Abstract
Purpose
Breast cancer patients often develop musculoskeletal pain, resembling that experienced by patients with rheumatoid arthritis (RA), during cancer treatment. This study aimed to investigate the causes of musculoskeletal pain, including RA, among breast cancer patients.Methods
This retrospective study included breast cancer patients experiencing new-onset arthralgia during cancer treatment along with age- and sex-matched controls without breast cancer, who were evaluated at the Rheumatologic clinic between 2004 and 2017. The causes of musculoskeletal pain were compared between breast cancer patients and controls. The effects of cancer treatment on arthralgia and factors associated with RA were examined.Results
A total of 146 breast cancer patients and 102 controls were included in the final analysis. The most common cause of arthralgia during breast cancer treatment was osteoarthritis (OA, 61.0%), followed by enthesopathy/tendinopathy (28.1%), which included tendinitis, adhesive capsulitis, and carpal tunnel syndrome. Overall, 50.0% of 72 breast cancer patients receiving aromatase inhibitors (AIs) satisfied the criteria of AI-induced musculoskeletal symptoms (AIMSS). The mean symptom duration (i.e., the time between pain onset and evaluation by a rheumatologist) was shorter in breast cancer patients than in controls (7.0 ± 12.1 vs. 14.8 ± 24.9 months, respectively; p = 0.004). RA was diagnosed in 3 (2.1%) breast cancer patients and 3 (2.9%) controls. All breast cancer patients with RA had an elevated erythrocyte sedimentation rate (ESR, 66.7 ± 25.0 mm/h), whereas those without RA had a normal ESR (20.4 ± 21.5 mm/h). Patients with breast cancer required more analgesics than the controls.Conclusion
OA and enthesopathy/tendinopathy are the most common causes of arthralgia in breast cancer patients, which may concurrently manifest as AIMSS. Patients with breast cancer did not have a higher prevalence of RA than those without breast cancer.Free full text

Musculoskeletal Pain and the Prevalence of Rheumatoid Arthritis in Breast Cancer Patients During Cancer Treatment: A Retrospective Study
Abstract
Purpose
Breast cancer patients often develop musculoskeletal pain, resembling that experienced by patients with rheumatoid arthritis (RA), during cancer treatment. This study aimed to investigate the causes of musculoskeletal pain, including RA, among breast cancer patients.
Methods
This retrospective study included breast cancer patients experiencing new-onset arthralgia during cancer treatment along with age- and sex-matched controls without breast cancer, who were evaluated at the Rheumatologic clinic between 2004 and 2017. The causes of musculoskeletal pain were compared between breast cancer patients and controls. The effects of cancer treatment on arthralgia and factors associated with RA were examined.
Results
A total of 146 breast cancer patients and 102 controls were included in the final analysis. The most common cause of arthralgia during breast cancer treatment was osteoarthritis (OA, 61.0%), followed by enthesopathy/tendinopathy (28.1%), which included tendinitis, adhesive capsulitis, and carpal tunnel syndrome. Overall, 50.0% of 72 breast cancer patients receiving aromatase inhibitors (AIs) satisfied the criteria of AI-induced musculoskeletal symptoms (AIMSS). The mean symptom duration (i.e., the time between pain onset and evaluation by a rheumatologist) was shorter in breast cancer patients than in controls (7.0 ± 12.1 vs. 14.8 ± 24.9 months, respectively; p = 0.004). RA was diagnosed in 3 (2.1%) breast cancer patients and 3 (2.9%) controls. All breast cancer patients with RA had an elevated erythrocyte sedimentation rate (ESR, 66.7 ± 25.0 mm/h), whereas those without RA had a normal ESR (20.4 ± 21.5 mm/h). Patients with breast cancer required more analgesics than the controls.
Conclusion
OA and enthesopathy/tendinopathy are the most common causes of arthralgia in breast cancer patients, which may concurrently manifest as AIMSS. Patients with breast cancer did not have a higher prevalence of RA than those without breast cancer.
