Abstract
Background and objective
Coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus has caused millions of deaths worldwide. The mRNA vaccines prevented the figure from being more severe. The objective of this retrospective study is to evaluate the safety of COVID-19 vaccines by analyzing the adverse events following immunization (AEFIs).Methods
A retrospective observational pharmacovigilance study was conducted, based on the collection of reports of suspected AEFIs reported between 1 January 2021 and 31 December 2021 at the Naples 3 local health authority. AEFIs were stratified and described according to mRNA vaccine, demographics, clinical status, description of AEFI, and degree of severity. In 2021, local health authority Asl Naples 3 South received 1164 reports of suspected adverse events that occurred following the administration of mRNA vaccines.Results
During the reporting period, 746 reports were related to the Comirnaty vaccine (64.1%), 281 to the Vaxzevria vaccine (24.1%), 107 to the Spikevax vaccine (9.2%), and 30 to the Jcovden vaccine (2.6%); 89.3% of the reports were classified as not serious (N = 1039 reports), the remaining 10.7% as serious (N = 125 reports).Conclusions
This retrospective pharmacovigilance study demonstrates that COVID-19 mRNA vaccines are safe in all population groups.Free full text

COVID-19 mRNA Vaccines: A Retrospective Observational Pharmacovigilance Study
Abstract
Background and Objective
Coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus has caused millions of deaths worldwide. The mRNA vaccines prevented the figure from being more severe. The objective of this retrospective study is to evaluate the safety of COVID-19 vaccines by analyzing the adverse events following immunization (AEFIs).
Methods
A retrospective observational pharmacovigilance study was conducted, based on the collection of reports of suspected AEFIs reported between 1 January 2021 and 31 December 2021 at the Naples 3 local health authority. AEFIs were stratified and described according to mRNA vaccine, demographics, clinical status, description of AEFI, and degree of severity. In 2021, local health authority Asl Naples 3 South received 1164 reports of suspected adverse events that occurred following the administration of mRNA vaccines.
Results
During the reporting period, 746 reports were related to the Comirnaty vaccine (64.1%), 281 to the Vaxzevria vaccine (24.1%), 107 to the Spikevax vaccine (9.2%), and 30 to the Jcovden vaccine (2.6%); 89.3% of the reports were classified as not serious (N = 1039 reports), the remaining 10.7% as serious (N = 125 reports).
Conclusions
This retrospective pharmacovigilance study demonstrates that COVID-19 mRNA vaccines are safe in all population groups.
Plain Language Summary
Pharmacovigilance is an activity that ensures the safety of health care treatments. The COVID-19 pandemic has accelerated the administration of vaccines whose efficacy and safety is to be evaluated. In the year 2021, an analysis of all reported adverse events following immunization (AEFIs) to the vaccine was conducted on a sample of about 1 million people with the aim of understanding efficacy and safety. All adverse events were divided by age, sex, type of reaction, and severity. Serious reactions were divided into subcategories to report the most common critical issues. At the conclusion of the work, it can be seen that COVID-19 mRNA vaccines are safe but can give serious cardiovascular (12% of the total number of serious reports) and neurological (one serious case that led to the development of Guillain Barré syndrome) side effects that need to be monitored by medical personnel.
Key Points
| In the year 2021 at local health authority Asl Naples 3 South, approximately 1,972,669 doses of COVID-19 vaccines were administered. |
| In 2021, Asl Naples 3 South received 1164 reports of suspected adverse events that occurred following the administration of COVID-19 vaccines. |
| Those who experienced adverse reactions comprise a small fraction and events were mostly non-serious. |
| The most reported suspected adverse events belong to the system organ class "general and site-related pathologies". |
Introduction
SARS-CoV-2 Infection and the COVID-19 Pandemic
The COVID-19 pandemic triggered by SARS-CoV-2 is still ongoing, and to date has caused about 550 million infections with over 6 million deaths [1–3]. SARS-CoV-2 is dangerous due to the wide variability with which it can affect people and due to its rapid human-to-human transmission [4]. An infection from SARS-CoV-2 can have an asymptomatic or slightly symptomatic course [5]. However, a small percentage of vulnerable people with advanced age or concomitant clinical conditions are at high risk of severe symptoms such as respiratory distress syndrome, which requires hospitalization [6, 7]. Moreover, SARS-CoV-2 has the ability to mutate. Indeed, the main SARS-CoV-2 variants Alpha, Beta, Gamma, Delta and Omicron have been responsible for the largest pandemic waves [8]. Scientists are continuing to work to identify ever safer and more effective preventive tools against the different variants of the virus [9, 10]; global vaccination appears to be the most effective weapon to overcome the pandemic.
