Abstract
Free full text

The dynamic lung microbiome in health and disease
Abstract
New methods and technologies within the field of lung biology are beginning to shed new light into the microbial world of the respiratory tract. Long considered to be a sterile environment, it is now clear that the human lungs are frequently exposed to live microbes and their by-products. The nature of the lung microbiome is quite distinct from other microbial communities inhabiting our bodies such as those in the gut. Notably, the microbiome of the lung exhibits a low biomass and is dominated by dynamic fluxes of microbial immigration and clearance, resulting in a bacterial burden and microbiome composition that is fluid in nature rather than fixed. As our understanding of the microbial ecology of the lung improves, it is becoming increasingly apparent that certain disease states can disrupt the microbial–host interface and ultimately affect disease pathogenesis. In this Review, we provide an overview of lower airway microbial dynamics in health and disease and discuss future work that is required to uncover novel therapeutic targets to improve lung health.
Introduction
Humans and microbes have symbiotically coevolved over hundreds of thousands of years, resulting in a human microbiome that is home to a wide range of microorganisms, including bacteria, yeasts, archaea, fungi, protozoa and viruses. These microbes, as well as their local environment, have a crucial role in immune system regulation and maturation, digestion, the production of certain vitamins, and the prevention of disease1. We now know that microbes occupy virtually all surfaces of the human body, and the respiratory tract is no exception.
The upper and lower respiratory tracts differ in their microbial composition and biomass (Fig. 1). The upper respiratory tract, which includes the nasal cavity, paranasal sinuses, pharynx and supraglottic portion of the larynx, is largely colonized with bacteria. Moreover, within the upper respiratory tract, there are topographical differences in microbial composition. For example, the dominant taxa in the nasal cavity and nasopharynx include Moraxella, Staphylococcus, Corynebacterium, Haemophilus and Streptococcus species, whereas the oropharynx exhibits high abundance of Prevotella, Veillonella, Streptococcus, Leptotrichia, Rothia, Neisseria and Haemophilus species2 (Fig. 1). By contrast, the lower respiratory tract, comprised of the trachea and lungs, exhibits a relatively low biomass, with a major role in lower airway mucosal immunology (discussed below). Such low biomass is maintained by rapid microbial clearance via a number of physiological mechanisms (Fig. 1), enabling the lower respiratory tract to perform its most crucial function: the exchange of oxygen and carbon dioxide.

The upper respiratory tract, comprised of the nasal cavity, paranasal sinuses, nasopharynx, oropharynx and the supraglottic portion of the larynx, exhibits a relatively high biomass with topographically distinct microbiota within each section. By contrast, the lower respiratory tract, comprised of the infraglottic portion of the larynx, trachea and lungs, exhibits a relatively low biomass consisting mostly of oral commensals. Several factors can contribute to dysbiotic changes along the respiratory tract, as shown in red boxes. Aspiration of oropharyngeal or gastro-oesophageal contents is the predominant means by which bacteria reach the lower airways (arrows). Three major lung clearance mechanisms are coughing, mucociliary transport and the innate immune system.
Dysbiosis, defined as deviation from a normal microbial composition, is associated with a number of adverse biological phenomena, sometimes with clinical consequences. In the lung, dysbiosis can have a significant impact on the development and progression of respiratory diseases, emphasizing the clinical need to understand the biology of the lung microbiome. Over the past decade, there has been a rapid expansion of studies applying culture-independent genomic methods to profile the lung microbial environment (Box 1). As we discuss below, several studies have now shown that certain microbial signals are associated with distinct lower airway inflammatory endotypes and disease processes.
The lung microbiome in health and disease is often conceptualized as a pendulum that swings between two static states — from a paucity of microbes in the lung airways to patterns of resilient microbial colonization. However, it is possible that these extremes are not mutually exclusive but rather snapshots of a dynamic microbial system. In this Review, we describe the evolutionary nature of the lung microbiome and the underlying mechanisms by which sustained dysbiosis might affect respiratory disease progression. We hope to challenge the conceptualization that the lung microbial environment is a static entity and emphasize the dynamic balance between microbial immigration and clearance. As discussed below, the dynamic nature of the lung microbiome has been documented by experimental mouse models of microaspiration3,4 and might be an important distinguishing feature from the microbial behaviour seen in other mucosae with high biomass, such as the oral cavity and gut, where microbial communities are highly resilient. We argue that focusing on elucidating the dynamic nature of the lung microbiome will be critical to understanding mechanisms of lung disease and for the development of novel therapeutic targets involving the microbial–host interface.
The origins of the lung microbiome
Once thought to be a sterile environment, it is now understood that the lungs are frequently exposed to a variety of microorganisms. Human data support that microbial exposures start early in life, within days to weeks after birth, and have important implications for immune system maturation5. In a study of lower airway samples from infants, differences in the composition of the microbial community were associated with distinct changes in the host immune tone5. Within the first few weeks of life, domination of the lower airway microbial community by either Staphylococcus or Ureaplasma species represents two distinct signatures of the lung microbiome in association with the mode of neonate delivery. Lower airway enrichment with Ureaplasma, a common genital tract commensal, is associated with vaginal delivery, whereas enrichment with Staphylococcus, a common skin commensal, is associated with caesarean delivery, providing an early example of the role of the environment on the lung microbiome5. As neonates mature, their lung microbiome shifts towards enrichment with a more diverse mixture of oral commensals such as Streptococcus, Prevotella, Porphyromonas and Veillonella.
These longitudinal changes in the lung microbiome affect the regulation of immunoglobulins and innate immune responses, and are thus postulated to be one of the mechanisms by which lower airway immune maturation occurs5. Microbial changes in other parts of the respiratory tract may also affect the immune tone in early life. For example, microbial diversity and enrichment of the neonatal hypopharynx with microbes such as Prevotella and Veillonella species have been linked to the subsequent development of reactive airway diseases such as asthma, suggesting that early changes in the microbial environment, even if occurring among commensal taxa rather than among obvious respiratory pathogens, may predispose infants to certain respiratory conditions later in life6.
