Abstract
Free full text

Toward a biomarker panel measured in CNS-originating extracellular vesicles for improved differential diagnosis of Parkinson’s disease and multiple system atrophy
Synucleinopathies are neurodegenerative diseases characterized by accumulation of misfolded α-synuclein (α-syn) inclusions in neuronal and/or glial cells. Despite differences in the underlying pathophysiology, synucleinopathies often are misdiagnosed, especially in early stages, due to the overlapping clinical symptoms [1].
Recently, our group has shown that α-syn measured in both neuronal extracellular vesicles (nEVs) and oligodendroglial EVs (oEVs) in the same samples, and in particular the oEV:nEV α-syn concentration ratio, yielded a discriminative model distinguishing multiple system atrophy (MSA) from healthy controls (HC) or Parkinson’s disease (PD) with high sensitivity and specificity. In contrast, the separation between PD and HC was moderate [2].
In this study, using remaining samples from the previous study, we evaluated whether adding nEV and oEV pS129-α-syn, total tau, tau phosphorylated at Thr 181 (pT181-tau), and/or serum neurofilament light chain (NfL) to the previously measured α-syn might improve the diagnostic power. Patient information is shown in Additional file 1: Tables S1–S3. Most results are presented as log-transformed values to facilitate normal distribution of the data. Non-transformed values are summarized in Additional file 1: Table S4.
The level of pS129-α-syn in normal brain is ~
~ 4% of total α-syn and may increase to
4% of total α-syn and may increase to ~
~ 90% in Lewy bodies [3]. pS129-α-syn is also enriched in glial-cytoplasmic inclusions though to a lesser degree [4], suggesting that its concentrations in CNS-originating EVs may help distinguish among the groups.
90% in Lewy bodies [3]. pS129-α-syn is also enriched in glial-cytoplasmic inclusions though to a lesser degree [4], suggesting that its concentrations in CNS-originating EVs may help distinguish among the groups.
Measurement of pS129-α-syn in 32 HC, 46 PD, and 30 MSA samples showed that in many cases its concentration constituted a small fraction of total α-syn (Additional file 1: Fig. S1a). The nEV pS129-α-syn concentrations decreased in the order HC >
> PD
PD >
> MSA, yet the differences were statistically insignificant (Fig. 1a). In contrast, the oEV concentrations of pS129-α-syn increased in the same order, HC
MSA, yet the differences were statistically insignificant (Fig. 1a). In contrast, the oEV concentrations of pS129-α-syn increased in the same order, HC <
< PD
PD <
< MSA, and were significantly higher in both disease groups (Fig. 1a). Thus, pS129-α-syn concentration in nEVs was not affected by synucleinopathy, whereas in oEVs it was increased in MSA and in a subgroup of patients with PD compared to HCs.
MSA, and were significantly higher in both disease groups (Fig. 1a). Thus, pS129-α-syn concentration in nEVs was not affected by synucleinopathy, whereas in oEVs it was increased in MSA and in a subgroup of patients with PD compared to HCs.
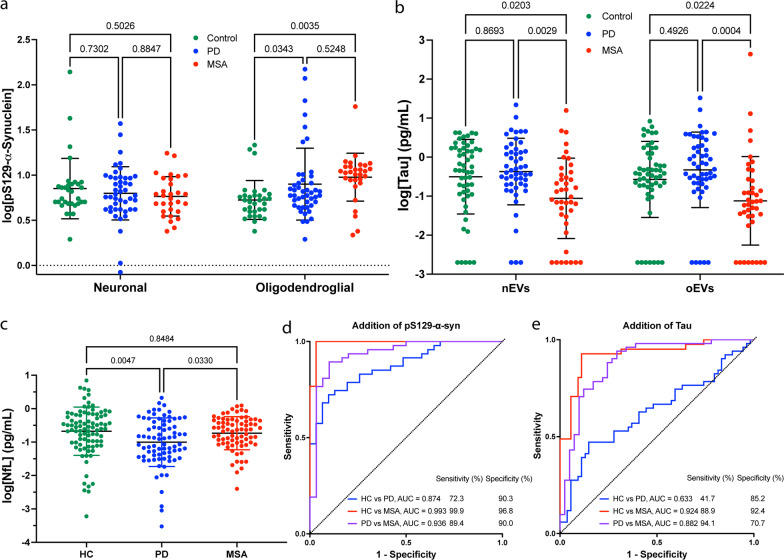
Biomarker analysis in HC, PD, and MSA. a, b pS129-α-Syn and Tau concentrations were measured using ECLIA, log-transformed, and analyzed by a mixed-effect analysis with post-hoc Tukey test. Data are represented as mean ±
± SD. Values below the lower limit of detection were imputed as the minimum value divided by 2. c NfL concentrations were measured using Simoa, log-transformed, and analyzed by a one-way ANOVA with post-hoc Tukey test. Data are represented as mean
SD. Values below the lower limit of detection were imputed as the minimum value divided by 2. c NfL concentrations were measured using Simoa, log-transformed, and analyzed by a one-way ANOVA with post-hoc Tukey test. Data are represented as mean ±
± SD. d, e ROC analyses of biomarker concentrations using a multinomial logistic regression model with LASSO variable selection (Additional file 1: Fig. S3). The model included nEV α-syn concentration, oEV:nEV α-syn concentration ratio, total EV concentration in the sample and d oEV pS129-α-syn or e oEV tau concentration. The presented models do not include NfL