COVID-19 Vaccines
In December 2020, after a pandemic year with no viable therapeutic alternatives, the first COVID-19 vaccines were approved and rapidly distributed around the world [11, 12]. To date, authorized COVID-19 vaccines are characterized by different mechanisms of operation: messenger RNA (mRNA), viral vector, protein subunit, and inactivated virus vaccines [13]. More than 892 million doses of vaccines have been given to people in the EU and European Economic Area (EEA) as of the end of June 2022. Currently, in Italy, the Italian Medicine Agency (AIFA) has authorized five different COVID-19 vaccines; two out of five use the mRNA method, two use the viral vector method, and one is characterized by a protein subunit composition [14]. Globally, COVID-19 mRNA vaccines have been the first mRNA vaccines to be developed and the most widely administered [11, 12, 15]. Compared with traditional vaccines, the main advantages that mRNA vaccines possess are the ability to be developed quickly, their low production cost, good clinical efficacy and a reliable safety profile based on in vivo data [16, 17].
Comirnaty
Comirnaty is indicated for active immunization for the prevention of COVID-19 in individuals 12 years of age and older. The nucleoside-modified mRNA present in Comirnaty (tozinameran) is formulated in lipid nanoparticles to allow release of the nonreplicating RNA within host cells and direct transient expression of the S antigen of SARS-CoV-2. The mRNA encodes for a membrane-anchored whole S protein with two point mutations at the central helix. Mutation of these two amino acids to proline stabilizes the S protein in the antigenically preferable prefusion conformation. The vaccine induces both a neutralizing antibody response and a cell-mediated immune response toward the spike (S) protein antigen, which may help protect against COVID-19 [18].
Vaxzevria
Vaxzevria is indicated for active immunization in the prevention of COVID-19 in individuals 18 years of age and older. Vaxzevria is a monovalent vaccine composed of a single recombinant replication-deficient chimpanzee adenovirus vector (ChAdOx1) that encodes for the S-glycoprotein of SARS-CoV-2. The SARS-CoV-2 S immunogen in the vaccine is expressed in trimeric prefusion conformation; the coding sequence was not modified to stabilize the S protein expressed in prefusion conformation. After administration, SARS-CoV-2 S glycoprotein is expressed locally stimulating neutralizing antibodies and cellular immune responses, which may contribute to protection against COVID-19 [19].
Spikevax
Spikevax is indicated for active immunization in the prevention of COVID-19 in individuals 18 years of age and older. Spikevax contains mRNA inserted into lipid nanoparticles. The mRNA encodes for the whole spike protein of SARS-CoV-2 modified via two proline substitutions within the seven-peptide repeat (S-2P) domain to stabilize it in pre-fusion conformation. Following intramuscular injection, cells at the site of injection and draining lymph nodes absorb the lipid nanoparticles, managing to release within them the mRNA sequence for translation into viral protein. This induces a response of both T cells and B cells generating neutralizing antibodies, which may contribute to protection against COVID-19 [20].
Jcovden
Jcovden is indicated for active immunization in the prevention of COVID-19 in subjects 18 years of age and older. Jcovden is a monovalent vaccine composed of a recombinant vector based on human adenovirus type 26 incompetent for replication, which encodes for the complete sequence of the spike glycoprotein (S) of SARS-CoV-2 in a stabilized conformation. After administration, the S glycoprotein of SARS-CoV-2 is transiently expressed, stimulating both neutralizing anti-S antibodies and other specific functional anti-S antibodies, as well as cellular immune responses directed against the S antigen, which may help protect against COVID-19 [21].
COVID-19 Adverse Events
As of 26 June 2022 [22],
649,000,000 doses of Comirnaty have been administered to people in the EU/EEA, associated with 848,204 reports of suspected side effects;
69,000,000 doses of Vaxzevria have been administered to people in the EU/EEA, associated with 297,917 reports of suspected side effects;
155,000,000 doses of Spikevax have been administered to people in the EU/EEA, associated with 230,524 reports of suspected side effects;
19,400,000 doses of Jcovden have been administered to people in the EU/EEA, associated with 54,475 reports of suspected side effects.
In Italy, according to the 12th report of the Italian Medicines Agency, as of 26 June 2022, 100 reports have been entered for every 100,000 doses administered, regardless of vaccine and dose [23]. The reports mainly concerned Comirnaty, which was the most widely used vaccine, and to a lesser extent Spikevax, whereas Vaxzevria and Jcovden were less used. Most of the reported adverse events have been classified as non-serious (about 81.8%) and to a lesser extent as serious (18.1%); the outcome was complete resolution or improvement in most cases. In the second quarter of 2022, however, reporting rates for the first dose remained higher than for subsequent doses and decreased significantly after the fourth dose for all vaccines. The most reported adverse events for all vaccines have been fever, headache, muscle/joint pain, chills, gastrointestinal disturbances, vegetative reactions, fatigue, local reaction or pain at the injection site. Serious correlated adverse reactions were rare and in most cases characterized by flu-like symptoms. Adverse events of special interest were very rare and the reporting rate has been stable over time.
This observational study was designed during the global pandemic of COVID-19, with the aim of identifying the main adverse effects due to the administration of the four available vaccines, retrospectively investigating the frequency of reporting, preventability and severity of the same, observed over a period of 1 year at the local health authority Naples 3. The classification of adverse reactions due to the administration of vaccines in our local situation has been compared with national data and with those published in the international literature, in order to assess their overlap or any differences.
