Abstract
Free full text

Diagnosis and management of CF exacerbations
Abstract
With the improving survival of cystic fibrosis (CF) patients and the advent of highly effective cystic fibrosis transmembrane conductance regulator (CFTR) therapy, the clinical spectrum of this complex multi-system disease continues to evolve. One of the most important clinical events for patients with CF in the course of this disease is an acute pulmonary exacerbation. Clinical and microbial epidemiology studies of CF pulmonary exacerbations continue to provide important insight into the disease course, prognosis, and complications. This work has now led to a number of large-scale clinical trials designed to clarify the treatment paradigm for CF pulmonary exacerbation. The primary goal of this review is to provide a summary and update of the pathophysiology, clinical and microbial epidemiology, outcome and treatment of CF pulmonary exacerbations, biomarkers for exacerbation and the impact of highly effective CFTR therapy (HEMT) on these events moving forward.
Cystic fibrosis (CF) is the most common life shortening inherited disease in Caucasians and affects approximately 30,000 individuals in the U.S.1 Advances in care for individuals with CF have resulted in dramatic improvements in survival, but people with CF (PWCF) still experience significant morbidity and premature mortality.2,3 Acute pulmonary exacerbations may result in permanent loss of lung function, worse quality of life, and shortened survival.4–8 Pulmonary exacerbations (PEx) are frequent and typically require a multifaceted approach including the use of antibiotics, given in various combinations of systemic and inhaled routes9.
Pathophysiology of CF Pulmonary Exacerbation
Despite our detailed understanding of the molecular basis of disease progression in CF, relatively little is known about the pathophysiology of CF exacerbations. Exacerbations present clinically with changes in cough, sputum purulence, volume and viscosity, dyspnea, fatigue, anorexia, weight loss, and spirometric decline. They reflect acute imbalances in the complex interplay between airway infection, chronic inflammation and CFTR dysfunction that culminate in muco-obstruction. Viral infections, including respiratory syncytial virus (RSV), may play a role in the initiation of these events10–12 although data regarding the impact of vaccination against viral infection are limited.13,14 Primarily, however, pulmonary exacerbations are associated with a change in the bacterial density of colonizing flora, in particular Pseudomonas aeruginosa.15,16 In affected PwCF, bacterial concentrations of Pseudomonas are high during an exacerbation and decrease with treatment; and treatment with antimicrobial agents reduces symptoms and improves lung function.15–17 Current data suggest that the majority of exacerbations are not due to acquisition of new strains of pathogen (i.e. Pseudomonas), but potentially a clonal expansion of existing strains.18 Aaron and colleagues have previously shown that among 80 individuals followed for 2 years with quarterly sputum cultures, 40 patients experienced a pulmonary exacerbation.18 Only 36 had isolates that could be genotyped and among those, only two subjects demonstrated acquisition of a new clone during exacerbation that had not been present during a period of clinical stability. Despite clear increases in airway inflammation, anti-inflammatory therapeutics have had limited effect on exacerbation rates in randomized controlled trials and observational studies.19–24
Definitions/diagnosis
Despite calls for a consensus diagnosis of pulmonary exacerbations by a CF Outcomes Group in 1994, no consensus diagnostic criteria exist.25 A prior review highlighted prior definitions employed26 – to date most clinical trials have employed the definition of Fuchs’ criteria, which were originally developed for trials of rhDNase.27 Other diagnostic tools include the Acute Respiratory Illness Checklist (ARIC)14 and the Respiratory and Systemic Symptoms Questionnaire (RSSQ©) 28 but neither has been widely adopted. Current definitions vary, but generally combine patient reported symptomatology, laboratory data, spirometry and clinical gestalt with the addition of a physician decision to treat making the diagnosis event-based. Components of these definitions have been examined to see which clinical characteristics best predict a pulmonary exacerbation.29–31 - symptoms rather than physical examination and laboratory values were found to be more predicted of a PEx. Given lack of a consensus definition of these events, it is not surprising that patterns of treatment of pulmonary exacerbation continue to vary.32
In addition to the aforementioned symptoms, severe drops in lung function may be accompanied by hypoventilation, hypoxemia and respiratory failure. Like the overall definition, no universally accepted gradation of exacerbation exists. What is clear is that severe events – in particular those requiring intensive care unit admission – are associated with a high one year mortality, ranging from 32% to upwards of 50%.33–36 Multivariate predictors of mortality from such an event include annual decline in FEV1, simplified acute physiology score II, and the use of invasive mechanical ventilation. Improved outcomes have been recently noted using non-invasive oxygen and bi-level ventilation.37 More recent data suggests that the mortality for CF patients requiring mechanical ventilation remains high but is decreasing.38
CF Pulmonary Exacerbation as a predictor and outcome variable
The annual rate of CF pulmonary exacerbation has clearly been associated with 2 year and 5 year survival in two separate prediction models evaluating the odds of death during follow-up in the US and recent models from the French and Canadian/UK registries.4,5,39–43 In a recent publication, the addition of PEx in CF has added substantively to the lung allocation score in transplant eligible CF patients in the US.44 CF pulmonary exacerbations requiring intravenous antibiotics have also been associated with later diminished lung function in children ages 1 to 6 years6, with CF related diabetes45, and with sleep disturbances and health related quality of life.7,8 Lastly, PEx rate has been an important marker of disease severity and as such has been used as an adjustment variable in studies looking at survival46,47, a study inclusion criterion48 and an important outcome measure when assessing the impact of socioeconomic status and environmental exposure on CF.49,50 PEx rate has also been used as an important variable to assess novel outcome measures like high resolution computed tomography (CT) of the chest or cough frequency.51,52 Improvements in PEx profile have been central to regulatory approval of a number of key therapies.17,27,53–55
