Abstract
Background
The welcome advent and subsequent development of interventional neuroradiology led to an important paradigm shift in the management of many cerebrovascular diseases. This paradigm shift is especially true for carotid cavernous fistula and, for some time now, endovascular techniques are the mainstay approach for these lesions. The neurosurgical intervention should be adopted when the endovascular treatment is not practicable.Case description
We present the surgical solution adopted to treat a patient with an indirect carotid cavernous fistula (CCF), with quickly progressive symptoms, in which it was not possible to treat using the currently standardized endovascular technique. A pretemporal craniotomy with peeling of the dura mater at the middle fossa and exposure of Parkinson's triangle on the lateral wall of the cavernous sinus was performed. Fibrin glue was injected by puncture of the lateral wall of the cavernous sinus for direct thrombosis of this sinus and the superior ophthalmic vein.Conclusion
In the now far 60s, Parkinson already treated patients with CCF effectively and elegantly through the lateral wall of the cavernous sinus. Revisiting techniques from the past, associating them with the supplies widely available today, can sometimes be the solution to some especially challenging cases that we face in our profession.Free full text

Revisiting Parkinson: After six decades, his triangle remains useful
Abstract
Background:
The welcome advent and subsequent development of interventional neuroradiology led to an important paradigm shift in the management of many cerebrovascular diseases. This paradigm shift is especially true for carotid cavernous fistula and, for some time now, endovascular techniques are the mainstay approach for these lesions. The neurosurgical intervention should be adopted when the endovascular treatment is not practicable.
Case Description:
We present the surgical solution adopted to treat a patient with an indirect carotid cavernous fistula (CCF), with quickly progressive symptoms, in which it was not possible to treat using the currently standardized endovascular technique. A pretemporal craniotomy with peeling of the dura mater at the middle fossa and exposure of Parkinson’s triangle on the lateral wall of the cavernous sinus was performed. Fibrin glue was injected by puncture of the lateral wall of the cavernous sinus for direct thrombosis of this sinus and the superior ophthalmic vein.
Conclusion:
In the now far 60s, Parkinson already treated patients with CCF effectively and elegantly through the lateral wall of the cavernous sinus. Revisiting techniques from the past, associating them with the supplies widely available today, can sometimes be the solution to some especially challenging cases that we face in our profession.

INTRODUCTION
“I see the future repeat the past;
I see a museum of great novelties;
Time doesn’t stand still.” (Arnaldo Brandão and Cazuza [1988], Brazilian musicians)
Human brain surgery is undoubtedly a professional exercise that requires from neurosurgeon extreme skills and competences, rigorous and continuous study, and an emotional stability which allow correct and accurate decisions in moments of great stress. Such professional complexity is one of the forces that drive this medical specialty to develop continuously and in an accelerated way in search of increasingly effective and safe solutions for patients. In this way, neurosurgery has undergone revolutionary changes.
If the past decades of the 20th century were a period of reinvention of the specialty with the development and/or technological improvement of several techniques (e.g., imaging, stereotactic guidance, navigation, microscopy, hemostasis, endoscopy, radiosurgery, neuromodulation, endovascular techniques, and molecular medicine),[2] the first two decades of the 21st century can be characterized as a period of refinement of all this technological acquire.
An obvious consequence of this progressive technical and technological development of neurosurgery is the progressive abandonment of more invasive surgeries in favor of the adoption of minimally invasive and increasingly effective techniques. It seems indisputable that we are walking on a no return path in the direction of a neurosurgery minimally invasive and maximally resolving. The welcome advent and subsequent development of interventional neuroradiology led to an important paradigm shift in the management of many cerebrovascular diseases. This paradigm shift is especially true for carotid cavernous fistula (CCF) and, for some time now, endovascular techniques are the mainstay approach for these lesions.[18]
However, as already said by the famous American pugilist Mike Tyson, “everyone has a plan until they get punched in the mouth.” What to do when our initial therapeutic plans fall into the ring and the illness “opens count” due to progressive symptoms? What to do when the modern and already standardized less invasive treatment techniques are not available? In these moments, it is interesting to review all the imaging examinations, re-discuss the challenging case with the multidisciplinary team, and revisit the techniques that our forerunners so elegantly developed in the past, and pairing them with a bit of creativity is often the solution available to winning some challenging “combats.”