INTRODUCTION
Breast cancer is one of the most common malignancies among women, accounting for over 2 million annual cases worldwide [1]. New treatment options have improved the survival rate; however, treatment-associated side effects have emerged as novel disease entities [2,3]. Burdensome sequelae of cancer treatment include musculoskeletal pain, which can be debilitating and significantly lower the quality of life [4,5,6]. This pain can also have detrimental effects on the treatment, reducing the patient’s treatment adherence and, subsequently, their overall survival [7,8]. New-onset arthralgia is a common musculoskeletal complaint after starting treatment with aromatase inhibitors (AIs) or tamoxifen for hormone receptor (HR)-positive breast cancer [9,10,11,12,13]. AI-induced musculoskeletal symptoms (AIMSS) have been noted in up to one-third of patients in clinical trials, and in as high as 80% of patients in a community-based survey [12,14]. While musculoskeletal symptoms are more prevalent in AI users, the Arimidex, Tamoxifen, Alone or in Combination study revealed that tamoxifen was also associated with musculoskeletal complaints in approximately 30% of the patients [12]. In addition to endocrine therapy, systemic chemotherapy, and taxane-based chemotherapy, in particular, can trigger severe arthralgia in breast cancer patients [11,15].
In a significant subset of patients with breast cancer, the severity and distribution of the musculoskeletal pain resemble that of rheumatoid arthritis (RA), which often results in a referral for a formal rheumatologic evaluation [16]. Data are conflicting regarding whether breast cancer, with or without endocrine therapy, increases the risk of developing RA [17,18,19,20].
In this retrospective study, we aimed to investigate the causes of musculoskeletal pain and the prevalence of RA among breast cancer patients referred to a rheumatology clinic for new-onset arthralgia and to identify factors associated with RA development in breast cancer patients.
METHODS
Patients
This retrospective study included breast cancer survivors who were referred to the Rheumatology Clinic at Seoul National University Hospital for evaluation and management of musculoskeletal pain between January 2004 and January 2018. Breast cancer was identified by the International Classification of Diseases 10th Revision codes for breast cancer (C509), regardless of the cancer stage or status of active treatment. The medical records of the identified patients were reviewed meticulously. Patients with musculoskeletal pain that had started before their breast cancer diagnosis were excluded. Patients with known autoimmune diseases, such as RA or bone metastasis, were excluded. Control subjects were chosen from patients without breast cancer who were newly evaluated for musculoskeletal pain at the Rheumatology Clinic during the same period. Controls and breast cancer patients were matched using propensity score matching based on age and sex.
The study was conducted as per the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (H-1803-013-925). Due to the study’s retrospective nature, the requirement for informed consent was waived.
Data collection
Data on demographics, breast cancer diagnosis, and clinical and laboratory parameters were retrieved from the electronic medical records. The data extraction focused on breast cancer staging, HR and human epidermal growth factor receptor 2 status, female hormonal status (menopause, oophorectomy, or hormone replacement therapy), and cancer treatment (surgery, radiation therapy, chemotherapy, or endocrine therapy).
Rheumatological evaluation of arthralgia
All patients underwent a standard rheumatological examination, which included a thorough joint and soft tissue examination. The assessed laboratory parameters included the erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hs-CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (ACPA), anti-nuclear antibody (ANA) titers, and radiographic studies of the joints. RA was defined according to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [21]. The final diagnosis and treatment for arthralgia were determined by the treating rheumatologist. Patients were treated at the physician’s discretion.
Evaluation for AIMSS
AIMSS was diagnosed based on the following criteria: 1) symmetrical joint pains, carpal tunnel syndrome, or morning stiffness that improves with movement or exercise, and 2) pain developed or worsened within 6 months of starting AI therapy [13,22,23,24].