A dynamic microbial–host interface
As with any mucosal surface in the human body, microbial exposures are not invisible to the host immune system. Indeed, the microbial products found in the lower airways derive from a balancing act between microbial immigration and microbial clearance7. Unlike other mucosae, however, the lungs must maintain a lower bacterial burden in order to facilitate gas exchange, their main physiological purpose. Functionally, the conditions of the lower airways are distinct from those of other mucosae with regard to oxygen tension, protein content, presence of surfactants and other environmental factors. Further, in order to maintain a low bacterial burden, there is a significant reliance on mechanisms that facilitate the clearance of microbes that reach the lower airways, including mechanical mechanisms, such as mucociliary clearance and cough, as well as innate and adaptive host immune responses (Fig. 1).
Unlike the oral cavity and gut lumen, both of which have a very high bacterial burden containing millions of viable and replicating microbes, the lower airways must maintain a thin bronchial epithelial fluid lining to facilitate effective gas transfer. Such unique conditions favour a more dynamic homeostatic state of the lung microbiome. Specifically, the microbiomes of the oral cavity and gastrointestinal tract tend to be much more static, dominated by reproducing site-specific microbial communities, changes in which are interpreted as a dysbiotic signature. However, the lower airway microbiome is frequently changing, with episodic exposure to microbes and their rapid clearance, resulting in a dynamic physiological process that defines eubiosis (Fig. 2). This is supported by several human cross-sectional investigations showing that traces of microbial DNA from oral commensals, despite a failure to isolate these bacteria by culture, are common in the healthy lung8–10. Genetic evaluation of the lower airway microbiota (by 16S rRNA gene sequencing, shotgun metagenomic sequencing and metatranscriptome sequencing) shows that a small proportion of individuals with detectable oral commensal microbial DNA also have detectable oral commensal microbial RNA3. As RNA is rapidly degraded after cell lysis, this suggests the transient presence of viable microbes after an aspiration event3. Experimental microaspiration with viable oral commensals in preclinical mouse models support these dynamic changes seen with culture-independent methods3.

a, A series of dynamic events promote eubiosis in the lower airways. Aspiration of a microorganism triggers the activation of leukocytes that orchestrate bacterial elimination by macrophage and neutrophil activity. After a transient upregulation of adaptive immunity, the immune tone returns to baseline with a low level of detectable residual microbial DNA. b, Steps commonly found in early dysbiotic events. Recurrent aspirations lead to changes in the lower airway microbial environment that can slowly become irreversible. c, Increased mucus production facilitates persistent colonization and increased bacterial burden and is associated with acute and chronic lower airway disease. Microbial immunomodulatory products, such as short-chain fatty acids (SCFAs), and host immune responses, such as a T helper 1 (TH1)/TH2 imbalance, an aggravated TH17 response and pro-inflammatory cytokines, can perpetuate neutrophilic inflammation, increased regulatory T (Treg) cell response and impaired immune surveillance. These changes can ultimately promote increased bacterial burden.
Lower airway immune tone: the lung–lung axis
Multiple investigations have shown the importance of gut microbial communities in setting the host immune tone, which affects lung immune responses as part of the gut–lung axis. Much less studied, lung microbial communities also affect lung immune responses. Indeed, existing cross-sectional human data, combined with data from murine models, suggest that there is frequent, episodic lung exposure to oral commensals that are subsequently rapidly cleared (Fig. 2). Models of microaspiration support that microbial clearance occurs at variable rates and is dependent on the species of the microbes aspirated4. Importantly, even with rapid lower airway clearance of aspirated oral commensals, such events lead to a durable and dynamic change in the lower airway immune tone4. For instance, mouse aspiration with a mixture of oral commensals (Prevotella melaninogenica, Veillonella parvula and Streptococcus mitis) leads to activation of CD4+ and CD8+ T cells, recruitment of IL-17-producing T cells (T helper 17 (TH17) and γδ T cells), and other counter-regulatory immunological responses such as an increase in regulatory T cells and immune-checkpoint inhibitor markers on T cells4. Importantly, this microbiome-dependent immunological priming is a durable phenomenon that persists beyond the transient presence of aspirated microbes and goes on to significantly impact innate immune defences against respiratory pathogens4. If such responses are representative of normal lung microbial dynamics, these dynamic changes may reflect true lung ‘eubiosis’.
The above experimental preclinical data is in line with multiple observations from cross-sectional studies, illustrating again that distinct lung microbial signatures are associated with lower airway immune tone10,11. For example, one study of mice with similar genetic backgrounds but heterogeneous microbial compositions found that the composition of the lower airway microbiome was associated with levels of several inflammatory markers in the lungs12. In humans, lower airway enrichment with supraglottic-predominant taxa, including Prevotella, Rothia and Veillonella, positively correlated with levels of multiple TH17 cytokines, such as IL-1α, IL-1β, IL-6, fractalkine and IL-17, as well as with the recruitment of both TH17 cells and neutrophils in the lung10. In another study of patients with severe asthma, Proteobacteria species were associated with activation of TH17-associated pathways11. Collectively, these data suggest that the lung microbiome, despite constantly being in flux with rapid clearance of microbes acquired via microaspiration or environmental exposures, leaves important signatures and imprints on the lower airway immune tone.
