Abstract
Introduction
Elevated plasma amino acid levels overload kidney function by increasing glomerular filtration rate (GFR). Inhibiting gut amino acid intake may have therapeutic benefits for patients with kidney dysfunction. For a prospective phase 2a trial, we carried out an exploratory evaluation of the safety and efficacy of SCO-792, an enteropeptidase inhibitor that blocks gut amino acid intake, in patients with type 2 diabetes mellitus (T2DM) and albuminuria.Methods
Seventy-two patients with T2DM, a urine albumin-to-creatinine ratio (UACR) of 200-5000 mg/g, and an estimated GFR >30 ml/min per 1.73 m2 were included. Patients were randomly assigned (1:2:2) to the following groups and received treatment for 12 weeks: placebo (n = 15), SCO-792 500 mg once daily (SCO-792 QD; n = 29), or SCO-792 500 mg 3 times daily (SCO-792 3 times a day (TID); n = 28) by following a double-blind approach. We evaluated UACR changes from the baseline along with safety as the primary end points and other parameters as secondary or exploratory end points.Results
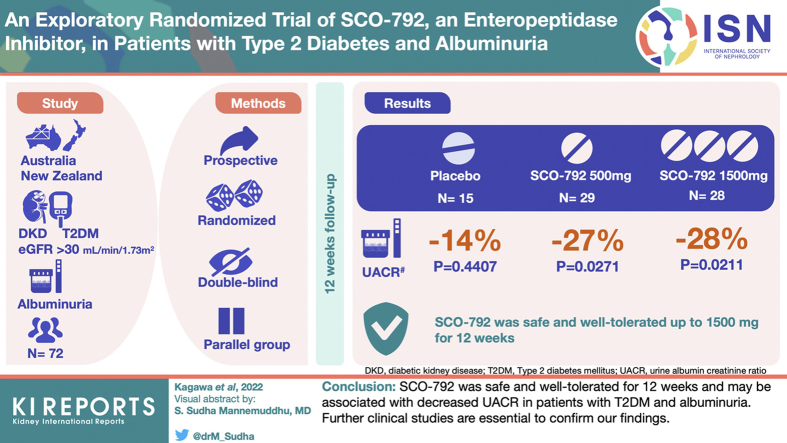
SCO-792 was safe and well tolerated up to 1500 mg/day for 12 weeks. UACR changes from baseline were -14% (P = 0.4407), -27% (P = 0.0271), and -28% (P = 0.0211) in placebo, SCO-792 QD, and SCO-792 TID, respectively, whereas UACR changes in SCO-792 groups were not statistically significant compared with placebo. The hemoglobin A1c (HbA1c) levels from baseline, an exploratory end point, decreased in the SCO-792 TID group.Conclusion
SCO-792 was safe and well tolerated for 12 weeks and may be associated with decreased UACR in patients with T2DM and albuminuria. Further clinical studies are essential to confirm our findings.Free full text

An Exploratory Randomized Trial of SCO-792, an Enteropeptidase Inhibitor, in Patients With Type 2 Diabetes and Albuminuria
Abstract
Introduction
Elevated plasma amino acid levels overload kidney function by increasing glomerular filtration rate (GFR). Inhibiting gut amino acid intake may have therapeutic benefits for patients with kidney dysfunction. For a prospective phase 2a trial, we carried out an exploratory evaluation of the safety and efficacy of SCO-792, an enteropeptidase inhibitor that blocks gut amino acid intake, in patients with type 2 diabetes mellitus (T2DM) and albuminuria.
Methods
Seventy-two patients with T2DM, a urine albumin-to-creatinine ratio (UACR) of 200–5000 mg/g, and an estimated GFR >30 ml/min per 1.73 m2 were included. Patients were randomly assigned (1:2:2) to the following groups and received treatment for 12 weeks: placebo (n = 15), SCO-792 500 mg once daily (SCO-792 QD; n = 29), or SCO-792 500 mg 3 times daily (SCO-792 3 times a day (TID); n = 28) by following a double-blind approach. We evaluated UACR changes from the baseline along with safety as the primary end points and other parameters as secondary or exploratory end points.
Results
SCO-792 was safe and well tolerated up to 1500 mg/day for 12 weeks. UACR changes from baseline were −14% (P = 0.4407), −27% (P = 0.0271), and −28% (P = 0.0211) in placebo, SCO-792 QD, and SCO-792 TID, respectively, whereas UACR changes in SCO-792 groups were not statistically significant compared with placebo. The hemoglobin A1c (HbA1c) levels from baseline, an exploratory end point, decreased in the SCO-792 TID group.
Conclusion
SCO-792 was safe and well tolerated for 12 weeks and may be associated with decreased UACR in patients with T2DM and albuminuria. Further clinical studies are essential to confirm our findings.
The incidence of T2DM has rapidly increased over the past decades, driven primarily by obesity and the increased prevalence of sedentary lifestyles.1 Although most diabetes-associated complications are related to cardiovascular health, diabetes remains one of the leading causes of kidney failure.2,3 Diabetic kidney disease (DKD) is defined as the presence of persistent albuminuria or an estimated GFR (eGFR) of <60 ml/min per 1.73 m2 in an individual with diabetes.4 Pharmacological inhibition of the renin-angiotensin system (RAS) is an effective treatment strategy for patients with DKD5; sodium-glucose cotransporter-2 inhibitors have been recommended as a new class of renoprotective agents for DKD.6,7 Recently, a mineralocorticoid receptor antagonist has been approved for patients with chronic kidney disease (CKD) associated with T2DM.8,9 Despite these available therapies, the residual risk of kidney disease progression remains a serious challenge. Therefore, new strategies are required for the treatment of DKD.
Elevated plasma amino acid levels induce kidney hyperfiltration in both healthy participants and patients with diabetes,10,11 which suggests that plasma amino acids may play a role in regulating kidney function. High protein diets in patients with CKD may induce glomerular hyperfiltration, leading to kidney dysfunction.12 These findings suggest that lowering plasma amino acid levels may improve kidney function in patients with kidney impairment.