Statistical analyses
Data were presented as the mean value and standard deviation (continuous variables) or as absolute numbers and percentages (categorical variables). The χ2 test or Fisher’s exact test was found to be appropriate to analyze categorical variables, while Student’s t-test was appropriate for continuous variables. A p-value < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics software version 26.0 (IBM Corp., Armonk, USA).
RESULTS
Patient characteristics
A total of 168 patients with breast cancer were referred to the Rheumatology clinic for evaluation of musculoskeletal pain during the study period. Finally, 146 patients and 102 age- and sex-matched control patients with new-onset arthralgia were included in the study (Figure 1). All breast cancer patients and 95 out of 102 control patients were internally referred from other departments within the medical center. The mean age of the breast cancer patients and controls was 53.3 ± 8.1 years and 51.8 ± 8.2 years, respectively (Table 1). All the patients were female. The mean symptom duration (i.e., the time between pain onset and evaluation by a rheumatologist) was shorter for the breast cancer group than for the control group (7.0 ± 12.1 vs. 14.8 ± 24.9 months, respectively; p = 0.004). The mean age at menopause was 48.8 ± 4.7 years for the breast cancer group and 48.2 ± 5.4 years for the control group. The proportion of patients with menopause did not differ between the groups (p = 0.775). The 2 groups did not differ in terms of oophorectomy status (p = 0.672), although fewer patients in the breast cancer group had received previous hormone replacement therapy (p = 0.002).
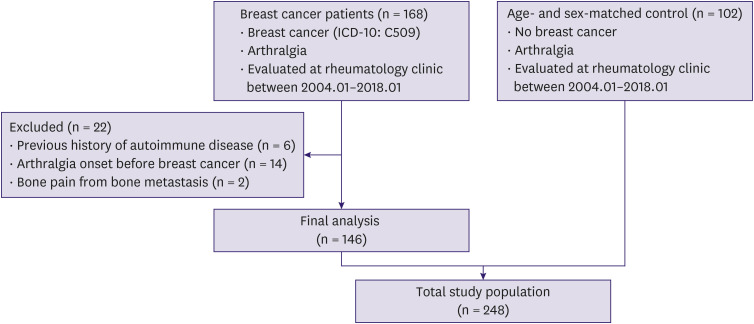
ICD-10 = International Classification of Diseases 10th Revision.
Table 1
| Characteristics | Breast cancer (n = 146) | Control (n = 102) | p-value | |
|---|---|---|---|---|
| Female sex | 146 (100) | 102 (100) | 1.000 | |
| Age at pain onset (yr) | 53.3 ± 8.1 | 51.8 ± 8.2 | 0.151 | |
| Pain duration (mo) | 7.0 ± 12.1 | 14.8 ± 24.9 | 0.004 | |
| Age at breast cancer diagnosis | 51.5 ± 8.3 | |||
| Ever smoked | 2/146 (1.4) | 1/102 (1.0) | 1.000 | |
| Median BMI (IQR) | 23.0 (18.4–27.6) | 22.9 (18.3–27.5) | 0.377 | |
| BMI > 23 | 69/140 (49.3) | 30/63 (47.6) | 0.826 | |
| Post-menopausal | 115/146 (78.8) | 82/102 (80.4) | 0.775 | |
| Age at menopause | 48.8 ± 4.7 | 48.2 ± 5.4 | 0.428 | |
| Early age at menopause* | 25/105 (23.8) | 21/79 (26.6) | 0.667 | |
| Oophorectomy | 9/146 (6.2) | 5/102 (4.9) | 0.672 | |
| Previous HRT | 15/146 (10.3) | 26/102 (25.5) | 0.002 | |
| Hormone-receptor positive | 114/146 (78.1) | |||
| ER positive | 110/146 (75.3) | |||
| PR positive | 81/146 (55.5) | |||
| ER and PR positive | 77/146 (52.7) | |||
| Anti-hormonal treatment | 117/146 (80.1) | |||
| Tamoxifen | 45/146 (30.8) | |||
| Aromatase inhibitor | 72/146 (49.3) | |||
| Surgery to symptom onset (mo) | 22.1 ± 23.6 | |||
| Endocrine therapy to symptom onset (mo) | 15.5 ± 14.9 | |||
| Chemotherapy to symptom onset (mo) | 22.1 ± 23.6 | |||
| HR-negative received only adjuvant chemo | 8.0 ± 6.1 | |||
| RA | 3/146 (2.1) | 3/102 (2.9) | 0.692 | |
| RF positivity | 19/140 (13.6) | 16/96 (14.2) | 0.511 | |
| ACPA positivity | 3/131 (2.3) | 3/85 (3.5) | 0.682 | |
| ANA (1:80) | 3/128 (2.3) | 4/89 (4.5) | 0.449 | |
| ESR (mm/hr) | 21.9 ± 23.1 | 17.0 ± 17.9 | 0.108 | |
| CRP (mg/dL) | 1.0 ± 5.0 | 0.2 ± 0.5 | 0.106 | |
Data are presented as the mean ± standard deviation or number (%). Numbers in bold indicate a statistical significance.