SD. d, e ROC analyses of biomarker concentrations using a multinomial logistic regression model with LASSO variable selection (Additional file 1: Fig. S3). The model included nEV α-syn concentration, oEV:nEV α-syn concentration ratio, total EV concentration in the sample and d oEV pS129-α-syn or e oEV tau concentration. The presented models do not include NfL
The oEV:nEV pS129-α-syn ratio increased similarly in the order HC <
< PD
PD <
< MSA (Additional file 1: Fig. S1b). In most patients with MSA this ratio was
MSA (Additional file 1: Fig. S1b). In most patients with MSA this ratio was >
> 1, as reported previously for total α-syn [2]. However, in the PD group roughly equal numbers of samples had a ratio
1, as reported previously for total α-syn [2]. However, in the PD group roughly equal numbers of samples had a ratio <
< 1 or
1 or >
> 1. Consequently, unlike the oEV:nEV total α-syn ratio, the oEV:nEV pS129-α-syn ratio separated the groups only moderately.
1. Consequently, unlike the oEV:nEV total α-syn ratio, the oEV:nEV pS129-α-syn ratio separated the groups only moderately.
Genome-wide association and other genetic studies have revealed a strong link of the MAPT gene to PD [5] and tau aggregates have been observed in ~
~ 50% of PD brains. In contrast, tau pathology is rare in MSA. Thr 181 is a common tau phosphorylation site in pathological conditions and CSF pT181-tau has been used as a biomarker for PD and atypical parkinsonian disorders [6].
50% of PD brains. In contrast, tau pathology is rare in MSA. Thr 181 is a common tau phosphorylation site in pathological conditions and CSF pT181-tau has been used as a biomarker for PD and atypical parkinsonian disorders [6].
Total tau was measured in 54 HC, 51 PD, and 41 MSA samples. The tau concentrations were approximately an order of magnitude lower than those of α-syn (Additional file 1: Table S4). A few samples yielded signal below the lower limit of detection, which was imputed as the minimum/2 value (Fig. 1b). Analysis without the imputed values did not change the significance or direction of the results. The average concentration in both nEVs and oEVs was significantly lower in the MSA group compared to HC and PD, in agreement with the scarce observation of tau pathology in MSA. The differences remained when the data were adjusted for age. The HC and PD groups had similar tau concentrations in both nEVs and oEVs. The differences between nEVs and oEVs were statistically insignificant in all three groups (Fig. 1b), suggesting that measurement of tau in one type of EV is sufficient.
In our first attempt to measure pT181-tau we isolated nEVs and oEVs from 9 HC, 17 PD, and 12 MSA samples. Unfortunately, pT181-tau was detectable only in 6 of the 76 EV lysates, suggesting that the pT181-tau levels were a small fraction of the total tau concentration and that at the present assay-sensitivity level this analyte is not useful for distinguishing among HC, PD, and MSA. Alternatively, the low levels may be due to sample age.
CSF NfL is a useful marker of neuroaxonal injury and neurodegeneration and plasma NfL concentrations have been shown to correlate well with CSF levels [7]. Significantly higher plasma NfL concentrations have been reported in MSA compared to HC and PD [8]. Therefore, we tested whether similar differences could be detected in our cohort, though we used serum rather than plasma. NfL concentrations in serum and plasma have been reported to correlate well in patients with multiple sclerosis [7] though we are not aware of a similar comparison in patients with synucleinopathies.
Here, as the volume needed for direct measurement of NfL (20 μl) is ~
~ 10% of the volume needed for EV isolation, we measured serum NfL in most of the samples—88 HC, 79 PD, and 74 MSA, using a Simoa assay to match most of the current literature. We found a significantly lower average NfL concentration in the PD group compared to HC and MSA (Fig. 1c), even after the data were adjusted for age. The lower concentration of NfL in PD compared to HC samples was in agreement with a recent paper by Chen et al. [9] who found that the difference was statistically significant only in females. Consistently, in our cohort, we found lower NfL concentrations in both PD and MSA samples from females but not from males (Additional file 1: Fig. S2).
