Abstract
Free full text

Role of microRNA-34b-5p in cancer and injury: how does it work?
Abstract
MicroRNAs (miRNAs or miRs) are a class of noncoding single-stranded RNAs that can regulate gene expression by binding to the untranslated sequences at the 3 ' end of messenger RNAs. The microRNA-34 family is dysregulated in various human diseases. It is considered as a tumor-suppressive microRNA because of its synergistic effect with the well-known tumor suppressor p53. As a member of the miRNA-34 family, miR-34b-5p serves as a powerful regulator of a suite of cellular activities, including cell growth, multiplication, development, differentiation, and apoptosis. It promotes or represses disease occurrence and progression by participating in some important signaling pathways. This review aimed to provide an overview and update on the differential expression and function of miR-34b-5p in pathophysiologic processes, especially cancer and injury. Additionally, miR-34b-5p‐mediated clinical trials have indicated promising consequences for the therapies of carcinomatosis and injury. With the application of the first tumor-targeted microRNA drug based on miR-34a mimics, it can be inferred that miR-34b-5p may become a crucial factor in the therapy of various diseases. However, further studies on miR-34b-5p should shed light on its involvement in disease pathogenesis and treatment options.
Introduction
MicroRNAs (miRNAs or miRs), 21–24 nucleotides in length, are small, single-stranded noncoding RNAs that regulate gene expression at the post-transcriptional level through target mRNA cleavage or translational inhibition. The process of their generaton is usually divided into two steps: (i) genomic DNA genetic information transcription by RNA polymerase II to produce primary miRNA(pri-miRNA) transcript, which contains one or a few stem-loop structures consisting of approximately 70 nucleotides each; and (ii) processing of pri-miRNA by a microprocessor, Dicer-like 1 protein, into precursor miRNA (pre-miRNA), which is also a stem-loop structure and finally becomes mature miRNA by modification [1]. The mature miRNA is incorporated into an RNA-induced silencing complex. They recognize target mRNAs through imperfect base pairing and commonly result in the translational inhibition or destabilization of the target mRNA.
Disclosing the biological functionality of miRNAs is generally implemented by animal knockout models and transgenic overexpression experiments [2]. Functional studies indicate that miRNAs regulate practically every cellular process investigated so far, such as cell proliferation, differentiation, immune response, metastasis, senescence, autophagy and apoptosis, via regulating housekeeping genes and involving in various cell signaling pathways [3]. The changes in their expression are associated with many human pathologies [4–6]. The interesting thing is that the functions of miRNAs depend on different pathological types and physiological environments [3]. When miRNA is located in the cell plasma, it can act on the mRNA 3′-untranslated region (UTR) like a fire extinguisher, blocking the translation of mRNA and then exerting the negative regulation of genes. In contrast, when it is located in the nucleus, it serves as an igniter that changes the chromatin state of enhancers by binding to enhancers, thereby activating the transcriptional expression of genes.
The miR-34 family has been extensively studied and considered as tumor suppressor RNA because of its synergistic effect with the tumor suppressor p53 [7]. It is a tiny fragment located in the sub-band 1 of band 3 in the long arm 2 region of chromosome 11, including three members of miR-34a, miR-34b, and miR-34c. It is highly conserved during the evolutionary process. MiR-34 family acts as an antitumor agent by participating in some important signaling pathways or regulating multiple target mRNAs and proteins [8], such as phosphatidylinositol 3-kinase–protein kinase B signaling pathway (PI3K–Akt), Notch signaling pathway, cyclin dependent kinase (Cdk), and silent mating type information regulation 2 homolog 1 (SIRT1), promoting tumor cell apoptosis, inhibiting the proliferation and differentiation of tumor cells, hindering the invasion and migration of tumor cells, and enhancing immune surveillance [9]. In addition, recent studies have put forward that miR-34 family members not only assume the function of repressors in the development of tumors but also contribute to the pathogenesis of other diseases, such as regulating reproductive and nervous system function, influencing inflammatory and immune responses [10–13].
As a member of the miR-34 family, the altered expression patterns of miR-34b-5p play a key role in a variety of human diseases. The genetic inactivation of miR-34b-5p can influence the repression effects on its target gene, mRNA, or protein, particularly if the targets are functionally linked. If these problems are not controlled, changes in protein expression and cellular dysfunction often ensue, which may lead to disease [14–19]. For example, one study showed the deregulation of miR-34b-5p in patients with bladder carcinoma of aggressive phenotype compared with nonaggressive participants [20]. Another study indicated that miR-34b-5p inhibited aquaporin-2 to promote lipopolysaccharide-induced injury in human renal tubular epithelial cells [21]. LncRNA is one of the upstream regulators of miR-34b-5p, which inhibits the downstream target genes by binding to miR-34b-5p through sponge action, thereby regulating biological processes such as cell proliferation and apoptosis [22]. In contrast, the autoregulatory feedback on microprocessor expression is instrumental for balancing the efficiency and specificity of its activity by tuning effectively the microprocessor levels to those of its pri-miRNA substrates [23].
Although studies suggest that miR-34b-5p regulates various diseases, its pathogenic mechanisms primarily focus on tumor and cell injury. Thus, in this review, we focused on cancer and injury to overview and update the changes in the functional regulation, cellular communication, and pathogenesis of miR-34b-5p. Besides, findings on its mechanisms might provide guidance and novel ideas for detecting, diagnosing, and treating miR-34b-5p-related diseases.
MiR-34b-5p in cancer
Role of miR-34b-5p in colorectal cancer
A study related to human colorectal cancer (CRC) proved that the expression of LINC02418 in CRC tissues was markedly higher than that in normal tissues; moreover, the patients with CRC having a high expressional level of LINC02418 had lower overall survival rates [24]. This long noncoding RNA contained a binding sequence for miR-34b-5p. Hence, it was deduced that LINC02418 might exert its biological effect via binding to miR-34b-5p [25]. The cell signaling pathway analysis revealed that LINC02418 adsorbed to miR-34b-5p via sponge action to hinder its binding to B-cell lymphoma-2 (Bcl-2), thus preventing the degradation of Bcl. It is well known that Bcl-2 inhibits the release of cytochrome c and pro-apoptotic factors so that the downstream caspase pathway is not able to activate, decreasing the expression of caspase 9 and caspase 3 [26]. Consequently, cell apoptosis is curbed, rendering cancer cells to growth, mobility, invasion, escape from cell death, and re-entry into abnormal cell cycles [27]. The study underlined that LINC02418 acted as a tumor driver by negatively regulating cell apoptosis through the miR-34b-5p/Bcl-2 axis, indicating that the LINC02418/miR-34b-5p/Bcl-2 axis was one potential indicator for prognosis prediction and a promising therapeutic target for CRC treatment (Fig. 1A) [16].

