Abstract
Free full text

Response to Capmatinib in a MET Fusion-positive Cholangiocarcinoma
Abstract
Cholangiocarcinoma is the second most common liver cancer after hepatocellular carcinoma. In case of metastatic or unresectable disease, the recommended first-line treatment is gemcitabine-based doublet, most commonly gemcitabine and cisplatin. There is no standard treatment for further lines. MET fusions are rare alterations described in many cancers. The efficacy of specific MET inhibitors is poorly studied. We present the case of a patient with chemotherapy-refractory metastatic cholangiocarcinoma harboring a CAPZA-2-MET fusion along with MET amplification who dramatically responded to capmatinib, a specific MET tyrosine kinase inhibitor.
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second most common liver cancer after hepatocellular carcinoma. In case of metastatic or unresectable disease, the recommended first-line treatment is a gemcitabine-based doublet, most commonly gemcitabine and cisplatin. FOLFOX regimen is the current standard of care in second-line setting, based on the ABC-06 trial with improved survival compared with active symptom management.1 The prognosis remains poor with a median survival of less than 12 months.2 Several targeted therapies are currently being evaluated including FGFR, IDH1, PARP, and BRAF inhibitors.3 MET inhibitors have also been tested in cholangiocarcinoma. Cabozantinib, a non-specific MET tyrosine kinase inhibitor (TKI), was evaluated as a single agent in a phase II trial in patients with chemotherapy-refractory cholangiocarcinoma, but showed poor efficacy (median PFS 1.7 months, median OS 5.2 months).4 In this study, one patient with 3 + MET expression in the tumor stayed on treatment for 278 days; however, MET expression did not correlate with outcome in the overall study population. Tivantinib, another non-specific MET TKI, was evaluated in a phase I trial in combination with gemcitabine in patients with solid tumor. Eight patients with cholangiocarcinoma were included and one partial response was observed.5 Circulating c-MET was measured in blood samples at baseline and after treatment and was not correlated with tumor response.5 These disappointing results may be due to the lack of specificity of these MET TKIs on the one hand, and the absence of any biomarker-based selection of patients on the other hand.
Here, we present the case of a patient with chemotherapy-refractory metastatic cholangiocarcinoma harboring a CAPZA-2-MET fusion along with MET amplification who dramatically responded to capmatinib, a specific MET TKI.
Patient Story
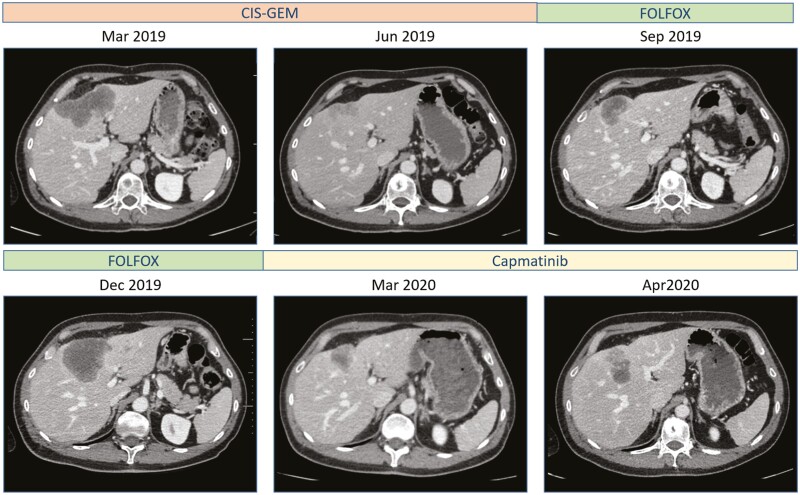
In January 2019, a 49-year-old male with no medical history was diagnosed with stage IV intrahepatic cholangiocarcinoma which was revealed by painful osteolytic metastases. MRI showed typical features of intrahepatic cholangiocarcinoma. He received radiation therapy on main bone metastases and a first-line chemotherapy cisplatin and gemcitabine (Gemcis protocol) which yielded an objective response that lasted 6 months. He then received a second-line chemotherapy with mFOLFOX but had disease progression at first radiological assessment.
Molecular Tumor Board
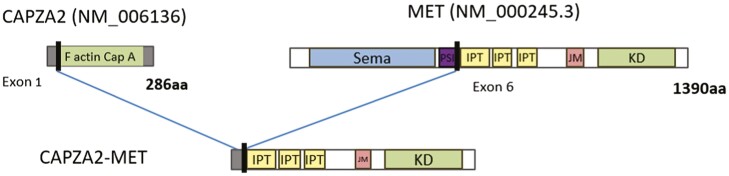
A liver rebiopsy was performed to obtain tissue for molecular analysis. A comprehensive molecular analysis was performed using the oncomine comprehensive assay (OCA V3) which revealed a fusion between exon 1 of CAPZA2 and exon 6 of MET (Fig. 1). The CAPZA2-MET fusion was predicted to lead to a chimeric protein with an intact MET kinase domain. Molecular analysis also detected a TP53 mutation (c.991C>T) and a RB1 mutation (c.[2087G>C;2099T>C])). FISH analysis revealed an elevated MET gene copy number (GCN) of 6.3, with a low ratio of GCN between MET and centromere of chromosome 7 of 2.1. IHC analysis revealed a high MET expression (100%).