Methods
We conducted a retrospective observational pharmacovigilance study by monitoring reports of suspected adverse events following immunization (AEFIs) between January 1, 2021 and December 31, 2021 that occurred in the local health authority Azienda Sanitaria Locale Napoli 3 Sud (Asl Naples 3 South) following administration of COVID-19 disease prophylaxis vaccines. All vaccine doses administered during the reporting period have been counted. We analyzed from the Italian pharmacovigilance network (RNF) all reports of suspected AEFIs, stratifying AEFI reports by patient demographic characteristics (age, sex). In addition, AEFI descriptions by diagnosis and symptoms were coded using the Medical Dictionary for Regulatory Activities (MedDRA) and organized by system organ class (SOC) [24]. AEFI severity was classified according to ‘outcome resolution’ criteria [25] as complete resolution, improvement, worsening, invariant situation, death or not available. For the assessment of the causality of vaccine-related AEFIs, the WHO-specific algorithm was used [26, 27]. This is called vaccinovigilance and encompasses all pharmacovigilance activities related to the collection, evaluation, analysis, and reporting of AEFIs. In broader terms, pharmacovigilance and vaccinovigilance represent a complex set of activities aimed at continuously assessing all information related to the safety of medicinal products and ensuring that the benefit/risk (B/R) ratio remains favorable over time. Thus, vaccinovigilance represents a tool for monitoring the safety of vaccines even after they have been approved and placed on the market. Passive vaccinovigilance is carried out through the collection and analysis of AEFI spontaneous reports (from physicians, health care workers, and vaccinated persons), through which signals may emerge that need further investigation (to be disproved or confirmed and quantified in terms of risk) by conducting pharmacoepidemiology studies. Reports can be made online (through the information technology platform of the National Drug Agency, Aifa), or by sending the completed paper form by mail (or fax/email) to the pharmacovigilance officer of the facility to which the reporter belongs. Reports are collected in the database of the National Pharmacovigilance Network (Rnf). The Rnf ensures not only the collection, management, and analysis of spontaneous reports, but also the rapid dissemination of information issued by Aifa on vaccine safety, through a network involving Aifa, regions and autonomous provinces, local health units, hospitals, scientific research and treatment institutes, and pharmaceutical industries. Aifa evaluates each reported event and forwards reports of serious ones to the European Eudravigilance database (which collects reports of suspected adverse reactions to drugs authorized in the European Economic Area). To learn more, see also the pages on pharmacovigilance data interpretation and adverse reactions [12–14]. National passive pharmacovigilance activities are also enhanced through linkage with Vigibase, the WHO's database for international drug monitoring, which collects reports that currently come from 125 countries around the world. The availability of all these data increases the size of the populations studied and allows specific issues that warrant further investigation to be quickly highlighted, making it possible to assess even very rare AEFIs that cannot be adequately studied using data derived from a limited geographic area or selected sample.
Vaccines used in national immunization programs can be considered among the most controlled and safe pharmaceuticals. Vaccines are administered to healthy people, often children, for the purpose of preventing disease; therefore, a higher standard of safety is expected for them than for drugs used to treat people who are already ill (such as antibiotics), and there tends to be a low tolerance for any adverse reaction. The risks of adverse reactions after vaccination must be compared with the risks associated with the preventable disease and also with the risks of taking treatments to relieve the symptoms of the disease should an unvaccinated person become infected. Vaccines make it possible to prevent thousands of cases of disease and consequently also the complications and deaths associated with them. The frequency of adverse reactions to the vaccine is always much lower than the frequency of complications of the disease being prevented, and the dangers of the diseases always far outweigh the risks associated with vaccines. Therefore, choosing not to vaccinate yourself or your children is not a risk-free choice; it is a choice to accept a different kind of risk.
Surveillance of adverse events arising following vaccination plays a key role in ‘on-the-ground’ knowledge of the effects of vaccines in use and the risk–benefit ratio of strategies implemented. Indeed, it makes it possible to highlight warning signs, such as the possible excess of reactogenicity of a particular batch or the existence of unexpected adverse reactions. The return of surveillance results also improves the work of operators and, consequently, allows them to inform families more adequately.
Results
In the year 2021, 844,548 people have been vaccinated in Asl Naples 3 South, with approximately 1,972,669 doses of COVID-19 vaccines administered (Table (Table1).1). In particular, 1,084,968 doses of Comirnaty, 295,900 doses of Vaxzevria, 572,074 doses of Spikevax, and 19,727 doses of Jcovden have been administered (Table (Table22).