The neurosurgical intervention should be adopted when the endovascular treatment is not practicable. In the present paper, we present the surgical solution adopted to treat a patient with an indirect CCF, with quickly progressive symptoms, in which it was not possible to treat using the currently standardized endovascular technique.
CASE DESCRIPTION
A 61-year-old male patient is admitted to our emergency department due to a complaint of progressive worsening of the previous left ocular symptoms. He reported having been diagnosed with CCF nearly 2 months ago and being under outpatient follow-up at another neurosurgical department. However, in the past 4 days before hospital admission, the patient worsened from of the previous proptosis and chemosis, in addition to the onset of ipsilateral eye ache and diplopia when looking to the left. He denied history of trauma and emphasized the spontaneous onset of the condition. He reported a personal medical history of hypertension, myocardial infarction, nondialysis chronic kidney disease, and smoking.
Admission brain computed tomography angiography (CTA) demonstrated the left superior ophthalmic vein engorgement and proptosis, suggesting the diagnosis reported by the patient. Brain angiography confirmed the diagnosis and showed that it was an indirect CCF (Barrow type D) whose arterial supply was through multiple small-caliber branches from the cavernous segment of the left internal carotid artery (ICA) and distal branches of the left maxillary artery. The arteriovenous shunt in the left cavernous sinus (CS) drained retrogradely into the ipsilateral superior ophthalmic vein (which was enlarged). During angiography, venous access was attempted to the CS for the endovascular treatment of the fistula; however, the thrombosed inferior and superior petrosal sinuses prevented the endovascular staff from reaching the CS.
After multidisciplinary discussion, a new endovascular approach to the fistula through the angular vein was chosen. Unfortunately, the transition from the angular to the superior ophthalmic vein proved to be too small and angled, preventing navigation with the microcatheter. On the 1st day after this new treatment attempt, the patient evolved with progressive worsening of symptoms and onset of visual deficit. This left visual deficit rapidly progressed to monocular blindness.
We assumed that such an unfavorable evolution would be due to thrombosis (post catheterization) of the angular and facial veins, and secondary increase in ocular venous hypertension. Given the impossibility of endovascular treatment and the unfavorable evolution of the case, we chose to perform a pretemporal craniotomy with peeling of the dura mater at the middle fossa and exposure of Parkinson’s triangle on the lateral wall of the CS. After surgically exposing the CS and observing slight arterial bleeding in the middle fossa peeling, we performed a temporary clipping of the common carotid artery to decrease arterial flow in the CS. Immediately after cervical clipping, a change in the appearance of bleeding (coloration) along the lateral wall of the CS was observed. Puncture of the lateral wall of the CS was performed in the Parkinson’s triangle and fibrin glue was injected (Tisseel Lyo, Baxter AG, Vienna, Austria) for direct thrombosis of the CS and superior ophthalmic vein (about 3 ml of fibrin glue was injected). The temporary clip from the common carotid was then removed and no bleeding was observed through the lateral wall of the CS.
Postoperative CTA showed no surgical complications and no arterial filling of the CS or retrograde venous filling of the superior ophthalmic vein [Figure 1]. The patient evolved with excellent postoperative clinical evolution and explicit regression of proptosis, chemosis, and diplopia. Hospital discharge on the 3rd postoperative day. At the outpatient consultation, 21 days after surgery, the patient had complete resolution of proptosis and chemosis, decreased of the VI left cranial nerve previous paresis, and persistence of ipsilateral blindness [Figure 2].