The studies described above give some insight into how the human lung might respond to sporadic exposure to certain microbes. However, numerous unanswered questions remain. It is unclear what happens when the frequency of microbial exposures increases. For example, it is unknown whether the balance between pro-inflammatory effects and counter-regulatory mechanisms becomes altered as aspiration events become more frequent. Whether recurrent exposure leads to chronic inflammation is also unclear. Despite a paucity of answers, data suggest that persistent exposure to certain microbes can trigger mechanisms leading not only to increased inflammation but also to immune exhaustion (Fig. 2). For example, in individuals where the lower airway microbiome is dominated by oral commensals, there is a blunting of the Toll-like receptor 4 (TLR4) response of alveolar macrophages10. In another human study, microbial products, such as short-chain fatty acids (SCFAs), had significant immunomodulatory properties and blunted IFNγ and IL-17 responses to pathogen-associated molecular patterns13. Finally, in mice, experimental exposure of the lower airways to oral commensals not only triggers an increase in inflammatory cytokines but also an increase in immune-checkpoint inhibitor markers, such as PD1, among T cells and recruitment of regulatory T cells4. These findings collectively suggest that the dynamic nature of microbial–host interactions in the lung likely changes as the frequency of exposure to microbes, rate of elimination and type of immune response change over time.
Dysbiosis in respiratory inflammatory diseases
Microbial dysbiosis is present in various lung diseases, such as cystic fibrosis, chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF), in which a reduction in the diversity of bacterial composition has been linked to disease progression14–16. However, whether microbial dysbiosis itself is the cause of disease or a result of the disease process is not clear. For example, in lung disease, pathophysiological changes in the lung architecture and impaired mucus clearance mechanisms might result in microbial dysbiosis rather than be caused by it. Alternatively, dysbiosis may play a causative role in disease through upregulation of inflammatory signals (such as NF-κB, Ras, IL-17 and PI3K) or blunting of TNF and IFNγ production in response to pathogens in the lower airways10,13,17,18. In this section, we review some examples of dysbiotic signatures identified among pulmonary diseases commonly described as inflammatory and discuss the potential role of lower airway microbes in their pathogenesis.
Chronic obstructive pulmonary disease
COPD serves as a useful example of the evolutionary nature of the lung microbiome and its impact on respiratory disease progression. Initial microbiome studies in patients with stable COPD demonstrated a clear association between the presence of potentially pathogenic microbes (PPMs), such as Haemophilus species, Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, Pseudomonas aeruginosa and gram-negative enteric bacteria, and the presence of neutrophilic inflammation and increased expression of cytokines, such as IL-8 and TNF, in the lower airways19–22. Lower airway colonization with PPMs has been correlated with increased severity of daily symptoms in COPD and progressive radiographic changes23. Nonetheless, precisely how these microbial events contribute to early stages of disease onset is poorly understood. Smoking alone does not seem to be associated with a significant change in the lower airway microbiome24,25; however, this situation changes as smokers start to develop early pathophysiological evidence of COPD. In a recent study of the lower airway microbiome in patients with early COPD, enrichment with Streptococcus, Staphylococcus, Prevotella and Gemella (strains that are part of the normal oral microbiota) was associated with lung function abnormalities, including an impaired response to bronchodilator therapy26. In support of a causative role of the microbiome in COPD, longitudinal enrichment with oral commensals in repeated lower airway samples results in the development of COPD-like changes in a macaque preclinical model27.
As COPD progresses, chronic inflammation results in impairment of the lung innate immune defences, which in turn paves the way for increased bacterial burden of both new and existing pathogens. Multiple investigations have shown that, in moderate-stage to advanced-stage COPD, there is enrichment with Gammaproteobacteria (which includes many PPMs such as Haemophilus and Moraxella species)14,28,29. In another study of patients with COPD, the presence of Proteobacteria-dominated microbes in sputum was associated with poorer lung function, worse scores on the Medical Research Council dyspnoea scale, and more frequent exacerbations30. In a multicentre trial involving longitudinal sampling from three large cohorts of patients with moderate to severe COPD, the airway microbiome (as assessed in sputum samples) showed clear associations between its composition and the inflammatory endotype31. Specifically, the Haemophilus-dominated sputum microbiota behaved rather stably over time and was associated with elevated IL-1β and TNF levels. These findings are supported by another study of patients with stable COPD, which similarly showed that colonization with Haemophilus influenzae was associated with increased airway inflammation32. It is therefore possible that these patients might benefit from targeted antimicrobial therapies to mitigate chronic inflammation. In contrast, patients with a ‘balanced’ microbiome profile, characterized by enrichment with several oral commensals such as Veillonella and Prevotella, exhibited a more dynamic microbiome over time and had elevations in serum and sputum IL-17A31. This subgroup was more susceptible to greater microbiome shifts during exacerbations. Thus, it is possible that these patients may benefit from a different, perhaps distinctly targeted, anti-inflammatory therapeutic approach or closer monitoring of airway ecology and pathogen acquisition, particularly at times of acute exacerbations. Importantly, increases in the relative abundance of some oral commensals, such as Campylobacter and Granulicatella, were associated with intra-patient switches from neutrophilic to eosinophilic inflammation31. Taken together, these findings are consistent with a model in which recurrent microaspiration of oral secretions starts occurring at a higher rate in early stages of disease, which contributes to lower airway inflammation; subsequently, further impairment of microbial clearance results in immune exhaustion and in eventual lower airway colonization with respiratory pathogens, a phenomenon that has been well documented in later stages of disease through both genomic-based and culture-based methods28,33–35. Thus, multiple forms of dysbiosis can be seen in patients with inflammatory airway diseases such as COPD (Fig. 3a).
