Abstract
Importance
Given the high risk of thrombosis and anticoagulation-related bleeding in patients with hypoxemic COVID-19 pneumonia, identifying the lowest effective dose of anticoagulation therapy for these patients is imperative.Objectives
To determine whether therapeutic anticoagulation (TA) or high-dose prophylactic anticoagulation (HD-PA) decreases mortality and/or disease duration compared with standard-dose prophylactic anticoagulation (SD-PA), and whether TA outperforms HD-PA; and to compare the net clinical outcomes among the 3 strategies.Design, settings, and participants
The ANTICOVID randomized clinical open-label trial included patients with hypoxemic COVID-19 pneumonia requiring supplemental oxygen and having no initial thrombosis on chest computer tomography with pulmonary angiogram at 23 health centers in France from April 14 to December 13, 2021. Of 339 patients randomized, 334 were included in the primary analysis-114 patients in the SD-PA group, 110 in the HD-PA, and 110 in the TA. At randomization, 90% of the patients were in the intensive care unit. Data analyses were performed from April 13, 2022, to January 3, 2023.Interventions
Patients were randomly assigned (1:1:1) to receive either SD-PA, HD-PA, or TA with low-molecular-weight or unfractionated heparin for 14 days.Main outcomes and measures
A hierarchical criterion of all-cause mortality followed by time to clinical improvement at day 28. Main secondary outcome was net clinical outcome at day 28 (composite of thrombosis, major bleeding, and all-cause death).Results
Among the study population of 334 individuals (mean [SD] age, 58.3 [13.0] years; 226 [67.7%] men and 108 [32.3%] women), use of HD-PA and SD-PA had similar probabilities of favorable outcome (47.3% [95% CI, 39.9% to 54.8%] vs 52.7% [95% CI, 45.2% to 60.1%]; P = .48), as did TA compared with SD-PA (50.9% [95% CI, 43.4% to 58.3%] vs 49.1% [95% CI, 41.7% to 56.6%]; P = .82) and TA compared with HD-PA (53.5% [95% CI 45.8% to 60.9%] vs 46.5% [95% CI, 39.1% to 54.2%]; P = .37). Net clinical outcome was met in 29.8% of patients receiving SD-PA (20.2% thrombosis, 2.6% bleeding, 14.0% death), 16.4% receiving HD-PA (5.5% thrombosis, 3.6% bleeding, 11.8% death), and 20.0% receiving TA (5.5% thrombosis, 3.6% bleeding, 12.7% death). Moreover, HD-PA and TA use significantly reduced thrombosis compared with SD-PA (absolute difference, -14.7 [95% CI -6.2 to -23.2] and -14.7 [95% CI -6.2 to -23.2], respectively). Use of HD-PA significantly reduced net clinical outcome compared with SD-PA (absolute difference, -13.5; 95% CI -2.6 to -24.3).Conclusions and relevance
This randomized clinical trial found that compared with SD-PA, neither HD-PA nor TA use improved the primary hierarchical outcome of all-cause mortality or time to clinical improvement in patients with hypoxemic COVID-19 pneumonia; however, HD-PA resulted in significantly better net clinical outcome by decreasing the risk of de novo thrombosis.Trial registration
ClinicalTrials.gov Identifier: NCT04808882.Free full text

Effects of Standard-Dose Prophylactic, High-Dose Prophylactic, and Therapeutic Anticoagulation in Patients With Hypoxemic COVID-19 Pneumonia
Key Points
Question
Does standard dose prophylactic anticoagulation (SD-PA), high-dose prophylactic anticoagulation (HD-PA), or therapeutic anticoagulation (TA) have a greater benefit in patients with hypoxemic COVID-19 pneumonia?
Findings
This randomized clinical trial including 334 patients with severe hypoxemic COVID-19 pneumonia found that HD-PA and TA did not improve the primary hierarchical criteria of death and time to clinical improvement compared with SD-PA; however, HD-PA provided a better net clinical benefit driven by a 4-fold reduction in de novo thrombosis rate (5.5% vs 20.2%; absolute difference, −14.7; 95% CI −6.2 to −23.2) with no increase in major bleeding compared with SD-PA. Also, TA did not provide additional benefit in comparison with HD-PA.
Meaning
The findings of this randomized clinical trial support the routine empirical use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia.
Abstract
Importance
Given the high risk of thrombosis and anticoagulation-related bleeding in patients with hypoxemic COVID-19 pneumonia, identifying the lowest effective dose of anticoagulation therapy for these patients is imperative.