Epidemiology of CF exacerbation and the Impact of CFTR Modulators
The incidence of PEx appears to be relatively constant over the life of a CF patient, but antibiotic treatments change as patient airway infections become more complex and lung disease advances.56 In adolescents and adults, the proportions of PEx that are treated with intravenous (IV) antibiotics steadily increases.56,57 Several studies have shown that in approximately 25% of exacerbations, patients do not return to within 90% of their baseline lung function following treatment for the exacerbation (Figure 1).58,59 One factor associated with poor response to exacerbation treatment may be longer time from symptom onset to exacerbation treatment; suggesting that delayed treatment results in worse treatment outcomes. There is also evidence suggesting that CF centers that see patients more frequently and treat patients more aggressively, e.g. more antibiotic use, have better clinical outcomes.32,60 PEx are also associated with more rapid lung function decline and increased healthcare costs.61 A recent secondary analysis of the Standardized Treatment of Pulmonary Exacerbation Study (STOP2) revealed that total costs were driven by duration of therapy in the trial where subjects had primarily shorter hospital stays.62,63
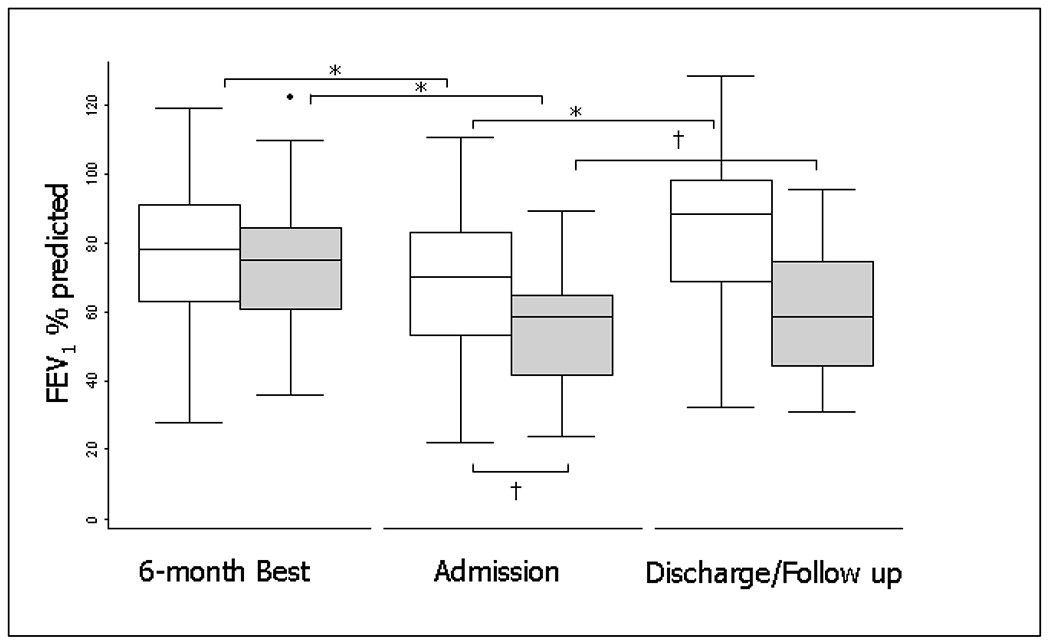
FEV1 for nonresponders and responders at baseline, treatment initiation, and the best FEV1 in the 3 months after treatment (n = 4,391). Responders are in white, nonresponders are in gray. The boxes represent the middle 50% of patients; the whiskers include all patients in each group. The horizontal line within the box represents the median FEV1. The x axis represents three time points: the best FEV1 in the 6 months before admission (baseline); FEV1 at treatment initiation (exacerbation); and the best FEV1 in the 3 months after treatment (follow-up). Reprinted with permission from the AJRCCM.58 * represent P<0.05
Pulmonary exacerbation in small children
PEx’s are also meaningful events in young children.64 Frequent PEx’s, especially in the first two years of life are associated with decreased spirometry (FEV1) at age 5; PEx’s treated with IV antibiotics in small children are associated with the development of bronchiectasis and decreased weight-for-age at 5 years. The challenge in CF infants and toddlers with exacerbations is that the frequency of PEx’s is very similar to the frequency of viral upper respiratory tract infections that occur in healthy children without CF.65 Children with CF are more likely to have prolonged viral infections of greater severity, so these event are intrinsically linked.66 Furthermore, the presence of rhinovirus (RV)67 and RSV68 may enable Pseudomonas to more easily infect airway epithelial cells from patients with CF. Despite recent advances in CF care, preventing exacerbations early in life remains a top priority. The role of hypertonic saline in achieving this was recently clarified by the recent Infant Study of Inhaled Saline in Cystic Fibrosis (ISIS), where it failed to decrease the rate of PExs in children ages 4-60 months with CF.69 However, patients who received inhaled hypertonic saline – and who could perform spirometry adequately – had a significantly larger mean improvement in forced expiratory volume in 0.5 seconds.69 Early introduction of azithromycin when added to inhaled tobramycin as part of a has been shown to decrease exacerbations in children infected with Pseudomonas; however, the effect of this combination on Pseudomonas eradication remains unclear.70
Given that spirometry cannot be reliably performed on very young children, alternative measures of restoration of lung function such as lung clearance index (LCI) have been explored. LCI involves a multiple-breath washout (MBW) and has been shown to be repeatable, reproducible, and sensitive in detecting the presence of lung disease in children with CF as young as 4 months of age.71 LCI in 3-5 year-olds with CF is also predictive of future LCI at 6-10 years of age.72
In recent studies, LCI has been used to evaluate changes in lung function during and after therapy for PEx. Baseline LCI has been shown to be a predictor of subsequent PEx in children with CF, including subgroups of children with normal spirometry.73 Prior studies have shown significant improvement in LCI (decrease) following antibiotic treatment of PEx and with symptom resolution, suggesting that LCI may be a promising tool to assess PEx treatment response.74 Additionally, LCI may be a more sensitive index than FEV1 to evaluate treatment response in IV antibiotic therapy for PEx.74 A systemic review of seven studies evaluating LCI in PEx showed LCI response to therapy for PEx but study results were heterogenous and LCI was discordant with FEV1 in a few studies.75 More recent data, however, does confirm the LCI association with pulmonary exacerbations, showing that worsening LCI is associated with PEx as well as incomplete recovery on follow up visits in school age children.76 These findings were confirmed in a recent study where LCI worsening corresponded with PEx as well as bacterial infections in patients with CF.77 While LCI may be a promising clinical tool for monitoring patients with CF, the minimal clinically important difference in LCI is not yet clear.
Microbiologic diagnosis
Chronic bacterial airway infections are characteristically seen in the majority of individuals with CF. These infections are commonly polymicrobial and are only rarely eradicated fully by antimicrobial therapy. Knowledge of the natural history of colonization and infection can be helpful in the management of CF PEx. Culture of respiratory tract specimens from individuals with CF can present challenges to microbiology laboratories unaccustomed to processing them, because of problems related to sample viscosity, the polymicrobial nature of infections, and slow bacterial growth.