Mechanism of miR-34b-5p in the development of cancer. A Role of miR-34b-5p in colorectal cancer: LINC02418 promoted colon cancer progression by suppressing apoptosis via interaction with miR-34b-5p/BCL2 axis. HuR bound to OIP5-AS1 and stabilized OIP5AS1 expression while miR-34b-5p inhibited the proliferation and invasion of CC cells by inhibiting the OIP5-AS1 and PI3K/Akt pathways. B Role of miR-34b-5p in bladder carcinoma: Exosomal LINC00355 derived from cancer-associated fibroblasts promoted bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. B Role of miR-34b-5p in prostate carcinoma: The process of enhancement of anticancer activity of docetaxel by combination with Fuzheng Yiliu decoction in a mouse model of castration-resistant prostate cancer involved the regulation of miR-34b-5p and PI3K/Akt signaling pathways. D Role of miR-34b-5p in colitis-associated cancer: Inflammatory factors influenced the expression of c-MYC and CRL4DCAF4 E3 ligase activity by downregulating of miR-34b-5p to cause colitis-associated cancer. Role of miR-34b-5p in pancreatic ductal adenocarcinoma: Circular-RNA circBFAR promoted the progression of pancreatic ductal adenocarcinoma via the miR-34b-5p/MET/Akt axis
In another study, the combination of miR-34b-5p and RNA-binding protein human antigen R (HuR) could decrease the level of lncRNA opa-interacting protein 5 antisense transcript 1 (OIP5-AS1) by combining with OIP5-AS1 and prevent the PI3K–Akt pathway. Consequently, the tumor growth rate and tumor weight significantly decreased, finally impeding colon cancer (CC) progression (Fig. 1A) [28]. OIP5-AS1 is elevated in various cancers serving as a sponge against HuR [29], which has been found to promote proliferation, migration, invasion, and apoptosis of CC [30]. HuR is a highly abundant protein in cancers, targeting mRNAs to encode proteins that promote different aspects of tumorigenesis, such as cell proliferation, survival, invasion, and metastasis [29].
Role of miR-34b-5p in bladder carcinoma
MiR-34b-5p overexpression or ABCB1 silencing enhanced the sensitivity of bladder carcinoma (BC) cells to cisplatin by inhibiting cell viability and facilitating cell apoptosis in cisplatin-exposed BC cells [31]. LINC00355 acts as a competing endogenous RNA by sponging miR-34b-5p, whose target mRNA is ABCB1, to prevent it from binding to ABCB1 mRNA and upregulate ABCB1 expression; this causes BC cell proliferation as well as the inhibition of BC cell apoptosis (Fig. 1B) [32]. ABCB1 inhibits the uptake of orally administered drugs and contributes to multidrug resistance in cancer cells via exporting various exogenous compounds [33]. A study aimed to determine candidate miRNAs as prognostic biomarkers for differentiating the aggressive type of BC, and verified the deregulation of miR-34b-5p in patients with BC of aggressive phenotype compared with nonaggressive participants to promote the proliferation of cancers [20]. As a consequence, miR-34b-5p may be used not only as a diagnostic marker to identify patients with BC, but also for treating BC via mimics transfection.
Role of miR-34b-5p in prostate carcinoma
The analysis of putative target genes of castration-resistant prostate cancer indicated that specific downregulation of miR-34b-5p after the combination therapy of docetaxel and Fuzheng Yiliu decoction might hinder the PI3K–Akt signaling pathway to suppress cell survival, growth, and proliferation, and facilitate apoptosis resulting in enhanced anticancer effects (Fig. 1C) [34]. Therefore, limiting the expression of miR-34b-5p and silencing PI3K–Akt could restrain the development of castration-resistant prostate cancer and present a promising therapeutic strategy for this cancer.
Role of miR-34b-5p in colitis-associated cancer
The findings of colitis-associated cancer (CAC) tumorigenesis demonstrated that the expression of miR-34b-5p was constrained due to intracellular inflammation and DNA hypermethylation, which restricted its inhibitory effect on transcription factor proto-oncogene proteins c-myc (c-Myc). They activated the downstream events, including the activation of cullin 4A and 4B (CUL4A/4B) and the induction of CRL4DCAF4E3 ligase activity. CRL4DCAF4E3 ligase ubiquitinated the suppression of tumorigenicity 7 and led to its degradation, eventually resulting in CAC tumorigenesis (Fig. 1D) [7, 35].
Role of miR-34b-5p in pancreatic ductal adenocarcinoma
An analysis of a cohort of 208 patients with pancreatic ductal adenocarcinoma (PDAC) showed that the expression of miR-34b-5p in PDAC tissues declined and was negatively associated with the tumor-node-metastasis stage. Besides, circular-RNA BFAR (circBFAR), which was highly expressed in PDAC, upregulated the expression of the mesenchymal–epithelial transition (MET) factor via sponging miR-34b-5p. It also activated the downstream phosphorylation of Akt and further activated the MET/PI3K/Akt signaling pathway to ensure the multiplication and differentiation of PDAC cells, which ultimately promoted the progression of PDAC (Fig. 1E) [17]. In brief, this consequence provided evidence to support that the binding of circBFAR and miR-34b-5p might be a promising site for clinical MET-targeted therapy in PDAC.