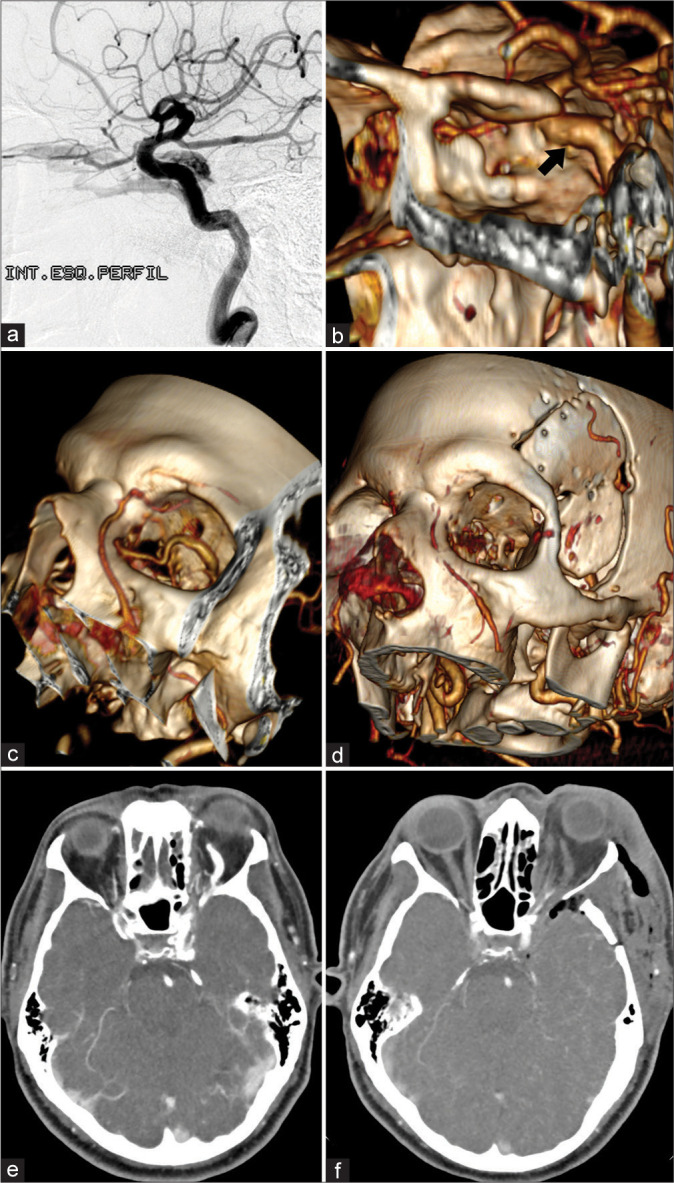
Imaging exams. (a) Diagnostic brain angiography (left ICA – lateral view) showing a CCF. The arteriovenous shunt in the left CS drains retrogradely into the ipsilateral superior ophthalmic vein (which is enlarged). (b) Postoperative CTA reconstruction shows normal-appearing ICA (black arrow), preserved flow, and excludes arterial lesion or dissection. (c) Preoperative CTA reconstruction shows an important dilation of the superior ophthalmic vein inside the orbit. (d) Postoperative CTA reconstruction demonstrates the absence of contrast in the left superior ophthalmic vein and the absence of flow in the ipsilateral facial vein (which was well visualized in the preoperative imaging exam). We believe that the rapidly progressive worsening of visual function was secondary to angular and facial veins thrombosis after attempted catheterization of the superior ophthalmic vein. (e) Preoperative CTA with extravasation of contrast within the CS in the arterial phase and demonstrating the superior ophthalmic vein dilated and precociously contrasted. (f) Postoperative CTA with contrast exclusively in the cavernous segment of the ICA (no extravasation into the CS) and no contrast in the superior ophthalmic vein. ICA: Internal carotid artery, CS: Cavernous sinus, CTA: Computed tomography angiography, CCF: Carotid cavernous fistula.