Objectives
To determine whether therapeutic anticoagulation (TA) or high-dose prophylactic anticoagulation (HD-PA) decreases mortality and/or disease duration compared with standard-dose prophylactic anticoagulation (SD-PA), and whether TA outperforms HD-PA; and to compare the net clinical outcomes among the 3 strategies.
Design, Settings, and Participants
The ANTICOVID randomized clinical open-label trial included patients with hypoxemic COVID-19 pneumonia requiring supplemental oxygen and having no initial thrombosis on chest computer tomography with pulmonary angiogram at 23 health centers in France from April 14 to December 13, 2021. Of 339 patients randomized, 334 were included in the primary analysis—114 patients in the SD-PA group, 110 in the HD-PA, and 110 in the TA. At randomization, 90% of the patients were in the intensive care unit. Data analyses were performed from April 13, 2022, to January 3, 2023.
Interventions
Patients were randomly assigned (1:1:1) to receive either SD-PA, HD-PA, or TA with low-molecular-weight or unfractionated heparin for 14 days.
Main Outcomes and Measures
A hierarchical criterion of all-cause mortality followed by time to clinical improvement at day 28. Main secondary outcome was net clinical outcome at day 28 (composite of thrombosis, major bleeding, and all-cause death).
Results
Among the study population of 334 individuals (mean [SD] age, 58.3 [13.0] years; 226 [67.7%] men and 108 [32.3%] women), use of HD-PA and SD-PA had similar probabilities of favorable outcome (47.3% [95% CI, 39.9% to 54.8%] vs 52.7% [95% CI, 45.2% to 60.1%]; P =
= .48), as did TA compared with SD-PA (50.9% [95% CI, 43.4% to 58.3%] vs 49.1% [95% CI, 41.7% to 56.6%]; P
.48), as did TA compared with SD-PA (50.9% [95% CI, 43.4% to 58.3%] vs 49.1% [95% CI, 41.7% to 56.6%]; P =
= .82) and TA compared with HD-PA (53.5% [95% CI 45.8% to 60.9%] vs 46.5% [95% CI, 39.1% to 54.2%]; P
.82) and TA compared with HD-PA (53.5% [95% CI 45.8% to 60.9%] vs 46.5% [95% CI, 39.1% to 54.2%]; P =
= .37). Net clinical outcome was met in 29.8% of patients receiving SD-PA (20.2% thrombosis, 2.6% bleeding, 14.0% death), 16.4% receiving HD-PA (5.5% thrombosis, 3.6% bleeding, 11.8% death), and 20.0% receiving TA (5.5% thrombosis, 3.6% bleeding, 12.7% death). Moreover, HD-PA and TA use significantly reduced thrombosis compared with SD-PA (absolute difference, −14.7 [95% CI −6.2 to −23.2] and −14.7 [95% CI −6.2 to −23.2], respectively). Use of HD-PA significantly reduced net clinical outcome compared with SD-PA (absolute difference, −13.5; 95% CI −2.6 to −24.3).
.37). Net clinical outcome was met in 29.8% of patients receiving SD-PA (20.2% thrombosis, 2.6% bleeding, 14.0% death), 16.4% receiving HD-PA (5.5% thrombosis, 3.6% bleeding, 11.8% death), and 20.0% receiving TA (5.5% thrombosis, 3.6% bleeding, 12.7% death). Moreover, HD-PA and TA use significantly reduced thrombosis compared with SD-PA (absolute difference, −14.7 [95% CI −6.2 to −23.2] and −14.7 [95% CI −6.2 to −23.2], respectively). Use of HD-PA significantly reduced net clinical outcome compared with SD-PA (absolute difference, −13.5; 95% CI −2.6 to −24.3).