10% of the volume needed for EV isolation, we measured serum NfL in most of the samples—88 HC, 79 PD, and 74 MSA, using a Simoa assay to match most of the current literature. We found a significantly lower average NfL concentration in the PD group compared to HC and MSA (Fig. 1c), even after the data were adjusted for age. The lower concentration of NfL in PD compared to HC samples was in agreement with a recent paper by Chen et al. [9] who found that the difference was statistically significant only in females. Consistently, in our cohort, we found lower NfL concentrations in both PD and MSA samples from females but not from males (Additional file 1: Fig. S2).
Finally, we used the same statistical approach as in [2] to construct new models (Additional file 1: Fig. S3) including both the previously selected parameters (nEV α-syn concentration, oEV:nEV α-syn concentration ratio, and total EV concentration) and the new biomarker measurements, to evaluate whether pS129-α-syn or tau might improve the separation when added separately, as the number of samples in which both pS129-α-syn and tau were measured was too small in this study to allow meaningful evaluation of both together. NfL was measured in most of the samples and therefore we tested it in combination with pS129-α-syn or tau.
For the subset of samples with pS129-α-syn measurements, addition of oEV pS129-α-syn concentration to the previous parameters in the ROC analysis resulted in improved predictions (Akaike information criterion [AIC] =
= 136.1 vs 138.7; AIC change
136.1 vs 138.7; AIC change =
= −
− 2.6). The improved model separated between the PD and HC groups with AUC
2.6). The improved model separated between the PD and HC groups with AUC =
= 0.874 (95% CI 0.82–0.96), MSA and HC with AUC
0.874 (95% CI 0.82–0.96), MSA and HC with AUC =
= 0.993 (95% CI 0.98–0.99), and PD and MSA with AUC
0.993 (95% CI 0.98–0.99), and PD and MSA with AUC =
= 0.936 (95% CI 0.88–0.99) (Fig. 1d, Additional file 1: Table S5). Addition of NfL did not improve the separation in this sample subset.
0.936 (95% CI 0.88–0.99) (Fig. 1d, Additional file 1: Table S5). Addition of NfL did not improve the separation in this sample subset.
For the tau measurement subset, addition of oEV tau did not improve the model predictions significantly (Fig. 1e, Additional file 1: Table S5). Adding NfL to oEV tau did improve the model predictions (AIC change =
= −
− 2.2) and the AUC value for separation between HC and PD increased from 0.633 to 0.720 (P
2.2) and the AUC value for separation between HC and PD increased from 0.633 to 0.720 (P =
= 0.055). α-Syn concentration in both nEVs and oEVs in all the groups combined correlated with oEV pS129-α-syn (rnEV
0.055). α-Syn concentration in both nEVs and oEVs in all the groups combined correlated with oEV pS129-α-syn (rnEV =
= 0.38, roEV
0.38, roEV =
= 0.35; P
0.35; P <
< 0.0001) but not with nEV pS129-α-syn. Analysis in each group showed no significant correlations in the control group between nEV or oEV total α-syn and nEV or oEV pS129-α-syn, indicating that this group did not contribute to the observed correlations. In the PD group, both nEV and oEV α-syn correlated positively with oEV pS129-α-syn (rnEV
0.0001) but not with nEV pS129-α-syn. Analysis in each group showed no significant correlations in the control group between nEV or oEV total α-syn and nEV or oEV pS129-α-syn, indicating that this group did not contribute to the observed correlations. In the PD group, both nEV and oEV α-syn correlated positively with oEV pS129-α-syn (rnEV =
= 0.37, P
0.37, P =
= 0.010; roEV
0.010; roEV =
= 0.33, P
0.33, P =
= 0.025). Similarly, positive correlations were found for the MSA group (rnEV = 0.38, P
0.025). Similarly, positive correlations were found for the MSA group (rnEV = 0.38, P =
= 0.037; roEV
0.037; roEV =
= 0.38, P
0.38, P =
= 0.036). None of the biomarkers correlated with disease duration or disease progression as assessed by Unified Parkinson’s Disease Rating Scale-III, Unified Multiple System Atrophy Rating Scale, or Hoehn and Yahr scale.
0.036). None of the biomarkers correlated with disease duration or disease progression as assessed by Unified Parkinson’s Disease Rating Scale-III, Unified Multiple System Atrophy Rating Scale, or Hoehn and Yahr scale.
Our study is the first to measure pS129-α-syn in nEVs and oEVs and assess tau concentrations in these types of EVs in patients with MSA. Though the results need validation in larger cohorts, the data support measurement of these and additional biomarkers in CNS-originating EVs toward constructing a panel comprising at a minimum nEV α-syn, oEV:nEV α-syn ratio, oEV pS129-α-syn, and total EV concentration for improved diagnosis of parkinsonian disorders.
