospital, Uganda. In PROMOTE, women who consented to study participation were randomly assigned to 1 of 3 malaria chemoprevention regimens containing sulfadoxine-pyrimethamine (SP) or dihydroartemisinin-piperaquine (DP): 3 doses of SP, 3 doses of DP (3DP), or monthly DP (MDP), from 16 weeks of gestation to birth [11]. Children were assigned treatment from 2 to 24 months of age as follows: Children of mothers who received SP were given DP every 3 months (DP3) and children of mothers who received 3 doses of DP or monthly DP were randomized to receive DP3 or MDP [18]. The 5 maternal and children treatment groups were SP/DP3, 3DP/DP3, 3DP/MDP, MDP/DP3, and MDP/MDP. Children were assigned to a treatment group at the time their mothers were randomized in the parent study. Details of the study drugs, randomization, and blinding are available in prior publications [11, 18].
Children in the PROMOTE study were eligible for the present study (PROTECT). Inclusion criteria for the participants in the present study were as follows: (i) enrolled in the parent clinical trial [11]; (ii) human immunodeficiency virus uninfected; (iii) 12 months of age at enrollment; (iv) living <30 km from the study clinic; (v) able to comply with study procedures; and (vi) not lost to follow-up or withdrawn from the primary study. Exclusion criteria for the present study included (i) cessation of study drug; (ii) active illness at enrollment; (iii) history of head trauma or coma; (iv) severe neurologic disease precluding neurodevelopmental testing; (v) known chronic illness requiring medical care; or (vi) major medical abnormalities on screening history. Enrollment in PROTECT began on 25 November 2015 and 36-month assessments were completed on 7 June 2018.
Assessment of Malaria
Placental malaria in mothers was assessed by detection of parasites in placental blood by microscopy and loop-mediated isothermal amplification (LAMP) and detection of parasites or malaria pigment in placental tissue by histopathology [19]. MIP was assessed by testing monthly for Plasmodium falciparum in dried blood spots using LAMP, and by testing all mothers seen at the clinic with fever for P. falciparum by blood smear [11]. Routine blood smears for malaria detection were performed on the children every 4 weeks. In addition, parents were asked to bring children in the study to the clinic for any illness in the first 3 years of life. All children with fever in the previous 24 hours or measured temperature ≥38°C were evaluated for malaria by microscopy testing and treated with ACT if microscopy positive [18].
Study Assessments
The Bayley Scales of Infant and Toddler Development, Third Edition (hereafter “Bayley”) were used to assess cognition, language (receptive and expressive language), and motor functions (fine and gross motor) [20]. The Color Object Association Test (COAT) was used to assess for associative memory [21]. Attention was assessed using the Early Child Vigilance Test (ECVT) [22]. Behavior and executive function were assessed using the Child Behavior Checklist (CBCL) [23] and the Behavior Rating Inventory for Executive Function, preschool version (BRIEF) [24] respectively, completed by the primary caregiver. Higher scores in the Bayley, COAT, and ECVT are better whereas lower scores in the CBCL and BRIEF are better. All tests have been previously used in Uganda [25–27]. The primary outcomes for this study were the Bayley cognition score, the ECVT attention score, and the total memory score from the COAT. Testers were blinded to study participant treatment arm.
Sample Size Determination
For the study's hypothesis comparing the mean scores of the 5 intervention arms, the sample size of 270 children (90, 45, 45, 45, and 45 children in respective arms) had >83% power to detect an effect size of 0.22 (variance of group means/error variance = 0.046) using a 2-sided 1-way analysis of variance (ANOVA) F-test at the .05 significance level.
Statistical Analysis
Variables measured on a continuous scale were compared across groups using a 1-way ANOVA test. Variables not normally distributed were tested with the Kruskal–Wallis test. The χ2 test or Fisher exact test were employed for categorical variables. Cognition, attention, memory, behavior, and executive functions scores were compared across the 5 treatment groups at each time point of assessment by modeling each of the neurodevelopmental outcomes in a linear mixed-effects regression model that incorporates measurements from all time points per participant (child) and accommodates the intraparticipant correlations using a participant-level random effect. The fixed-effect predictors of this model are indicator variables representing different treatment groups (SP in mother and DP every 3 months in child designated as the reference level), time dummies indicating the assessment periods, and interactions between treatments and assessment periods. The significant main effects were followed up with Tukey test to evaluate pairwise differences in mean scores among the 5 treatment groups at each time point of assessment, adjusting for multiple comparisons. The same statistical approach was employed to compare neurodevelopmental outcomes according to measures of MIP at each assessment period. The only modification here was that the linear mixed-effects models included 5 additional covariates—maternal age, gravidity (first pregnancy vs other), socioeconomic status, gestational age at delivery, and birthweight—which differed significantly by MIP status.