Conclusions and Relevance
This randomized clinical trial found that compared with SD-PA, neither HD-PA nor TA use improved the primary hierarchical outcome of all-cause mortality or time to clinical improvement in patients with hypoxemic COVID-19 pneumonia; however, HD-PA resulted in significantly better net clinical outcome by decreasing the risk of de novo thrombosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT04808882
Introduction
Patients with COVID-19 have heightened thrombotic risk associated with inflammation, platelet activation, and endothelial dysfunction that may eventually lead to organ dysfunction (mostly respiratory distress) and a greater probability of death.1,2 Given that observational data show macrovascular thrombosis in 10% to 30% of patients with COVID-19 receiving standard-dose prophylactic anticoagulation (SD-PA),2,3,4 several institutions have recommended the use of high-dose prophylactic anticoagulation (HD-PA) and therapeutic anticoagulation (TA), either empirically or according to certain criteria, such as body mass index (BMI) or D-dimer concentration.5,6,7
Meta-analyses of randomized clinical trials (RCTs) of noncritically ill8 and critically ill patients9 reported that TA significantly reduced venous thromboembolic risk, with no significant effect on death or organ support. In contrast, escalating heparin dose with HD-PA showed no benefit to thrombosis or mortality in an RCT of noncritically ill patients10 and 2 RCTs of critically ill patients.11,12 However, to our knowledge, no published trials have compared the efficacy of the 3 strategies (SD-PA, HD-PA, TA) in separate groups. In the most recent RCTs that tested TA, the control group consisted of a combination of patients receiving both SD-PA and HD-PA.13,14,15,16,17,18,19
Microvascular thrombosis is another important issue among patients with COVID-19.20 Prompted by endotheliitis and coagulopathy, microvascular thrombosis may lead to respiratory distress and other organ dysfunctions.21,22 Therefore, the use of HD-PA or TA in patients with severe COVID-19 uncomplicated by macrovascular thrombosis could limit the propagation of microvascular thrombosis and forestall lung and multiorgan microcirculatory dysfunction. Conversely, an increase in bleeding with TA in critically ill patients has been reported,19 and autopsy studies have shown evidence of alveolar hemorrhage that may be worsened by escalating the dose of anticoagulant.23
To address these uncertainties, we conducted an RCT of patients with severe COVID-19 who had no macrovascular thrombosis on initial chest computed tomography with pulmonary angiogram (CTPA) to compare outcomes among patients receiving SD-PA, HD-PA, or TA. Our 2 primary hypotheses were (1) TA and HD-PA use would decrease mortality and/or disease duration (measured by respiratory function) compared with SD-PA use, and (2) TA use would outperform HD-PA use in this setting. A secondary objective was to compare the net clinical outcome (a composite of thrombosis, bleeding, and death) among the 3 groups receiving the different dosing levels.
Methods
Study Design
The Anticoagulation COVID-19 (ANTICOVID) trial was an open-label, multicenter, randomized clinical trial that enrolled patients with COVID-19 being treated in 23 health centers in France from April 14 to December 13, 2021 (details on the health centers are available in the eAppendix in Supplement 2). The trial rationale and design have been described previously.24 The first and final versions of the protocol and a summary of changes are available in the Trial Protocol in Supplement 1. A central institutional ethics review board (Comité de Protection des Personnes, Ile-de-France VII, Paris, France) approved this trial (No. 21-019 and No. ID-RCB: 2020-A03531-38) in accordance with the Declaration of Helsinki. Each patient or next of kin provided written informed consent (eMethods 1 in Supplement 2). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patient Sample
All consecutively hospitalized adult (≥18 years) patients with hypoxemic COVID-19 pneumonia per the study definition were eligible for inclusion. The study definition of hypoxemic COVID-19 was a new pulmonary parenchymal infiltrate with a positive reverse transcription polymerase chain reaction for SARS-CoV-2 in the respiratory tract and a score of 5 or higher on the World Health Organization ordinal severity scale (WHO scale; 5 =
= supplemental oxygen required).25 Exclusion criteria included admission to hospital (with WHO scale score of 5) or intensive care unit (ICU; with WHO scale score was ≥6) more than 72 hours prior to being considered for study inclusion, body weight less than 40 kg or more than 100 kg, calculated creatinine clearance rate less than 30 mL/min, clinical need for TA, contraindication to TA, and long-term oxygen supplementation. The full list of eligibility criteria is available in the eMethods 2 in Supplement 2.
supplemental oxygen required).25 Exclusion criteria included admission to hospital (with WHO scale score of 5) or intensive care unit (ICU; with WHO scale score was ≥6) more than 72 hours prior to being considered for study inclusion, body weight less than 40 kg or more than 100 kg, calculated creatinine clearance rate less than 30 mL/min, clinical need for TA, contraindication to TA, and long-term oxygen supplementation. The full list of eligibility criteria is available in the eMethods 2 in Supplement 2.