Polymicrobial infections are the norm in CF airway infections and can be problematic since the organisms in the specimen may have very different growth requirements. Pseudomonas aeruginosa is often present and, because of its mucoid phenotype, frequently overgrows both Gram-positive bacteria such as Staphylococcus aureus and more fastidious or slower-growing Gram-negative organisms such as Haemophilus influenzae and Burkholderia cepacia complex. The use of selective media, which inhibits the growth of P. aeruginosa, is very useful for the isolation of S. aureus and H. influenzae and is mandatory for the isolation of B. cepacia complex.78–81 In addition, multiple subcultures may need to be performed in order to isolate pure bacterial cultures for identification and susceptibility testing. Slow bacterial growth also requires that culture plates receive prolonged incubation. Laboratories specializing in CF microbiology frequently use incubation times of 48 hours for cultures expected to yield P. aeruginosa, and up to 96 hours before reporting a culture negative for B. cepacia complex.80
Microbiologic sampling
Sampling of lower airway secretions is considered essential for determining the infectious etiology of PEx in CF. This is most readily accomplished using expectorated sputum. However, some individuals with CF are unable to expectorate. In addition, with the advent of highly effective CFTR modulators, expectoration has been noted to decrease substantially.82 Oropharyngeal swabs have served as a surrogate but may not be representative of lower airway infection83,84; oropharyngeal cultures for P. aeruginosa had a sensitivity of 44% and a specificity of 95%84. H. influenzae was similar, but the specificity was significantly lower for S. aureus. Oropharyngeal swabs obtained after chest physiotherapy were found to have increased sensitivity and specificity for the detection of both P. aeruginosa and S. aureus compared with swabs obtained prior to physiotherapy.85 Hypertonic saline induction of sputum has been reported to be a good surrogate for lower airway sampling in CF.86,87 Several studies suggest that induced sputum may be more sensitive in detecting bacteria in the lower airway compared with expectorated sputum and even bronchoalveolar lavage.87–89 Sputum induction has been used to monitor both inflammation and infection after intravenous antibiotic therapy for pulmonary exacerbations in CF.90
Antibiotic resistance and choice of antibiotics
Susceptibility testing of CF isolates of P. aeruginosa is difficult, for many of the same reasons that impact organism isolation and identification. Slow growth and mucoidy may impact the utility of automated systems for susceptibility testing of P. aeruginosa as well as for organism identification.91,92 When compared with broth microdilution methodology, agar diffusion methodologies including disk diffusion (Kirby-Bauer) and Etest performed well for the majority of antibiotics tested.93
Early infections with P. aeruginosa are commonly susceptible to anti-pseudomonal β-lactam antibiotics, aminoglycosides and fluoroquinolones. However, antibiotic resistance increases with age.94 Multiple antibiotic resistance, defined as in vitro susceptibility to only a single class of antimicrobial agents, has been reported in up to 11.6% of P. aeruginosa isolates from individuals with CF in the United States and up to 17.4% in Italy.81,95 Unfortunately, in patients with polyresistant Pseudomonas isolates, synergy testing and multiple combination bactericidal testing (MCBT) has not been demonstrated to improve clinical or microbiological outcomes compared to usual susceptibility testing.96,97 Interestingly, even standard susceptibility testing has not been clearly demonstrated to improve patient outcome noted by evaluation of observational data.98 Moreover, more elaborate testing of P. aeruginosa grown in biofilms also failed to have superior impacts on lung function in two separate studies.99,100
A recent systematic review of antimicrobial susceptibility testing in CF by the Antimicrobial Resistance in Cystic Fibrosis International Working Group101 concluded that there is little evidence that antibiotic susceptibility testing “predicts the clinical outcome of CF antimicrobial treatment, suggesting a need for careful consideration of current AST use by the CF community.”
B. cepacia complex organisms are often highly antibiotic resistant. All are intrinsically resistant to the aminoglycosides102 and the rate of in vitro resistance to the β-lactam antibiotics, with the exception of meropenem, is also quite high.103,104 The quinolones have variable activity, but resistance can be readily induced.103 In vitro susceptibility testing suggests that there are combinations of antibiotics that act synergistically against B. cepacia complex using either synergy testing or MBCT.104,105 Synergy testing, using two drug combinations, found that for 57% of isolates tested, no active combination could be identified.105 The most active combinations were chloramphenicol plus minocycline (49% of isolates) and chloramphenicol plus ceftazidime (26% of isolates). MBCT testing using two or three drug combinations determined that at least one combination could be identified for all isolates tested.106 The majority of active combinations included meropenem. It was not possible to predict for a given isolate whether a drug combination would be synergistic, additive or antagonistic.
Other antibiotic resistant Gram-negative CF isolates include S. maltophilia and A. xylosoxidans. Treatment of these organisms is often complicated by resistance to the aminoglycosides and variable susceptibility to the β-lactams and quinolones. The most active single drugs in vitro against S. maltophilia are ticarcillin/clavulanate and trimethoprim/sulfamethoxazole; the most active combination in synergy studies is ticarcillin/clavulanate plus aztreonam.107 In a study of 106 CF isolates of A. xylosoxidans, the most active drugs were imipenem (59% susceptible), piperacillin/tazobactam (55%), meropenem (51%) and minocycline (51%).108 The most active additive or synergistic combinations were chloramphenicol plus minocycline, ciprofloxacin plus imipenem, and ciprofloxacin plus meropenem.
Use of combination IV aminoglycoside and β-lactams to treat Pseudomonas
Use of two antipseudomonal agents was originally rationalized as reducing emergence of acquired antimicrobial resistance and/or providing a potential for synergy of action,109 but the former has not been routinely observed among the few clinical trials comparing antipseudomonal monotherapies to combination therapies110–114 and in vitro data suggest that the latter occurs in a small proportion of instances and is dependent on the agent combinations.115 In fact, some randomized trials have reported that combination antipseudomonal therapy was associated with increased antimicrobial resistance112,114 and if/when synergy occurs, it has not been adequate to be associated with a detectable difference in mean clinical responses.111–114 In addition, there are no data to suggest that selecting antimicrobial combinations with greater in vitro efficacy is associated with improved PEx treatment response;116 these data have justified recommendations for a reduced emphasis on in vitro antimicrobial synergy testing for choosing CF antimicrobial treatments.96,117
Although multiple studies have compared two versus one antibiotic to treat Pa infection, no consensus algorithm is available. As noted above, a systematic review was unable to make a strong recommendation in regards to two antipseudomonal agents to treat PEx events.118 Today, justification for β-lactams combined with an aminoglycoside includes leveraging the post-antibiotic effect of aminoglycosides, reduction of development of resistant Pa strains (still in the absence of supportive data) and delaying the time to next PEx. Pa strains in CF sputum specimens can have very heterogeneity resistance, and this can arise spontaneously in clonal bacterial cultures (e.g. from high-frequency mutations), or can come from pre-existing variants in single or mixed-strain infections.119 Investigators have showed that Pseudomonas aeruginosa from CF patients exhibit marked population-level resistance heterogeneity.120–123 Combination therapy has not shown a benefit with respect to standard clinical endpoints – resolution of symptoms and improvement in lung function have not demonstrated superiority.111–113 One trial has had an outsized impact in demonstrating the value of adding an aminoglycoside to the treatment regimen. Smith et al. randomized 76 PwCF to either azlocillin/placebo or azlocillin/tobramycin. They found reduced Pa density in the sputum of people treated with the tobramycin regimen but no difference in any other outcome 26 days after treatment; however they noted a reduced time to next PEx rate in the azlocillin group compared to azlocillin/tobramycin.114 Unfortunately, that study did not stratify by prior-year PEx number at randomization and the associated time-to-next PEx analysis did not include adjustment for covariates known to be associated with future PEx hazard, including sex, lung disease stage, number of prior-year PEx, and other CF comorbidities (allergic bronchopulmonary aspergillosis, gastric reflux, CF-related diabetes, and liver disease).124 For this reason, when formally evaluated in a systematic review, the number of antibiotics chosen (commonly two for Pa – primarily β-lactam plus aminoglycoside) received a grade of insufficient data to make a recommendation in CF guidelines.118
Overall approach to Treatment
A recent systematic reviewed evaluating the management of PEx found insufficient evidence for most treatment decisions, including those related to antibiotic prescribing.118 STOP2 (see detailed description below) has provided convincing evidence that, in adult PwCF with early treatment improvement during exacerbation, spirometric improvement after 10 days of IV antibiotics is non-inferior to 14 days.63 For those with less improvement after one week, 21 days is not superior to 14 days (Figure 2a, ,2b2b and and2c2c).63 Additional key aspects of PEx treatment approaches other than antibiotics include identifying and monitoring CF related diabetes, frequent airway clearance, nutritional support and reducing risks of new organism acquisition if hospitalized. The role of oral steroids in pulmonary exacerbation remains unknown. In a recent US multicenter observational study of 220 CF adolescent and adults treated with IV antibiotics, 18.2% were treated with oral steroids for the exacerbation and 20% were treated with mucolytic agents.125 The only randomized controlled trial (RCT) of prednisone as an adjunct to treatment for as PEx demonstrated a non-statistically significant improvement in lung function, but no clear improvement in symptoms or sputum inflammatory markers compared to placebo.126 The STOP2 study included steroid use as a stratification variable to reduce the potential to large RCT. Steroids were used in 9.3% to 12.1% of each treatment arm. An ongoing secondary analysis is evaluating the potential impact of steroids on clinical outcome. A largescale RCT is also underway in Canada to address the role of prednisone in patients with PEx who have do not have not recovered to their baseline lung function with standard treatment by day 7 (NCT03070522).