BMI = body mass index; IQR = interquartile range; HRT = hormone replacement therapy; ER = estrogen receptor; PR = progesterone receptor; HR = hormone receptor; RA = rheumatoid arthritis; RF = rheumatoid factor; ACPA = anti-citrullinated protein antibodies; ANA = anti-nuclear antibody; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
*Defined as menopause before 45 years of age.
Among the 146 breast cancer patients, 117 (80.1%), 45 (30.8%), and 72 (49.3%) patients were treated with endocrine, tamoxifen, and AI therapy, respectively, while 29 (19.9%) patients did not receive endocrine therapy (Table 2). The median (interquartile range [IQR]) time from initiation of endocrine therapy to arthralgia onset was 10.1 (3.6–24.1) months in those who had received endocrine treatment at some point. Ninety-four patients (80.3% of those treated with endocrine therapy at any point) were receiving endocrine therapy at the time of symptom development. Among these 94 patients, 32 (34.0%) and 62 (66.0%) received tamoxifen and AIs (35 and 27 patients received anastrozole and letrozole, respectively), respectively. Sixty-two patients who were treated with AIs at the onset of symptoms developed symptoms at a median (IQR) of 6.9 (3.1–20.6) months after the initiation of endocrine therapy, while 32 patients on tamoxifen developed symptoms after a median (IQR) of 14.8 (8.1–31.2) months (Supplementary Figure 1).
Table 2
| Characteristics | Breast cancer without RA (n = 143) | Breast cancer with RA (n = 3) | p-value | Control without RA (n = 99) | Control with RA (n = 3) | p-value | |
|---|---|---|---|---|---|---|---|
| ESR (mm/hr) | 20.4 ± 21.5 | 66.7 ± 25.0 | < 0.001 | 16.5 ± 17.3 | 33.0 ± 29.5 | 0.133 | |
| CRP (mg/dL) | 0.9 ± 5.1 | 2.8 ± 2.4 | 0.536 | 0.9 ± 5.1 | 2.8 ± 2.4 | 0.267 | |
| RF positivity | 17 (12.4) | 2 (66.7) | 0.048 | 13 (13.1) | 3 (100) | 0.003 | |
| ACPA positivity | 0 (0) | 3 (100.0) | < 0.001 | 1 (1.0) | 2 (66.7) | 0.002 | |
| ANA (≥ 1:80) | 2 (1.4) | 1 (33.3) | < 0.001 | 4 (4.0) | 0 (0) | 1.000 | |
| Surgery | 143 (100) | 3 (100) | 1.000 | ||||
| Radiation therapy | 108 (75.5) | 1 (33.3) | 0.158 | ||||
| Anti-hormonal treatment | 115 (80.4) | 2 (66.7) | 0.488 | ||||
| Tamoxifen | 45 (31.5) | 0 (0) | 0.553 | ||||
| Aromatase inhibitor | 72 (50.3) | 2 (66.7) | 0.617 | ||||
| Chemotherapy | 113 (79.0) | 3 (100) | 1.000 | ||||
| Trastuzumab | 28 (19.6) | 2 (66.7) | 0.107 | ||||
Data are presented as the mean ± standard deviation or number (%). Bold font indicates statistical significance.