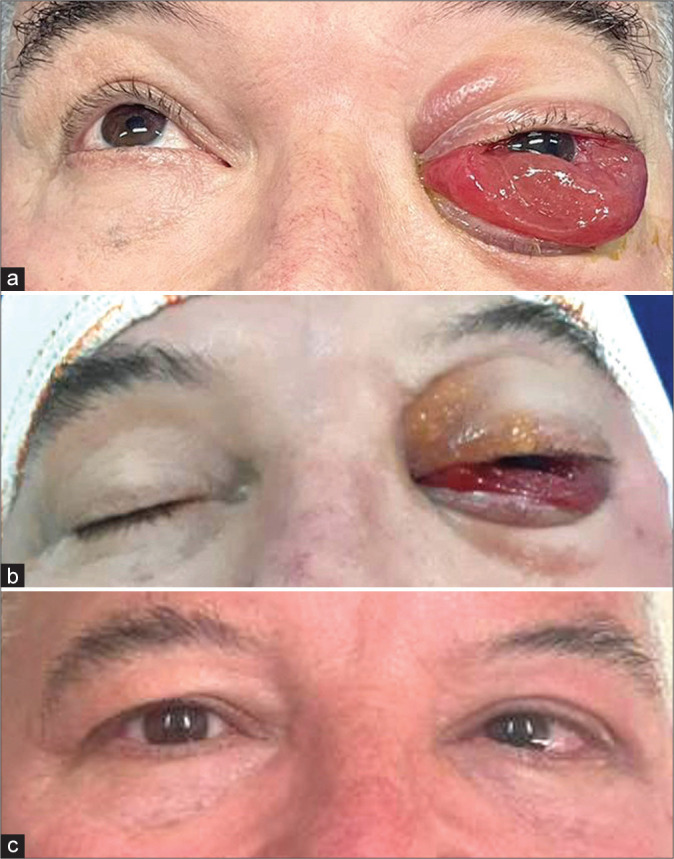
(a) Patient on the operating table just before surgery. Amaurosis and paralysis of the cranial nerves responsible for extrinsic ocular movement (III, IV, and VI) of the left eye. Significant chemosis is noted. (b) Patient on the operating table immediately after surgery. Significant decrease in chemosis is observed. (c) At the outpatient consultation, 21 days after surgery, the patient had complete resolution of proptosis and chemosis, decreased of the VI left cranial nerve previous paresis, and unfortunately persistence of ipsilateral blindness.
DISCUSSION
According to Barrow et al.,[3] CCFs are abnormal communications between the carotid artery or its branches and the CS. Angiographically, these lesions can be classified into direct or indirect (dural) fistulas and be placed into one of four categories of abnormal communications: type A fistulas are direct shunts between the ICA and the CS; type B are dural shunts between meningeal branches of ICA and the CS; type C are dural shunts between meningeal branches of the external carotid artery (usually distal branches of the maxillary and/or middle meningeal arteries) and the CS; and type D are dural shunts between meningeal branches of both the internal and external carotid arteries and the CS.[3] The type A classification is the direct fistula and types B, C, and D are dural shunts (indirect fistulas). This classification is the most widely used system that categorizes the CCFs according to their arterial supply.[18] Our patient had a type D fistula that is the most common type of fistula among the indirect CCFs.
It is well known that indirect fistulas have a relatively high incidence of spontaneous resolution or with conservative management due to their lower flow rate.[3,18] This incidence ranges from 10% to 73% in various series in the literature.[3,11,18] Interestingly, in several cases, patients improved shortly after the diagnostic angiography.[3] Thus, an initial conservative approach may be indicated in patients with indirect fistulas.[3,18] If conservative management of the fistula is adopted, in addition to local eye care (prism or patching therapy for diplopia, topical agents for glaucoma, lubrication for keratopathy, and ophthalmic ointment during sleep period), the patient must be carefully followed for worsening of ocular symptoms, which often warrants treatment to prevent serious complications including intracranial hemorrhage.[18] The main indications for treatment for indirect fistulas are as follows: (i) visual deterioration (this may result from a combination of reduced arterial perfusion and venous hypertension); (ii) obtrusive diplopia related to vascular engorgement and enlargement of the extraocular muscles or to neural compression within the CS; (iii) intolerable ocular bruit or headache; (iv) “malignant proptosis” with untreatable corneal exposure; (v) venous reflux to cortical veins; and (vi) annoying symptomatology that decrease the patient’s quality of life or cosmetic disfigurement (e.g., ocular bruit, pulsatile exophthalmos, or conjunctival chemosis). These indications are not absolute and depend on the general physical condition of the patient, the severity of the symptoms, and the fistula anatomy that fundamentally determine the treatment modality.