Table 1
People vaccinated and number of doses of COVID-19 vaccines administered in 2021
| Key elements of the analysis | N |
|---|---|
| Population of Asl Naples 3 in 2021 | 1,044,024 |
| People vaccinated in Asl Naples 3 South in 2021 | 844,548 |
| Doses administered | 1,972,669 |
Table 2
Doses of various COVID-19 vaccines administered in the year 2021 in Asl Naples 3 South
| Vaccine | N (%) |
|---|---|
| Comirnaty | 1,084,968 (55.0) |
| Vaxzevria | 295,900 (15.0) |
| Spikevax | 572,074 (29.0) |
| Jcovden | 19,727 (1.0) |
Asl Naples 3 South (Azienda Sanitaria Locale Napoli 3 Sud) is a local health authority in Naples, Italy
In 2021, Asl Naples 3 South received 1164 reports of suspected adverse events that occurred following the administration of COVID-19 vaccines. Of these, 746 reports have been related to the Comirnaty vaccine (64.1%), 281 to the Vaxzevria vaccine (24.1%), 107 to the Spikevax vaccine (9.2%), and 30 to the Jcovden vaccine (2.6%) (Table (Table33).
Table 3
Number of adverse events (AEs) reported in the year 2021 in Asl Naples 3 South, stratified by vaccine
| Vaccine | AEs, N (%) |
|---|---|
| Comirnaty | 746 (64.1) |
| Vaxzevria | 281 (24.1) |
| Spikevax | 107 (9.2) |
| Jcovden | 30 (2.6) |
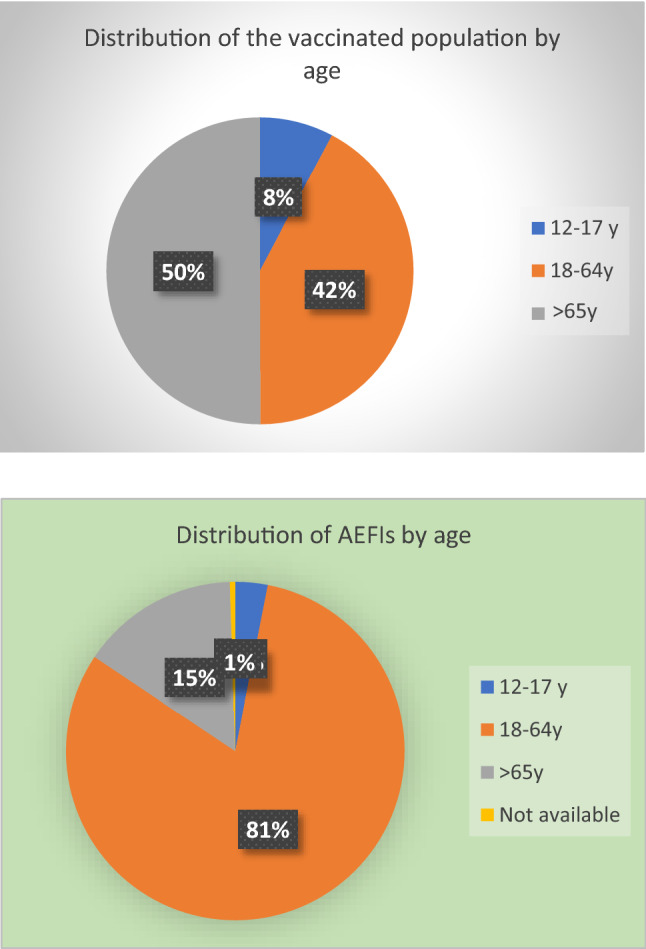
The distribution of reports by age group shows that 3.1% of reports are related to the age group 12–17 years (n = 36 reports), 81.3% are related to the age group 18–64 years (n = 946 reports), and 15.1% are related to patients aged 65 years and older (n = 176 reports); for the remaining 0.5% of the reported events, age has not been available (n = 6 reports). Regarding sex, 31.8% of the reports referred to males (n = 370 reports), 67.8% to females (n = 789 reports), while the remaining 0.4% of the reports did not report sex information (n = 5 reports) (Table (Table44 and Fig. Fig.11).
Table 4
AEFIs reports stratified by sex and age
| Classification | Vaccinated population, N (%) | AEFIs, N (%) |
|---|---|---|
| Age group | ||
| 12–17 years | 65,746 (7.8%) | 36 (3.1%) |
| 18–64 years | 356,213 (42.2%) | 946 (81.3%) |
| ≥ 65 years | 422,589 (50.0%) | 176 (15.1%) |
| Not available | 6 (0.4%) | |
| Sex | ||
| Male | 370 (31.8%) | |
| Female | 789 (67.8%) | |
| Not available | 5 (0.4%) |
AEFIs adverse events following immunization
Of all the reports, 89.3% have been classified as not serious (n = 1039 reports) and the remaining 10.7% as serious (n = 125 reports). The severity criterion was reported as “other clinically relevant condition” for 53.6% of the reports (n = 67 reports), “hospitalization or prolongation of hospitalization” for 27.2% (n = 34 reports), “severe or permanent disability” for 11.2% (n = 14 reports), “life-threatening” for 7.2% (n = 9 reports), and death for 0.8% (n = 1 report). In 62.1% of cases, the outcome was complete resolution of the suspected adverse events (n = 723 reports), 20.6% of cases reported improvement (n = 240 reports), for 12.2% of cases the events had not yet resolved at the time of reporting (n = 142 reports), 1.6% of reports showed resolution with sequelae, one report reported a fatal outcome, and for 3.4% of reports the outcome was not available (n = 39 reports) (Table (Table55).