All analyses were intention-to-treat analyses that included all participants randomly assigned to treatment, regardless of treatment eventually received. Statistical analyses were carried out using SAS software version 9.4 (SAS Institute, Cary, North Carolina). All tests were 2-sided with significance level of .05.
RESULTS
Sociodemographic and Clinical Characteristics Between the Treatment Arms
Of the 305 mothers enrolled in the parent PROMOTE chemoprevention study, 293 children were enrolled in PROTECT. Figure 1 outlines enrollment and follow-up numbers and the reasons children were not enrolled in or did not have follow-up visits in PROTECT. At the first study visit (12 months of age), 272 children with a mean gestational age at delivery of 39.2 weeks (standard deviation, 1.9 weeks) were tested. Sex distribution, maternal age, parental education, and wealth index were similar across the groups (Supplementary Table 1). Exposure to indoor residual spraying insecticide did not differ between treatment groups (Supplementary Table 2). As expected, prevalence of MIP and presence of placental malaria were reduced with 3DP and MDP regimens compared to SP, and frequency of clinical malaria episodes in children were lower in the children receiving MDP compared to 3DP (Supplementary Table 3).

Flow diagram of enrollment and testing of study participants. *An additional child was assigned monthly dihydroartemisinin-piperaquine but withdrew from study before neurocognitive assessments. Abbreviations: C, could not comply with study protocol; DC, declined participation; DE, death; DP, dihydroartemisinin-piperaquine; HIV, human immunodeficiency virus; L, could not be located; M, moved out of study area; SP, sulfadoxine-pyrimethamine; W, withdrew consent.
Cognitive Outcomes and Malaria Chemoprevention
272, 251 and 242 children were tested at ages 12, 24, and 36 months, respectively (Figure 1). Cognitive composite scores for the MDP/MDP group were higher than the 3DP/MPD group at 36 months, but there were no differences in cognitive scores between malaria chemoprevention regimens at 12, 24, or 36 months, and no differences in memory or attention scores between chemoprevention regimens at 24 or 36 months (Figure 2). Secondary evaluation of the Bayley language and motor scores according to malaria chemoprevention regimen showed worse motor scores in children in the 3DP/DP3 compared to the SP/DP3 or MDP/DP3 arms at 12 months (Figure 2). Cognitive, language, and motor scores decreased in all children, regardless of treatment group, in years 2 and 3 compared to year 1 (Figure 2).
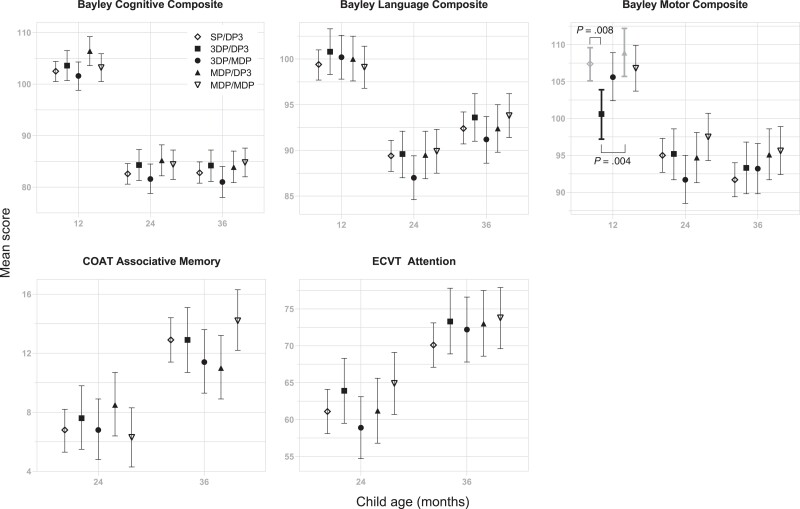
Cognitive outcomes at 12, 24, and 36 months, according to malaria chemoprevention regimen. Mean and 95% confidence interval for each chemoprevention regimens are shown. Within each assessment and time point, a regimen pair with significantly different means at a significance level of .05 is shown with a bracket with the corresponding Tukey P values adjusted for multiple comparisons. Abbreviations: 3DP, 3 doses of dihydroartemisinin-piperaquine; COAT, Color Object Association Test; DP3, dihydroartemisinin-piperaquine every 3 months; ECVT, Early Childhood Vigilance Test; MDP, monthly dihydroartemisinin-piperaquine; SP, 3 doses of sulfadoxine-pyrimethamine.