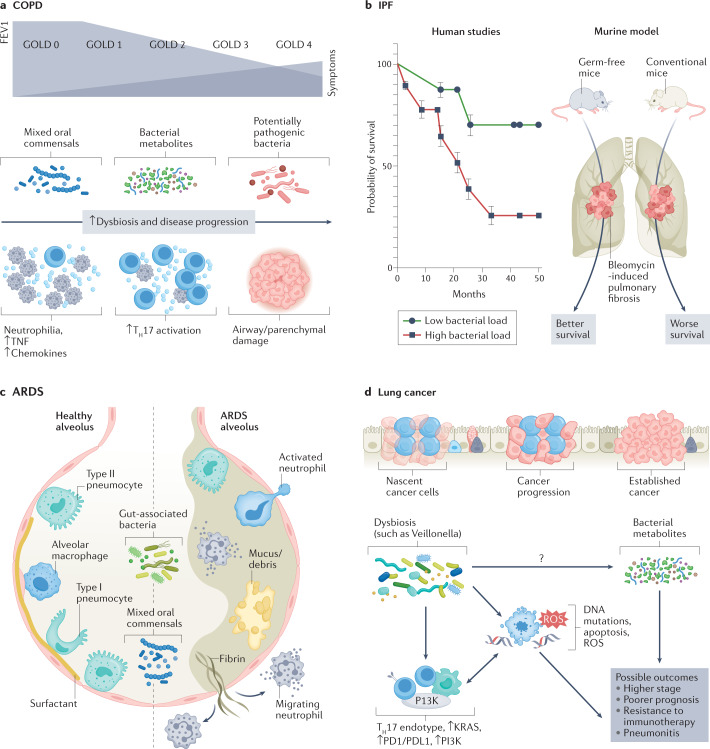
Different forms of dysbiosis coexist with different immune endotypes and pathological phenotypes in various disease states. a, In chronic obstructive pulmonary disease (COPD), progression to advanced stages, characterized by decrease in forced expiratory volume in one second (FEV1) and higher Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, is hallmarked by the presence of potentially pathogenic microbes, neutrophilia, and increased inflammatory cytokines and chemokines. Newer investigations are identifying early dysbiotic events involving some oral commensals and bacterial metabolites that might precede the development of advanced-stage COPD. b, Increased bacterial burden has been shown to be associated with a poorer prognosis in patients with idiopathic pulmonary fibrosis (IPF). In animal studies, germ-free mice had a survival benefit after the induction of bleomycin-induced pulmonary fibrosis as compared to conventional mice with normal bacterial burden. c, In acute respiratory distress syndrome (ARDS), there is a relationship between gut-associated bacteria and mixed oral commensals with alveolar filling, increased mucus or cellular debris, and immune activation. d, Mixed oral commensals can play a role in the progression of lung cancer by perpetuating a T helper 17 (TH17) endotype, increased PI3K signalling and immune-checkpoint inhibition. These events, in conjunction with the more direct carcinogenesis precursors (such as DNA mutations and cell apoptosis, which further contribute to a pro-inflammatory tumour microenvironment), can have serious effects on the prognosis of lung cancer. It is likely that metabolites regulated by microbial metabolism play a significant role in cancer pathogenesis, which deserves further investigation. ROS, reactive oxygen species.
Asthma
Several investigations have focused on the airway microbiome in asthma. Although studies in early childhood with lower airway samples are lacking, multiple studies have shown associations between changes in the microbiome and development of wheezing and asthma diagnosis36–38. Even among children with an established asthma diagnosis, compositional changes in the upper airway microbiome are associated with disease control39. Evaluation of the lower airway microbiome of patients with asthma has been performed more extensively in adults. Several studies have now shown that the composition of the lower airway microbiome is associated with distinct clinical features among patients with asthma, including airway bronchial responsiveness and severity11,40. It is now understood that asthma comprises a group of very heterogeneous endotypes with a large proportion of patients with eosinophilic or type 2 (T2)-high inflammation that is more susceptible to steroids and targeted therapies. In contrast, treatment is less effective in patients with a non-T2-high inflammatory endotype (that is, patients with mostly neutrophilic airway inflammation) for reasons that remain unknown41. One intriguing hypothesis is that the lung microbiome may have a major role specifically among patients with a non-T2-high inflammatory neutrophilic endotype. For example, evaluation of the lower airway microbiota using airway brushings obtained from asthmatics showed that bacterial composition was not associated eosinophilic inflammation. In contrast, significant associations were found between the enrichment with Proteobacteria and other taxa and TH17 inflammatory markers, likely resulting in neutrophilic recruitment11. Thus, better understanding of the molecular mechanism affecting these associations may allow for the discovery of novel treatable traits among patients with asthma presenting with more refractory forms of the disease.
Idiopathic pulmonary fibrosis
The lung microbiome has important implications for disease progression and survival in various other pulmonary diseases. There are now several studies showing an association between lower airway bacterial burden and disease progression-free survival among patients with IPF42–44 (Fig. 3b). In particular, one study reported a correlation between alveolar inflammatory and fibrotic cytokines and lung microbiota diversity and composition, and lower Shannon diversity indices (within-sample diversity) were associated with higher concentrations of IL-1Ra, IL-1β, CXCL8, MIP1α, G-CSF and EGF42. In addition, alveolar concentrations of IL-6 were positively correlated with relative abundance of the lung Firmicutes phylum, whereas alveolar IL-12p70 was negatively correlated with relative abundance of the lung Proteobacteria phylum42. Other IPF studies have also shown clear associations between lung dysbiosis and both peripheral blood mononuclear cell transcriptomic expression of immune pathways and progression-free survival, suggesting that microbial–host interactions have an important role in the pathogenesis of pulmonary fibrosis44,45. However, a major limitation of these human studies is the inability to test the directionality of observed associations. In a bleomycin murine model of pulmonary fibrosis, microbial dysbiosis preceded peak lung injury and persisted until the development of fibrosis42. In contrast, germ-free mice exposed to bleomycin-induced pulmonary fibrosis had a mortality benefit compared to conventional mice42 (Fig. 3b). In another preclinical murine model, Bacteroides and Prevotella species were associated with increased fibrotic pathogenesis through IL-17R signalling46. Taken together, these data suggest that certain microbial exposures may act as persistent stimuli for repetitive alveolar injury, ultimately contributing to pulmonary fibrosis and continual inflammatory injury in this disease.