The fact that indirect fistulas are generally associated with lower mortality and risk for intracranial complications emphasizes the importance of minimizing the morbidity and mortality of any therapeutic procedure.[3] The recent progress in the field of interventional neuroradiology has introduced multiple treatment modalities for the management of CCFs and the mainstay of therapy for these lesions is endovascular embolization (transarterial or transvenous), while other treatment options such as microsurgery or radiosurgery are still utilized as second-line or adjuvant therapeutic options.[18]
In general, due to rapid advances in techniques and devices, endovascular treatment is considered the primary treatment for CCFs and the transvenous embolization is the mainstay of treatment for indirect fistulas.[9,18] Our initial therapeutic option was precisely the transvenous route, but it was not possible to catheterize the inferior or superior petrosal sinuses to reach the CS. Subsequently, a second attempt was performed to treat the fistulous lesion using an alternative route through the superior ophthalmic vein (through the facial and angular veins). Unfortunately, in addition to the failure to reach the superior ophthalmic vein (the transition from the angular to the superior ophthalmic vein proved to be very small and angled), the patient progressed with worsening ocular symptoms and rapidly progressive onset of ipsilateral visual deficit. Our diagnostic hypothesis was facial vein thrombosis and, consequently, increased ocular venous hypertension due to decreased retrograde venous redistribution from the superior ophthalmic vein to the facial veins. Direct surgical catheterization of the superior ophthalmic vein was considered to be, in our service, a risky procedure due to venous hypertension of the vessel and risk of profuse bleeding during the procedure. Given the challenging scenario, we opted for a direct surgical approach to the fistulous communications to treat the lesion while preserving the ICA because, as already exposed in the past by Hamby, “as in other parts of the body the fistula itself should be attacked rather than attempting piecemeal progressive ligation of its feeding arteries.”[14]
Knowledge of the anatomy of the CS and the cavernous portion of the ICA is essential to an understanding of the etiology and treatment of CCF.[3,15] This anatomy has been studied and reported in detail by Parkinson.[12-15] He described a triangle within the lateral wall of the CS through which the cavernous portion of the ICA and its branches might be exposed for the surgical treatment of CCFs [Figure 3].[12,16] The Parkinson’s triangle (infratrochlear triangle) is located between the lower margin of the trochlear nerve, the upper margin of the ophthalmic nerve, and a third margin that are formed by a line connecting the point of entry of the fourth nerve into the dura to the site where the trigeminal nerve enters the Meckel’s cave.[16] The origin of the meningohypophyseal trunk in the posterior bend of the cavernous portion of the ICA is located in this triangle.[12,16] While Parkinson has advocated a direct surgical approach through the infratrochlear triangle to the cavernous carotid for the treatment of these fistulas (lateral transdural approach),[12-14] Hosobuchi[5] and Mullan[10] have developed and modified a technique of CCF occlusion with carotid artery preservation by inserting various thrombogenic materials (copper wires) into the venous side of the fistula. The goal of the treatment is the complete occlusion of the fistula with preservation of normal flow of blood in ICA, and we chose to adopt a mixture between the different techniques reported in the literature to achieve this therapeutic goal. The surgical intervention is adopted when the endovascular treatment is not practicable.