Behavioral and Executive Function Outcomes and Malaria Chemoprevention
At 24 months, children in the 3DP/DP3 had less internalizing or externalizing problems than the 3DP/MDP or MDP/DP3 groups, respectively (Figure 3). Similarly, children in the 3DP/DP3 group had fewer total behavioral problems than the 3DP/MDP and MDP/DP3 groups at 24 months. No differences were seen in behavioral scores between treatment groups at 36 months or in executive function scores between treatment groups at 24 or 36 months (Figure 3). For all neurodevelopmental assessments, no trend was seen in terms of better scores with more effective malaria chemoprevention in mothers and children.

Behavioral and executive function scores at 24 and 36 months, according to malaria chemoprevention regimen. Mean and 95% confidence interval for each chemoprevention regimen are shown. Within each assessment and time point, a regimen pair with significantly different means at a significance level of .05 is shown with a bracket, along with the corresponding Tukey P values adjusted for multiple comparisons. Abbreviations: 3DP, 3 doses of dihydroartemisinin-piperaquine; BRIEF, Behavior Rating Inventory for Executive Function; CBCL, Child Behavior Checklist; DP3, dihydroartemisinin-piperaquine every 3 months; MDP, monthly dihydroartemisinin-piperaquine; SP, 3 doses of sulfadoxine-pyrimethamine.
Cognitive Outcomes After MIP or Malaria in the First Year of the Child's Life
Children born to mothers who had MIP had lower cognitive scores at 24 months, but not 12 or 36 months (Figure 4). However, MIP did not affect associative memory or attention scores at any age, and placental malaria and malaria in childhood had no effect on cognitive, memory, or attention scores at any age (Figure 4 and Supplementary Figure 1). There was no effect of MIP, placental malaria, or malaria at enrollment on Bayley language or motor scores, but children who had childhood malaria in the first year of life (hereafter, “childhood malaria”) had worse language scores at 24 and 36 months and worse motor scores at 36 months (Figure 4, Supplementary Table 4). In addition, children whose mothers had MIP who also malaria in the first year of life consistently had worse cognitive outcomes than children with neither MIP exposure nor childhood malaria (Supplementary Table 5).
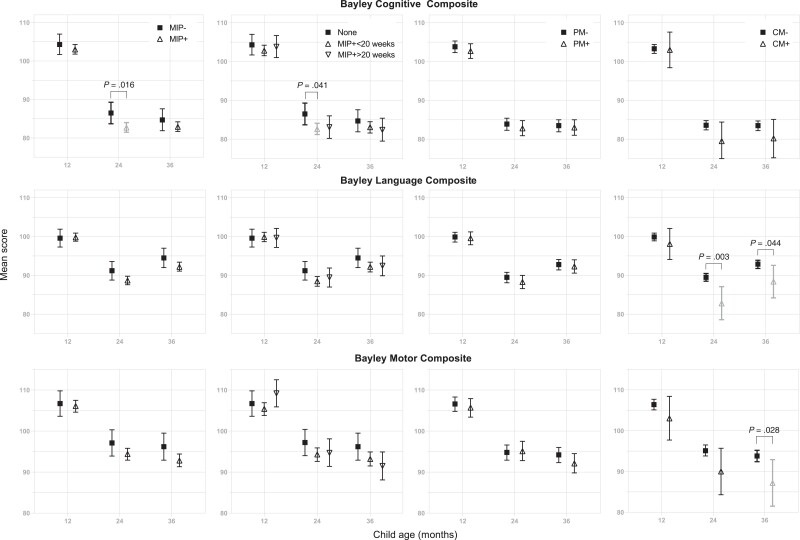
Bayley cognitive, language, and motor composite scores at 12, 24, and 36 months, according to malaria risk factors. Mean and 95% confidence interval for each risk factor are shown. First column: malaria in pregnancy (MIP) assessed by loop-mediated isothermal amplification (LAMP). Second column: timing of MIP assessed by LAMP. Third column: presence of placental malaria. Fourth column: presence of clinical malaria in the child in the first 12 months of life. Within each assessment and time point, a regimen pair with significantly different means at a significance level of .05 is shown with a bracket, along with the corresponding Tukey P values adjusted for multiple comparisons. Abbreviations: CM, clinical malaria; MIP, malaria in pregnancy; PM, placental malaria.