Acknowledgements
We thank Dr. Andrea Hevener for the use of the MSD QuickPlex SQ 120 at the UCLA Metabolic & Molecular Physiology Core.
Author contributions
HBT, SD and GB conceptualized and designed the study. HBT and GB wrote the manuscript; HBT, LF, SH, and OL acquired the data; HBT, SH, DM, and GB analyzed the data; DYW, ADF, JAP, UJK, RNA, MS, HK, BLF, JMB, and BR provided samples and critical comments on the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by grants from the MSA Coalition 2017-10-007 (GB), California Department of Public Health 18-10926 (GB), The Alzheimer’s Association, The Michael J. Fox Foundation, Weston Brain Institute, and Alzheimer’s Research UK Biomarkers Across Neurodegenerative Diseases (BAND 3) 17990 (GB), CurePSP 665-2019-07 (GB), The Michael J. Fox Foundation 18303 (GB), The National Ataxia Foundation 20201551 (GB and BLF), Cure Sanfilippo Foundation 20215318 (GB), NIH/NIEHS ES10544 (BR), and by generous gifts from the Karen Toffler Charitable Trust and the Binder Foundation.
Availability of data and materials
All the data generated or analyzed during this study are included in the published article.
Declarations
Blood-collection procedures were approved by the respective Human Subjects Committees at UCLA, New York University, Columbia University, and University of North Carolina Chapel Hill and informed consent was obtained from all participants.
Not applicable.
The authors declare that they have no competing interests.
References
Articles from Translational Neurodegeneration are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s40035-023-00346-0
Read article for free, from open access legal sources, via Unpaywall:
https://translationalneurodegeneration.biomedcentral.com/counter/pdf/10.1186/s40035-023-00346-0
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/144127332
Article citations
CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases.
Front Neurosci, 18:1426700, 20 Jun 2024
Cited by: 0 articles | PMID: 38966760 | PMCID: PMC11222337
Review Free full text in Europe PMC
Single-molecule characterization of salivary protein aggregates from Parkinson's disease patients: a pilot study.
Brain Commun, 6(3):fcae178, 21 May 2024
Cited by: 1 article | PMID: 38863577
Extracellular vesicles from bodily fluids for the accurate diagnosis of Parkinson's disease and related disorders: A systematic review and diagnostic meta-analysis.
J Extracell Biol, 2(11):e121, 13 Nov 2023
Cited by: 2 articles | PMID: 38939363
Review
Analysis of biomarkers in speculative CNS-enriched extracellular vesicles for parkinsonian disorders: a comprehensive systematic review and diagnostic meta-analysis.
J Neurol, 271(4):1680-1706, 16 Dec 2023
Cited by: 7 articles | PMID: 38103086 | PMCID: PMC10973014
Review Free full text in Europe PMC
Evaluation of α-synuclein in CNS-originating extracellular vesicles for Parkinsonian disorders: A systematic review and meta-analysis.
CNS Neurosci Ther, 29(12):3741-3755, 07 Jul 2023
Cited by: 7 articles | PMID: 37416941 | PMCID: PMC10651986
Review Free full text in Europe PMC
Go to all (9) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism.
J Neurol Neurosurg Psychiatry, 91(7):720-729, 09 Apr 2020
Cited by: 122 articles | PMID: 32273329 | PMCID: PMC7361010
A patient with a parkinsonian syndrome illustrating the difficult differential diagnosis between multisystem atrophy and Parkinson's disease.
Mov Disord, 23(14):2103-2104, 01 Oct 2008
Cited by: 0 articles | PMID: 18709674
Differentiating multiple-system atrophy from Parkinson's disease.
Clin Radiol, 70(5):555-564, 07 Mar 2015
Cited by: 14 articles | PMID: 25752581
Review
Early distinction of Parkinson-variant multiple system atrophy from Parkinson's disease.
Mov Disord, 34(3):440-441, 20 Feb 2019
Cited by: 15 articles | PMID: 30788854
Funding
Funders who supported this work.
California Department of Public Health (1)
Grant ID: 18-10926
Cure Sanfilippo Foundation (1)
Grant ID: 20215318
CurePSP (1)
Grant ID: 665-2019-07
Michael J. Fox Foundation for Parkinson's Research (2)
Grant ID: 18303
Grant ID: 17990
Multiple System Atrophy Coalition (1)
Grant ID: 2017-10-007
NIH/NIEHS (1)
Grant ID: ES10544
National Ataxia Foundation (2)
Grant ID: 20201551
Grant ID: 686288

 1,12,13
1,12,13