Acute respiratory distress syndrome
Both in preclinical models of sepsis and in humans with established acute respiratory distress syndrome, the lung microbiome likely plays a major role in the pathogenesis of the disease (Fig. 3c). Using culture-independent methods, a study from 2016 demonstrated that enrichment of the lower airways with gut-associated bacteria correlated with concentrations of alveolar TNF, a key mediator of alveolar inflammation in acute respiratory distress syndrome47. In addition, a recent study of patients with COVID-19 on mechanical ventilation demonstrated worse clinical outcomes among patients whose lower airway microbiome was enriched with Mycoplasma salivarium, another oral commensal48. Additionally, a recent large study of over 300 patients on mechanical ventilation demonstrated that enrichment of the lower airways with Staphylococcus or Pseudomonadaceae was associated with increased lower airway inflammation and worse clinical outcomes, including poorer 30-day survival and longer time to liberation from mechanical ventilation49. Taken together, these data suggest that microbial dysbiosis may have an important role in the dysregulation of local and systemic inflammation that consequently impacts clinical outcomes in acute respiratory failure.
The lung microbiome and immune surveillance
Although much research has focused on how the lung microbiome promotes inflammatory states, its effects on immune surveillance are much less understood but are likely very important. Among patients with cystic fibrosis, a reduction in alpha diversity, which quantifies microbial diversity within a sample, is directly correlated with disease severity and colonization with bacterial pathogens50. In many cases, this is likely due to the emergence of a dominant pathogen that increases in relative abundance. In addition, decreased microbial diversity sometimes precedes the development of cystic fibrosis exacerbations51. These data suggest that a reduction in biodiversity over time can render the lower airways more susceptible to colonization with a dominant pathogen such as Pseudomonas or Burkholderia species.
However, there are different possible scenarios in which dysbiosis might affect host susceptibility to pathogens. Figure 4 depicts some of these scenarios in which pathogens become either dominant or non-dominant in the lower airway microbiome. For example, in patients with bronchiectasis, those with Pseudomonas aeruginosa lower airway colonization experience more frequent exacerbations and often have more severe disease. Colonization with Pseudomonas, which, when present, tends to dominate the airway microbiota, has been associated with a distinct lower airway host immune profile characterized by severe neutrophilic inflammation, including high levels of neutrophil elastase52. However, the microbial events that occur prior to acquisition of Pseudomonas are unknown (Fig. 4a). Moreover, it remains to be determined whether we can identify the lower airway dysbiosis that precedes Pseudomonas colonization. Such investigations would require longitudinal sampling of the lower airways prior to the emergence of a dominant pathogen. In contrast, there are other respiratory pathogens that, when present, frequently do not dominate the lower airway microbial community (Fig. 4b,c). For example, non-tuberculous mycobacterium (NTM) is frequently a non-dominant infectious pathogen, thus potentially allowing for the identification of dysbiotic signatures beyond the presence of the pathogen itself53. In that case, it is possible that an initial lower airway dysbiosis that favours host susceptibility to NTM persists after acquisition of this pathogen, possibly contributing to the inflammatory injury in this disease (Fig. 4b). Interestingly, in a small case series of patients with NTM disease, levels of inflammatory cytokines measured in the lower airways correlated with relative abundances of several oral commensals in the lower airways rather than with the abundance of Mycobacterium53. Alternatively, in the case of non-dominant pathogens, a pre-pathogen lower airway dysbiosis may lead not only to acquisition of the non-dominant pathogen but also to a replacement by another dysbiotic signature, which may be important for the survival of the pathogen itself (microorganism–microorganism interaction) and affect the inflammatory injury (Fig. 4c).
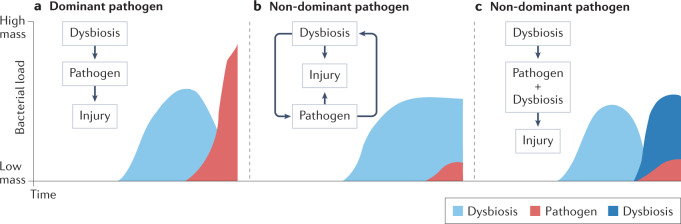
a, In cases of acquisition of a dominant pathogen, dysbiotic changes occurring prior to pathogen seeding may promote host susceptibility. b, In cases of non-dominant respiratory pathogens, changes in the lower airway microbiome might continue as a dysbiotic signature once pathogens are established in the lower airways causing lung injury. c, Alternatively, non-dominant pathogens could lead to a new dysbiotic signature that favours pathogen survival and contributes to lung injury.
HIV
As another example of dysbiosis that might result in decreased host immune surveillance, people living with HIV continue to experience higher rates of respiratory infections and lung cancer despite widespread use of antiretroviral therapy (ART). It is hypothesized that residual levels of HIV in deep tissues leads to chronic immune activation and lung inflammation. This longstanding pro-inflammatory state can eventually contribute to immune cell exhaustion and pulmonary immune dysregulation, thereby making people living with HIV be at higher risk for pulmonary infections and malignancies54. Several studies have tried to identify dysbiotic signals present in HIV, including a multicentre trial that described the presence of high levels of Tropheryma whipplei in the lungs of some individuals with HIV55; of note, however, this has also been observed in smokers and other cohorts, and thus may not be unique to HIV24. Further, the lung microbiome in people living with HIV, especially among those with advanced HIV, shows an increased burden of Prevotella and Veillonella species56. Even with the immune reconstitution that occurs after initiation of ART, there is persistent enrichment of the lower airway microbiota with these oral commensals56. This pattern of microbial dysbiosis is associated with subclinical inflammation, characterized by an increase in neutrophils and lymphocytes9,10. Further, patients with HIV who are treatment naive have been shown to have decreased biodiversity and greater inter-sample diversity compared to healthy controls, suggesting that this dysbiotic signature may be prominent in patients with HIV and may confer increased susceptibility to pneumonia57. In patients with HIV treated with ART, enrichment of the lower airways with oral taxa was associated with increased production of microbial SCFAs13. As shown in one longitudinal cohort study of South African patients with HIV treated with ART, SCFAs have direct inhibitory effects on T cell function via the suppression IFNγ and IL-17 production and are associated with an increased risk of tuberculosis. Given the well-known immunoregulatory properties of SCFAs and the evidence that these molecules could be produced through fermentation of aspirated oral anaerobes3, it is plausible that this may be one potential mechanism by which the lower airway microbiome impairs host immune surveillance to pathogens.
