Abstract
Free full text

Unprecedented long‐term survival in a patient with malignant pleural mesothelioma treated with subsequent systemic chemo‐ and immunotherapeutic regimens
Abstract
Pleural mesothelioma is a rare disease with a dismal prognosis and few therapeutic options. Until recently the median overall survival for a pleural mesothelioma patient was up to 2 years, with few exceptional cases of patients achieving a longer survival. Here, we report the clinical case of a patient whose survival spanned over 10
years, with few exceptional cases of patients achieving a longer survival. Here, we report the clinical case of a patient whose survival spanned over 10 years. The patient underwent several systemic treatments, including three different chemotherapy lines (cisplatin‐pemetrexed, vinorelbine and platinum rechallenge) and two immunotherapy regimens using immune checkpoint inhibitors (anti CTLA‐4 tremelimumab and anti PD‐1 nivolumab). At the time this report was written, the patient was off‐treatment, asymptomatic and with a stable radiological disease. Our case demonstrates that a prolonged survival with a preserved quality of life may be reached in selected patients through the exploitation of the available treatments in an expertise setting.
years. The patient underwent several systemic treatments, including three different chemotherapy lines (cisplatin‐pemetrexed, vinorelbine and platinum rechallenge) and two immunotherapy regimens using immune checkpoint inhibitors (anti CTLA‐4 tremelimumab and anti PD‐1 nivolumab). At the time this report was written, the patient was off‐treatment, asymptomatic and with a stable radiological disease. Our case demonstrates that a prolonged survival with a preserved quality of life may be reached in selected patients through the exploitation of the available treatments in an expertise setting.
Abstract
Pleural mesothelioma is a disease with a dismal prognosis. Here, we show an unusual case of a patient with pleural mesothelioma with adenomatoid histology (1), with nonsurgically operable disease. This patient was managed with multiple lines of systemic therapy, achieving long lasting survival from 2012 (2) to date (3). Disease control is still ongoing and the patient is asymptomatic with a good performance status.
INTRODUCTION
Mesothelioma is a rare and aggressive tumor of the mesothelial surfaces. Ninety percent of mesothelioma cases arise from the pleura. At least 80% of pleural mesothelioma (PM) cases are related to asbestos exposure, after a long latency period which can exceed 40 years.
1
years.
1
The major histological subtypes of PM are epithelioid, sarcomatoid, and biphasic.
2
Epithelioid PM represents more than half of the pleural mesothelioma diagnoses, with median patient survival of 14 months, whereas the biphasic and sarcomatoid histology account overall for 30%–40% of cases and are associated with a shorter survival time of less than 10 months.
3
At present, surgery is recommended only in carefully selected patients with localized disease and within a multimodal treatment approach.
4
,
5
Specifically, in a retrospective study, conservative surgery through pleurectomy/decortication was proven to prolong median survival mainly in epithelioid mesothelioma patients up to 19
months, whereas the biphasic and sarcomatoid histology account overall for 30%–40% of cases and are associated with a shorter survival time of less than 10 months.
3
At present, surgery is recommended only in carefully selected patients with localized disease and within a multimodal treatment approach.
4
,
5
Specifically, in a retrospective study, conservative surgery through pleurectomy/decortication was proven to prolong median survival mainly in epithelioid mesothelioma patients up to 19 months.
3
months.
3
For around 20 years, no major advances in the systemic treatment of PM have been made.
5
Indeed, since 2003,
6
the standard first‐line recommended systemic treatment has been platinum‐based regimens plus pemetrexed.
5
,
7
The addition of bevacizumab to standard first‐line platinum‐pemetrexed significatively prolonged overall survival (OS) in PM in a phase III trial,
8
but due to regulatory issues the combination is not available in every country. Only recently the combination of ipilimumab and nivolumab has been proven to prolong OS in PM patients, when compared to standard first‐line platinum pemetrexed,
9
especially in those patients with nonepithelioid histology. No second‐line treatment is currently approved for PM and single agent gemcitabine or vinorelbine are commonly used in this setting. Only recently, a randomized trial confirmed the efficacy of single agent vinorelbine over placebo in those patients,
10
while a recent phase II trial evaluating the combination of gemcitabine and ramucirumab over placebo in pretreated mesothelioma patients demonstrated an improvement in OS.
11
Over the last few years, immune checkpoint inhibitors (ICIs) have showed promising results in terms of both disease control and response rate in patients failing a first‐line platinum pemetrexed regimen,
12
,
13
,
14
,
15
despite the negative results in the phase III randomized trial PROMISE Meso trial.
16
years, no major advances in the systemic treatment of PM have been made.