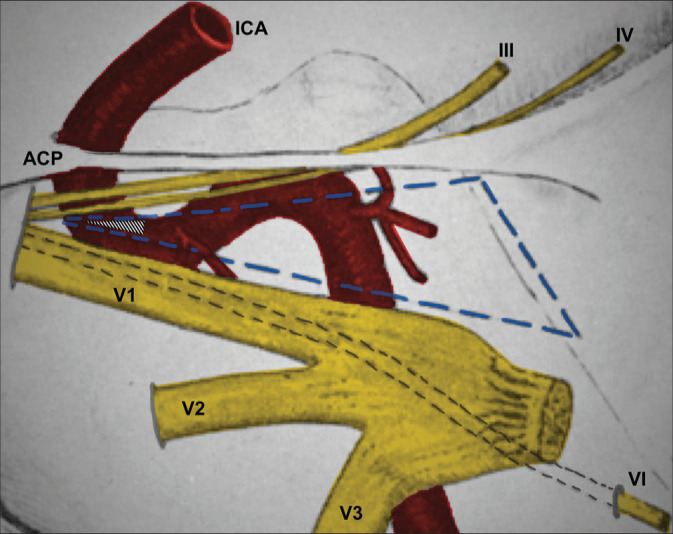
Schematic drawing adapted and modified from Parkinson’s original publication.[12] We chose to perform a puncture in the lateral wall of the CS in the most anterior portion of Parkinson’s triangle (hatched triangle). This choice was based on the greater proximity to the superior ophthalmic vein, lesser need for dural peeling of the middle fossa, lesser retraction of the temporal lobe, and inspired by Parkinson’s publications. The Parkinson’s triangle is outlined in dotted blue lines. According to Rhoton, this triangle measures on average 13 × 14 × 6 mm.[16] However, depending on anatomical variations, it could be very small.[16] III: oculomotor nerve, IV: Trochlear nerve, VI: Abducens nerve; ACP: Anterior clinoid process, ICA: Internal carotid artery, V1: Ophthalmic nerve, V2: Maxillary nerve, V3: Mandibular nerve.
In the present case, our surgical strategy was: (i) dissect the common carotid artery and perform proximal cervical control to temporarily decrease arterial flow at the moment of fistula treatment; (ii) perform a pretemporal craniotomy and a microsurgical dural peeling of the middle fossa to expose the lateral wall of the CS; (iii) check the swollen CS with multiple points of small arterial bleeding on its side wall; (iv) identification of cranial nerves in the lateral wall of the CS for the identification of Parkinson’s triangle; (v) temporary clipping of the common carotid artery and verification of changes in the appearance and color of the bleeding by the middle fossa peeling; (vi) Parkinson’s triangle puncture and biological glue injection (initially at a higher speed [about 1 ml] and then at a lower speed [about 2 ml]); and (vii) removal of the temporary surgical clip from the common carotid artery and microscopically verified the total absence of bleeding through the lateral wall of the CS. Our surgery was entirely performed extradural and under microscopy. The patient evolved with clear and immediate improvement in proptosis, chemosis, and diplopia [Figure 2]. Parkinson conducted his surgeries under hypothermia with the heart exposed and cardiac arrest to control hemorrhage.[12-14] The measures we adopted proved to be appropriate for hemorrhage control when handling within the CS.