Cancer
Beyond associations with susceptibility to pathogens, several investigations have suggested a link between lower airway dysbiosis and lung cancer. Epidemiological studies and culture-dependent techniques have shown that chronic or recurrent bacterial infections are common in patients with lung cancer over the course of their disease, and post-obstructive pneumonia can negatively impact their response to therapy and overall survival58. Studies have reported a correlation between certain respiratory pathogens, such as Mycobacterium tuberculosis, with lung cancer, and a systematic review found a significantly increased lung cancer risk associated with pre-existing tuberculosis, independent of tobacco exposure59. This might be due to tuberculosis being associated with inflammatory mediators, such as TNF, and excessive extracellular matrix turnover. Mechanistically, the increase in pathogenic bacteria can lead to chronic inflammation through the persistent generation of inflammatory mediators, thereby affecting cellular apoptosis and increasing risk for DNA mutations60. Bacterial metabolites, such as reactive oxygen and nitrogen species, can cause direct DNA damage and disrupt a variety of signalling pathways, thereby generating a pro-carcinogenic environment60–62 (Fig. 3d). A number of bacterial metabolites, including SFCAs, have also been implicated in both the regulation of host metabolism in the lungs and in signalling pathways63. Thus, it is possible that alterations in bacteria-derived molecules can affect the tumour microenvironment and oncogenic signals. This delicate balance between tolerance and immune activation in the lung microenvironment can be disrupted by the overuse of antibiotics for the treatment of infections. For example, preclinical models using broad-spectrum antibiotics demonstrated the importance of commensal bacteria in supporting the host immune response against cancer, defined an important role for γδ TH17 cell responses in the mechanism and showed deleterious effects of antibiotic treatment on cancer progression64.
With the growing use of culture-independent methods, we can now appreciate microbiome differences between patients with and without lung cancer diagnoses. The microbiota of lung tumours is characterized by a lower alpha diversity compared to non-malignant lung tissue samples, an association that seems to be independent of tobacco use, environmental exposures and clinical staging65. Among taxonomic signatures, associations have been described between lung malignancy and increased relative abundance of oral commensals such as Streptococcus, Prevotella, Veillonella, Rothia, Megasphaera and Acidovorax17,66. Other less common respiratory pathogens, such as Staphylococcus and bacteria from the Firmicutes and Saccharibacteria (formerly known as TM7) phyla, have also been found in lower airway samples from patients with lung cancer67. Microbial differences have also been noted among various types of cancer. One study found that Brevundimonas, Acinetobacter and Propionibacterium were more enriched in lung adenocarcinoma whereas Enterobacter was more enriched in squamous cell carcinoma68. Another study demonstrated an association between the Acidovorax genus and squamous cell carcinoma among smokers, particularly in tumours harbouring TP53 mutations69; it is also likely that some of these microbial exposures contribute to a host immune response that may affect carcinogenesis. Indeed, human data show that enrichment with supraglottic-predominant taxa, such as Streptococcus, Prevotella and Veillonella, is associated with upregulation of transcriptomic pathways that are known to have a major pathogenic role in carcinogenesis such as ERK and PI3K17. Upregulation of the PI3K pathway occurs early in lung carcinogenesis and may affect proliferation, apoptosis and cell survival as well as the induction of PDL1 expression70,71. Thus, these data demonstrate how a dysregulated microbiota might increase the risk of lung cancer development. Furthermore, exposure of KRAS-mutated bronchial epithelial cells to either the supernatant of these taxa or their heat-killed products leads to similar upregulation of transcriptomic signatures to those seen in human data17. Overall, although the specific bacterial taxa identified varied somewhat from one study to another depending on the sample type and patient cohort, the consistent observation is that lung cancer is associated with a dysregulated local microbiome characterized by reduced alpha diversity and altered bacterial composition (Fig. 3d).