ACPA = anti-citrullinated protein antibodies; ANA = anti-nuclear antibody; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; RA = rheumatoid arthritis; RF = rheumatoid factor.
Overall, 116 (79.5%) out of 146 breast cancer patients received chemotherapy at least once. The median (IQR) time between systemic chemotherapy and pain development was 12.3 months (6.4–31.8). Among these, 67 (57.8%) patients received taxane-based chemotherapy. Among those who received chemotherapy, 16 (13.8%) developed musculoskeletal pain whilst on active chemotherapy at a median (IQR) of 3.0 (1.2–4.5) months after the initiation of the chemotherapy. Out of these 16 patients, 8 were treated with taxane-based chemotherapy (Supplementary Figure 1). Among 146 breast cancer patients, 2 developed musculoskeletal pain without receiving chemotherapy or endocrine therapy.
Cause of musculoskeletal pain
The most common cause of musculoskeletal pain was osteoarthritis (OA). The prevalence of OA did not differ between the breast cancer patients and the controls (63.7% vs. 65.7%, p = 0.748). However, more patients in the breast cancer group than in the control group developed tendinopathy/enthesopathy (diseases of the tendons and/or adjacent structures), which includes tendinitis, adhesive capsulitis, carpal tunnel syndrome, and bursitis (28.1% vs. 12.7%, respectively; p = 0.004). Thirty-six (50.0%) out of 72 patients on AI developed AIMSS as well (Supplementary Table 1).
Three patients (2.1%) in the breast cancer group were diagnosed with fibromyalgia, polymyalgia rheumatica, and polymyositis, respectively. In the control group, 10 patients (9.8%) were diagnosed with rheumatic diseases, including systemic lupus erythematosus (n = 1), Bechet’s syndrome (n = 2), fibromyalgia (n = 6), and reactive arthritis (n = 1). The etiology of arthralgia could not be classified in 6 patients (4.1%) in the breast cancer group and 14 patients (13.7%) in the control group (Table 3).
Table 3
| Characteristics | Breast cancer (n = 146) | Control (n = 102) | p-value | |
|---|---|---|---|---|
| Rheumatoid arthritis | 3 (2.1) | 3 (2.9) | 0.692 | |
| Osteoarthritis | 93 (63.7) | 67 (65.7) | 0.748 | |
| Enthesopathy/Tendinopathy | 41 (28.1) | 13 (12.7) | 0.004 | |
| Tendinitis | 33 (22.6) | 11 (10.8) | ||
| Adhesive capsulitis | 6 (4.1) | 0 (0) | ||
| Carpal tunnel syndrome | 4 (2.7) | 4 (4.1) | ||
| Bursitis | 1 (0.7) | 0 (0) | ||
| Sinus tarsi syndrome | 1 (0.7) | 0 | ||
| Plantar fasciitis | 1 (0.7) | 0 | ||
| AI-induced musculoskeletal symptoms | 36 (24.7) | |||
| Infection | 2 (1.4) | 0 (0) | 0.514 | |
| Uremic syndrome | 0 | 1 (1.0) | 1.000 | |
| Chronic regional pain syndrome | 1 (0.7) | 0 | 1.000 | |
| Other rheumatic disease | 3 (2.1) | 10 (9.8) | 0.007 | |
| Fibromyalgia | 1 (0.7) | 6 (5.9) | ||
| Bechet's disease | 0 | 2 (2.0) | ||
| Systemic lupus erythematosus | 0 | 1 (1.0) | ||
| Polymyalgia rheumatic | 1 (0.7) | 0 | ||
| Polymyositis | 1 (0.7) | 0 | ||
| Reactive arthritis | 0 | 1 (1.0) | ||
| Unclassified | 6 (4.1) | 14 (13.7) | ||
Data are presented as number (%). Bold font indicates statistical significance.