Enteropeptidase, a transmembrane serine protease localized at the brush border of the duodenal and jejunal mucosa, regulates protein digestion in mammals.13, 14, 15 Enteropeptidase converts inactive trypsinogen into its active form, trypsin, thereby activating digestive enzyme precursors produced in the pancreas.13, 14, 15 These activated enzymes, in turn, facilitate protein breakdown, thereby increasing amino acid absorption in the gut.13, 14, 15 Consequently, enteropeptidase is pivotal for gut amino acid absorption in mammals.
SCO-792, an orally available enteropeptidase inhibitor that mitigates postprandial plasma amino acid levels,16,17 has been shown to inhibit gut amino acid intake, improve glycemic control, ameliorate glomerular hyperfiltration, decrease markers of kidney fibrosis and injury, and induce a sustained decrease in UACR in Wistar fatty rats, a DKD model.18 In addition, SCO-792 decreases gut amino acid intake, prevents GFR decline, improves albuminuria, and ameliorates glomerulosclerosis and kidney fibrosis in spontaneously hypercholesterolemic rats, a nondiabetic CKD model.19 These findings suggest that the SCO-792–mediated inhibition of gut amino acid intake, which reduces postprandial plasma amino acid levels, may exert therapeutic effects against diseases associated with kidney dysfunction.
In this study, we conducted a phase 2a exploratory study to investigate the potential of SCO-792 as a new candidate for treating DKD and evaluated the change in UACR from the baseline after 12 weeks of treatment as well as safety of SCO-792 (primary end points). We also evaluated eGFR and responder rate of at least 30% UACR decrease (secondary end points) in patients with T2DM and albuminuria. Metabolic end points were also measured as exploratory end points to evaluate the potential effects of SCO-792 in patients with T2DM and albuminuria.
Methods
Study Design
No clinical trials for SCO-792 on UACR reduction were conducted before this study. Owing to the exploratory nature of this trial, the number of participants was decided based on an overall feasibility assessment including trial cost and clinical trial implementation. Formal statistical power calculation was not conducted. This was a multicenter, prospective, randomized, double-blind, parallel-group, placebo-controlled trial of SCO-792 (SCOHIA PHARMA, Inc) administered to patients with DKD in Australia (7 sites) and New Zealand (9 sites) from June 24, 2020, to May 7, 2021. The study duration consisted of a screening period of 4 weeks, a treatment period of 12 weeks, and a follow-up period of 1 week (Supplementary Figure S1). The trial was designed and conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with the International Council for Harmonization and Good Clinical Practice. Before initiation of the study, the study protocol, written study participant information, informed consent form, investigator’s brochure, and all other relevant documents were reviewed and approved by the Human Research Ethics Committee at each study site. The trial is registered at JapicCTI (Study Number JapicCTI-205109). Participants who provided consent were screened during the screening period (week −4 to week 0). Those who met the eligibility criteria were randomized in a ratio of 1:2:2 to 1 of the 3 treatment groups on day 1 of week 0: placebo TID, SCO-792 500 mg once daily plus placebo twice daily (SCO-792 QD; 500 mg/day), or SCO-792 500 mg 3 times a day (SCO-792 TID; 1500 mg/day). The number of placebo-allocated patients amounted to almost half of the SCO-792–allocated patients; thus, the study was not designed to confirm a difference between placebo and SCO-792. The SCO-792 and placebo tablets were not distinguishable by their appearance. Stratified permuted block randomization was implemented using the screening or eligibility UACR value as a stratification factor as follows: Strata 1 is UACR <500 mg/gCr, and Strata 2 is UACR ≥500 mg/gCr. A computer-generated randomization schedule was prepared by an unblinded statistician before the start of the study. Stratification and randomization were performed via the Interactive Web Response System, and sites were required to enter the UACR measurement in mg/gCr. The investigational product was administered in accordance with the randomization list. During the treatment period, site staff and participants were blinded to the treatment allocation. All participants were administered the study drug orally approximately 10 minutes before breakfast, lunch, and dinner. Participants assigned to QD dosing received placebo tablets for 2 of the 3 doses, and the active dose was administered at dinner time.
Study Participants
Patients with T2DM and albuminuria were eligible if they met the following criteria: aged between 18 and 75 years, had been treated with stable doses of the same RAS inhibitors and antihyperglycemic agents (AHAs) either in monotherapy or combination therapy for at least 3 months before the first visit to the end of treatment, had a UACR of the first morning urine of ≥200 to <5000 mg/gCr as assessed in the screening period, and had an eGFR according to creatinine >30 ml/min per 1.73 m2 at week −4.
Participants with a history of type 1 diabetes mellitus or other specific types of diabetes (e.g., genetic syndromes, secondary pancreatic diabetes, diabetes due to endocrinopathies, drug-induced or chemical-induced, and postorgan transplant) with a confirmed diagnosis of renal disease other than type 2 DKD (e.g., lupus nephritis and primary focal segmental glomerular sclerosis), significant gastrointestinal disorders or abdominal surgery, or cardiovascular disease were excluded.
Changes in dose or dosing regimen were prohibited during the study period. Medications for T2DM, hypertension, dyslipidemia, psychoactive agents such as monoamine oxidase inhibitors and antidepressive agents, hormonal contraception or hormone replacement therapy, supplements, vitamins, over-the-counter products, herbal preparations, and thyroid replacement were allowed during the study, but their use was restricted and maintained at stable levels throughout the study.
Outcomes
The primary end points included the safety and tolerability of SCO-792 dosing and changes in log-transformed UACR from baseline to the end of treatment. Changes in eGFR from baseline and responder rate of at least 30% UACR decrease from baseline to the end of treatment were analyzed as secondary end points. Other observed values and changes from baseline to the end of treatment were also analyzed as exploratory end points.
Blood and urine samples were collected by a central laboratory at >8-hour fasting condition to test for UACR, urinary protein-to-creatinine ratio, eGFR, HbA1c, fasting plasma glucose, fasting insulin, homeostasis model assessment for insulin resistance, high-sensitivity C-reactive protein, branched-chain amino acids, and lipid profile.