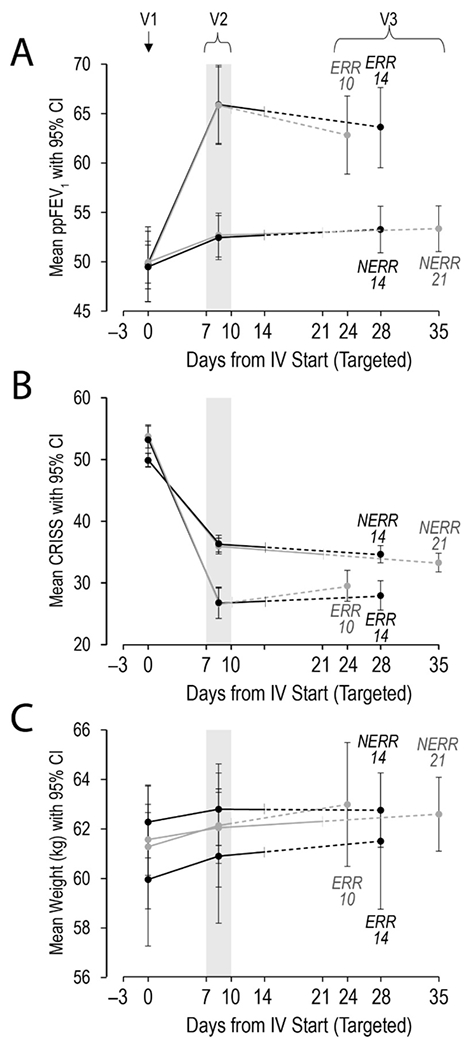
STOP2 study outcomes by Visit, allocation and antimicrobial treatment duration: A) primary outcome ppFEV1 B) Chronic Respiratory Infection Symptom Score (CRISS), C) weight. V1=Visit 1at start of IV antimicrobials; V2=Visit 2 when randomization occurs, V3=Visit 3 was targeted for 14 days after scheduled end of IV antimicrobial treatment. Treatment arms are the Early Robust Responders (ERR) and Non-Early Robust Responders (NERR). Treatment response was assessed at day 7-10 of IV antibiotic treatment and the cut point to determine ERR was ≥ 8% improvement in FEV1 and ≥ 11 points on the CRISS. Reprinted with permission from the AJRCCM.63
In one recent study of exacerbation, delineating the complexity of antibiotics used in a prospective multi-center cohort of 220 CF adolescents and adults, 51.8% received 2 IV antibiotics and 32.7 received 3 or more IV antibiotics.125 IV Tobramycin was most commonly used (in 59.5% of the cases) with meropenem, piperacillin/tazobatam/ceftazidime and vancomycin all prescribed in about 25% of the patients. Over 25% of patients also received an oral antibiotic with only 9% receiving an inhaled antibiotic. To address the question of whether multiple antipseudomonal antibiotics are required for PEx in which Pa is implicated, vanDevanter and colleagues retrospectively studied treatment responses for STOP2 participants with a history of Pa infection, with sensitivity analyses limited to the most common one-, two-, and three-class regimens, to only IV/oral antipseudomonal treatments, and with more stringent Pa infection definitions applied. The investigators did not find evidence of additional benefit when multiple antipseudomonal classes are used to treat PEx in Pw CF and Pa.The role of inhaled antibiotics for treatment of PEx is not fully clear, but early data are encouraging. In a recent open label RCT, inhaled aztreonam lysine was substituted for IV antibiotic in the treatment of an acute PEx in 16 patients.127 The combination of inhaled antibiotics with IV antibiotics significantly improved lung function and symptom score despite the small sample size. This study suggests that a larger trial is warranted.
Regarding dosing intervals, daily vs three times a day aminoglycosides has been studied in RCTs. The best known trial is the TOPIC study; this study reported equal efficacy between once and three-times daily tobramycin given with ceftazidime, with a trend to less nephrotoxicity in CF children.128 Based on this study, many centers now use one daily IV tobramycin. The use of IV gentamycin has markedly decreased since an observational study in the UK suggested increased toxicity compared to tobramycin.129 Another key topic of dosing of antibiotics in CF PEx has been the use of continuous infusions of beta lactams, combination penicillins/beta-lactamase inhibitors (i.e. piperacillin/tazobactam), or aminoglycosides.130–132 The rationale has been to optimize pharmacokinetic/pharmacodynamic (PK/PD) properties. Despite the PK/PD properties, studies to date have not been powered to demonstrate clinical superiority to routine dosing.
Treatment and treatment response
Several cohort prospective studies have clearly delineated the treatment response to IV antibiotics in CF PEx. These newer studies support earlier studies that note that an acute drop in pulmonary function is highly associated with the diagnosis and treatment of PEx31 and that treatment with IV antibiotics has been shown to result in improved lung function in CF patients experiencing PEx.15 Sagel et al. enrolled 103 patients and collected clinical data (symptom scores, spirometry) and inflammatory markers; 84% recovered at least 90% of their baseline FEV1 within 3 months of the exacerbation (see biomarker section below).133 An extension of this cohort study (n=123) demonstrated that 33% experienced <10% relative improvement in FEV1 during treatment. Symptom improvement was observed but was not associated with subsequent lung function or time to next antibiotic therapy, which had a median recurrence time of 143 days.134 More recently, the Standard Treatment of Pulmonary exacerbation (STOP) study prospectively enrolled 220 CF subjects admitted to the hospital for treatment of a PEx to evaluate the variability of treatment durations and to identify the clinical outcome measures deemed most important to care-givers in determining treatment success.125,135–137 these data show that respiratory symptoms as measured with the Cystic Fibrosis Respiratory Symptom Diary Chronic Respiratory Infection Symptom Scale (CFRSD-CRISS) and lung function typically improve with IV treatment but later declines. Of note, other patient reported outcome measures such as CFQ-R can be used to assess response to treatment of a pulmonary exacerbation.138 These outcomes have been replicated in the STOP2 trial noting consistent improvement in both lung function and symptoms with treatment.63
Duration of treatment for a pulmonary exacerbation
The most common duration used to treat exacerbations is 14 days but there is considerable variance, including 10% of patients receiving >23 days. Systematic reviews of the literature found there was insufficient evidence upon which to develop guidelines for most treatment decisions, including antibiotic selection and duration139 and there is little evidence of additional clinical benefit in registry analyses.140,141 Studies of PEx’s assessing response to treatment have shown that lung function improves up to about day 8-10 with little additional improvement with longer treatments.142 A recent single-center retrospective study demonstrated that some patients continue to experience improvement in symptoms and FEV1 after cessation of treatment with longer antibiotic courses143, but it is not clear that this improvement was related to extending treatment, or that similar improvements would not have been observed with shorter treatment durations. The total duration of IV antibiotic therapy for PEx has large implications on clinical outcomes, but also increased resource utilization, treatment burden, and potential for toxicity associated with extended treatment.