RA prevalence
RA was diagnosed in 3 (2.1%) patients in the breast cancer group and 3 (2.9%) patients in the control group (p = 0.692). There was no difference between the groups with respect to serological features of RA (i.e., RF, ACPA, and ANA), hs-CRP levels, or ESR (Table 1).
All patients with RA in the breast cancer group had elevated ESR and hs-CRP levels. Breast cancer patients with RA had a higher ESR than those without RA (66.7 ± 25.0 mm/h vs. 20.4 ± 21.5 mm/h, p < 0.001). Patients with RA had higher levels of hs-CRP. Additionally, RF, ACPA, and ANA were more common in breast cancer patients with RA than those without RA (Table 2).
Among the 3 patients with a recent RA diagnosis in the breast cancer group, one (33.3%) did not receive any endocrine therapy, while 2 received AI therapy (66.7%) (Supplementary Table 2). RA was not associated with any specific cancer treatment modality, such as endocrine treatment, surgery, chemotherapy, Trastuzumab, or radiation therapy (Table 2).
Treatment and outcome
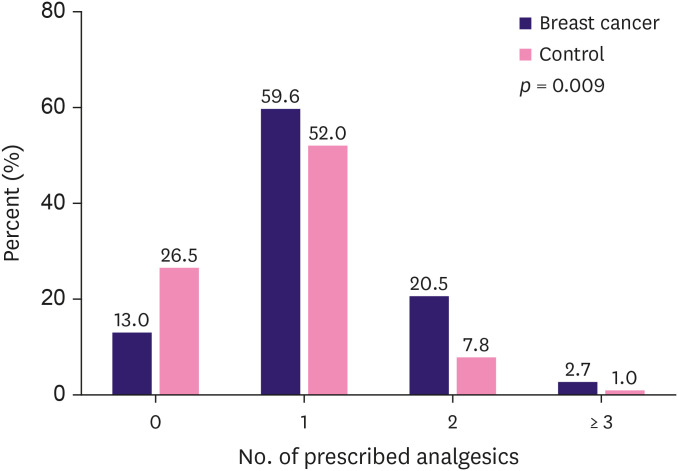
More breast cancer patients required analgesics compared to the controls (89.7% vs. 75.5%, respectively) (Table 4). Furthermore, breast cancer patients required more intensive treatment than controls (p = 0.009); more patients in the breast cancer group were treated with 2 or more analgesics (including nonsteroidal anti-inflammatory drugs [NSAIDs], tramadol, and tricyclic antidepressants (TCAs)/gamma-aminobutyric acid [GABA] analogs) than in the control group (20.5% vs. 7.8%, p = 0.024). Four patients in the breast cancer group (2.7%) were prescribed a combination of NSAIDs, tramadol, and TCA or GABA analogs, whereas one patient (1.0%) in the control group required a triple combination (Figure 2). Moreover, more patients in the control group than in the breast cancer group experienced spontaneous improvements in pain (25.8% vs. 10.3%, respectively; p = 0.007).
Table 4
| Characteristics | Breast cancer (n = 146) | Control (n = 102) | p-value |
|---|---|---|---|
| NSAID | 113 (77.4) | 60 (58.8) | 0.002 |
| Tramadol | 34 (23.3) | 14 (13.7) | 0.061 |
| TCA/GABA analogue | 12 (8.2) | 7 (6.9) | 0.693 |
| Glucocorticoid | 9 (6.2) | 9 (8.8) | 0.427 |
| DMARDs | 6 (4.1) | 7 (6.9) | 0.097 |
| AAP | 1 (0.7) | 7 (6.9) | 0.009 |
| Local injection | 19 (13.0) | 7 (6.9) | 0.126 |
| None | 15 (10.3) | 25 (25.5) | 0.003 |
Data are presented as number (%). Bold font indicates statistical significance.