Body weight, trough sitting diastolic blood pressure, and systolic blood pressure were measured as exploratory efficacy assessments at each clinical site.
Urinary biomarker (UACR and urinary protein-to-creatinine ratio) concentrations were measured from the first morning void urine sample collected for 3 consecutive days before the baseline visit (week 0) and the end of treatment (week 12). The first morning void urine sample on the day of the visit was measured at screening and at weeks 4 and 8. The urine biomarker or creatinine ratio was calculated by the central laboratory, as were the eGFR values for each visit using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.20
Safety was assessed using treatment-emergent adverse events (AEs) after randomization identified by investigators based on clinical evaluations, including vital signs (systolic and diastolic blood pressure, pulse rate, and body temperature), 12-lead electrocardiograms, and laboratory tests. The severity (mild, moderate, or severe) and causality of AEs were recorded. AEs were coded using the Medical Dictionary for Regulatory Activities version 22.1.
Data and Statistical Analysis
In this study, 72 participants were deemed sufficient to investigate the 2 different dose levels of SCO-792 compared with the placebo, and the descriptive statistics of each parameter were calculated.
The intent-to-treat population included all randomized participants and was based on the randomized treatment, regardless of which treatment the participant received. The per-protocol population included all randomized participants who received any amount of investigational product, had at least 1 postbaseline efficacy value, and completed the study without any major protocol deviations and was based on the randomized treatment. Safety analyses were performed on the safety analysis set, which included all randomized patients who received at least 1 dose of the study drug.
Efficacy data for participants who received treatment and were withdrawn from the study before week 12 were imputed using the last observation carried forward method. For these participants, the last available value recorded at a protocol-scheduled point was carried forward to the end of the study. No imputation was performed for missing baseline values; only the postbaseline values were carried forward. Baseline data were not carried forward to postbaseline visits. Statistical comparisons of the changes from baseline to the end of treatment were performed on the data obtained from week 12 and on week 12 last observation carried forward data. There was no imputation for any other missing data unless otherwise stated. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
An analysis of covariance model of log-transformed UACR change from baseline with treatment and screening or eligibility UACR (<500, ≥500 mg/gCr) as fixed factors and log-transformed baseline UACR value as a continuous covariate was used to determine treatment differences in SCO-792 versus placebo groups. Geometric least squares mean, 2-sided 95% confidence intervals (CIs), and P-values for change from baseline to the end of treatment were reported for each treatment group. For the treatment difference between each active dose and placebo, the geometric least square mean ratio, 2-sided 95% CIs, and P values were reported. Urine samples collected during menstruation, after extreme dietary changes, after excessive exercise, or when the participant had a fever due to a cold or urinary tract infection were excluded from the UACR analysis.
Results
Characteristics of Study Participants
A total of 153 patients were screened, of which 72 were enrolled after meeting the eligibility criteria and randomly assigned to 1 of the 2 treatment groups (Figure 1). Of these, 65 completed the double-blind study drug administration, and 7 prematurely discontinued the double-blind study drug administration; 3 subjects withdrew because of AEs, and 2 subjects withdrew their consent to participate in the study; the other reasons for study discontinuation were failure to follow-up (1 subject) and death (1 subject, unrelated to the study drug). All participants received at least 1 dose of the study drug and were therefore included in the safety analysis. A total of 56 participants (77.8%) completed the study without major protocol deviations and were included in the per-protocol population set. The demographic and other baseline characteristics were generally balanced among all treatment groups (Table 1).
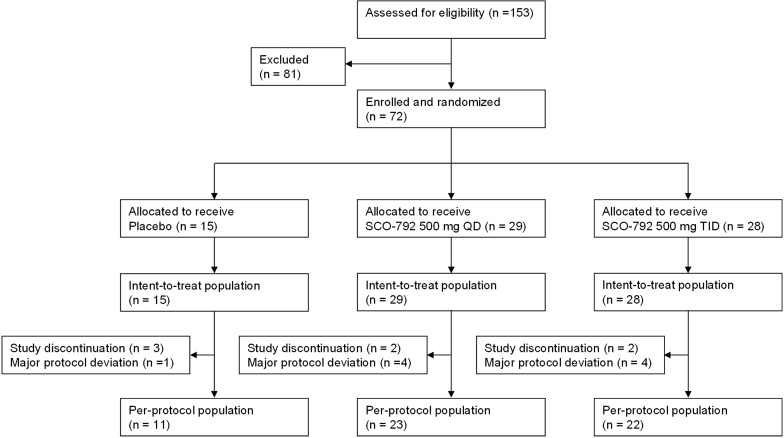
Patient disposition. Of the 153 patients with diabetic kidney disease who satisfied the eligibility criteria, only 72 were enrolled and allocated to 1 of the 3 treatment groups: (i) Placebo, (ii) SCO-792 500 mg once daily, or (iii) SCO-792 500 mg thrice a day. During the treatment period, 16 subjects were removed from the study because of either protocol deviation or treatment discontinuation. QD, once a day; TID, thrice a day.