An acute drop in pulmonary function is highly associated with the diagnosis and treatment of PEx31, and treatment with IV antibiotics has been shown to result in improved lung function in CF patients experiencing PEx.15 In 2012, 35.1% of patients of all ages followed in the Cystic Fibrosis Foundation Patient Registry (CFFPR, 9,516 patients) were treated at least once with IV antibiotics for PEx, with the median number of IV antibiotic treatment days per PEx varying greatly by CF care center, from 3 days to 24.2 days (Figure 3).48,144 There are two clear peaks in duration of antibiotic duration – 14 and 21 days but very dramatic variation across US centers.48 Overall, in the CFFPR in these years, the median IV antibiotic treatment duration was 13.5 days for children and 14.5 days for adults (≥ 18 years).144 Unfortunately, it has been estimated that as many as a quarter of patients with PEx treated with IV antibiotics fail to return to even 90% of the lung function they had prior to exacerbation (Figure 1).59,145
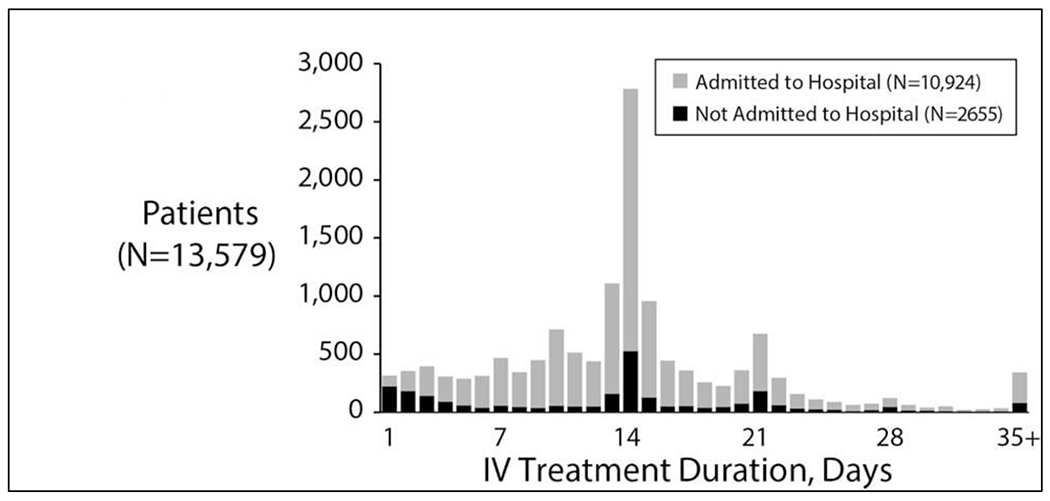
IV Antibiotic Treatment Durations(hospitalized and not hospitalized) in the US CFFPR. Distribution of IV antibiotic treatment durations. Black bars show numbers of patients of a given treatment duration who were never admitted to hospital as part of their exacerbation treatment. Reprinted with permission of the Journal of Cystic Fibrosis.48
Studies that have focused on clinical outcomes after PEx have noted poorer short-term outcomes, including reduced recovery of lung function in a substantial fraction of events (up to 50%).58,146 It is possible that some PEx treatment decisions may account for poorer outcomes.48 For example, in the US, treatment with intravenous (IV) antibiotics for less than 9 days and treatment entirely as an outpatient have both been associated with an increased risk of retreatment with IV antibiotics within 30 days of PEx treatment completion, despite similar patient characteristics at IV antibiotic initiation.48
Given the strong evidence and successful implementation in other diseases, it is very possible for an optimal standard duration of IV antibiotic therapy to be defined in CF. For this reason, the STOP study (NCT02109822) was conducted to refine key clinical endpoints and variance for treatment response for an IV exacerbation in CF as noted above.125 This study led to the initiation of the STOP2 study (NCT02781610).137 The STOP2 trial was a divergent trial design that evaluates subjects’ interim improvement in lung function as measured by FEV1 and symptoms as measured by a CF specific respiratory symptom diary to tailor randomization to IV treatment duration (10 vs. 14 days for early responders, 14 vs. 21 days for delayed responders).
The STOP2 trial enrolled 982 PwCF to 4 different treatment arms.63 The study noted that 10 days of IV antibiotics was not inferior to 14 days for an acute exacerbation as measured by improvement to lung function in early robust responders (improvement of 8% predicted FEV1% predicted and an 11-point improvement in the CRISS symptom scale after 7-10 days of treatment). For those patients who were not early robust responders after 7-10 days of treatment, 21 days of IV antibiotics was not superior to 14 days of treatment (Figure 2a, ,2b2b and and2c).2c). Symptom or weight change was also not different by treatment arm along with time to next exacerbation or need for early treatment failure.63 This does not mean that 10 days would also be equivalent to 21 days of therapy, as the STOP2 team tested durations in two different populations based on their initial response to treatment. Adverse events were relatively rare and did not differ among treatment durations. Furthermore, this is the first such study to address treatment duration in a chronic infection, in which the goal of treatment is not eradication of the causal bacteria. Future studies of treatment of PEx can use a fixed duration of IV antimicrobials to limit confounding by treatment duration and ensure proper interpretation of the results.
Site of Care
As noted above, systematic reviews of the literature found there was insufficient evidence upon which to develop guidelines for most treatment decisions, including site of treatment (home or hospital).118 Only one comparative trial exists which demonstrated similar outcomes for the home and hospital setting.118,147 Observational data has suggested that optimal treatment responses may not be achieved by treated acute PEx’s in the outpatient setting. A recent analysis of a national data registry found a 10% greater likelihood of FEV1 returning to ≥90% of baseline comparing complete inpatient treatment with no inpatient treatment.148 The conclusions of this paper differed from another observational study using the CFFPR which found that site of treatment did not impact long-term outcomes of exacerbations.141 Thus, site of treatment remains a key unresolved question in the management of acute pulmonary exacerbations. In the 2016 CFFPR report, the median treatment for PEx in adults was 12 days with a median hospitalization of 10 days,149 demonstrating that at least part of the treatment for PEx commonly occurs at home.