Following multidisciplinary discussion in a molecular tumor board, and taking into account the lack of any approved alternative treatment and the efficacy of MET TKIs in cancer patients harboring a MET fusion, it was decided to propose the patient for a compassionate use of capmatinib.
Patient Update
Treatment with capmatinib (400 mg bid) was started on January 2020 in the context of an expanded access program. The first radiological assessment performed 2 months later showed a partial response (−36%) (Fig. 2). Tolerance was good. However, the following radiological assessment performed 4 months after starting capmatinib showed progression of the primary tumor with stable bone lesions. Radiation therapy on the primary tumor was performed. Capmatinib was withdrawn during radiation therapy and then resumed. At the same time, a new liver biopsy and a circulating free DNA analysis were performed. Analysis of the tumor biopsy did not reveal the MET fusion anymore, but the TP53 and RB1 mutations were still detected. Circulating free DNA revealed the presence of TP53 and RB1 mutations and an additional MET domain kinase mutation D1228N. A new CT scan performed 2 months after the end of radiation therapy showed stable disease.
Discussion
Here we describe for the first time a case of response to MET TKI in a cholangiocarcinoma patient with a CAPZA-2-MET fusion. This is the second case describing a partial response of capmatinib in intrahepatic cholangiocarcinoma. The case report published by Lefler et al. represents a partial response and nearly 6 months of improved quality of life in a patient diagnosed with an inoperable iCCA and unable to tolerate conventional cytotoxic chemotherapies.6
MET alterations are rare in cholangiocarcinoma. MET amplifications are found in 2% of iCCA cases.7 MET fusion-positive cholangiocarcinoma has been described only once in a 41-year-old patient with a EHBP1-MET fusion.8 Clinical studies evaluating MET inhibitors have failed so far. However, none of these studies has selected patients according to their molecular status. However, MET alterations have been associated with efficacy of MET TKI in other types of cancer. In non-small cell lung cancer (NSCLC), MET mutations affecting the splice sites of exon 14 are predictive of efficacy of capmatinib or tepotinib, 2 new-generation-specific MET TKIs.9,10 MET amplifications have also been found to predict efficacy of capmatinib in NSCLC, only in case of high-level amplification (GCN ≥10). In the present case, although GCN was increased, the ratio between MET and CEP7 was only 2.1, indicating a low-level of MET amplification. Such levels of MET amplification are usually associated with the presence of other oncogenic driver mutations and are not predictive of activity of MET inhibitors, suggesting that this amplification had no role in the response to capmatinib in the present case.9
MET fusions have been described at a low frequency in various cancers such as NSCLC, glioma, melanoma, colorectal cancer, and hepatocellular carcinoma. To date, more than 10 MET fusion partners have been identified. The most frequent fusion gene partners described are HLA-DRB1 (HLA class II histocompatibility antigen, DRB1 beta chain), CAPZA2 (F-actin-capping protein subunit alpha-2) and KIF5B (Kinesin-1 heavy chain). Clinical cases have reported clinical activity of MET TKI in MET fusion-positive tumors.11
We have identified 2 potential mechanisms of resistance in this patient. First, we found the emergence of an MET D1228N kinase domain mutation on circulating free DNA. MET kinase mutations have already been described as a potential mechanism of resistance to MET TKIs in MET-driven cancers. Preclinical data have demonstrated a variable sensitivity to MET TKI according to the type of MET kinase mutation.12 The D1228X mutations are predicted to be resistant to type I MET TKIs but sensitive to type II MET TKIs. However, clinical data are still sparse which prevented us to use a type II TKI in this patient.12
The second potential mechanism of resistance in this patient was the loss of MET fusion based on the biopsy performed at progression. Loss of the fusion has been previously proposed as a mechanism of resistance to targeted therapies but has never been reported with MET fusions. In this patient, MET fusion was initially detected at a low frequency and was not detected at progression anymore, suggesting that the fusion could be present as a sub-clonal variant. This hypothesis could explain the short response observed on capmatinib.
Finally, we did not observe any adverse due to capmatinib in this patient, apart from a grade I asthenia. Contrary to multikinase inhibitors, capmatinib is usually well tolerated. The most common adverse events reported in METex14 NSCLC patients treated with capmatinib were peripheral edema, nausea, and vomiting. However, these patients were mostly elderly and had comorbidities, which may explain why tolerance was better in this patient.
Overall, these results support molecular testing in cholangiocarcinoma patients, as recently recommended.13 The low rate of MET alterations in this setting could justify a basket clinical trial.