Role of miR-34b-5p in lung cancer
Since benzo(a)pyrene (BaP) exposure results in lung cancer, several studies discovered prominent upregulation of miR-34b-5p in mice exposed to BaP. The consequences also revealed that high levels of miR-34b-5p in the lungs might cause changes in the expression of critical downstream target cyclin-dependent kinase inhibitor (Cdkn)1 of p53 to repair the damage caused by BaP in the lungs. Further, it was concluded that the activation of miR-34b-5p was related to the change in the cell cycle because Cdkn1 was an indispensable factor in controlling the cell cycle (Fig. 2A) [36, 37]. It was realized that miR-34b/c methylation was a collective alteration in small-cell lung cancer (SCLC). As a consequence, decreased normal miR-34b/c expression might confer tumor cell proliferation and invasiveness [38]. Another study showed that the aberrant DNA methylation of miR-34b/c was associated with a high probability of recurrence (P =
= 0.026) and correlated with low overall survival (P
0.026) and correlated with low overall survival (P =
= 0.010) and disease-free survival (P
0.010) and disease-free survival (P =
= 0.017) in lung cancer [39, 40]. These results strongly indicated that miR-34b/c was part of the pathogenesis of SCLC and presumably played a vital role as the therapeutic target in SCLC. Nevertheless, the detailed mechanism underlying the role of miR-34b-5p in SCLC is not clear. Hence, further studies are required to identify whether these molecular signatures can be used as markers for screening or another biomarker of immunotoxicity to diagnose lung cancer and forecast the prognosis.
0.017) in lung cancer [39, 40]. These results strongly indicated that miR-34b/c was part of the pathogenesis of SCLC and presumably played a vital role as the therapeutic target in SCLC. Nevertheless, the detailed mechanism underlying the role of miR-34b-5p in SCLC is not clear. Hence, further studies are required to identify whether these molecular signatures can be used as markers for screening or another biomarker of immunotoxicity to diagnose lung cancer and forecast the prognosis.

Mechanism of miR-34b-5p in the development of cancer. A Role of miR-34b-5p in lung cancer: High levels of miR-34b-5p in the lungs inhibited Cdkn1 of p53, Then the appearance of p21 protein, cyclin and CDK complex caused the decrease in RB protein phosphorylation, thereby inhibiting the expression of E2F and cyclin A and E; CNA downregulation led to the cell cycle arrest. B Role of miR-34b-5p in renal cancer: The expression of miR-34b-5p was upregulated, which decreased the level of IGF1R. Also, the PI3K/Akt signal transduction pathway was inhibited, thereby reducing the proliferation, invasion, and metastasis of renal cancer cells. C Role of miR-34b-5p in thyroid carcinoma: MiR-34b-5p could influence angiogenesis in thyroid carcinoma by changing endothelial cell proliferation through reduced VEGF-A level, arrest cell cycle and induce apoptosis. D Role of miR-34b-5p in diffuse large B-cell lymphoma: Nuclear paraspeckle assembly transcript (NEAT)1 inhibited cell proliferation and promoted cell apoptosis through decreasing miR-34b-5p level and upregulating GLI1
Role of miR-34b-5p in renal cancer
The results of an experiment displayed that the upregulation of miR-34b-5p significantly reduced the proliferation and invasion of renal cancer cells, suggesting that it might play a vital role as a tumor suppressor gene in renal cancer. The potential target gene of miR-34b-5p is the type 1 insulin-like growth factor receptor (IGF1R), whose abnormal expression is closely related to the occurrence and development of a variety of malignant tumors [41]. When the expression of miR-34b-5p was upregulated in renal carcinoma cells, the expression of the IGF1R gene significantly decreased. The PI3K/Akt signal transduction pathway was inhibited after IGF1R gene decreased. Further, the levels of PI3K, p-Akt and p-extracellular regulated protein kinases, as the PI3K/Akt signal transduction pathway proteins, decreased, thereby reducing the proliferation, invasion, and metastasis of cells. At the same time, the levels of Ki-67 and N-cadherin act, as proliferation-related and invasion-related proteins, reduced suggesting that renal cancer cells had reduced proliferation and invasion (Fig. 2B) [42].
Through gathering preoperative urine samples from patients who had histologically certified clear cell renal cell carcinoma(ccRCC), it was found that miR-126-3p combined with miR-34b-5p could prominently distinguish patients with ccRCC from healthy participants and was also able to recognize small renal masses (pT1a, ≤
≤ 4 cm) more sensitively[43, 44]. Therefore, we believed that miR-34b-5p might become a predictor and treatment direction of renal malignant renal tumors.
4 cm) more sensitively[43, 44]. Therefore, we believed that miR-34b-5p might become a predictor and treatment direction of renal malignant renal tumors.
Role of miR-34b-5p in thyroid carcinoma
The expression of miR-34b was low in anaplastic thyroid carcinoma cells. However, its apparent overexpression was perceived with transfected liposome-loaded miR-34b in thyroid carcinoma cells and mouse xenografts. Also, the diminution in VEGF-A, Bcl-2, and Notch1 protein translation, declined cell proliferation, retarded wound healing, impeded cell cycle progression, and aggrandized apoptosis occurred. Further, the expression of miR-34b were obviously related to T-stages of thyroid carcinomas (P =
= 0.042) [45]. Some experimental results suggested that miR-34b-5p could influence angiogenesis in thyroid carcinoma by changing endothelial cell proliferation through reduced VEGF-A secretion in the extracellular matrix. Also, miR-34b-5p caused cell cycle arrest via accumulation of cells in the G0–G1 phase, blocked their entry into the S transitional phase, and induced apoptosis in anaplastic thyroid carcinoma cells (Fig. 2C) [46, 47]. These results indicated that miR-34b-5p inhibition might play an indispensable role in the proliferation and metastasis of thyroid carcinoma. Multiple targeted agents are currently available for treating malignancies [48, 49]. The surveys verified the tumor suppressor properties of the miR-34b family by VEGF-A regulation in thyroid carcinoma. It is expected that the delivery of miR-34b-5p using cationic liposomes may be a useful, targeted therapeutic strategy in thyroid carcinoma.