5
Indeed, since 2003,
6
the standard first‐line recommended systemic treatment has been platinum‐based regimens plus pemetrexed.
5
,
7
The addition of bevacizumab to standard first‐line platinum‐pemetrexed significatively prolonged overall survival (OS) in PM in a phase III trial,
8
but due to regulatory issues the combination is not available in every country. Only recently the combination of ipilimumab and nivolumab has been proven to prolong OS in PM patients, when compared to standard first‐line platinum pemetrexed,
9
especially in those patients with nonepithelioid histology. No second‐line treatment is currently approved for PM and single agent gemcitabine or vinorelbine are commonly used in this setting. Only recently, a randomized trial confirmed the efficacy of single agent vinorelbine over placebo in those patients,
10
while a recent phase II trial evaluating the combination of gemcitabine and ramucirumab over placebo in pretreated mesothelioma patients demonstrated an improvement in OS.
11
Over the last few years, immune checkpoint inhibitors (ICIs) have showed promising results in terms of both disease control and response rate in patients failing a first‐line platinum pemetrexed regimen,
12
,
13
,
14
,
15
despite the negative results in the phase III randomized trial PROMISE Meso trial.
16
Here, we present the case of a PM patient treated with multiple anticancer treatments including both chemotherapy and ICIs.
CASE REPORT
After a long history of recurrent pleuritis, which started in 2004 and continued to 2012, a CT scan was performed on a 59 year‐old man which showed pleural effusion and thickening in the right hemithorax. The patient was a nonsmoker, general practitioner in full activity, without any known history of asbestos exposure, or familiar history of cancer or any other relevant comorbidity (PS ECOG 0). He underwent video‐assisted thoracoscopy for biopsies and talc pleurodesis.
year‐old man which showed pleural effusion and thickening in the right hemithorax. The patient was a nonsmoker, general practitioner in full activity, without any known history of asbestos exposure, or familiar history of cancer or any other relevant comorbidity (PS ECOG 0). He underwent video‐assisted thoracoscopy for biopsies and talc pleurodesis.
The histological examination led to a diagnosis of epithelioid diffuse PM. Morphological assessment showed that the tumor tissue was composed of flat to cuboidal cells lining small glandular structures or microcystic spaces invading the fibrous and adipose stromal tissue. The infiltrating tumoral stroma was assessed as low to moderate. Clearly invasive areas with a tubulopapillary growth pattern were associated (Figure 1). These morphological aspects corresponded to the adenomatoid variant of epithelioid mesothelioma. 17 No necrosis was found so the grade rating (according to WHO 2021) was established as low (low nuclear atypia mitotic count). 17 Immunohistochemical staining showed diffuse positivity for calretinin. As the case was diagnosed in 2012, no additional markers were found and weak antigenicity currently found of the tissue had prevented further staining.
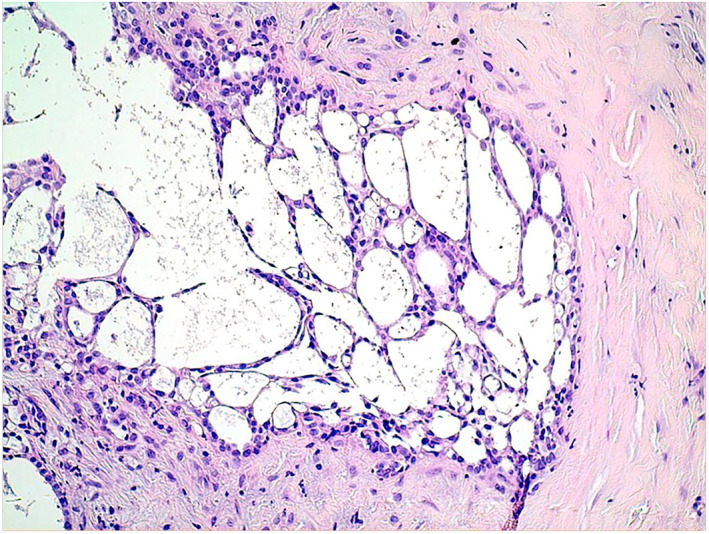
Histological image showing diffuse mesothelioma with adenomatoid features (hematoxylin & eosin, 100x) and dilated structures lined by cuboidal cells mixed with small glandular‐like structures invading the stromal tissue
The patient had a non surgically resetable disease and thus received a combination of cisplatin and pemetrexed with a G3 hematological toxicity, stopped after the fifth course because of a deep venous thrombosis, but achieved a partial response.