Behavioral and Executive Function Outcomes After MIP or Malaria in the First Year of the Child's Life
In contrast, children born to mothers with MIP and children who had malaria in the first year of life had more internalizing, externalizing, and total behavioral problems at 24 months of life but not at 36 months (Figure 5). Similarly, MIP and malaria in the first year of the child's life were both associated with worse (higher) composite scores of behavior-related executive function at 24 months but not 36 months of age (Figure 5). Similar findings were noted when malaria at maternal enrollment was compared to behavior and executive function outcomes (Supplementary Table 4). In addition, the combination of MIP and childhood malaria was associated with worse behavioral outcomes at 24 months than either alone or neither exposure (Supplementary Table 5). Asymptomatic parasitemia, measured by microscopy or LAMP monthly, was affected in a similar way to symptomatic parasitemia (ie, the most effective chemoprevention regimen led to the least asymptomatic parasitemia Supplementary Table 6), so unexpected differences in asymptomatic parasitemia did not confound the effects of chemoprevention regimens.

Behavioral and executive function scores at 24 and 36 months, according to malaria risk factors. Mean and 95% confidence interval for each risk factor are shown. First column: malaria in pregnancy (MIP) assessed by loop-mediated isothermal amplification (LAMP). Second column: timing of MIP assessed by LAMP. Third column: presence of placental malaria. Fourth column: presence of clinical malaria in the child in the first 12 months of life. Within each assessment and time point, a regimen pair with significantly different means at a significance level of .05 is shown with a bracket, along with the corresponding Tukey P values adjusted for multiple comparisons. Abbreviations: BRIEF, Behavior Rating Inventory for Executive Function; CBCL, Child Behavior Checklist; CM, clinical malaria; MIP, malaria in pregnancy; PM, placental malaria.
DISCUSSION
Pathways established during fetal neurodevelopment are critical to subsequent cognition, behavior, and executive function in a child [28, 29]. MIP is known to affect multiple factors that can affect neurotransmission and brain function, including inflammation, angiogenesis, and metabolism [8]. However, few studies have assessed the effect of MIP, malaria chemoprevention in pregnancy, or malaria chemoprevention in the child's first year of life on child cognitive and motor outcomes [9, 10], and none to date on behavioral and executive function outcomes. In the present study, we found that MIP, as detected by monthly LAMP assay for malaria in mothers and clinical surveillance in febrile mothers, was associated with worse overall cognitive scores, behavior, and behavior-related executive function at 24 months but not 36 months of age in children. Malaria in the first year of childhood was also associated with worse behavior and worse behavior-related executive function. Finally, the combination of MIP and malaria in the first year of childhood was associated with lower scores in cognitive and language outcomes at 24 and 36 months and worse behavioral outcomes at 24 months, compared to having neither exposure. However, although MIP and malaria in childhood were associated with worse cognition, behavior, and behavior-related executive function, chemoprevention regimens that decreased malaria during pregnancy [11] and childhood [18] did not lead to better cognitive, behavioral, or executive function outcomes, likely due to the high prevalence of P falciparum parasitemia in mothers at enrollment. Together the findings suggest a need, prior to and early in pregnancy, to decrease the effects of MIP on child neurodevelopmental outcomes, and for even more effective measures to prevent malaria in the first year of life in children, to prevent malaria in childhood from affecting neurodevelopment.