0.042) [45]. Some experimental results suggested that miR-34b-5p could influence angiogenesis in thyroid carcinoma by changing endothelial cell proliferation through reduced VEGF-A secretion in the extracellular matrix. Also, miR-34b-5p caused cell cycle arrest via accumulation of cells in the G0–G1 phase, blocked their entry into the S transitional phase, and induced apoptosis in anaplastic thyroid carcinoma cells (Fig. 2C) [46, 47]. These results indicated that miR-34b-5p inhibition might play an indispensable role in the proliferation and metastasis of thyroid carcinoma. Multiple targeted agents are currently available for treating malignancies [48, 49]. The surveys verified the tumor suppressor properties of the miR-34b family by VEGF-A regulation in thyroid carcinoma. It is expected that the delivery of miR-34b-5p using cationic liposomes may be a useful, targeted therapeutic strategy in thyroid carcinoma.
Role of miR-34b-5p in diffuse large B-cell lymphoma
In an experiment, Qian et al. found that nuclear paraspeckle assembly transcript (NEAT)1 and GLI family zinc finger (GLI)1 were upregulated while miR-34b-5p was downregulated in diffuse large B-cell lymphoma (DLBCL) tissues and cell lines compared with the control group. The other finding was that the knockdown of NEAT1 or the overexpression of miR-34b-5p inhibited cell proliferation and promoted cell apoptosis [50]. Moreover, the expression of proliferation-related proteins, such as GLI1, cyclin D1, and CDK4 [51], decreased accompanied by increased p27 expression after miR-34b-5p overexpression. It was inferred that NEAT1 acted as a competing endogenous RNA, regulating the miR-34b-5p–GLI1 axis through declining the miR-34b-5p level and increasing GLI1 level, further accelerating the proliferation of DLBCL cells, which was often associated with disease progression and poor prognosis (Fig. 2D) [52]. In other words, the NEAT1–miR-34b-5p–GLI1 axis played an important role in the progression of DLBCL and could provide a novel therapeutic target for DLBCL.
Role of miR-34b-5p in leukosis
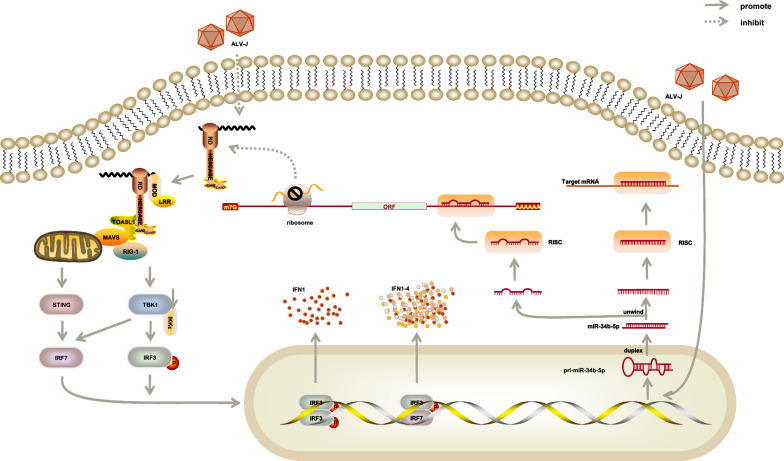
Avian Leukosis Virus Subgroup J (ALV-J) can induce myeloid leukosis, various tumors, growth retardation, and serious immunosuppression [53]. Previous data showed that miR-34b-5p was significantly upregulated to accelerate the proliferation of ALV-J-infected cells by inducing the progression from the G2 phase to the S phase and promote cell migration in ALV-J-infected chicken spleens. However, melanoma differentiation −
− associated gene 5 (MDA5) had the opposite expression pattern. The ectopic expression of MDA5 inhibited cell proliferation, cell cycle, and cell migration in ALV-J infection. A previous study proved that the MDA5 signaling pathway inhibited the mRNA and protein expression of the ALV-J env gene and suppressed virion secretion by activating the interferon (IFN) signaling pathway (Fig. 3) [54]. On the contrary, the abnormal expression of miR-34b-5p suppressed the function of MDA5, which triggered the signal transduction cascade to induce an IFN-β response and, in turn, upregulated downstream antiviral genes, such as interferon promoter stimulator 1 (IPS-1), IFN-β, oligoadenylate synthetase (OAS), myxovirus resistance gene (Mx)-1, and major histocompatibility complex (MHC) class I. MiR-34b-5p inhibited the expression of MDA5, led to increased proliferation and migration of the ALV-J-infected cells, and promoted ALV-J replication [55].
associated gene 5 (MDA5) had the opposite expression pattern. The ectopic expression of MDA5 inhibited cell proliferation, cell cycle, and cell migration in ALV-J infection. A previous study proved that the MDA5 signaling pathway inhibited the mRNA and protein expression of the ALV-J env gene and suppressed virion secretion by activating the interferon (IFN) signaling pathway (Fig. 3) [54]. On the contrary, the abnormal expression of miR-34b-5p suppressed the function of MDA5, which triggered the signal transduction cascade to induce an IFN-β response and, in turn, upregulated downstream antiviral genes, such as interferon promoter stimulator 1 (IPS-1), IFN-β, oligoadenylate synthetase (OAS), myxovirus resistance gene (Mx)-1, and major histocompatibility complex (MHC) class I. MiR-34b-5p inhibited the expression of MDA5, led to increased proliferation and migration of the ALV-J-infected cells, and promoted ALV-J replication [55].
The expression of miR-34b-5p was significantly upregulated in ALV-J-infected chicken spleens while melanoma differentiation-associated gene 5 had the opposite expression pattern. It could activate the interferon signaling pathway and the MDA5 signaling pathway to inhibit the mRNA and protein expression of the ALV-J env gene and suppress virion secretion. In addition, miR-34b-5p significantly inhibited the expression of genes in the MDA5 signaling pathway, including MDA5, IPS-1, IFNβ, OAS, Mx1, and MHC class I, to accelerate the proliferation and migration of ALV-J-infected cells.