Table 1
Demographic and other baseline characteristics
| Variable (unit) category | Statistic | Placebo (n = 15) | SCO-792 500 mg QD (n = 29) | SCO-792 500 mg TID (n = 28) | All participants (N = 72) |
|---|---|---|---|---|---|
| Age (yr) | Mean (SD) | 65.1 (9.12) | 59.7 (10.80) | 56.2 (9.93) | 59.5 (10.53) |
| Sex | |||||
| Male | n (%) | 11 (73.3) | 21 (72.4) | 12 (42.9) | 44 (61.1) |
| Female | n (%) | 4 (26.7) | 8 (27.6) | 16 (57.1) | 28 (38.9) |
| Race | |||||
| Asian | n (%) | 2 (13.3) | 3 (10.3) | 0 | 5 (6.9) |
| White | n (%) | 8 (53.3) | 14 (48.3) | 13 (46.4) | 35 (48.6) |
| Native Hawaiian or Other Pacific Islander | n (%) | 2 (13.3) | 9 (31.0) | 10 (35.7) | 21 (29.2) |
| Australian Aborigine or Torres Strait Islander | n (%) | 1 (6.7) | 2 (6.9) | 1 (3.6) | 4 (5.6) |
| Other | n (%) | 2 (13.3) | 1 (3.4) | 4 (14.3) | 7 (9.7) |
| Baseline UACR (mg/gCr) | Geo Mean (Geo SD) | 402.2 (1.97) | 430.0 (2.14) | 378.5 (2.81) | 403.5 (2.35) |
| Baseline UACR group (mg/gCr) | |||||
| ≤300 | n (%) | 5 (33.3) | 11 (37.9) | 13 (46.4) | 29 (40.3) |
| >300–≤1000 | n (%) | 8 (53.3) | 13 (44.8) | 10 (35.7) | 31 (43.1) |
| >1000 | n (%) | 2 (13.3) | 5 (17.2) | 5 (17.9) | 12 (16.7) |
| eGFR (ml/min per 1.73 m2) | Mean (SD) | 66.9 (23.94) | 72.7 (26.43) | 68.1 (23.25) | 69.7 (24.50) |
| eGFR group (ml/min per 1.73 m2) | |||||
| <30.0 | n (%) | 1 (6.7) | 0 | 1 (3.6) | 2 (2.8) |
| 30.0–<45.0 | n (%) | 2 (13.3) | 5 (17.2) | 5 (17.9) | 12 (16.7) |
| 45.0–<60.0 | n (%) | 3 (20.0) | 5 (17.2) | 4 (14.3) | 12 (16.7) |
| 60.0–<90.0 | n (%) | 6 (40.0) | 11 (37.9) | 12 (42.9) | 29 (40.3) |
| ≥90.0 | n (%) | 3 (20.0) | 8 (27.6) | 6 (21.4) | 17 (23.6) |
| HbA1c (%) | Mean (SD) | 7.5 (0.92) | 7.9 (1.21) | 7.8 (1.42) | 7.8 (1.24) |
| Weight (kg) | Mean (SD) | 101.2 (22.14) | 107.8 (23.88) | 107.1 (21.28) | 106.2 (22.37) |
| BMI (kg/m2) | Mean (SD) | 34.9 (7.25) | 37.7 (7.96) | 38.0 (7.51) | 37.3 (7.63) |
| Trough sitting DBP (mm Hg) | Mean (SD) | 82.3 (10.42) | 81.2 (8.29) | 84.4 (7.55) | 82.7 (8.50) |
| Trough sitting SBP (mm Hg) | Mean (SD) | 137.6 (12.13) | 139.8 (16.75) | 144.9 (21.18) | 141.3 (17.87) |
| Concomitant medications | |||||
| Antihyperglycemic agents | n (%) | 15 (100.0) | 29 (100.0) | 28 (100.0) | 72 (100.0) |
| Renin-angiotensin system inhibitors | n (%) | 15 (100.0) | 29 (100.0) | 28 (100.0) | 72 (100.0) |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Geo, geometric; N, total number of participants in the relevant group; n, number of participants in each category; QD, once a day; SBP, systolic blood pressure; TID, thrice a day; UACR, urine albumin-to-creatinine ratio.
All participants received at least 1 AHA and RAS inhibitor (Table 1). The most frequently reported AHAs were metformin hydrochloride (69.4%), gliclazide (20.8%), insulin glargine (18.1%), empagliflozin (11.1%), and metformin hydrochloride/vildagliptin (11.1%) (Supplementary Table S1). Meanwhile, the most frequently reported RAS inhibitor agents were cilazapril (27.8%), candesartan cilexetil (12.5%), quinapril hydrochloride (12.5%), and losartan potassium (11.1%) (Supplementary Table S2).
Safety
Most participants (70.8%) experienced at least 1 treatment-emergent AE, with a frequency comparable across all groups (Table 2). One-third of the participants experienced treatment-emergent AEs associated with the study drug, with similar frequencies reported in the SCO-792 QD and SCO-792 TID groups. No notable dose-related trends were observed among the SCO-792 treatment groups. Overall, 6 participants (1 in the placebo group, 3 in the SCO-792 QD group, and 2 in the SCO-792 TID group) had a total of 7 severe AEs, namely respiratory failure and acute myocardial infarction (both events in 1 participant), renal colic, asthma, pyomyositis, acute cardiac failure, and pneumonia (1 participant each). No severe AEs associated with the study drug were observed. One severe AE (acute myocardial infarction) in the placebo group was fatal. Overall, 6 participants (2 in the placebo group, 3 in the SCO-792 QD group, and 1 in the SCO-792 TID group) experienced treatment-emergent AEs that led to treatment discontinuation. The events in the SCO-792 groups (diarrhea in 3 participants in the SCO-792 QD group and flatulence in 1 participant in the SCO-792 TID group) were considered treatment related. Notably, more participants in the SCO-792 groups (27.6% in the SCO-792 QD group and 32.1% in the SCO-792 TID) experienced diarrhea, which was not observed in the placebo group. Among the investigational product-related diarrhea events, 7 of the 11 participants experienced mild diarrhea, and the remaining 4 experienced moderate diarrhea; no severe diarrhea was reported, and all individuals were able to continue the trial. No significant differences in laboratory or electrocardiogram parameters were observed between the treatment groups.