A prespecified STOP2 secondary analysis evaluated the impact of site of care (home vs hospital); inpatient care for exacerbations noted improvement in key clinical outcomes.150 Roughly a third (33%) of the trial participants received all their treatment in the hospital with 46% receiving a combination of inpatient and outpatient treatment. Treatment solely at home was associated with a lower lung function treatment response and smaller gains in symptoms recovery or nutritional improvement. In a propensity matched analysis, similar results were noted suggesting that indication bias may not explain these results.150 In fact, participants treated solely in the hospital has worse lung function at the time of exacerbation and lower lung function in the prior 6 months. The services to which patients have access are very different in the hospital compared to at home; differences may include nutritional support, regular chest physiotherapy and monitored antibiotic delivery. A separate cost analysis of the STOP2 data notes that costs were driven by inpatient days.62 Given the costs of inpatient care, a clinical trial definitively addressing the best treatment setting for an IV exacerbation would be both ethically challenging and prohibitively costly, thus further study will likely need innovative trial designs to definitively address this question.
Use of home monitoring for exacerbation
A recent prospective randomized controlled pilot trial using home monitoring to detect exacerbations enrolled 88 PwCF (44 in each arm) comparing twice weekly home assessments with spirometry and symptom measurement versus routine CF care much as in the earlier Early Intervention in CF Exacerbation (eICE) study.151,152 Much as in the eICE study, home monitoring was able to identify exacerbations and reduced healthcare utilization costs but did not lead to fewer inpatient bed days or improved health related quality of life.151,152 Yet another large-scale randomized trial (N=607) randomized PwCF to improve adherence with a digital platform and behavioral change sessions or usual care with the goal of improving adherence and reducing exacerbation rates.153 This study did improve measures of adherence and nutrition (body mass index) over the yearlong intervention period but did not reduce exacerbation rate, lung function or quality of life. In all of this prior work, we do not know the impact/implications in the setting of the novel CFTR modulator elexacaftor/tezacaftor/ivacaftor.
Molecular biomarkers for CF pulmonary exacerbations (PEX)
In assessing PEx, prospective biomarkers may bring value in diagnosis of PEx, predicting duration, severity and outcome, charting resolution, and providing a gauge for the effectiveness of therapeutic interventions. Ideally, biomarkers should also be biologically relevant to the pathogenesis of PEx, which in turn stands to increase their specificity. They must demonstrate feasibility, with satisfactory reproducibility of measurement between centers and within individual patients its measurement should be feasible with respect to sample collection and laboratory analysis, demonstrating measurement reproducibility both within individuals and among laboratories. They must finally be practical with ease of acquisition and quick turnaround of results and ideally be widely available in hospital laboratories.
At the time of writing, no molecular-level CF-specific exacerbation biomarker has been integrated into routine clinical practice, with only nonspecific biomarkers such as serum C-reactive protein (CRP) used to guide management in some centers. The relative scarcity of biomarker data from well-designed, prospective studies of PEx, coupled with the inconsistent definitions applied to define exacerbations have hindered biomarker development, as have clinical practice factors such as duration of antibiotic therapy and uncertainty regarding PEx etiology.63,125 Outside the realm of molecular biomarkers, FEV1, an effort-dependent measurement that is difficult to obtain reliably in children under 6 years, is a good example of an imperfect biomarker that has proved very useful in CF. Additional challenges with biomarker development include variable host responses to infection and varying magnitude of response in individual patients from one exacerbation to the next. Another confounder encountered by studies attempting to develop diagnostic biomarkers for PEx is that exacerbating PwCF have frequently commenced antibiotics prior to the biomarker sample being obtained making it very challenging to compare biomarker levels at the start and end of a PEx.
Interpreting biomarkers in context
When interpreting cytokine levels in inflammatory diseases, a distinction must be made between absolute level and biological activity. Similarly, investigators and clinicians should be aware that many of these mediators display substantial pleiotropy and exert physiological as well as pathological effects. IL-6, for example, is implicated in the development of fever, amplification of cytokinemia, induction and activation of chemokines, metalloprotesases, anemia, and B-cell dysregulation. However, it is also required for efficient bacterial clearance, macrophage polarization, lipid homeostasis and generation of the acute phase response.154 The latter includes the production and release of alpha-1 antitrypsin (AAT), the key inhibitor of neutrophil elastase (NE). Moreover, differential glycosylation of this key antiprotease, which increases its and anti-inflammatory and pro-resolution properties, occurs via IL-6-induced upregulation of ST6GAL1.155,156 This protective screen is lost when the IL-6R is blocked non-specifically by the monoclonal antibody tocilizumab.157 For signal transduction to occur, IL-6 must first bind its membrane-bound cognate receptor (IL-6R); the resultant IL-6/sIL-6R complex must then associate with another protein, gp130 before activation of JAK/STAT and other downstream pathways can ensue. This process of IL-6 signaling via the membrane-bound IL-6R – known as classical signalling – is restricted to cell types that express the IL-6R, predominates in healthy states and controlled inflammation, and governs the physiological effects of the cytokine. The pathological effects of IL-6 are mediated via trans-signalling, whereby cleavage of the IL-6R by ADAM-17 produces a soluble receptor (sIL-6R) capable of binding free IL-6 to generate a complex that can in turn signal to tissue systems that were previously IL-6-unresponsive. A soluble form of gp130 (sgp130) is also generated, albeit at a slower rate than sIL-6R. This sgp130 acts as a buffer, neutralizing Il-6/sIL-6R complexes before they reach IL-6R-negative cells. Therefore, the biological activity of IL-6 is not governed by its level in plasma, serum, or epithelial lining fluid (ELF) but rather by the relative abundance of sIL-6R above sgp130. The same principle of checks and balances can be applied to most of the inflammatory biomarkers studied in CF to date. Indeed, distinguishing clinically between an aggressive – but appropriate – response to infection and a harmful dysregulated inflammatory response is notoriously challenging in patients with advanced disease and/or severe exacerbations, further highlighting the important difference between an inflammatory biomarker and a reliable therapeutic target.