MiR-34b-5p in injury
Role of miR-34b-5p in respiratory system injury
MiR-34b-5p is predominantly expressed in the lung tissue [36]. The level of miR-34b-5p increased 7.3-fold in acute lung injury (ALI), which was the time point where the progranulin (PGRN) expression was greatly diminished. It was inferred that the upregulated miR-34b-5p might play a significant role by downregulating the expression of PGRN in ALI. The universal comprehension of PGRN manifested that it could increase survival by decreasing lung inflammation and relieving apoptosis [56]. Another study found that by declining the PGRN level, the augmentation of miR-34b-5p could recruit inflammatory cells, produce inflammatory stroma, regulate epithelial cell apoptosis, and damage lung tissue [57]. Taurine upregulated 1 (TUG1) is an RNA gene, which interacts with the polycomb repressor complex and is involved in the epigenetic regulation of transcription, promoting cell proliferation. A previous study showed that TUG1 was involved in the inflammatory response of ALI via declining the expression of the downstream target named miR-34b-5p [58]. Additionally, lncRNA TUG1 protected alveolar epithelial cells against inflammation by attenuating the transcriptional activity of miR-34b-5p and increasing the expression of Grb2-associated binding protein indirectly [59]. These findings indicated that miR-34b-5p regulated by TUG1 could play an important role in treating sepsis-induced ALI.
The results of a study revealed that the ratio of wet to dry lung tissue weight decreased, and the apoptosis rate was also significantly lower in rats with acute respiratory distress syndrome (ARDS) after the upregulation of miR-34b-5p. The results also showed that miR-34b-5p decreased interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels in the peripheral blood of rats with ARDS. As early inflammatory factors, IL-6 and TNF-α may reflect the severity of the inflammation and induce inflammatory chemokine production in the lung epithelium, which promotes inflammatory cell infiltration, increases cell permeability, exacerbates pulmonary edema, and ultimately leads to lung consolidation. These findings suggested that miR-34b-5p attenuated inflammatory cell infiltration and lung injury of rats with ARDS, reduced pulmonary edema, and inhibited the apoptosis of alveolar epithelial cells [60].
The results of a trial suggested that miR-34b-5p levels decreased significantly in human bronchial epithelial cells after respiratory syncytial virus infection [61]. Also, these changes mediated the induction regulation of the mucin expression gene MUC5AC through the activation of the c-Jun signaling pathway, DNA methylation, and histone modifications resulting in mucus deposition and airway obstruction, hence leading to further infection and even increasing bacterial colonization [62, 63]. A study suggested that miR-34b-5p could be a useful biomarker for influenza B detection [64]. The discovery of miR-34b-5p and its unique expression profile in patients with influenza offered a new way for the early diagnosis of influenza. It is expected that a noninvasive approach using the throat swab miRNAs may be an effective way for influenza diagnosis in the future.
An investigation suggested that miR-34b-5p played a versatile role in developing inflammation in bleomycin-induced fibrotic lung tissue in mice. To be specific, miR-34b-5p restricted extracellular matrix (ECM) degradation and enlarged the alveolar spaces continually via inhibiting the expression of tissue inhibitor of metalloproteinases-3 (TIMP3), which has been acknowledged as a pivotal regulator in lung homeostasis, to inhibit the progression of pulmonary fibrosis [65, 66]. TIMP3 was recognized as a direct target of miR-34b-5p in numerous studies since enhanced expression of miR-34b-5p led to a decline in TIMP3 expression and its knockdown was responsible for TIMP3 elevation. The research suggested that miR-34b-5p boosted bleomycin resistance by decreasing the expression of TIMP3 and further facilitated the fibrotic course of lung tissue [67].
A previous study showed a connection between idiopathic pulmonary arterial hypertension (IPAH) and and miR-34b-5p, which was associated with most of the declinable target differentially expressed genes, cell multiplication, and adhesion-independent growth [68]. The target genes of miR-34b-5p are basically related to immune and inflammatory reactions, for instance, neutrophil chemotaxis and migration, integrin binding, and Toll-like receptor binding [69, 70]. Besides, they are also involved in inflammation pathways such as the IL-17 signaling pathway, indicating that miR-34b-5p might play crucial roles in the pathogenesis of IPAH through enhanced inflammatory response [71].
Role of miR-34b-5p in reproductive system injury
MiR‐34b‐5p might be involved in alternative splicing of the KIT proto-oncogene (kit)-l pre‐mRNA in murine ovarian granulosa cells through retrovirus-associated DNA sequences (Ras) signaling pathway, Rap1 GTP-binding protein (Rap)1 signaling pathway, FOXO protein (Foxo) signaling pathway, Hippo signaling pathway, mitogen-activated protein kinases (MAPK) signaling pathway, and carcinogenic pathway to affect biological processes of cell metabolism regulation, post-transcriptional regulation of mRNA, interleukin-6-mediated signal transduction, cell cycle, cell proliferation, differentiation, and migration [72]. The downstream target genes and proteins of these pathways are closely involved in abnormal cellular processes, such as cell proliferation, growth and differentiation, apoptosis regulation, oxidative stress adaptation, and inflammatory response, which are manifested as cytotoxic effects that cause ovarian granulosa cell injury and ovarian dysfunction [73]. Besides the aforementioned cellular pathways, a large number of genes, signal channels, and proteins affected by miR-34b-5p have been found in multifarious reproductive system diseases, which are the basic macromolecules of biology, regulating practically all cellular activities and functions (Table (Table11).