The lung microbiome may have a role in the progression of lung cancer or its risk for recurrence. Among patients with surgically resected early-stage non-small-cell lung cancer (NSCLC), recurrence was associated with a higher diversity and enrichment of Bacteroidaceae, Lachnospiraceae and Ruminococcaceae in the lung tissue microbiome72. In another study of patients who underwent surgical resection of NSCLC, bronchoalveolar lavage enrichment with Sphingomonas, Psychromonas and Serratia and a reduced abundance of Cloacibacterium, Geobacillus and Brevibacterium were observed in patients who had early lung cancer recurrence compared to those without recurrence in the subsequent 3 years73. Importantly, pre-surgery bronchoalveolar lavage microbiome was associated with the tumour expression of genes involved in cell proliferation, immunity and signal transduction. Distinct microbial differences can also be noted among patients with different stage of disease. For example, the genus Thermus was reported to be more abundant in tumour tissues from patients with advanced stage disease (TNM stages IIIB–IV), and Legionella abundance was higher in patients who developed metastases65. Further, in a recent study, a lower airway dysbiotic signature characterized by enrichment with oral commensals was more prevalent in individuals with TNM stage IIIB–IV NSCLC compared to those with the disease at earlier stages, and a similar microbial signal was associated with poor prognosis and increased risk for mortality74. Mechanistically, multi-omics integration of the microbiome and host transcriptomic data from the lower airways of patients with lung cancer showed that several of these oral commensals, including Veillonella and Streptococcus species, were associated with upregulation of the IL-17, PI3K, MAPK and ERK pathways in the airway transcriptome75. Preclinical mouse models of lung adenocarcinoma show that lower airway dysbiosis, induced by intratracheal aspiration of some of these oral commensals, can lead to TH17 inflammation, increased expression of immune-checkpoint inhibitor molecules (PD1) by T cells, accelerated tumour progression and mortality74. Using germ-free preclinical lung cancer models, others have shown that commensal taxa stimulate Myd88-dependent IL-1β and IL-23 production from myeloid cells, activate IL-17 production by γδ T cells and lead to a pro-inflammatory state that induces tumour proliferation76. Furthermore, preclinical evidence suggests that direct blocking of the IL-17 pathway through anti-IL-17 monoclonal antibodies can slow tumour growth in the setting of lower airway dysbiosis induced by aspiration of oral commensals74. These findings clearly link local microbiota–immune crosstalk to lung tumour development and thereby identify candidate cellular and molecular mediators that can be targeted for anticancer therapy.
The evidence described above shows some lack of consistency in the specific microbial taxa associated with pulmonary malignancy. Inconsistencies between studies may be due to socio-demographic differences inpatient cohorts or differences in research techniques employed, a phenomenon similarly noted in studies of gut microbiome signals associated with malignancies. For example, while gut microbiomic signatures have been found to be associated with inflammatory tone and susceptibility to immunotherapy in melanoma and NSCLC, the specific taxa identified have been inconsistent among different studies77–80. It is also possible that taxonomic signals are markers of functional microbial differences but evaluation of this hypothesis is prevented by a lack of studies focusing on microbial metabolism in the lung (for example, using approaches such as metagenome, metatranscriptome, metabolome or proteome). However, despite these different taxonomic signals, the host signals associated with lower airway dysbiosis seem to similarly point towards a dysregulated immune response with a pro-inflammatory TH17 state and increased expression of immune-checkpoint inhibition, which might impair effective immune surveillance of nascent malignant cells81. Importantly, tumour microbiome studies are often limited by a very low microbial biomass and are thus prone to sequencing noise and DNA contamination from kits and reagents, although various methods have been proposed on how to effectively deal with both of these issues82. Thus, although recent studies indicate that the lung microbiome may soon become a critical diagnostic and preventive biomarker for lung cancer stage, genotype and risk factor stratification83, further mechanistic and large-scale clinical studies are required to dissect its precise role in tumorigenesis.
Conclusions and future directions
Studying the lung microbiome in varying disease states has identified microbial signatures associated with diagnosis, illness severity and prognosis. However, a lack of consistent findings across studies makes it difficult to clearly define lung dysbiosis. It is quite possible that even within a single disease process there are many forms of dysbiosis, a concept that has previously been referred to as the ‘Anna Karenina principle’ in reference to Tolstoy’s writings that “happy families are all alike; every unhappy family is unhappy in its own way.”84 Even less established is how we define lung microbial eubiosis. Part of the difficulty may reside in the nature of the lung microbiome, with its low biomass resulting in considerable background noise in sequencing data85,86, and the significant impact that the dynamic pendulum between immigration and elimination of microbes in the lung environment has on our assessments. Thus, contrasting with the Anna Karenina principle, all happy families (eubiosis) may not look alike, at least based on static snapshots of the lung microbiome. To add further complexity, even within the same individual, topographical differences in the lung microbiome exist9,14. Defining microbial resilience in such a dynamic environment is thus inherently difficult. As the use of culture-independent and multi-omics methods becomes more widespread, understanding these principals is essential for the successful development of novel diagnostic and therapeutic modalities targeting microbial–host interactions.
In Fig. 5, we illustrate an overarching speculative view of how the delicate balance between microbial immigration (driven by inhalation, microaspiration and mucosal dispersion) and microbial elimination (driven by cough, mucociliary transport and immune mechanisms) establishes a complex microbial–host interface. This interface is likely affected by many factors, including those related to both the host and the environment. The exact effects that each of these factors has on the microbiome are relatively unknown, although we can speculate about the existence of some beneficial and other detrimental factors that might change throughout the course of our lives (Fig. 5). We postulate that factors such as vaginal delivery87, exercise and a healthy diet contribute towards lower airway eubiosis, either directly or via the gut–lung axis. Conversely, obesity, an unhealthy diet and frequent antibiotic use could disrupt the lower airway microbiome in an unfavourable manner. The effect of breastfeeding versus formula feeds is also unknown, with only a single randomized controlled trial showing no difference in the incidence of gastrointestinal and respiratory infections in breastfed versus formula-fed infants, although this study did not specifically investigate the microbiome88. Finally, we surmise that lower airway immune resilience and the diversity of the lung microbiome likely change as we age, and the effect of external factors on the lung microbiome may therefore change across the lifespan. Well-designed controlled studies are needed to evaluate the potential effects of these environmental factors on the lung microbiome and host immune response. Careful consideration of age, sex and other important clinical conditions are key to a better understanding of how the lower airway microbiota influences lower airway immune tone. Thus, future investigations will need to move away from cross-sectional designs and correlative studies towards longitudinal sampling approaches that account for topographical characteristics and experimental conditions (Box 2). Further, although understanding the human lung microbiome requires human studies, preclinical models allow for better control of microbial and host conditions as well as the implementation of experimental approaches under controlled circumstances. These mechanistic approaches are needed to understand how the lung microbiome affects host susceptibility to inflammatory processes, infections and malignancy.