Table 2
Summary of TEAE, participants who experienced at least 1 TEAE, and TEAE occurring in ≥2 participants in any treatment group (safety analysis set)
| Preferred term | Placebo (n = 15), n (%) E | SCO-792 500 mg QD (n = 29), n (%) E | SCO-792 500 mg TID (n = 28), n (%) E | All participants (N = 72), n (%) E |
|---|---|---|---|---|
| At least one TEAE | 12 (80.0) 30 | 21 (72.4) 81 | 18 (64.3) 50 | 51 (70.8) 161 |
| TEAE occurring in ≥2 participants in any treatment group | ||||
| Diarrhea | 0 | 8 (27.6) 12 | 9 (32.1) 10 | 17 (23.6) 22 |
| Flatulence | 1 (6.7) 1 | 2 (6.9) 2 | 1 (3.6) 1 | 4 (5.6) 4 |
| Frequent bowel movements | 0 | 2 (6.9) 2 | 1 (3.6) 1 | 3 (4.2) 3 |
| Abdominal pain | 0 | 2 (6.9) 2 | 0 | 2 (2.8) 2 |
| Vomiting | 0 | 2 (6.9) 2 | 0 | 2 (2.8) 2 |
| Urinary tract infection | 1 (6.7) 1 | 2 (6.9) 3 | 1 (3.6) 1 | 4 (5.6) 5 |
| Bronchitis | 0 | 0 | 2 (7.1) 3 | 2 (2.8) 3 |
| Upper respiratory tract infection | 0 | 2 (6.9) 2 | 0 | 2 (2.8) 2 |
| Hyperglycemia | 2 (13.3) 2 | 1 (3.4) 1 | 3 (10.7) 4 | 6 (8.3) 7 |
| Fatigue | 1 (6.7) 1 | 2 (6.9) 2 | 0 | 3 (4.2) 3 |
| Edema peripheral | 1 (6.7) 1 | 2 (6.9) 2 | 0 | 3 (4.2) 3 |
| Headache | 0 | 2 (6.9) 2 | 0 | 2 (2.8) 2 |
| Skin abrasion | 0 | 1 (3.4) 2 | 2 (7.1) 2 | 3 (4.2) 4 |
| Muscle spasms | 0 | 2 (6.9) 2 | 1 (3.6) 1 | 3 (4.2) 3 |
AE, adverse event; E, number of TEAEs in each category; n, number of participants with at least one TEAE in each category (participants with multiple TEAEs in each category were counted only once in each category); N, total number of participants in the group; QD, once a day; TEAE, treatment-emergent adverse event; TID, thrice a day.
TEAEs were defined as AEs that commenced or worsened in severity during or after administration of the investigational product. Adverse events were coded using MedDRA version 22.1.
Effect of SCO-792 Treatment on UACR
The predefined setting for UACR analysis excluded urinary albumin values that exceeded the lower limit of assay detection, such as normoalbuminuria, as well as the upper limit of detection. This setting unexpectedly affected the UACR values for the efficacy analysis. A remission to normoalbuminuria is observed in patients with T2DM and microalbuminuria during treatment,21 indicating that predefinition data handling for urinary albumin measurement was unlikely reasonable. Based on the exploratory nature of this study, an additional analysis using lower limit urinary albumin value (when the value shown was below the lower limit) and upper limit urinary albumin value (when the value shown was over the upper limit) was conducted for the calculation of UACR, and the results are presented in Figure 2 and Table 3. The UACR change from baseline decreased following administration of SCO-792 and was sustained for 12 weeks (Figure 2). The geometric least square mean change in UACR from baseline was more evident at 0.73 (–27%; 95% CI 0.56, 0.96) in the SCO-792 QD group and 0.72 (–28%; 95% CI 0.54, 0.95) in the SCO-792 TID group compared with 0.86 (–14%; 95% CI 0.58, 1.27) in the placebo group (Table 3). The UACR reductions from baseline were associated with SCO-792 dosing (P = 0.0271 and 0.0211 for SCO-792 QD and TID, respectively), whereas it was not significant in the placebo group (P = 0.4407 for placebo) (Table 3). In contrast, when compared with the placebo group, SCO-792 QD and TID were not associated with the UACR reductions (geometric least squares mean ratios to placebo in SCO-792 QD and TID groups were 0.85 [95% CI 0.53, 1.37; P = 0.5018] and 0.83 [95% CI 0.52, 1.35; P = 0.4532], respectively; Table 3). The results of per-protocol populations for these parameters are included in Supplementary Figure S2A and Supplementary Table S3. Changes in UACR from baseline to end of treatment obtained using the predefined setting are included in Supplementary Table S4.
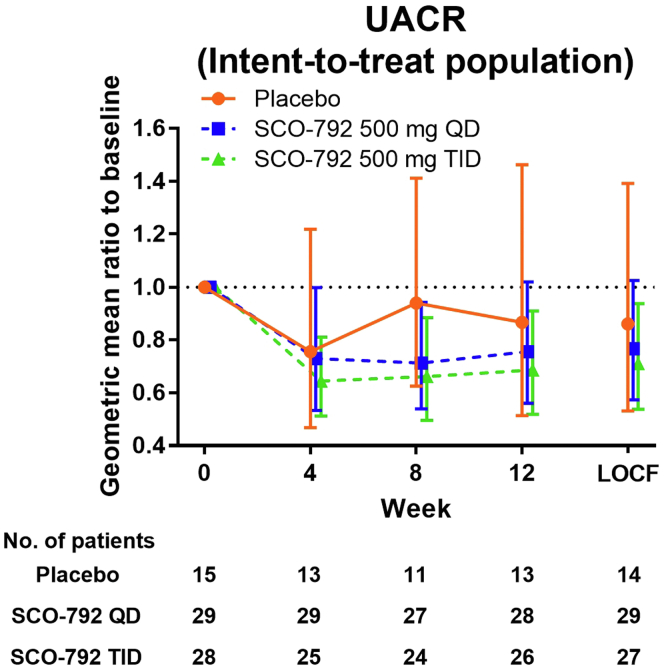
Geometric mean ratio in UACR from baseline. Values are presented as mean ± 95% CI. The number of patients is indicated in the figure. CI, confidence interval; LOCF, last observation carried forward; QD, once a day; TID, thrice a day; UACR, urine albumin-to-creatinine ratio.