AAP = acetaminophen; DMARD = disease-modifying antirheumatic drug; GABA = gamma aminobutyric acid; NSAID = nonsteroidal anti-inflammatory drug; TCA = tricyclic antidepressants.
DISCUSSION
This retrospective study showed that OA was the most common cause of arthralgia in both the breast cancer patients and controls, while tendinopathy/enthesopathy originating in extra-articular structures (e.g., tenosynovitis and adhesive capsulitis) was significantly more common in the breast cancer patients than in the controls. Among those who were treated with AI, as many as 50.0% of the patients had arthralgia associated with AIMSS. The prevalence of RA in breast cancer patients during breast cancer treatment was low, and the level was comparable with that in the age- and sex-matched control patients without breast cancer. Overall, breast cancer patients required more intensive pain treatment than controls.
Arthralgia in a patient with breast cancer can be caused by cancer treatment, including anti-hormonal treatment and chemotherapy, aging-associated OA or tendinopathy, or both. AIMSS is common in patients receiving AI treatment [23,24]. Significantly more patients in the breast cancer group developed tendinitis and adhesive capsulitis than in the control group, whereas the prevalence of symptomatic (painful) OA did not differ between the groups (Table 3). Previous studies using ultrasound or magnetic resonance imaging have demonstrated an association between AI and tenosynovitis [25,26], although one prospective study reported that these changes were commonly present before the initiation of AI therapy [27]. This suggests that pre-existing tendinitis may be aggravated during breast cancer treatment. Further studies should be conducted to investigate the mechanism by which cancer treatment induces or aggravates musculoskeletal pain in patients with breast cancer.
The patients in the breast cancer group had a shorter time duration between pain onset and evaluation by a rheumatologist. This temporal relationship suggests that breast cancer, its treatment, or both, induce arthralgia (Table 1). We confirmed that this effect was not due to a difference in accessibility, as all breast cancer patients and most control patients were referred within our medical center. The breast cancer patients required more intensive and combination treatments for pain management than the controls (Table 4). The concomitant anxiety and depression associated with a cancer diagnosis may render individuals more susceptible to pain [28,29]. It is unclear whether patients with high baseline pain levels respond differently to cancer treatment in terms of pain response [30].
Middle-aged women who experience new-onset or aggravation of existing joint pain should be evaluated for possible RA, especially when pain involves multiple small joints, such as the wrists and hands [16]. Since AIMSS shares similar clinical symptoms with RA, it is critical to differentiate between the 2 [13,22,24]. While sex (i.e., female) is a clear risk factor for both RA and breast cancer, the association between RA and breast cancer treatment has not yet been fully elucidated [20]. Studies based on Taiwan’s National Health Insurance Research Database and a Swedish nationwide registry reported that women with breast cancer had a lower risk of RA than women without breast cancer, independent of endocrine therapy status (tamoxifen, anastrozole, or letrozole) [18,20]. Other studies, however, found a dose-dependent relationship between endocrine therapy and the risk of RA [17,19]. According to the current study, the prevalence of RA among breast cancer patients with new-onset arthralgia was comparable to that among arthralgia patients without breast cancer.
We further attempted to identify factors associated with RA. We found that the risk of RA was unrelated to cancer treatment modality; all 3 breast cancer patients with recently diagnosed RA showed elevated inflammatory markers. This suggests that inflammatory markers are not usually elevated during cancer treatment. Unlike OA, RA is a systemic inflammatory disease in which inflammatory markers are elevated [31]. In this study, RA patients were defined as those positive for RF or ACPA (Supplementary Table 2). Although RF and ACPA are reliable laboratory markers of RA, the clinical significance of ANA is less clear with respect to an RA diagnosis in breast cancer patients [32,33]. One prospective study showed that ANA was positive in 16.2% of 97 breast cancer patients with AI-induced arthralgia, although no definitive autoimmune disease was diagnosed [34]. Taken together, this suggests that RA is not more common in breast cancer patients but should be considered as a possibility in breast cancer patients with arthralgia and elevated inflammatory markers.