The CS has previously been considered a “no man’s land.” This was mainly due to the poor understanding of its surgical anatomy and the difficultness in establishing hemostasis within the CS.[7] The use of fibrin glue to perform hemostasis in the CS was recently revisited by some authors and the popularization of this hemostatic technique has greatly facilitated the surgical approach to this challenging cranial base area.[7,8,17,19] In addition to its hemostatic use to facilitate surgery in the CS, fibrin glue was also described in the 1980s as an embolic agent for the treatment of CCF. According to Parkinson, Isamat was the first to use this methodology to treat CCFs with ICA preservation by injection of a fibrin glue and muscle fragments into the venous channels of the CS after temporarily slowing the arterial flow.[6,15] Fibrin glue, despite some variations in the surgical technique, is also reported as one of the emboligenic materials adopted for the treatment of these fistulas by Hasegawa et al.[4]
Fibrin glue is a two-component fibrin sealant made from pooled human plasma. The first component (sealant protein) is a highly concentrated fibrinogen solution, and the second component is a solution of thrombin, human albumin, and sodium chloride. It is applied with a dual-syringe delivery system with a single plastic tube that admixes the two components immediately before application by spraying or dripping onto the surgical target.[17] Once the components are combined, the thrombin catalyzes the conversion of the fibrinogen to fibrin, producing a fibrin clot (mimicking the final stage of the blood clotting cascade), which adheres to the tissue and can be used to achieve hemostasis, sealing, or adhesion according to the surgical need.[17] Our strategy was initially to inject the biological glue at a higher speed so that it reached the retrograde drainage veins (mainly the superior ophthalmic vein) before solidifying. After this first injection in the anterior direction, the injection speed was significantly slowed down so that the glue began to solidify locally, occupying the anterior and posterior venous spaces of the CS (changing the needle direction during injection), and thus achieving the desired thrombosis of this venous space. The mechanism of hemostasis by fibrin glue in the various venous spaces is presumably achieved through a combination of mechanical obliteration of the space, as well as by promotion of thrombosis (space-occupying substances with a thrombotic character).[1,17] We believe that the temporary clipping of the common carotid artery decreased the flow of the CCF and allowed, in addition to a safer puncture of the lateral wall of the CS, the dispersion of the biological glue to the desired targets.
The safety of fibrin glue injection into the CS for hemostasis is generally accepted and the venous flow has been shown to be re-established as early as 2–3 months postoperatively.[7,19] However, it is important to highlight that despite the safety and low complication rates reported in the literature, CS hemostasis with fibrin glue does have inherent risks.[1] The most catastrophic complication is the ICA thrombosis, with subsequent serious ischemia.[1] Our follow-up, to date, is approximately 6 months and we have not observed any complications.
CONCLUSION
In the now far 60s, Parkinson already treated patients with CCF effectively and elegantly through the lateral wall of the CS. His in-depth study of the anatomy of the CS is one of the most important bases that allowed a progressive construction of knowledge in the following decades. In the 1970s, Isamat was already using fibrin glue as an emboligenic agent for the treatment of CCF. Revisiting techniques from the past, associating them with supplies widely available today, can sometimes be the solution to some especially challenging cases that we face in our profession.
Footnotes
How to cite this article: Alves RV, Sousa LC, Rodrigues JP, Laube KA. Revisiting Parkinson: After six decades, his triangle remains useful. Surg Neurol Int 2022;13:483.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
Articles from Surgical Neurology International are provided here courtesy of Scientific Scholar
Full text links
Read article at publisher's site: https://doi.org/10.25259/sni_764_2022
Read article for free, from open access legal sources, via Unpaywall:
https://surgicalneurologyint.com/wp-content/uploads/2022/10/11942/SNI-13-483.pdf
Citations & impact
Impact metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.25259/sni_764_2022
Article citations
The rule of five: A novel anatomical landmark for approaching cavernous sinus content.
Surg Neurol Int, 14:269, 28 Jul 2023
Cited by: 0 articles | PMID: 37560588 | PMCID: PMC10408645
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Combined pretemporal and endovascular approach to the cavernous sinus for the treatment of carotid-cavernous dural fistulae: technical case report.
Neurosurgery, 44(2):415-418, 01 Feb 1999
Cited by: 21 articles | PMID: 9932900
Endoscope-assisted transsphenoidal puncture of the cavernous sinus for embolization of carotid-cavernous fistula in a neurosurgical hybrid operating suite.
J Neurosurg, 127(2):327-331, 05 Aug 2016
Cited by: 5 articles | PMID: 27494822
Surgical Sparing and Pairing Endovascular Interventions for Carotid-Cavernous Fistula: Case Series and Review of the Literature.
World Neurosurg, 140:18-25, 11 May 2020
Cited by: 4 articles | PMID: 32437988
Review
The evolution of endovascular treatment of carotid cavernous fistulas: a single-center experience.
World Neurosurg, 80(5):538-548, 09 Feb 2013
Cited by: 33 articles | PMID: 23402868