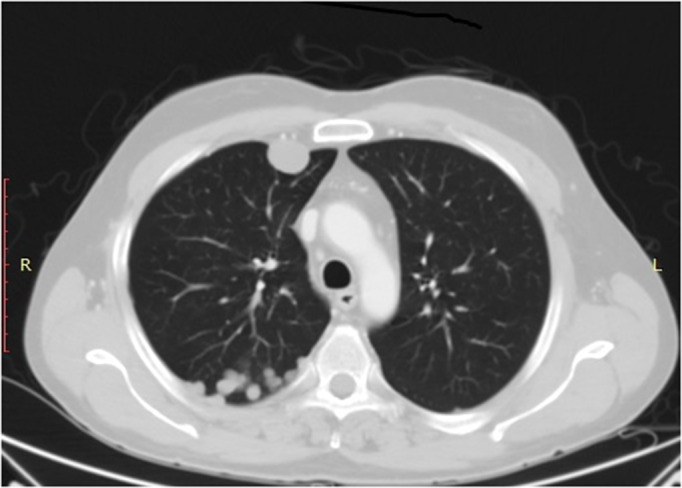
Nine months after the end of treatment, CT scan showed progressive disease (Figure (Figure2).2). At that time, a monoinstitutional phase II trial with anti‐CTLA‐four antibody tremelimumab was ongoing at another Italian institution. The patient was enrolled in the trial and received four cycles of monthly tremelimumab 10 mg/kg,
18
achieving a fast shrinkage of the neoplastic lesions. Subsequently a G4 CTCAE haemorragic colitis occurred at the fourth cycle of the treatment and tremelimumab was therefore discontinued. Twenty months later, the disease progressed again. Considering the previous toxicity reported to platinum doublet and the patient's will to continue his life in full activity a shared decision of treatment with vinorelbine was made. The patient received six courses, with stable disease as best radiological response. Eighteen months later, the CT scan revealed a further pleural progression. Due to the long interval from platinum treatment and valuing the partial response obtained after first line, in this condition a rechallenge with the less toxic association of carboplatin plus pemetrexed was administered for six courses and disease stabilization was achieved. The disease remained then stable for 16 months, when a further progression occurred. The patient was finally treated with nivolumab for 27 courses within a special fund available in Italy for drugs with an orphan indication, between January 2020 and April 2021. The CT scans performed during and after nivolumab treatment showed a substantial volumetric reduction of the pleural lesion with a subsequent stability of the disease.
months, when a further progression occurred. The patient was finally treated with nivolumab for 27 courses within a special fund available in Italy for drugs with an orphan indication, between January 2020 and April 2021. The CT scans performed during and after nivolumab treatment showed a substantial volumetric reduction of the pleural lesion with a subsequent stability of the disease.
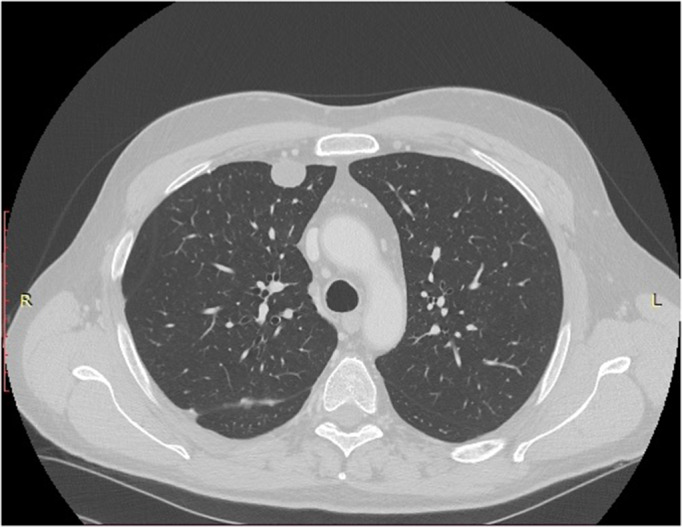
At the last CT and PET scans, performed in March 2022 (Figure 3), the disease stability was confirmed. The patient is currently asymptomatic and in good clinical condition, and is performance status is ECOG 0.
DISCUSSION
Here, we report an unusual case of a patient affected by advanced stage pleural mesothelioma with an exceptionally long survival exceeding 10 years. Although the histology can be listed among the favorable prognostic factors, according to its cytoarchitectural features
19
,
20
it proved to be exceptionally sensitive to multiple systemic treatments. The patient underwent multiples lines of therapy, including different types of chemotherapy and immunotherapy, each followed by both clinical benefit and radiological disease control. Each chemotherapy treatment was exploited up to recommended guidelines, and no maintenance treatment was performed. Thus, the patient's quality of life was preserved, with no significant impact on their work activity and wellbeing.
years. Although the histology can be listed among the favorable prognostic factors, according to its cytoarchitectural features
19
,
20
it proved to be exceptionally sensitive to multiple systemic treatments. The patient underwent multiples lines of therapy, including different types of chemotherapy and immunotherapy, each followed by both clinical benefit and radiological disease control. Each chemotherapy treatment was exploited up to recommended guidelines, and no maintenance treatment was performed. Thus, the patient's quality of life was preserved, with no significant impact on their work activity and wellbeing.