Table 3
Treatment differences in change in UACR from baseline to end of treatment
| Statistics | Intent-to-treat population | ||
|---|---|---|---|
| Placebo | SCO-792 500 mg QD | SCO-792 500 mg TID | |
| n | 14 | 29 | 27 |
| Geo LS mean | 0.86 | 0.73 | 0.72 |
| (95% CI) | (0.58, 1.27) | (0.56, 0.96) | (0.54, 0.95) |
| P valuea | 0.4407 | 0.0271 | 0.0211 |
| Geo LS mean ratio (ref. placebo) | 0.85 | 0.83 | |
| (95% CI) | (0.53, 1.37) | (0.52, 1.35) | |
| P valueb | 0.5018 | 0.4532 | |
CI, confidence interval; Geo, geometric; LS, least squares; n, number of participants in each category; QD, once a day; ref, reference; TID, thrice a day; UACR, urine albumin-to-creatinine ratio.
Geometric mean, geometric LS mean diff, 95% CI, and P value were determined using the analysis of covariance model of log-transformed UACR values.
Secondary Outcomes
No marked changes in eGFR from baseline were detected in placebo and SCO-792 groups (−0.2, −0.7, and −0.6 ml/min per 1.73 m2 in placebo, SCO-792 QD, and SCO-792 TID, respectively) (Figure 3, Table 4). The responder rate of ≥30% UACR decreases from baseline to the end of treatment were 20.0%, 24.1%, and 28.6% in placebo, SCO-792 QD, and SCO-792 TID, respectively (Table 4). The results of per-protocol populations for these parameters are included in Supplementary Figure S2B and Supplementary Table S5.
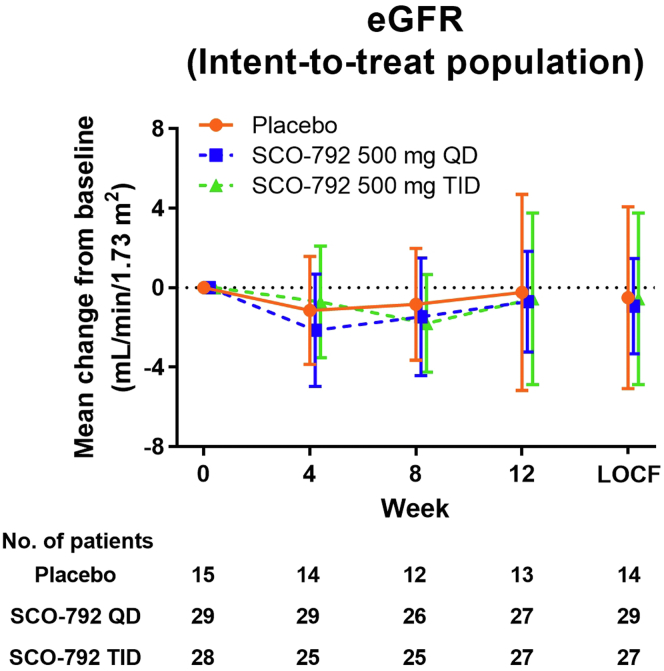
Mean change in eGFR from baseline. Values are presented as mean ± 95% CI. The number of patients is indicated in the figure. CI, confidence interval; eGFR, estimated glomerular filtration rate; LOCF, last observation carried forward; QD, once a day; TID, thrice a day.
Table 4
Changes in secondary and exploratory end points
| Parameters/statistics | Intent-to-treat population | ||
|---|---|---|---|
| Placebo (n = 15) | SCO-792 500 mg QD (n = 29) | SCO-792 500 mg TID (n = 28) | |
| eGFR (ml/min per 1.73 m2) Change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | −0.2 | −0.7 | −0.6 |
| (95% CI) | (−5.2, 4.7) | (−3.2, 1.8) | (−4.9, 3.8) |
| SD | 8.18 | 6.41 | 10.93 |
| Urinary albumin-to-creatinine ratio response rate ≥ 30% reduction | |||
| n | 3 | 7 | 8 |
| % | 20.0 | 24.1 | 28.6 |
| (95% CI) | (4.33, 48.09) | (10.30, 43.54) | (13.22, 48.67) |
| Hemoglobin A1c (%) Change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | 0.38 | −0.24 | −0.51 |
| (95% CI) | (0.15, 0.60) | (−0.61, 0.13) | (−0.79, −0.23)a |
| SD | 0.370 | 0.944 | 0.700 |
| Fasting plasma glucose (mmol/l) Change to week 12 | |||
| n | 11 | 24 | 26 |
| Mean | 0.62 | −0.42 | −0.98 |
| (95% CI) | (−2.59, 3.82) | (−1.56, 0.73) | (−2.05, 0.08) |
| SD | 4.770 | 2.705 | 2.633 |
| Fasting insulin (mU/l) Change to week 12 | |||
| n | 11 | 22 | 26 |
| Mean | 2.54 | −5.75 | 8.14 |
| (95% CI) | (−19.96, 25.03) | (−16.56, 5.06) | (−1.53, 17.82) |
| SD | 33.485 | 24.385 | 23.958 |
| HOMA-IR Change to week 12 | |||
| n | 13 | 25 | 27 |
| Mean | 11.456 | −4.370 | 1.478 |
| (95% CI) | (−15.046, 37.958) | (−12.134, 3.394) | (−1.964, 4.919) |
| SD | 43.8565 | 18.8095 | 8.7003 |
| Body weight (kg) % change to week 12 | |||
| n | 14 | 28 | 27 |
| Mean | 0.990 | −0.363 | −0.202 |
| (95% CI) | (−0.145, 2.125) | (−1.360, 0.633) | (−1.190, 0.786) |
| SD | 1.9657 | 2.5696 | 2.4977 |
| Urinary protein-to-creatinine ratio (mg/gCr) Week 12 | |||
| n | 13 | 28 | 26 |
| Geo Mean | 0.917 | 0.841 | 0.841 |
| (95% CI) | (0.598, 1.408) | (0.653, 1.085) | (0.698, 1.014) |
| Geo SD | 2.0324 | 1.9253 | 1.5868 |
| Systolic blood pressure (mm Hg) Change to week 12 | |||
| n | 14 | 28 | 27 |
| Mean | 7.8 | −3.4 | −5.6 |
| (95% CI) | (1.2, 14.3) | (−8.5, 1.7) | (−9.5, −1.7) |
| SD | 11.34 | 13.18 | 9.90 |
| Diastolic blood pressure (mm Hg) Change to week 12 | |||
| n | 14 | 28 | 27 |
| Mean | −1.1 | −2.7 | −2.1 |
| (95% CI) | (−7.0, 4.7) | (−5.8, 0.5) | (−4.3, 0.2) |
| SD | 10.17 | 8.12 | 5.64 |