Table 5
Outcomes of adverse events following immunization in the population
| Outcome | Total | Comirnaty | Vaxzevria | Spikevax | Jcovden |
|---|---|---|---|---|---|
| Complete resolution | 723 (62.1) | 498 (66.7) | 147 (52.3) | 54 (50.5) | 24 (80.0) |
| Improvement | 240 (20.6) | 123 (16.5) | 76 (27.0) | 39 (36.4) | 2 (6.7) |
| Worsening | 19 (1.6) | 14 (1.9) | 2 (0.8) | 3 (2.8) | |
| Invariant situation | 142 (12.2) | 92 (12.3) | 35 (12.5) | 11 (10.3) | 4 (13.3) |
| Death | 1 (0.1) | 1 (0.2) | |||
| Not available | 39 (3.4) | 18 (2.4) | 21 (7.4) |
Values are expressed as N (%)
The most prevalent reporting source was individual citizens (70%, n = 814 reports), followed by physicians (24.9%, n = 289 reports), other health care providers (3%, n = 35 reports), and pharmacists (2.2%, n = 26 reports). Of the reports, 61% were classified as ‘consistent’ (n = 709 reports), following the application of the WHO algorithm for vaccines to establish the causal link between the reported event and the administered medicinal specialty, 35.9% indeterminate (n = 417 reports), 1.1% unclassifiable (n = 13 reports), and 0.7% indeterminate (n = 8 reports). The outcomes from applying the Naranjo logarithm are shown in Tables Tables66 and and7.7. In addition, a table is provided to highlight the distribution of AEFIs into severe and non-severe (Table (Table88).
Table 6
Causality assesment of AEFIs
| Causality | Total number of AEFIs | Comirnaty | Vaxzevria | Spikevax | Jcovden |
|---|---|---|---|---|---|
| Consistent | 709 | 457 | 175 | 55 | 22 |
| Indeterminate | 417 | 268 | 98 | 44 | 7 |
| Unconsistent | 8 | 2 | 2 | 3 | 1 |
| Unclassificable | 13 | 11 | 2 |
AEFI adverse events following immunization
Table 7
Most frequently reported AEFIs for all the four COVID-19 vaccines, in relation to the system organ class
| System organ class | Total number of AEFIs | Comirnaty | Vaxzevria | Spikevax | Jcovden |
|---|---|---|---|---|---|
| Renal and urinary disorders | 3 | 3 | |||
| Metabolism and nutrition disorders | 7 | 4 | 3 | ||
| Injury, poisoning and procedural complications | 8 | 3 | 2 | 1 | 2 |
| Immune system disorders | 9 | 5 | 2 | 2 | |
| Eye disorders | 22 | 10 | 8 | 3 | 1 |
| Infections and infestations | 24 | 18 | 4 | 2 | |
| Reproductive system and breast disorders | 25 | 20 | 3 | 2 | |
| Investigations | 27 | 9 | 15 | 2 | 1 |
| Psychiatric disorders | 28 | 17 | 7 | 4 | |
| Respiratory, thoracic and mediastinal disorders | 43 | 25 | 11 | 7 | |
| Blood and lymphatic system disorders | 45 | 33 | 8 | 4 | |
| Cardiac disorders | 46 | 31 | 4 | 6 | 5 |
| Vascular disorders | 53 | 27 | 17 | 7 | 2 |
| Ear and labyrinth disorders | 67 | 44 | 18 | 3 | 2 |
| Skin and subcutaneous tissue disorders | 93 | 78 | 10 | 3 | 2 |
| Gastrointestinal disorders | 85 | 70 | 6 | 9 | |
| Musculoskeletal and connective tissue disorders | 124 | 96 | 14 | 11 | 3 |
| Nervous system disorders | 176 | 88 | 69 | 18 | 1 |
| General disorders and administration site conditions | 279 | 165 | 80 | 23 | 11 |
| Total | 1164 | 746 | 281 | 107 | 30 |
AEFI adverse events following immunization
Table 8
Classification of AEFIs into serious and non-serious
| Vaccine | Non-serious | Serious |
|---|---|---|
| Comirnaty | 25 | 5 |
| Vaxzevria | 96 | 11 |
| Spikevax | 245 | 36 |
| Jcovden | 673 | 73 |
| Total | 1039 | 125 |
AEFI adverse events following immunization
In conclusion, vaccine preventability and relative benefit/risk is high and has helped the Asl Naples 3 South population to avoid developing Covid-19 disease and its associated high health risks. The adverse reactions experienced are a small fraction and mostly concern non-serious events. Only 125 serious reactions were reported compared with the total of 1164 in a population of more than one million people with nearly one million vaccinees. This means that the risk from COVID immunization is acceptable compared with the risk from the disease itself. Furthermore, among the serious reactions, the majority were excessive reactogenicity of the vaccine that led to cardiovascular complications in young subjects with an intact and active immune system.