Table 1
Role of miR-34b-5p in multifarious reproductive system diseases
| Diseases | Gene, mRNA, signal channels, or proteins that may be involved | Variation of miR-34b-5p | Influencing mechanism, effect, or role of miR-34b-5p | References | |
|---|---|---|---|---|---|
| Ovarian function damage | Ras signaling pathway Rap1 signaling pathway Foxo signaling pathway Hippo signaling pathway MAPK signaling pathway Carcinogenic pathway | Down | Cell metabolism regulation post‐transcriptional Regulation of mRNA interleukin‐6‐mediated Signal transduction cell cycle Cell proliferation Differentiation and migration | Wang, W., et al., [72] | |
| Ovarian cancer | Met gene c-met gene Bcl-2 gene p53 gene Myc gene Cdk6 gene | MET protein BCL-2 protein CDK4 protein | Down | Regulation of cell death Occurrence of epithelial ovarian cancer Development of epithelial ovarian cancer Metastatic clinical stage Proliferation and invasion Cell Proliferation Cell adhesion-independent growth | |
| Spermatogenesis | c-Kit gene cdk6 gene Rbm44 gene Cdh3 gene | CDK6 protein C-KIT protein | Up in spermatogenesis Down in decreases in spermatocytes | Cell developmental processes mRNA transcription and regulation Cell cycle regulation Signal transduction Protein modification Semen concentration and motility regulation | Sree, S., et al., [76] Smorag, L., et al., [77] Eikmans, M., et al., [79] |
| Placental function | BCL2 gene TP53 gene MYC gene CDKN1B/C gene VEGFA gene TNFSF10 gene ZEB1 gene | Up | Trophoblast proliferation and apoptosis Abnormalities in nutrient transport Endocrine function in adolescent damage Tissue remodeling Angiogenesis Placental development | Baker, B.C., et al., [74] | |
| Endometrial endometrioid carcinoma | ESRRB mRNA SP7 mRNA GABRB2 mRNA B4GALNT mRNA | Down | Predict lymph node metastasis in endometrial Endometrioid carcinoma | ||
| Minimal deviation adenocarcinoma of uterine cervix | Notch1 gene Notch2 gene miR-34b-5p/Notch1 pathway | Down | Promote development of cervical cancer Diagnostic biomarkers | Lee, H., et al., [55] | |
A large number of genes, signaling pathways, and proteins affected by miR-34b-5p have been found in multifarious reproductive system diseases, which are the basic macromolecules of biology, regulating practically all cellular activities and functions
A prospective study found that miR-34b-5p was upregulated during the low folate status, and the levels of Bcl-2, myc, vascular endothelial growth factor-A (VEGF-A), and zinc finger E‐box-binding homeobox 1 (ZEB1) were reduced as predicted by bioinformatics analysis. These factors invariably caused a high incidence of small-for-gestational-age infants, placental dysfunction, trophoblast apoptosis, amino acid transport reduction, and altered placental hormones [74, 75]. Therefore, it was speculated that miR-34b-5p was an underlying factor in regulating the expression of Bcl-2, myc, VEGF-A, and ZEB1, which maintained placental function.
Many studies investigated the effect of miR-34b-5p on spermatogenesis. MiR-34b-5p showed a progressive increase from prepubertal through pubertal to adolescent [76]. MiRNA signature of spermatogonial stem cell (SSC), and premeiotic (PrM) and meiotic cells revealed that specific miRNAs of SSC (miR-221), PrM (miR-203), and meiotic (miR-34b-5p) and their targets, c-Kit, Rbm44, and Cdk6, showed evidence for their functional relevance during spermatogenesis [77]. In certain observational studies, the levels of miR-34b-5p acquired from the seminal plasma in the control group were significantly higher than those in the asthenozoospermia and oligozoospermia groups. In addition, the miR-34b-5p level of testicular biopsies from patients with nonobstructive azoospermia was downregulated. These results confirmed that the downregulation of miR-34b-5p resulted in decreased sperm motility and quantity [78, 79]. The testicular miR-34b-5p level was downregulated in the ethylene glycol monomethyl ether (EGME)-induced testicular toxicity model in cynomolgus monkeys, suggesting that these spermatogenic cells were damaged by EGME treatment [80]. In the testicular–hyperthermia (TH) treatment–induced testicular injury model characterized by decreased numbers of spermatocytes and spermatids, the level of miR-34b-5p located in meiotic cells was decreased [81]. All these studies showed that miR-34b-5p was a crucial factor in spermatogenesis.
Role of miR-34b-5p in nervous system injury
While testing the expression of miRNAs in the offspring rat hippocampus after exposure to fluorine combined with aluminum (FA), it was found that miR-34b-5p was higher in the exposed group. At the same time, the protein levels of brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine receptor kinase B (TrkB), were markedly downregulated in the hippocampus [82]. The combination of BDNF with TrkB causes the phosphorylation of TrkB and activation of intracellular signaling pathways, thus increasing the synthesis and release of neurotransmitters conducive to learning and memory [83]. Hence, researchers believed that miR-34b-5p might mediate FA-induced developmental neurotoxicity by downregulating the protein levels of BDNF and TrkB, thus participating in the mechanisms of hippocampal damage, which is key to the offspring's learning and memory damage caused by FA exposure during the embryonic stage and into adulthood [84]. In addition, numerous genes involved in the regulation of miR-34b-5p, such as Grm1, Syk, and BDNF, were closely related to learning and memory abilities (Fig. 4A).