| High-sensitivity C-reactive protein (mg/l) Change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | 2.547 | −3.603 | 2.074 |
| (95% CI) | (−3.087, 8.181) | (−9.260, 2.053) | (−1.119, 5.268) |
| SD | 9.3235 | 14.2984 | 8.0736 |
| Triglycerides (mmol/l) % change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | 30.450 | 16.425 | 14.209 |
| (95% CI) | (−3.043, 63.943) | (−4.520, 37.370) | (−1.888, 30.305) |
| SD | 55.4250 | 52.9465 | 40.6903 |
| Low-density lipoproteins (mmol/l) % change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | 6.174 | −0.228 | 25.401 |
| (95% CI) | (−4.159, 16.507) | (−7.488, 7.032) | (−15.807, 66.609) |
| SD | 17.0996 | 18.3530 | 104.1692 |
| High-density lipoprotein (mmol/l) % change to week 12 | |||
| n | 13 | 27 | 27 |
| Mean | 0.315 | 2.036 | −2.223 |
| (95% CI) | (−6.875, 7.505) | (−4.532, 8.604) | (−6.339, 1.894) |
| SD | 11.8981 | 16.6038 | 10.4062 |
| Valine (μmol/l) Change to week 12 | |||
| n | 7 | 21 | 26 |
| Mean | 19.4 | 1.0 | −6.9 |
| (95% CI) | (−40.3, 79.2) | (−30.6, 32.7) | (−33.8, 19.9) |
| SD | 64.60 | 69.55 | 66.49 |
| Leucine (μmol/l) Change to week 12 | |||
| n | 7 | 21 | 26 |
| Mean | 9.0 | −8.5 | −9.1 |
| (95% CI) | (−32.0, 50.0) | (−25.9, 9.0) | (−20.9, 2.8) |
| SD | 44.36 | 38.37 | 29.30 |
| Isoleucine (μmol/l) Change to week 12 | |||
| n | 7 | 21 | 26 |
| Mean | 7.6 | 1.0 | −2.2 |
| (95% CI) | (−20.5, 35.6) | (−9.1, 11.0) | (−10.0, 5.7) |
| SD | 30.33 | 22.14 | 19.38 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; Geo, geometric; HOMA-IR, homeostatic model assessment for insulin resistance; N, total number of participants in the relevant group; n, number of participants in each category; QD, once a day; SD, standard deviation; TID, thrice a day.
HOMA-IR = fasting insulin level (μU/ml) × fasting blood glucose level (mg/dl) / 405.
Exploratory Outcomes
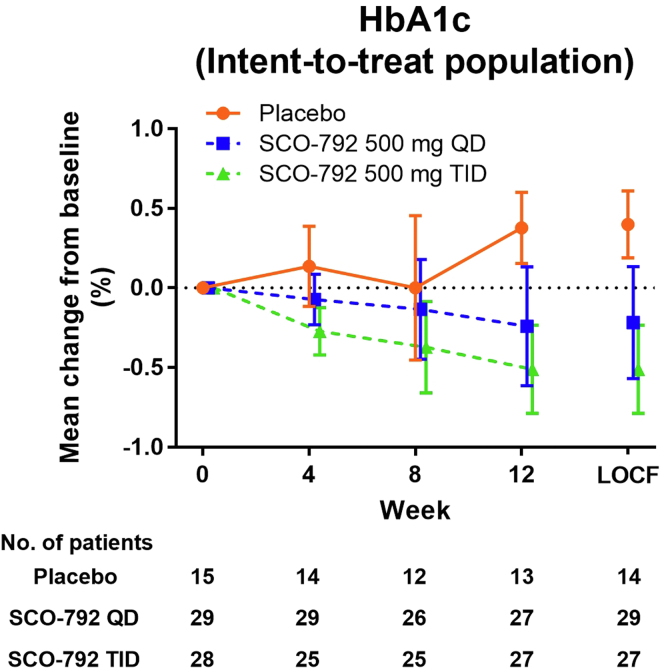
A summary of the results is shown in Table 4. HbA1c levels from baseline were elevated by 0.38% in the placebo group and decreased by 0.24% and 0.51% in SCO-792 QD and SCO-792 TID, respectively (Figure 4, Table 4). SCO-792 TID was associated with decreases in HbA1c levels from baseline (−0.51% [95% Cl –0.79, –0.23]) (Figure 4, Table 4). Associations were not clear for other measurements (Table 4). The results of per-protocol populations for these parameters are included in Supplementary Figure S2C and Supplementary Table S5.
Discussion
To our knowledge, this is the first study to report the clinical profile of SCO-792 in patients with T2DM and albuminuria. SCO-792 administration for 12 weeks was safe and well tolerated and may decrease UACR from baseline in patients with T2DM and albuminuria receiving stable AHA and RAS inhibitor therapies. Further clinical studies are essential to confirm our findings given the limitations of this study, including inadequate statistical power.
Based on the current results, SCO-792–induced enteropeptidase inhibition is likely associated with UACR reduction from baseline in patients with T2DM and albuminuria. Since the 2 SCO-792 regimens, QD and TID, induced a similar magnitude of reduction in UACR from the baseline, SCO-792 QD is likely sufficient to induce this effect. In this exploratory study, a smaller number of patients were enrolled in the placebo group, in which we unexpectedly observed a large variation in the UACR change from baseline to the end of the study. The limited numbers of patients included in the exploratory study may explain the lack of statistical differences in UACR change between the SCO-792 and placebo groups. In addition, the magnitude of the therapeutic effect of SCO-792 on UACR change is unclear. Further clinical studies are therefore warranted to elucidate the therapeutic potential of SCO-792 with respect to UACR changes in patients with T2DM and albuminuria.