Comirnaty Vaccine
There were 746 reported events related to the Comirnaty vaccine (64.1% out of a total of 1164 reports that referred to COVID-19 vaccines); 4.8% of the reports referred to the age group 12–17 years (n = 36 reports), 83.4% to the age group 18–64 years (n = 622 reports), and 11.3% (n = 84 reports) to age 65 years or older; in the remaining 0.5% of the cases, age was not available. The proportion of reports involving males was 30.2% (n = 225 reports) and 69.4% involved females (n = 518 reports); 0.4% (n = 3 reports) of the records did not report this information. The outcome of AEFIs is distributed as follows: 66.7% complete resolution (n = 498), 16.5% improvement (n = 123 reports), 1.9% resolution with sequelae (n = 14 reports), 12.3% not yet cured (n = 92 reports), and one death (0.1% of cases); in the remaining 2.4% of cases, the outcome was not available (n = 18 reports). Of the reports, 90.2% were classified as non-serious (n = 673 reports), and 73 reports were classified as serious. Of these, 52% were classified as ‘other clinically relevant condition’ (n = 38 reports), 26% required hospitalization or prolonged hospitalization (n = 19 reports), 13.7% resulted in severe or permanent disability (n = 10 reports), and 6.8% were classified as life-threatening (n = 5 reports); one case of death was also reported.
Vaxzevria Vaccine
There were 281 reported events related to the Vaxzevria vaccine (24.1% out of a total of 1164 reports that referred to COVID-19 vaccines); 70.5% of the reports referred to the age group 18–64 years (n = 198 reports) and 29.2% (n = 82 reports) to age 65 years or older; in the remaining 0.4% of the cases, age was not available (n = 1 reports). Of the reports, 30.6% involved males (n = 86 reports) and 68.7% females (n = 193 reports); 0.7% (n = 2 reports) of the records did not report this information. The outcome of AEFIs was distributed as follows: complete resolution 52.3% (n = 147 reports), improvement 27% (n = 76 reports), 0.7% resolution with sequelae (n = 2 reports), 12.4% not yet cured (n = 35 reports); in the remaining 7.5% of cases the outcome was not available (n = 21 signals). Reports were classified as not serious in 87.2% (n = 245 reports); 36 reports were classified as serious. Of these, 7.5% were classified as ‘other clinically relevant condition’ (n = 21 reports), 3.6% required hospitalization or prolonged hospitalization (n = 10 reports), 0.7% resulted in severe or permanent disability (n = 2 reports), and 1.1% were classified as life-threatening (n = 3 reports).
Spikevax Vaccine
There were 107 reported events related to the Spikevax vaccine (9.2% out of a total of 1164 reports that referred to COVID-19 vaccines); 90.6% of the reports referred to the age group 18–64 years (n = 97 reports) and 8.4% (n = 9 reports) to age 65 years or older; in the remaining 0.9% of cases, age was not available. Of the reports, 38.3% involved males (n = 41 reports) and 61.7% females (n = 66 reports). The outcome of AEFIs is distributed as follows: 50.5% complete resolution (n = 54 reports), 36.4% improvement (n = 39 reports), 2.8% resolution with sequelae (n = 3 reports), and 10.3% not yet cured (n = 11 reports). Reports were classified as non-serious in 89.7% (n = 96 reports); 11 reports were classified as serious. Of these, 36.4% were classified as ‘other clinically relevant condition’ (n = 4 reports), 36.4% required hospitalization or prolonged hospitalization (n = 4 reports), 18.1% resulted in severe or permanent disability (n = 2 reports), 9.1% were classified as life-threatening (n = 1 report).
Jcovden Vaccine
There were 30 reported events related to the Jcovden vaccine (2.6% out of a total of 1164 reports that referred to COVID-19 vaccines); 96.7% of the reports referred to the age group 18–64 years (n = 29 reports) and 3.3% (n = 1 report) to age 65 years or older. Sixty percent of the reports related to males (n = 18 reports) and 40% to females (n = 12 reports). The outcome of AEFIs is distributed as follows: 80% complete resolution (n = 24 reports), 6.7% improvement (n = 2 reports), and 13.3% not yet cured (n = 4 reports). Reports were classified as not serious in 83.3% (n = 25 reports) and five reports were classified as serious. Of these, 80% were classified as ‘other clinically relevant condition’ (n = 4 reports), 20% required hospitalization or prolonged hospitalization (n = 1 report). The 1164 reports received corresponded to 2421 suspected adverse events that arose after administration of COVID-19 vaccines; multiple AEFIs may be present for any single report, and thus the total number of suspected adverse events is greater than the number of reports received.