Airway biomarkers
Given that the main source of morbidity and mortality in CF is lung disease, it stands to reason that the airway is the most likely source of biomarkers specific for PEx. Although regarded as a gold standard for acquisition of airway samples, bronchoalveolar lavage (BAL) brings its own challenges. It is invasive and cannot be performed on a regular basis during a PEx admission in the same PwCF. These limitations make sputum a more attractive alternative for use in PEX assessments on feasibility grounds. Sputum has been used to confirm increased levels of inflammatory biomarkers in CF versus healthy controls158, correlation of biomarkers with neutrophilia158 and changes in biomarker levels following IV antibiotics.90 It is also a rich source of biomarkers of infection159–161 – in addition to identifying lower airway microbial pathogens and quantitative bacterial counts by standard culture, samples can be used to detect bacterial species and bacterial proteins of interest.159–165
While the reproducibility issues with sputum are undoubtedly influenced by the inherent variability of the matrix itself, other factors include the acquisition, handling and processing of samples, which in many cases involves the use of the reducing agent dithiothreitol (DTT).166–183 When designing PEX biomarker studies, there is also the possibility that sampling only PwCF who spontaneously expectorate sputum may result in a biased, especially in the context of decreased overall sputum expectoration in patients receiving highly effective modulator therapy (HEMT). On the other hand, it is clearly not feasible to conduct large-scale PEX studies using BAL, biasing the population to a cohort with a higher FEV1, less severe bronchiectasis and better overall health who are fit for repeated procedures.184 With this as background, it seems intuitive that induced sputum (IS) would be a better biologic specimen in a relatively non-invasive fashion, and that there are well-established protocols available for the induction process. The limitations to IS include similar questions of reproducibility and reliability, the time-consuming nature of sputum induction and the need for appropriate equipment and trained personnel.
Airway serine proteases are central to the inflammatory response to infection and bacterial killing. NE, cathepsin G (Cath G) and proteinase 3 (PR3) are the 3 most prominent serine proteases found in the CF lung. They belong to the chymotrypsin superfamily, and are stored in the primary granules of mature neutrophils, having been produced in the bone marrow.185,186 NE is, by some distance, the most widely studied airway biomarker in CF, both in the context of exacerbations and disease progression.
Airway NE activity is associated with increased exacerbation frequency187–189 has been shown to decrease in response to PEX treatment and resolution90,190,191 in parallel with neutrophil count and P. aeruginosa density.90 The biological plausibility of NE as an exacerbation biomarker is clear. It increases mucus production192, hinders ciliary clearance193, and cleaves immunoglobulin, complement, complement receptors, and the C-X-C motif chemokine receptor CXCR1 on neutrophils194–197, a process implicated in impaired bacterial phagocytosis and killing of Pseudomonas. NE is also implicated in cleavage of CFTR and loss of channel function in the airway.198 In addition to inducing and activating cysteinyl cathepsins and matrix metalloproteases (MMPs)199, NE also upregulates the key neutrophil chemoattractants IL-8200 and LTB4201, establishing a cycle of inflammation in the CF airway and promoting the development of bronchiectasis202, which in turn provides a reservoir for pooled secretions and bacterial growth. The inverse relationship between airway NE activity and FEV1 in CF203 is evidence of the clinical relevance of this protease burden. Measurement of NE activity is generally considered superior to absolute levels, since conventional assays for the latter do not discriminate between active NE and NE that is inactivated by AAT and other antiproteases. At present, the preferred method of assessing NE activity levels is by fluorescence resonance energy transfer (FRET).204,205 Point-of-care assays that do not require trained scientist operators are attractive, and have been used to predict exacerbations of bronchiectasis206 and for the purpose of stratifying patients for clinical trials 207, though they are less precise.
Matrix metalloproteases (MMPs) are metalloendopeptidases that degrade extracellular matrix and drive tissue remodeling, release cytokines, growth factors and chemokines, and control cell mobility and migration.208 At the transcription level, MMPs are induced by IL-1β and TNF-α, both of which are elevated in the inflamed CF lung. Overall airway levels of MMP-2, MMP-7, MMP-8, MMP-9 and MMP-12 have also been shown to be elevated in CF and change in response to intervention.199,209–211 Tetracycline antibiotics such as doxycycline can inhibit MMP-9 activity, with the observed reduction in total sputum MMP-9 activity predicting an increased in time-to-next-exacerbation.212 In addition to MMP-9, treatment of PEX with combined antipseudomonal antibiotic therapy has also been shown to decrease MMP-1 and MMP-8.213 There are several downstream effects of this protease-antiprotease imbalance. MMP-2, for example, disrupts Cl− current, while MMP-8 and MMP-9 cleave collagen and modify C-X-C chemokines. MMP-12 cleaves elastin, which may result in loss of lung tissue and activation of airway macrophages. Other proteases, such as the cysteinyl cathepsins have not been extensively used as PEx biomarkers, but could potentially be of use given the success of cathepsin inhibition in non-CF bronchiectasis.207
While a wide range of airway cytokines have been shown to correlate with severity and progression of CF lung disease, data in PEx have been mixed, with IL-8 90,214 and IL-1β 191 appearing to be the most conceivable candidates based on their relationship to both NE and airway infection.189,215–219 Additional sputum inflammatory biomarkers that have been associated with clinical events such as exacerbations include the calprotectin220, high-mobility group box 1 (HMGB-1)221, zinc222, the elastin breakdown product desmosine and YKL-40223,224, a chitinase-like protein produced by neutrophils, monocytes, macrophages and other immune cells, and a regulator of cell proliferation and survival. Levels of these markers are elevated in stable PwCF compared to healthy controls and rise further during exacerbations. Both calprotectin and YKL-40 levels in blood have previously correlated well with levels observed in sputum.220 These increases appear to be driven primarily by inflammation due to infection, in particular P. aeruginosa, rather than CFTR dysfunction, making them potentially useful markers for exacerbating PwCF on HEMT.
Circulating biomarkers
Blood-based biomarkers are an area of increasing interest given the effect of HEMT on sputum production. A wide variety of circulating candidate biomarkers have been investigated in actively exacerbating PwCF to monitor resolution and response to intervention. The most commonly studied to date are CRP, cytokines including IL-1β, IL-6, IL-8, IL-10 and TNF-α, ESR, procalcitonin, and markers reflective of increased neutrophil degranulation such as MPO, lactoferrin and NE:AAT complexes. It is worth noting that, although pronounced cytokinemia may be observed in critical illness217, levels of circulating cytokines in PwCF are typically modest, even in the context of severe exacerbations. Aspergillus-specific IgE can be used to diagnose allergic bronchopulmonary aspergillosis (ABPA) and monitor response to treatment, but antipseudomonal antibodies and immunoglobulins against other common CF pathogens remain understudied.
Serum CRP have previously been shown in multiple studies to decrease with successful treatment of PEx in hospitalized PwCF225, with this effect mirrored by calprotectin and vascular endothelial growth factor (VEGF).220 However, this generally only held for the CRP trend in a given patient in these studies, and was not consistently observed in interventional trials in CF. Indeed, though Log10 CRP two weeks after completion of treatment was associated with the odds of retreatment in 30 days and time to next exacerbation, recent analyses of STOP2 suggest that CRP changes have limited utility as a marker of treatment response.226 Overall, the variability of CRP within and between studies suggests that establishing a clinically applicable cutoff for diagnosis and/or resolution of PEx is unrealistic without the fold-change being applied.