Gastrointestinal disorders, including diarrhea, were observed in the SCO-792 group. Because SCO-792 increases fecal protein levels,17 inhibiting protein digestion inside the gut might have contributed to this event.
The decrease in HbA1c was associated with SCO-792 TID dosing, suggesting the potential use of this drug for the treatment of hyperglycemia in patients with DKD. The efficacy of SCO-792 TID was superior to that of SCO-792 QD, suggesting that TID-induced sustained inhibition of enteropeptidase may have a therapeutic benefit in treating diabetes in patients with T2DM and albuminuria. SCO-792 has been reported to inhibit gut amino acid intake and decrease blood glucose levels by improving insulin resistance in preclinical diabetes models.17,18 Plasma amino acids induce insulin resistance in humans22,23; thus, the improvement of these glycemic parameters observed with SCO-792 dosing might have been attributed to the inhibition of gut amino acid intake.
Although the average fasting plasma levels of valine, leucine, and isoleucine were lower after SCO-792 treatment, this change was not conspicuous compared with the results from a preclinical study, wherein SCO-792 highly decreased plasma amino acid levels post oral protein loading in rats.16 In addition, we did not observe an initial drop in eGFR, generally seen in drugs that improve glomerular hyperfiltration,24 after treatment initiation of SCO-792. In this study, blood samples for laboratory tests were collected after more than 8 hours of fasting. An infusion of amino acids is known to induce glomerular hyperfiltration in humans.10 Considering that SCO-792 inhibits gut lumen enteropeptidase, thereby inhibiting postprandial amino acid elevation in blood, SCO-792–induced effects on blood amino acids and eGFR may be more evident in postprandial conditions, which remains to be confirmed.
Roux-en-Y gastric bypass, which bypasses enteropeptidase-expressing duodenal function, can help to improve kidney function and metabolic control in humans.25 Considering the similarity between Roux-en-Y gastric bypass and enteropeptidase inhibition, both of which inhibit gut amino acid intake,17,26 the therapeutic benefits of SCO-792 observed in this study may also be attributed to this mechanism.
This study has some limitations. Based on its exploratory nature, this study included a limited number of patients over a relatively short period, which likely decreased the detection power of SCO-792–induced differences. To demonstrate conclusive therapeutic effects on kidney parameters in patients with DKD, longer study durations with a larger number of patients are required. UACR reduction of 30%, or eGFR slope reduction by 0.5 to 1.0 ml/min per 1.73 m2 per year, shows clinical promise for advancing to outcome trials that will demonstrate the renoprotective effect of SCO-792,27 and these studies should be considered for the development of this compound.
In conclusion, our study demonstrated that treatment with up to 1500 mg/day SCO-792 in patients with T2D and albuminuria receiving stable AHA and RAS inhibitor therapy was safe and well tolerated for 12 weeks. SCO-792 may be associated with decreased UACR from baseline in patients with T2DM and albuminuria. Taken together, these results suggest SCO-792 as a potential treatment option for DKD, although further clinical studies are essential to confirm our findings.
Disclosure
TK, JS, HN, YM, and MW are/were employees of SCOHIA PHARMA Inc. No other potential conflicts of interest relevant to this article are reported.
Acknowledgments
The authors thank the patients, all investigators who participated in the study, and the IQVIA Inc. staff. The authors also thank Takuya Saiki for discussions. The trial is registered at JapicCTI (study number JapicCTI-205109).
Funding
This trial was funded by SCOHIA PHARMA, Inc.
Support
Implementation of the study (development of monitoring, data management, and statistical analysis) was performed by IQVIA Inc. based on an outsourcing contract with SCOHIA PHARMA Inc.
Data Sharing
The data underlying this article are available in the article and online supplemental material, and no additional data are available. The reagents presented in this study are available on reasonable request under the Material Transfer Agreement.
Author Contributions
TK, JS, HN, YM, and MW contributed to study design. TK and HN collected data. TK, JS, HN, YM, and MW analyzed and interpreted data. All authors contributed important intellectual content during manuscript drafting or revision and agreed to be personally accountable for their own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even those in which they were not directly involved, were appropriately investigated and resolved.
Footnotes
Figure S1. Study design.
Figure S2. UACR, eGFR, and HbA1c (per-protocol population).
Table S1. Concomitant medications in AHA.
Table S2. Concomitant medications in RAS inhibitors.
Table S3. Treatment differences in change in UACR from baseline to end of treatment (per-protocol population).
Table S4. Changes in UACR from baseline to end of treatment (predefined setting).
Table S5. Changes in secondary and exploratory end points (per-protocol population).
Supplementary Material
Figure S1. Study design
Figure S2. UACR, eGFR, and HbA1c (per-protocol population)
Table S1. Concomitant medications in AHA
Table S2. Concomitant medications in RAS inhibitors
Table S3. Treatment differences in change in UACR from baseline to end of treatment (per-protocol population)
Table S4. Changes in UACR from baseline to end of treatment (predefined setting)
Table S5. Changes in secondary and exploratory end points (per-protocol population)
References
Articles from Kidney International Reports are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ekir.2022.10.006
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9831944
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/137141671
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enteropeptidase inhibitor SCO-792 effectively prevents kidney function decline and fibrosis in a rat model of chronic kidney disease.
Nephrol Dial Transplant, 36(4):631-640, 01 Mar 2021
Cited by: 3 articles | PMID: 33351150 | PMCID: PMC8008362
Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial.
Lancet Diabetes Endocrinol, 5(8):610-621, 27 Jun 2017
Cited by: 175 articles | PMID: 28666775
Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial.
Lancet Diabetes Endocrinol, 9(11):755-766, 04 Oct 2021
Cited by: 66 articles | PMID: 34619106
Effect of Intensive Urate Lowering With Combined Verinurad and Febuxostat on Albuminuria in Patients With Type 2 Diabetes: A Randomized Trial.
Am J Kidney Dis, 77(4):481-489, 29 Oct 2020
Cited by: 27 articles | PMID: 33130235 | PMCID: PMC8045740

 and
and