Main AEFIs
As illustrated in Table Table7,7, regardless of vaccine type, the most reported suspected adverse events belong to the SOC ‘general and site-related pathologies’ (32.7%, n = 791 reports). The most frequent AEFIs in this class were pyrexia, injection-site pain, asthenia, and chills. This was followed by ‘nervous system pathologies’ (20%, n = 485 reports), including headache, paresthesia, drowsiness, and tremor, and ‘disorders of the musculoskeletal system and connective tissue’ (18%, n = 435 reports), including limb pain, myalgia, and arthralgia. Less frequent were AEFIs referable to the class ‘gastrointestinal disorders’ (7.8%, n = 190 reports) and the class ‘skin and subcutaneous tissue disorders’ (4.2%, n = 103 reports).
Cardiac Disorders
In this study, 48 reports of medical events referable to the SOC ‘cardiac pathologies’ were received; among them, 15 showed cases of pericarditis and/or myocarditis (12% of the total number of serious reports), specifically five myocarditis, nine pericarditis, and one report of myopericarditis. The predominantly reported symptomatology was fever, chest pain, chest tightness, and asthenia. No patients had any special predisposing medical conditions or were following drug therapies. Eleven reports involved the Comirnaty vaccine (3 myocarditis, 1 myopericarditis, 7 pericarditis) and four involved the Spikevax vaccine (2 myocarditis, 2 pericarditis). They accounted for 15.1% and 36.4% of the serious reports regarding each of these two vaccines, respectively. In five cases (33.3%), patients were aged 20–29 years old (3 Comirnaty, 2 Spikevax), five (33.3%) were aged 30–39 years old (3 Comirnaty, 2 Spikevax), four (26.7%) belonged to the 40–49 years age group (4 Comirnaty), and one (6.7%) belonged to the 50–59 years age group (Comirnaty). Eight patients were male and seven were female (53.3% and 46.7%, respectively); five female patients were given the Comirnaty vaccine and the remaining two were given the Spikevax vaccine, while five male patients were given the Comirnaty vaccine and two were given the Spikevax vaccine.
The severity criterion was ‘other clinically relevant condition’ in six cases (40%), ‘severe or permanent disability’ in two (13.3%), three cases required ‘hospitalization or prolongation of hospitalization’ (20%), and four were classified as ‘life-threatening’ (26.7%). The outcome for two cases was improvement of clinical condition (13.3%), eight patients were still not cured at the time of reporting (53.3%), two had complete resolution (13.3%), two had resolution with sequelae (13.3%), and for one case the outcome is not available. AEFIs appeared for eight cases after one dose administration (53.3%, 6 cases after immunization with Comirnaty, 2 with Spikevax), six cases after the second dose (40%, 5 Comirnaty and 1 Spikevax), with one case of a second dose heterologous with Comirnaty, and finally one case after a third dose with Spikevax.
Nervous System Disorders
A report was received at the Asl Naples 3 South regarding a case similar to Guillain Barré syndrome in a 76-year-old male patient after administration of a second dose of the Vaxzevria vaccine, specifically 12 days after immunization. The condition required hospitalization of the patient, who was not yet cured at the time of reporting. Application of the WHO algorithm for vaccines to establish causality between adverse event and drug specialty yielded a correlated result.
Discussion
In total, 80% of Asl Naples 3 South patients were vaccinated against Sars-Cov-2 in January–December 2021. Of these, 84% received an mRNA vaccine and 16% a viral vector vaccine. In line with the largest number of doses administered, the administration of Comirnaty has seen the highest number of adverse reaction reports (64.1%) compared with the total number of reports received, followed by Vaxzevria (24.1%), Spikevax (9.2%), and Jcovden (2.6%). Reactions and health impacts were reported more frequently in female than in male recipients, and in individuals younger than 65 years old than in older individuals. We found that reported reactions to COVID-19 vaccination were mostly mild in severity and transient in duration, and most reports were non-serious. We received one case of suspected death, in a patient with comorbidity and on drug polypharmacy; this may represent a bias, causation was undefined. We also observed specific severe cases for the following SOCs: cardiac disorders, in particular myocarditis cases with rare frequency, and for Neurologic disorders, Guillain Barré cases, again with very rare reporting frequency. The results of reactogenicity following vaccination with the COVID-19 vaccines examined are similar to those found in the literature [28]. The observed patterns could be explained in part by host characteristics known to influence reactogenicity, including age, sex, and the presence of underlying medical conditions. The rate of AEFI reporting compared with the number of people vaccinated in 2021 is in line with data reported by EMA and large clinical trials [29, 30]. The data collected at the local health authority Asl Naples 3 and described in our study are in line with the most recent vaccine surveillance report published by AIFA at a national level. The reports mainly concern Comirnaty in view of the fact that it was the most widely used vaccine; most of the reported adverse events are classified as non-serious (about 82.7%) and to a lesser extent as serious (1.7%). The most reported adverse events for all vaccines are fever, pain at the injection site, fatigue, headache, muscle/joint pain and chills. Furthermore, our study showed, in line with some recently published research, that vaccination against COVID-19 provides benefits that are significantly greater than the risks of morbidity and mortality that may be expressed after COVID-19 infection and that these benefits involve all types of population groups [31–33]. However, further unknown side effects of these vaccines need to be investigated through the development of further studies that continue to investigate the existence of causality between COVID-19 vaccines and the reported adverse events. Finally, it is noteworthy that the occurrence of cardiac side effects and the reported adverse reactions have been matched by case reports published in the literature [34].