Almost 60% of the pregnant mothers enrolled in the study had malaria parasites detected by LAMP on enrollment, before chemoprevention was started at 16–20 weeks of gestation [11]. Malaria may already have affected fetal and therefore child neurodevelopment pathways by the time malaria chemoprevention was started in mothers. Since all mothers and children received malaria chemoprevention, the effects of having any chemoprevention versus none, which were potentially substantial, could not be evaluated. The lack of progressive worsening of scores with increased doses of ACT argues against artemisinin-related neurotoxicity as a reason for the differences seen. The reasons for the decrease in cognitive, language, and motor scores in years 2 and 3 of life across all treatment groups are not clear and require further study. Previous studies have shown a similar trend, with younger ages having higher scores than older children, which was attributed to lower test precision at 1 year of age, varying development across the ages, and item difficulty [30, 31].
A recent study in Benin showed associations between MIP or placental malaria on gross motor outcomes but not overall cognition at 1 year of life, and of malaria at the second antenatal visit and the nonverbal index of the Kauffman Assessment Battery for Children–2 at 6 years of life [10], whereas a study in Malawi showed an association of malaria late in pregnancy (33–37 weeks) and impaired language development through 2 years of age [9]. We did not find effects on motor or language function with MIP alone at any time, but did find an effect of MIP on cognition in the second year of life only. Differences in study findings could relate to different tests used and time points studied.
The present study is to our knowledge the first to assess the effects of mother and child malaria episodes and malaria chemoprevention regimens on behavioral and executive function outcomes in children. The effects of MIP, placental malaria, and malaria in the first year of the child's life on behavior and executive function only at 24 months of age were strikingly consistent. The findings suggest that processes undergirding behavior and executive function at 24 months are vulnerable to malaria exposure in utero and in early childhood. The similar behavior and executive scores at 36 months in children with and without MIP exposure suggest that there is resolution of differences at this age, so the long-term consequences of the differences seen at 24 months are unclear. In childhood, behavioral problems and poor executive function measured by tests like the CBCL and BRIEF are associated with poor productivity in adulthood in areas such as academic performance, occupational function, and mental health [32–34]. Future studies should assess these skills directly as the child reaches an age where executive function can be directly assessed rather than parentally reported.
Maternal immune activation disrupts the immune balance between maternal and fetal environments and is associated with fetal and child structural and functional brain changes, behavioral changes, and cognitive deficits in animal studies [35]. In 1 human study, elevated soluble receptor for tumor necrosis factor type II (sTNFRII) in maternal plasma collected during pregnancy was associated with lower language development [9]. Future studies will assess how maternal immune activation markers relate to neurodevelopmental and behavioral outcomes.
Overall, the differences of 4–6 points for MIP or childhood malaria on child cognitive outcomes, analogous to an IQ scale, would translate into substantial effects on a population basis, though modest on an individual basis. Differences in behavior scores are less well studied, and the significance of the differences in behavior is likely best assessed with additional follow-up, since these differences resolved at 36 months.
In summary, in this study we show that MIP and malaria in the first year of childhood are associated with worse neurodevelopment, including worse cognitive, behavioral, and executive function scores, particularly at 24 months of age. Although cognitive and behavioral outcomes did not improve with increasingly effective chemoprevention in mothers and children, the additional effect of the more effective regimens may not have been sufficient to achieve a change in these outcomes, and the effects of chemoprevention versus no chemoprevention could not be assessed. In addition, chemoprevention started at 16–20 weeks of pregnancy, when >50% of mothers already had malaria parasitemia. Future studies should evaluate the effects of chemoprevention given earlier in pregnancy on neurodevelopment and behavior through early and later childhood. In conjunction with other recent studies, the present study strongly supports the need for highly effective and early malaria chemoprevention in pregnancy and childhood to improve long-term neurobehavioral outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
ciac815_Supplementary_Data
Contributor Information
Paul Bangirana, Department of Psychiatry, Makerere University College of Health Sciences, Kampala, Uganda.
Andrea L Conroy, Ryan White Center for Pediatric Infectious Diseases and Global Health, Indiana University School of Medicine, Indianapolis.
Robert O Opoka, Department of Pediatrics and Child Health, Makerere University College of Health Sciences, Kampala, Uganda.