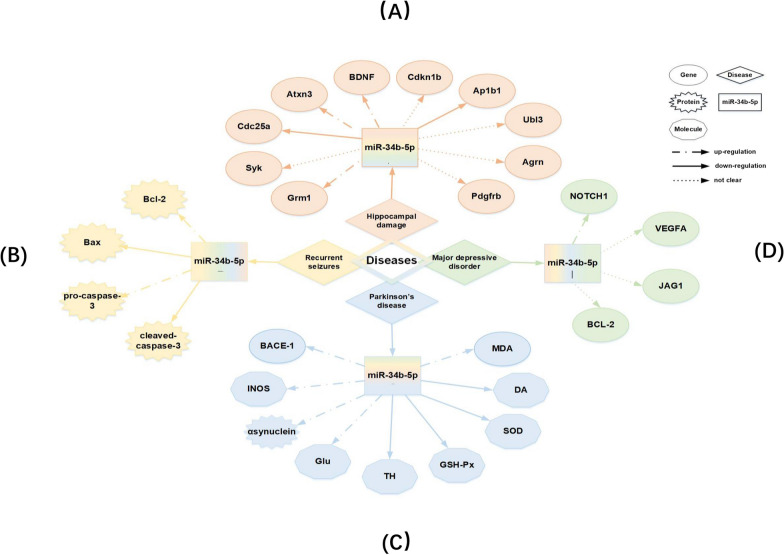
Relationship of neurological diseases, genes and miR-34b-5p. A The target genes of miR-34b-5p in hippocampal damage. Grm1 Glutamate receptor, metabotropic 1. Syk Spleen tyrosine kinase. Cdc25a Cell division cycle 25 homolog A. Atxn3 Ataxin 3. BDNF Brain-derived neurotrophic factor. Cdkn1b Cyclin-dependent kinase inhibitor 1B. Ap1b1 Adaptor-related protein complex 1,beta 1 subunit. Ubl3 Ubiquitin-like 3. Agrn Agrin. Pdgfrb Platelet-derived growth factor receptor,beta polypeptide. B The target genes of miR-34b-5p in recurrent seizures. C The target genes of miR-34b-5p in Parkinnson’s disease. BACE1 β-Site amyloid precursor protein cleaving enzyme 1. iNOS inducible nitric oxide synthase. Glu glutamic acid. TH tyrosine hydroxylase. GSH-Px glutathione peroxidase. SOD superoxide dismuta. DA dopamine. MDA malondialdehyde. D The target genes of miR-34b-5p in major depressive disorder. VEGF-A vascular endothelial growth factor
A study based on a rat model of recurrent seizures induced by flurothyl treatments showed that miR-34b-5p expression was boosted significantly in the experimental group [85]. More surprisingly, this upregulation occurred simultaneously with astrocyte apoptosis, implying the involvement of miR-34b-5p in seizures by causing astrocyte apoptosis [86]. Further, miR-34b-5p targeted Bcl-2 mRNA directly and caused a reduction of Bcl-2 and pro-caspase-3 in astrocytes but an accumulation of Bax and cleaved caspase-3. This finding indicated that miR-34b-5p exerted a pro-apoptotic function by increasing Bax/Bcl2 ratio [85]. Additionally, miR-34b-5p modulated astrocyte apoptosis in response to kainic acid (KA) glutamate receptors, causing ROS production, and affecting mitochondria function, and induce cell death in astrocytes [87]. These data indicated that miR-34b-5p was involved in modulating neuronal injury in the early stage of convulsion-induced damage and played an important role in seizure (Fig. 4B) [88, 89].Therefore, considering that preventing neuronal death in early disease stages would greatly improve therapeutic outcomes, the therapies targeting miR-34b-5p might serve as a novel therapeutic way of curing neurological diseases.
A study using rat models of Parkinson’s disease (PD) established through the injection of 6-hydroxydopamine revealed that the downregulation of lncRNA BACE1-AS inhibited inducible nitric oxide synthase, α-synuclein, and glutamic acid activation and elevated dopamine and TH levels to improve oxidative stress injury in rats with PD by upregulating miR-34b-5p via integrating with it and indirectly downregulating BACE1 [90]. This restricted apoptosis and improved oxidative stress injury in the substantia nigra neurons of rats with PD. It was observed that the substantia nigra neurons contracted into a round shape, and the intracellular substances were centralized obviously when BACE1 expression increased [90]. It indicated that the suppression of BACE1 activity could negatively work on AD progression by controlling miR-34b-5p production. This was because it was a direct target gene of miR-34b-5p [91]. A previous study also demonstrated that the repression of miR-34b and miR-34c improved α-synuclein expression in PD, which further promoted PD pathogenesis (Fig. 4C) [92].
The expression levels of miR-34b-5p in patients with major depressive disorder (MDD) were significantly higher than those in control participants, while they were significantly lower in patients with suicidal tendencies. Nevertheless, its mechanism is not clear to date. It might be related to the regulation of the Notch1 gene and cognitive function because the levels of the Notch1 gene were significantly lower in patients with MDD at the same point when the levels of miR-34b-5p increased [93]. The Notch signaling pathway induced proliferation, differentiation of neural stem cells, and growth of nerve cell axons and dendrites to affect neuronal plasticity and be involved in MDD [94, 95]. Therefore, it was presumed that miR-34b-5p might promote MDD by inhibiting the Notch signaling pathway. In a global analysis relative to the healthy controls, the miR-34b-5p level prominently declined in depressed patients. Also, its targets, such as DNA-methyltransferase 3 beta, Bcl2, and vascular endothelial growth factor-A (VEGF-A), changed, all of which were previously implicated in depression [96]. Further, multiple studies proved that the differential expression of miR-34b-5p aggravated brain injury leading to depression-like phenotypic manifestations as it was associated with neuronal regeneration and astrocytic death (Fig. 4D) [85, 90].
Role of miR-34b-5p in renal injury
A recent study showed that miR-34b-5p upregulation could suppress aquaporin-2 (AQP2) expression by binding to the 3′-UTR of AQP2, and increase the expression of pro-apoptotic proteins and proinflammatory cytokines to aggravate apoptosis and inflammation in LPS-induced renal tubular epithelial cells (HK-2) [21, 97]. Moreover, it was also found that AQP2 silencing abolished the negative effects of miR-34b-5p suppression on LPS-induced apoptosis and inflammatory response in HK-2 cells [98]. Both of them uncovered the mechanism of miR-34b-5p-mediated AQP2 in sepsis-induced injury, which is the crucial mechanism in renal injury.
Role of miR-34b-5p in skin injury
Recently, a study showed that the transfection of miR-34b-5p mimics decreased the expression of both collagen type I alpha 1 chain and elastin but abundantly increased matrix metallopeptidase (MMP)-1 expression. These factors were mainly involved in events such as cell adhesion, collagen synthesis, positive regulation of transcription and gene metabolism, and metabolism of collagen, synapse, and cytoplasmic vesicles [99], as well as insulin signaling pathway, erythroblastic leukemia viral oncogene homolog signaling pathway, and focal adhesion pathway. In addition, miR-34b-5p mimics in human dermal fibroblasts significantly induced cell cycle arrest, leading to abnormal cellular morphology, thus indicating negative impacts on the molecular markers for skin aging [100]. So far, the signaling pathway mostly investigated involves the interaction of these mimics with the target genes, including SIRT1, c-Myc, c-Met, and E2F3, which were regarded as genes for pro-longevity and cell cycle progression [101–103]. Therefore, considering the importance of miR-34b-5p in skin aging, further studies on the role of miR-34b-5p in skin aging should be conducted, providing possible biomarkers for skin aging research as well as potential targets for anti-aging therapies.