To the best of our knowledge, no other similar cases have previously been reported in literature.
This report confirms how mesothelioma patients, especially with good prognostic histological or clinical features, 21 , 22 may largely benefit from treatments, as already demonstrated in previously published meta‐analysis in which the authors show the OS benefit produced by anti‐PD‐1 and chemotherapy treatments as subsequent therapeutic regimens for mesothelioma. 23 While investigation on the identification of reliable predictive factors in mesothelioma is still at a primordial stage, we believe that the exploitation of all systemic treatment available in clinical practice and clinical trials, following the indications of an experienced center, may be crucial for optimal patient care. Indeed, the patient was managed at three different Italian referral centers along his clinical course with a profound intercenter collaboration. This allowed the enrolment of the patient in clinical trials with drugs otherwise not accessible, potentially playing a significant role in the survival extension.
AUTHOR CONTRIBUTION
FG contributed to the study conceptualization. LC, SD, AMD, SC and AC contributed to data collection. LC and FG wrote the article. All authors reviewed and contributed to the final full text, have approved the submitted version of the manuscript and agreed to be accountable for any part of the work.
CONFLICT OF INTEREST
FG reports, outside the submitted work, in the last 5 years personal fees for advisory role, speaker engagments, travel and accomodation expenses from Merck Sharp & Dhome, Bristol Myers Squibb, Novocure, PharmaMar, Novartis, Pierre Fabre.
years personal fees for advisory role, speaker engagments, travel and accomodation expenses from Merck Sharp & Dhome, Bristol Myers Squibb, Novocure, PharmaMar, Novartis, Pierre Fabre.
ACKNOWLEDGMENTS
We acknowledge the patient for his consent to case report and for his help in retrieving all the information needed for the compilation of this case report. We also acknowledge Alessandria section of "Lega Italiana per la Lotta contro i Tumori" for their financial support in manuscript publication.
Notes
Cerbone L, Delfanti S, De Angelis AM, Crivellari S, Boccuzzi F, Cimorelli A, et al. Unprecedented long‐term survival in a patient with malignant pleural mesothelioma treated with subsequent systemic chemo‐ and immunotherapeutic regimens. Thorac Cancer. 2023;14(5):524–527. 10.1111/1759-7714.14789 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
REFERENCES
Articles from Thoracic Cancer are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1111/1759-7714.14789
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/1759-7714.14789
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/141975264
Article citations
The Pleural Mesothelioma Cases and Mortality in Portugal in 2014-2020: A Descriptive Study.
Healthcare (Basel), 12(11):1103, 28 May 2024
Cited by: 0 articles | PMID: 38891178 | PMCID: PMC11171679
Zimberelimab plus chemotherapy as the first-line treatment of malignant peritoneal mesothelioma: A case report and review of literature.
World J Clin Cases, 11(22):5296-5302, 01 Aug 2023
Cited by: 0 articles | PMID: 37621601 | PMCID: PMC10445078
Unprecedented long-term survival in a patient with malignant pleural mesothelioma treated with subsequent systemic chemo- and immunotherapeutic regimens.
Thorac Cancer, 14(5):524-527, 04 Jan 2023
Cited by: 3 articles | PMID: 36599413 | PMCID: PMC9925339
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Treatment patterns and outcomes of patients with advanced malignant pleural mesothelioma in a community practice setting.
Future Oncol, 17(19):2439-2448, 26 Mar 2021
Cited by: 1 article | PMID: 33769073
What's Current and What's New in Mesothelioma?
Clin Oncol (R Coll Radiol), 34(11):771-780, 22 Sep 2022
Cited by: 2 articles | PMID: 36155156
Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): a multicentre, single-arm, phase 2 trial with a safety run-in.
Lancet Oncol, 21(9):1213-1223, 01 Sep 2020
Cited by: 77 articles | PMID: 32888453
Malignant Pleural Mesothelioma: Is Tailoring the Second-Line Therapy Really "Raising the Bar?"
Curr Treat Options Oncol, 20(3):23, 21 Feb 2019
Cited by: 3 articles | PMID: 30790063
Review

 1
1