Margaret Semrud-Clikeman, Department of Pediatrics, University of Minnesota, Minnesota, USA.
Jeong H Jang, Underwood International College and Department of Applied Statistics, Yonsei University, Seoul, Korea.
Claire Apayi, Infectious Diseases Research Collaboration, Kampala, Uganda.
Abel Kakuru, Infectious Diseases Research Collaboration, Kampala, Uganda.
Mary K Muhindo, Infectious Diseases Research Collaboration, Kampala, Uganda.
Michael K Georgieff, Department of Pediatrics, University of Minnesota, Minnesota, USA.
Grant M Dorsey, Department of Medicine, University of California, California, USA.
Moses R Kamya, Infectious Diseases Research Collaboration, Kampala, Uganda. Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda.
Diane Havlir, Department of Medicine, University of California, California, USA.
Chandy C John, Ryan White Center for Pediatric Infectious Diseases and Global Health, Indiana University School of Medicine, Indianapolis.
Notes
Acknowledgments. The authors thank the caregivers and children who participated in the study; the study team; George Okwakol, Evaline Oenen, Jemima Nayebare, and Richard Kaluma who collected the data; and the management and staff of Infectious Diseases Research Collaboration and Tororo District Hospital.
Disclaimer. The content is solely the responsibility of the authors and does not represent the views of the National Institutes of Health (NIH).
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers R01HD086124 to C. C. J. and P. B. and P01HD059454 to G. M. D., D. H., and M. R. K.).
References
Articles from Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/cid/ciac815
Read article for free, from open access legal sources, via Unpaywall:
https://escholarship.org/content/qt8227k389/qt8227k389.pdf?t=rxdq4l
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/137043185
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/cid/ciac815
Article citations
Exploring the hidden mental health consequences of malaria beyond the fever.
Front Hum Neurosci, 18:1432441, 18 Jul 2024
Cited by: 0 articles | PMID: 39091401 | PMCID: PMC11291252
Review Free full text in Europe PMC
Beyond TORCH: A narrative review of the impact of antenatal and perinatal infections on the risk of disability.
Neurosci Biobehav Rev, 153:105390, 13 Sep 2023
Cited by: 3 articles | PMID: 37708918 | PMCID: PMC10617835
Review Free full text in Europe PMC
The kidney-brain pathogenic axis in severe falciparum malaria.
Trends Parasitol, 39(3):191-199, 02 Feb 2023
Cited by: 2 articles | PMID: 36737313
Review
Developmental origins of disease highlight the immediate need for expanded access to comprehensive prenatal care.
Front Public Health, 10:1021901, 24 Nov 2022
Cited by: 2 articles | PMID: 36504964 | PMCID: PMC9730730
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Monthly sulfadoxine/pyrimethamine-amodiaquine or dihydroartemisinin-piperaquine as malaria chemoprevention in young Kenyan children with sickle cell anemia: A randomized controlled trial.
PLoS Med, 19(10):e1004104, 10 Oct 2022
Cited by: 0 articles | PMID: 36215323 | PMCID: PMC9591057
Randomized Noninferiority Trial of Dihydroartemisinin-Piperaquine Compared with Sulfadoxine-Pyrimethamine plus Amodiaquine for Seasonal Malaria Chemoprevention in Burkina Faso.
Antimicrob Agents Chemother, 59(8):4387-4396, 27 Apr 2015
Cited by: 47 articles | PMID: 25918149 | PMCID: PMC4505196
Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: a systematic review and meta-analysis.
Lancet Infect Dis, 17(2):184-193, 17 Nov 2016
Cited by: 62 articles | PMID: 27865890 | PMCID: PMC5266794
Review Free full text in Europe PMC
Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial.
PLoS Med, 11(8):e1001689, 05 Aug 2014
Cited by: 76 articles | PMID: 25093754 | PMCID: PMC4122345
Funding
Funders who supported this work.
Eunice Kennedy Shriver National Institute of Child Health and Human Development (2)
Grant ID: R01HD086124
Grant ID: P01HD059454
NICHD NIH HHS (1)
Grant ID: R01 HD086124
NINDS NIH HHS (1)
Grant ID: R01 NS055349