Role of miR-34b-5p in vascular injury
The most key modules associated with vascular aging are triglyceride and free fatty acid–related genes, which are considered significant determinants of age-related vascular dysfunction. Interrelated research demonstrated that the hub genes for Enpp5, Fez1, Kif1a, and F3 with their interacting miRNAs, including miR-34b-5p, miR-449a, and miR-449c, exhibited the maximum connectivity with external lipid-related traits [104, 105]. Thus, their interactions may occur in age-related vascular dysfunctions, and hence they might work as potential biomarkers.
Conclusion and future perspectives
On the one hand, miR-34b-5p could be downregulated by sponge adsorption, which relieved the inhibitory effect on downstream binding targets and promoted the proliferation, differentiation and invasion of tumor cells. On the other hand, when upregulating miR-34b-5p, tumor development was delayed by downregulating cell cycle-related proteins and increasing the expression of antitumor genes. An interesting finding is that via generating protective factors, participating in signaling pathways, or regulating gene expression, miR-34b-5p seems to act as a suppressor in cancers but as a stimulator in injury. However, there are few reports on the role its mechanism of miR-34b-5p in other systemic injuries. This may be because the function of miR-34b-5p has not yet been fully mined so that its role in other injuries has not received sufficient attention.
In this review, we provided an overview and update on different biological aspects and individual functions of miR-34b-5p. Most of the current studies on miR-34b-5p focus on the detection of expression levels and preliminary exploration of pathogenic mechanisms, while no standardized detection methods have yet been developed. In addition, the upstream and downstream regulatory network of miR-34b-5p remains unclear. It is speculated that the transfection of tumor cells with miR-34b-5p mimics to inhibit their proliferation and invasion and to repair cell damage using miR-34b-5p inhibitors may be new directions for future exploration, and the findings may translate into effective regimens for the treatment of tumors and injuries. This study was novel in summarizing the role of miR-34b-5p in the pathogenesis of a variety of cancer and injury, and mapping the miR-34b-5p related mechanistic pathways in graphical form so that the relevant research results were more clearly displayed. However, the manuscript fails to elucidate the specific protocol of miR-34b-5p in disease therapy because we need further studies to identify upstream and downstream mRNA signals associated with cancer as well as the background environment required for their interaction.
Abbreviations
| miRNAs or miRs | MicroRNAs |
| nt | Nucleotides |
| pri-miRNA | Primary miRNA |
| pre-miRNA | Precursor miRNA |
| PDAC | Pancreatic ductal adenocarcinoma |
| TNM | Tumor-node-metastasis |
| circBFAR | Circular-RNA BFAR |
| MET | Mesenchymal–epithelial transition factor |
| OSCC | Oral squamous cell carcinoma |
| CAC | Colitis-associated cancer |
| CRC | Colorectal cancer |
| BCL2 | B-cell lymphoma-2 |
| ALI | Acute lung injury |
| PGRN | Progranulin |
| LPS | Lipopolysaccharide |
| ECM | Extracellular matrix |
| TIMP3 | Tissue inhibitor of metalloproteinases-3 |
| BaP | Benzo(a)pyrene |
| SCLC | Small-cell lung cancer |
| BC | Bladder carcinoma |
| AQP2 | Aquaporin-2 |
| HK-2 | Human renal tubular epithelial cells |
| SSC | Spermatogonial stem cell |
| PrM | Premeiotic |
| EGME | Ethylene glycol monomethyl ether |
| TH | Testicular–hyperthermia |
| VEGF-A | Vascular endothelial growth factor-A |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
| EEC | Endometrial endometrioid carcinoma |
| LNM | Lymph node metastasis |
| MDA | Minimal deviation adenocarcinoma |
| DEMs | Differentially expressed miRNAs |
| CORT | Corticosterone |
| DLBCL | Diffuse large B-cell lymphoma |
| ALV-J | Avian leukosis virus subgroup J |
| MDA5 | Melanoma differentiation-associated gene 5 |
| IFN | Interferon |
| FA | Fluorine combined with aluminum |
| BDNF | Brain-derived neurotrophic factor |
| PD | Parkinson’s disease |
| SI | Social isolation |
| MDD | Major depressive disorder |
| IPAH | Idiopathic pulmonary arterial hypertension |
| DEGs | Differentially expressed genes |
Author contributions
ZLW contributed to the conception and design of the study. XY and LY organized the database. BXC wrote the first draft of the manuscript. LDD wrote sections of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Natural Science Foundation of Jilin Province (Grant No.20200201438JC).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Cancer Cell International are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s12935-022-02797-3
Read article for free, from open access legal sources, via Unpaywall:
https://cancerci.biomedcentral.com/counter/pdf/10.1186/s12935-022-02797-3
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/139571045
Article citations
Unraveling the Regulatory Role of HuR/microRNA Axis in Colorectal Cancer Tumorigenesis.
Cancers (Basel), 16(18):3183, 18 Sep 2024
Cited by: 0 articles | PMID: 39335155 | PMCID: PMC11430344
Review Free full text in Europe PMC
Characterization of the SARS-CoV-2 Genome 3'-Untranslated Region Interactions with Host MicroRNAs.
ACS Omega, 9(34):36148-36164, 16 Aug 2024
Cited by: 0 articles | PMID: 39220490 | PMCID: PMC11360049
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Analysis of the miR-34 family functions in breast cancer reveals annotation error of miR-34b.
Sci Rep, 7(1):9655, 28 Aug 2017
Cited by: 17 articles | PMID: 28848235 | PMCID: PMC5573726
MicroRNA-34b functions as a tumor suppressor and acts as a nodal point in the feedback loop with Met.
Int J Oncol, 42(3):957-962, 10 Jan 2013
Cited by: 40 articles | PMID: 23314612
MicroRNA-34b-5p inhibits proliferation, stemness, migration and invasion of retinoblastoma cells via Notch signaling.
Exp Ther Med, 21(3):255, 25 Jan 2021
Cited by: 7 articles | PMID: 33603862 | PMCID: PMC7851672
MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential.
Cancers (Basel), 15(19):4723, 26 Sep 2023
Cited by: 13 articles | PMID: 37835417 | PMCID: PMC10571940
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Natural Science Foundation of Jilin Province (1)
Grant ID: 20200201438JC