Glossary
Genomic terms and nomenclature
| ALK | anaplastic lymphoma kinase |
| BRAF | B-rapidly accelerated fibrosarcoma |
| CAPZA2 | F-actin-capping protein subunit alpha-2 |
| FGFR | fibroblast growth factor (receptor) |
| FISH | fluorescence in situ hybridization |
| FOLFOX | leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin |
| HLA-DRB1 | HLA class II histocompatibility antigen; DRB1: beta chain |
| IDH1 | isocitrate dehydrogenase 1 |
| IHC | immunochemistry |
| KIF5B | kinesin-1 heavy chain |
| NSCLC | non-small cell lung cancer |
| OCA | oncomine comprehensive assay |
| PARP | poly(ADP-ribose) polymérase |
| PTPRZ1 | receptor-type tyrosine-protein phosphatase zeta 1 |
| TKI | tyrosine kinase inhibitor. |
Contributor Information
Anthony Turpin, Medical Oncology Department, CHU Lille, University of Lille, Lille, France.
Clotilde Descarpentries, Department of Biochemistry and Molecular Biology, Hormonology Metabolism Nutrition Oncology, CHU Lille, University of Lille, Lille, France.
Valérie Grégoire, Pathology Department, CHU Lille, University of Lille, Lille, France.
Olivier Farchi, Department of Biochemistry and Molecular Biology, Hormonology Metabolism Nutrition Oncology, CHU Lille, University of Lille, Lille, France.
Alexis B Cortot, Thoracic Oncology Department, CHU Lille, University of Lille, Lille, France.
Philippe Jamme, Department of Dermatology, Hopital Claude Huriez, CHU Lille, University of Lille, France.
Conflict of Interest
Anthony Turpin reported consulting/advisory role and/or received honoraria from Merck, Servier, Viatris, Pierre Fabre, and AstraZeneca, as well as travel, accommodation, and other expense reimbursement from AstraZeneca and BMS. Clotilde Descarpentries reported consulting or advisory role with AstraZeneca, as well as travel and accommodations expenses from Roche, AstraZeneca, and Boehringer Ingelheim. Valérie Grégoire and Olivier Farchi indicated no financial relationships. Alexis Cortot reported honoraria from Takeda, Bristol Myers Squibb, AstraZeneca, Roche, Novartis, Pfizer, and MSD Oncology; consulting or advisory role with AstraZeneca, Boehringer Ingelheim, Pfizer, Roche, Novartis, and Takeda; research funding to institution from Merck Serono, Novartis, Roche; and travel anda ccommodations expenses from Roche, AstraZeneca, Pfizer, and Novartis. Philippe Jamme reported consulting or advisory role with Pierre Fabre, BMS, and Novartis, as well as travel and accommodations expenses from Pierre Fabre.
Author Contributions
Conception/design: A.T., A.B.C., P.J. Provision of study material or patients: A.T., C.D., O.F. Collection and/or assembly of data: A.T., P.J. Data analysis and interpretation: All authors. Manuscript writing: A.T., A.B.C., P.J. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Articles from The Oncologist are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/oncolo/oyac194
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9847551
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/139035825
Article citations
The development of a custom RNA-sequencing panel for the identification of predictive and diagnostic biomarkers in glioma.
J Neurooncol, 167(1):75-88, 16 Feb 2024
Cited by: 0 articles | PMID: 38363490 | PMCID: PMC10978676
Remarkable response to capmatinib in a patient with intrahepatic cholangiocarcinoma harboring TFG-MET fusion.
Int Cancer Conf J, 13(3):199-203, 09 Mar 2024
Cited by: 0 articles | PMID: 38962049
Emerging Therapies in Management of Cholangiocarcinoma.
Cancers (Basel), 16(3):613, 31 Jan 2024
Cited by: 0 articles | PMID: 38339363 | PMCID: PMC10854763
Review Free full text in Europe PMC
MET alterations detection platforms and clinical implications in solid tumors: a comprehensive review of literature.
Ther Adv Med Oncol, 16:17588359231221910, 18 Jan 2024
Cited by: 0 articles | PMID: 38249331 | PMCID: PMC10798113
Review Free full text in Europe PMC
MET fusions are targetable genomic variants in the treatment of advanced malignancies.
Cell Commun Signal, 22(1):20, 09 Jan 2024
Cited by: 1 article | PMID: 38195556 | PMCID: PMC10775437
Review Free full text in Europe PMC
Go to all (7) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Partial treatment response to capmatinib in MET-amplified metastatic intrahepatic cholangiocarcinoma: case report & review of literature.
Cancer Biol Ther, 23(1):112-116, 01 Dec 2022
Cited by: 5 articles | PMID: 35129063 | PMCID: PMC8820818
Review Free full text in Europe PMC
Molecular targeted and systemic therapy for intrahepatic cholangiocarcinoma: a multi-disciplinary approach.
Future Oncol, 19(39):2607-2621, 18 Dec 2023
Cited by: 1 article | PMID: 38108100
Review
Infigratinib for cholangiocarcinoma.
Drugs Today (Barc), 58(7):327-334, 01 Jul 2022
Cited by: 1 article | PMID: 35851868
Review
Effect of FGFR2 Alterations on Overall and Progression-Free Survival in Patients Receiving Systemic Therapy for Intrahepatic Cholangiocarcinoma.
Target Oncol, 17(5):517-527, 17 Sep 2022
Cited by: 14 articles | PMID: 36114955 | PMCID: PMC9512879