Other circulating mediators known to be increased in parallel with respiratory symptoms include human neutrophil lipocalin, MMP-1, -8, and -9, IL-6, IL-1 receptor a (IL-1Ra), serum amyloid A (SAA) and NE:AAT.133,213,225,227 Although concentrations of TNF-α, and IL-1β are known to be increased in CF airways and are associated with increased PEx frequency, data regarding their ability of circulating levels of these master proinflammatory cytokines to diagnose PEx are limited. Serum IL-6, IL-1Ra, NE:AAT, SAA, G-CSF, TGF-β, myeloperoxidase (MPO) and lactoferrin have previously been shown to correlate with either responses to treatment or resolution of PEx, as has total leukocyte count.133,228,229
Protease activity – and in particular NE activity – is usually undetectable in the blood of exacerbating PwCF. This is largely because the majority of the NE activity is contained within the lung, where it overwhelms the antiprotease protection provided by AAT, SLPI and elafin. Although there is local production of these antiproteases within the airway, over 95% of AAT is produced by the liver, from which it must travel via the bloodstream and migrate to the airway via the lung interstitium. As a result, circulating concentrations of AAT are greater than those found in epithelial lining fluid, even in the context of a PEx, so the relatively small amount of NE that escapes the lung (as well as the NE released by primed circulating neutrophils) is more easily inhibited. Therefore, measurement of AAT:NE complexes are more readily detectable than active NE in blood. That being said, indirect markers of airway protease activity can be measured systemically, and present a potential opportunity to non-invasively assess lung damage. Circulating levels of desmosine and isodesmosine correlate directly with sputum NE activity and can also be detected in urine, though their use in blood has been hindered thus far by cost and poor reproducibility. The matrikine proline-glycine-proline (PGP) may offer an alternative surrogate for airway protease activity.230 The value of developing high quality biomarkers for PEx – particularly in the blood – may be essential to evaluating and treating PEx now that the majority of PwCF in the North America and Europe are treated with elexacaftor/tezacaftor/ivacaftor.
Future Directions: Challenges with Studying Exacerbation in the Post-modulator world
With the advent of highly effective modulators like ivacaftor53 and now elexacaftor/tezacaftor/ivacaftor231, clinicians do not know how the landscape of PEx will change. Based on clinical trial data, ivacaftor decreased exacerbation rates by 55% over 48 weeks.53 In post-marketing data from the Irish CF Registry, ivacaftor has the potential to reduce exacerbation rates treated with intravenous antibiotic courses by 46% (95% CI: −62.5% to −23.3%) and oral antibiotics by 49%( (95% CI, −61.1% to −32.1%).232 ETI is now available to roughly 90% of PwCF in the US and smaller but significant proportions of PwCF in Europe, Canada, Australia and New Zealand233,234 and has changed the face of CF care. The impact of ETI on the need for intravenous antibiotics to treat PEx appears to be significant in the registry data from 2021.235 Moreover, there appears to be a significant impact of delayed access to ETI, an important consideration for health policymakers.236 Even further data suggests that the future may be very bright for our patients; in a recent publication supporting earlier work, ivacaftor appears to reduce the incidence of new Pseudomonas aeruginosa acquisition.237,238 Despite the potential for radical changes in PEx rates, the majority of CF patients will still have moderate to extensive bronchiectasis complicated by lower airway infection and like non-CF bronchiectasis, will continue to have PEx’s managed by CF physicians. These events however may be different, with milder presentations. Much of what we know about PEx in CF may change.
Given the reduction in PEx rate and improvement in lung health (and likely longevity) in the setting of novel CFTR therapies,233,234,239 some key aspects of our treatment approaches for PEx may need to be re-evaluated. The validity of earlier studies demonstrating marginal benefit for combination antibiotic IV treatments (a β-lactam plus aminoglycoside) needs to be questioned. Given the well-known toxicities of IV aminoglycosides (vestibular, ototoxicity and renal toxicity),240 a superiority trial assessing the impact of adding aminoglycosides to a β-lactam is essential to ensure the risk of toxicity is associated with clear clinical benefit, particularly in those without advanced lung disease. A trial is now starting in the US, Standardizing Treatments for Pulmonary Exacerbations: A platform for evaluating treatment decisions to improve outcomes Trials (STOP360). This trial will enroll up to 730 PwCF ages 6 years or older experiencing a physician defined PEx requiring IV anti-Pseudomonal antibiotics in over 60 centers in the US and Canada (NCT05548283). This trial will randomized PwCF to either β-lactam plus aminoglycoside versus β-lactam alone and followed for 2 weeks after the end of 14 days of IV antibiotics. The study will test if the addition of aminoglycoside to β-lactam is superior to β-lactam alone. This study is just one example about how we may need to rethink the management of PEx.
Conclusions
Research regarding pulmonary exacerbations in cystic fibrosis continues to evolve improving our understanding the natural history of disease of CF patients. Pulmonary exacerbations continue to significantly impact the lives of both children and adults with CF. Improving our understanding of these events will have implications for both basic research and clinical research in CF. We are now entering a new era first of large scale clinical trials to provide high quality evidence to treatment of PEx. We are also about to enter an era when >90% of our patients will likely have highly effective CFTR therapy that could alter the severity and the rates of PEx in CF. Despite these advances, many questions remain about basic aspects of pathophysiology and treatment of PEx that will likely persist. More work is desperately needed to further the science of PEx.
Funding:
Dr. Goss’ research time is supported by the Cystic Fibrosis Foundation Therapeutics (GOSS13A0) and NIH (P30 DK089507, UL1TR000423). There was no role of funding sources in writing of this manuscript, or the decision to submit for publication.
References:
Citations & impact
Impact metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1055/s-0042-1760250
Article citations
Predicting lung function decline in cystic fibrosis: the impact of initiating ivacaftor therapy.
Respir Res, 25(1):187, 27 Apr 2024
Cited by: 0 articles | PMID: 38678203 | PMCID: PMC11056050
Polymerized cyclodextrin microparticles for sustained antibiotic delivery in lung infections.
J Biomed Mater Res A, 112(8):1305-1316, 21 Feb 2024
Cited by: 0 articles | PMID: 38380736
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Clinical Trials (4)
- (1 citation) ClinicalTrials.gov - NCT02781610
- (1 citation) ClinicalTrials.gov - NCT02109822
- (1 citation) ClinicalTrials.gov - NCT03070522
- (1 citation) ClinicalTrials.gov - NCT05548283
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acute Pulmonary Exacerbations in Cystic Fibrosis.
Semin Respir Crit Care Med, 40(6):792-803, 28 Oct 2019
Cited by: 39 articles | PMID: 31659730 | PMCID: PMC7528649
Review Free full text in Europe PMC
[Chinese experts consensus statement: diagnosis and treatment of cystic fibrosis (2023)].
Zhonghua Jie He He Hu Xi Za Zhi, 46(4):352-372, 01 Apr 2023
Cited by: 4 articles | PMID: 36990700
Clinical utility of C-reactive protein to predict treatment response during cystic fibrosis pulmonary exacerbations.
PLoS One, 12(2):e0171229, 08 Feb 2017
Cited by: 16 articles | PMID: 28178305 | PMCID: PMC5298271
[Evidence-based treatment of cystic fibrosis].
Internist (Berl), 61(12):1212-1229, 01 Dec 2020
Cited by: 3 articles | PMID: 33201261
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000423
NIDDK NIH HHS (1)
Grant ID: P30 DK089507