Strengths and Limitations
This study has several strengths, including the large surveillance population and comprehensive data acquisition; also, knowing the number of population vaccinated during the baseline period, it was possible to calculate reporting rates. A new study is underway for the baseline period year 2022. In some cases, adverse reaction reports were not complete with all data such as age, sex, and outcome researched, especially for reports filled out by citiziens or people without pharmacovigilance skills. Moreover, it is probable that some AEFIs were not reported, a recurring element in pharmacovigilance studies that should be strengthened.
Conclusions
COVID-19 vaccines represent a key weapon in preventing higher numbers of deaths from SARS-CoV-2. Vaccines with mRNA methodology have represented a preventive drug innovation with few precedents in the history of modern medicine, demonstrating an excellent tolerability profile since the first pre-registration clinical trials. In this retrospective observational pharmacovigilance study we have shown that data from more than 844,548 doses of COVID-19 vaccine administered during 2021 in Asl Naples 3 South demonstrated that adverse events have been mild and short in duration, confirming the vaccines’ safety. However, continuous monitoring of the safety profile of COVID-19 vaccines needs to be continued with great care.
Declarations
Not applicable.
Not applicable.
Not applicable.
FF: conceptualization, writing—original draft, methodology, supervision, validation. CM: supervision, validation. AV: conceptualization, writing—original draft. SS: conceptualization, writing—original draft. AZ: writing—review and editing. EN: supervision, validation. UT: supervision, validation. MB: supervision, validation. AV: writing—review and editing.
No funding was received to conduct this study.
Full availability of data and materials. All stated data can be provided to the reader on request.
Not applicable.
The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work reported in this paper. The authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the Administrations to which they belong.
Contributor Information
Francesco Ferrara, Email: ti.orebil@rfararref, Email: [email protected].
Carolina Mancaniello, Email: [email protected].
Alessia Varriale, Email: [email protected].
Sarah Sorrentino, Email: [email protected].
Andrea Zovi, Email: [email protected].
Eduardo Nava, Email: [email protected].
Ugo Trama, Email: [email protected].
Mariarosaria Boccellino, Email: [email protected].
Antonio Vitiello, Email: ti.liamtoh@olleitiva.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40261-022-01216-9
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s40261-022-01216-9.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/137613008
Article citations
Pharmacovigilance in Vaccines: Importance, Main Aspects, Perspectives, and Challenges-A Narrative Review.
Pharmaceuticals (Basel), 17(6):807, 19 Jun 2024
Cited by: 0 articles | PMID: 38931474
Review
Trend analysis of proton pump inhibitor consumption and expenditure: The real-world evidence.
Indian J Gastroenterol, 43(3):645-651, 17 Jan 2024
Cited by: 1 article | PMID: 38231298
COVID-19 Pandemic: Therapeutic Strategies and Vaccines.
Int J Mol Sci, 25(1):556, 31 Dec 2023
Cited by: 1 article | PMID: 38203727 | PMCID: PMC10778581
The diabetic patient between sustainability and effectiveness of new treatments.
J Diabetes Metab Disord, 22(2):1635-1643, 16 Sep 2023
Cited by: 0 articles | PMID: 37975093 | PMCID: PMC10638228
Adverse Events Following mRNA COVID-19 Vaccine in 2021 and 2022: A Retrospective Analysis in Costa Rica and Italy.
Cureus, 15(10):e47834, 27 Oct 2023
Cited by: 0 articles | PMID: 38021647 | PMCID: PMC10676764
Go to all (9) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands.
Drug Saf, 45(4):319-331, 21 Mar 2022
Cited by: 27 articles | PMID: 35314943 | PMCID: PMC8936041
Capillary leak syndrome following COVID-19 vaccination: Data from the European pharmacovigilance database Eudravigilance.
Front Immunol, 13:956825, 13 Sep 2022
Cited by: 8 articles | PMID: 36177033 | PMCID: PMC9513245
Adverse Events of COVID-19 Vaccines in the United States: Temporal and Spatial Analysis.
JMIR Public Health Surveill, 10:e51007, 15 Jul 2024
Cited by: 1 article | PMID: 39008362 | PMCID: PMC11287098
Serious neurological adverse events following immunization against SARS-CoV-2: a narrative review of the literature.
Ther Adv Drug Saf, 14:20420986231165674, 21 May 2023
Cited by: 2 articles | PMID: 37223456 | PMCID: PMC10201278
Review Free full text in Europe PMC

 1
1