The data presented herein suggest that breast cancer patients suffer from more severe musculoskeletal pain during cancer treatment. Those with new-onset arthralgia should first be empirically and conservatively managed with pain medications. A formal rheumatological evaluation should be reserved for those patients with elevated inflammatory markers (Supplementary Figure 2).
This retrospective study had several limitations. First, the causal relationship between cancer treatment and arthralgia causes is unknown. For example, it is particularly difficult to distinguish AIMSS from OA/tendinopathy since AIMSS can induce de novo arthralgia or aggravate preexisting OA pain. The effect of confounding factors such as depression or other comorbidities on pain perception or intensity also requires independent investigation. Since the RA prevalence was low, the study with a relatively small number of patients is not sufficient to detect differences in RA prevalence between breast cancer patients and control patients. In addition, the clinical characteristics of RA patients in this study may not represent the RA population in general. Since only breast cancer patients with significant musculoskeletal pain who were referred for a rheumatologic evaluation were included, the causes of arthralgia and RA prevalence among all breast cancer patients during treatment cannot be generalized to all breast cancer patients. Finally, pain intensity had to be compared indirectly between breast cancer patients and control patients because accurate measurements of pain intensity were not available. A larger prospective study is warranted to confirm the findings of this investigation.
In conclusion, musculoskeletal pain due to OA and tendinopathy/enthesopathy is common in breast cancer patients. The prevalence of RA in breast cancer patients with new-onset arthralgia during cancer treatment is low and comparable to that in patients without breast cancer. Breast cancer patients with new-onset pain and elevated inflammatory markers may benefit from rheumatological evaluation.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
Author Contributions:
Conceptualization: Kim JY, Kim MJ, Park JK.
Data curation: Kim JY, Kim MJ.
Formal analysis: Kim JY, Kim MJ.
Methodology: Kim TY, Lee KH, Im SA, Park JK.
Supervision: Lee EB, Park JK.
Writing - original draft: Kim JY, Kim MJ, Park JK.
Writing - review & editing: Lee EB, Kim TY, Lee KH, Im SA, Park JK.
SUPPLEMENTARY MATERIALS
Causes of musculoskeletal pain in 146 breast cancer patients, stratified by treatment
Characteristics of patients with recent RA diagnosis
Cancer treatment for breast cancer patients.
Management of musculoskeletal pain in breast cancer patients.
References
Articles from Journal of Breast Cancer are provided here courtesy of Korean Breast Cancer Society
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The CIRAS study: a case control study to define the clinical, immunologic, and radiographic features of aromatase inhibitor-induced musculoskeletal symptoms.
Breast Cancer Res Treat, 131(2):699-708, 11 Nov 2011
Cited by: 10 articles | PMID: 22076476 | PMCID: PMC3664236
Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia.
J Clin Oncol, 27(30):4955-4960, 14 Sep 2009
Cited by: 42 articles | PMID: 19752344
Aromatase inhibitor-associated musculoskeletal symptoms: incidence and associated factors.
J Korean Surg Soc, 85(5):205-211, 25 Oct 2013
Cited by: 9 articles | PMID: 24266010 | PMCID: PMC3834018
Aromatase and CDK4/6 Inhibitor-Induced Musculoskeletal Symptoms: A Systematic Review.
Cancers (Basel), 13(3):465, 26 Jan 2021
Cited by: 7 articles | PMID: 33530456 | PMCID: PMC7865932
Review Free full text in Europe PMC

 1
1