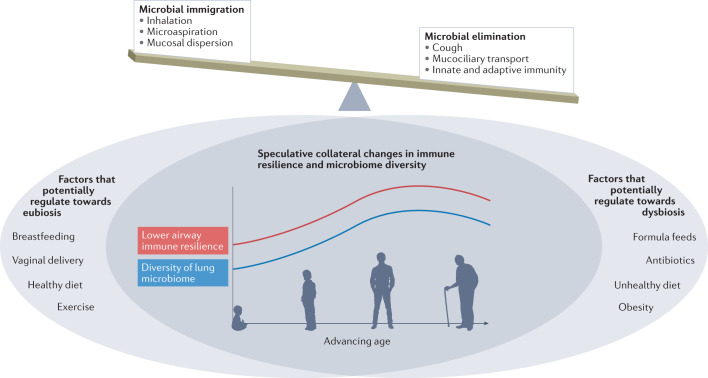
The dynamic nature of the lower airway microbiome is ever evolving. The overarching factors that control the balance of the microbiota are microbial immigration via inhalation and microaspiration and microbial elimination via cough, mucociliary transport and immune responses. We postulate that factors such as vaginal delivery87, exercise and a healthy diet may contribute towards eubiosis in the lower airway, whereas obesity, an unhealthy diet and frequent antibiotic use could disrupt the lower airway microbiome in an unfavourable manner. Finally, we surmise that lower airway immune resilience and the diversity of the lung microbiome (a symbol of health in its own right) follows a quasi-sinusoidal path, peaking in adulthood.
Next, we must consider the potential therapeutic impact that modulating the lung microbiome might have on respiratory diseases. Currently, many existing and widely used interventions beyond antibiotics, such as inhaled corticosteroids and cystic fibrosis transmembrane conductance regulator modulators, already alter the respiratory microbiota89–91. In fact, there are now several potential mechanisms that have been proposed to explain how corticosteroid therapy might impact epithelial barrier function and consequently alter airway ecology, including the increased expression of epithelial genes involved in tight junction promotion and the upregulation of IL-17 and TNF inflammatory pathways92. Beyond corticosteroids and cystic fibrosis transmembrane conductance regulator modulators, several other interventions have been trialled to modulate the lung microbiota with varied success. For example, oral probiotics have been studied in the context of both cystic fibrosis and ventilator-associated pneumonia, although these likely target shifts in the gut microbiome and their effects on the respiratory microbiome remain unknown93. Bacteriophage therapy has similarly been trialled, although its use is still in its infancy94. Other studies have employed both inhaled and systemic antibiotics to modulate the respiratory microbiota but results are mixed across various pulmonary diseases95.
Unlike the gut microbiome, in which probiotics can be used to restore normal gastrointestinal flora, no such respiratory probiotic currently exists. However, in a preclinical model, episodic instillation of oral commensals into the lower respiratory tract modified host susceptibility to respiratory pathogens4. While these types of investigations may provide the biological plausibility that microbiome modifications in the lung could provide health benefits, mechanistic studies that include both preclinical work and individuals with respiratory diseases are needed. These type of investigations in turn might allow us to identify microbial signatures associated with less inflammation or decreased pathogen susceptibility. Until we understand what a beneficial ecological target might look like — a so-called ‘ideal’ respiratory microbiome — we are limited in our ability to implement safe and meaningful clinical trials.
In conclusion, there is a need to embrace the dynamic nature of microbes visiting the lower airways, particularly as it relates to host immune tone and the pathogenesis of multiple different disease processes. Novel clinical applications need to account for the evolutionary nature of the lung microbiome where eubiosis and dysbiosis truly exist on a spectrum.
Peer review
Peer review information
Nature Reviews Microbiology thanks Christopher Brightling, Michael Surette and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41579-022-00821-x
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41579-022-00821-x.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/138528212
Article citations
Air pollution, dysbiosis and diseases: pneumonia, asthma, COPD, lung cancer and irritable bowel syndrome.
Future Microbiol, 19(17):1497-1513, 30 Sep 2024
Cited by: 0 articles | PMID: 39345043
Review
A systematic framework for understanding the microbiome in human health and disease: from basic principles to clinical translation.
Signal Transduct Target Ther, 9(1):237, 23 Sep 2024
Cited by: 0 articles | PMID: 39307902 | PMCID: PMC11418828
Review Free full text in Europe PMC
Microbiome and metabolome patterns after lung transplantation reflect underlying disease and chronic lung allograft dysfunction.
Microbiome, 12(1):196, 09 Oct 2024
Cited by: 1 article | PMID: 39385282 | PMCID: PMC11462767
How oxygenation shapes immune responses: emerging roles for physioxia and pathological hypoxia.
Nat Rev Immunol, 30 Sep 2024
Cited by: 0 articles | PMID: 39349943
Review
Microbiota in tumors: new factor influencing cancer development.
Cancer Gene Ther, 28 Sep 2024
Cited by: 0 articles | PMID: 39342031
Review
Go to all (99) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Lung Microbiome.
J Immunol, 212(8):1269-1275, 01 Apr 2024
Cited by: 1 article | PMID: 38560811
Review
A brave new world: the lung microbiota in an era of change.
Ann Am Thorac Soc, 11 Suppl 1:S21-7, 01 Jan 2014
Cited by: 55 articles | PMID: 24437400 | PMCID: PMC3972973
Interactions between microbiome and lungs: Paving new paths for microbiome based bio-engineered drug delivery systems in chronic respiratory diseases.
Chem Biol Interact, 310:108732, 02 Jul 2019
Cited by: 7 articles | PMID: 31276660
Review
The role of the bacterial microbiome in lung disease.
Expert Rev Respir Med, 7(3):245-257, 01 Jun 2013
Cited by: 236 articles | PMID: 23734647 | PMCID: PMC4007100
Review Free full text in Europe PMC

 1
1