Randomization and Masking
The CTPA had to be performed during the 72 hours before or up to 24 hours after inclusion (eFigure 1 in Supplement 2). If the CTPA did not show pulmonary artery thrombosis, the patient was randomized to 1 of the 3 anticoagulation regimens. Centralized 1:1:1 ratio block randomization using random block sizes of 6, 9, and 12 was stratified by hospital and according to the following criteria at randomization: invasive mechanical ventilation (yes/no), D-dimer levels (> or ≤3000 ng/mL; to convert to nmol/L, multiply by 4.476), and BMI (> or ≤30; calculated as weight in kilograms divided by height in meters squared).
Trial Interventions
Immediately after randomization, patients began receiving SD-PA, HD-PA (2 ×
× SD-PA dose), and TA using low-molecular-weight heparin (tinzaparin preferred, if available) for 14 days, or until hospital discharge or weaning off of supplemental oxygen for 48 consecutive hours, whichever came first. Details of doses and heparin available products are provided in eMethods 1 in Supplement 2. In the 3 groups, predefined modifications of the anticoagulant were advised in case a patient developed kidney failure, a clinical indication for TA, or required a high-risk bleeding procedure (eMethods 1 in Supplement 2).24 Patient compliance was defined as adherence to assigned treatment for 75% or more of the time recommended by the protocol or until occurrence of thrombosis, major bleeding, death, or hospital discharge, whichever occurred first. In all groups, current recommendations for the management of hypoxemic COVID-19 pneumonia were followed, including the use of dexamethasone.26
SD-PA dose), and TA using low-molecular-weight heparin (tinzaparin preferred, if available) for 14 days, or until hospital discharge or weaning off of supplemental oxygen for 48 consecutive hours, whichever came first. Details of doses and heparin available products are provided in eMethods 1 in Supplement 2. In the 3 groups, predefined modifications of the anticoagulant were advised in case a patient developed kidney failure, a clinical indication for TA, or required a high-risk bleeding procedure (eMethods 1 in Supplement 2).24 Patient compliance was defined as adherence to assigned treatment for 75% or more of the time recommended by the protocol or until occurrence of thrombosis, major bleeding, death, or hospital discharge, whichever occurred first. In all groups, current recommendations for the management of hypoxemic COVID-19 pneumonia were followed, including the use of dexamethasone.26
Primary and Secondary Outcomes
The primary efficacy outcome was a hierarchical criterion assessed at day 28, which included all-cause mortality followed by the time to clinical improvement, and calculated so that death constituted an outcome worse than delay of clinical improvement. The time (days) to clinical improvement was defined as the time from randomization to an improvement of 2 points on a 7-category ordinal scale focused on respiratory function and derived from the WHO scale,25 as proposed by Coa and colleagues27 (eMethods 3 in Supplement 2). For patients discharged from the hospital before day 28, we checked the use of nasal oxygen at home and assumed no other organ support when alive through day 28.
The secondary combined efficacy and safety outcome was the net clinical outcome at day 28, defined as a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter-related deep venous thrombosis), major bleeding (as defined by the International Society on Thrombosis and Hemostasis definition28) or all-cause death (eTable 3 in Supplement 2). The secondary efficacy outcomes at day 28 were the individual components of the hierarchical primary end point, thrombosis, organ failure (assessed by ventilator-, oxygen-, and vasopressors-free days), and hospital and ICU length of stay. The secondary efficacy outcomes at day 90 included all-cause death, hospital and ICU length of stay, and quality of life assessed using a quality-of-life questionnaire (EuroQol 5-Dimension 5-Level29). The exploratory efficacy outcomes at day 7 were D-dimer level and Sepsis-Induced Coagulopathy Score.30 The safety outcomes at day 28 included major bleeding (as defined), life-threatening bleeding,31 and heparin-induced thrombocytopenia. Complete definitions of study outcomes are available in eMethods 3 in Supplement 2.
After randomization, diagnostic investigations were left to the discretion of the treating clinicians. In particular, CTPA was performed on the basis of 1 or more of the following signs: worsening hypoxemia, occurrence or worsening of shock, occurrence or worsening of cor pulmonale, and/or increase of D-dimer level. An independent committee, blinded to the treatment groups, adjudicated thrombosis and safety outcomes.
Statistical Analysis
Sample size calculation for the primary hierarchical end point and all statistical analyses including those used to compare secondary outcomes are detailed in eMethods 4 in Supplement 2. We aimed to include 353 patients so that 300 (100 per group) could be randomized.
Primary end point analyses were performed according to randomization group on intention-to-treat basis and additional supportive analyses were performed on the per-protocol population who did not deviate from the protocol, as defined in eMethods 4 in Supplement 2. For the ranked composite primary end point analysis, each patient was compared with every other patient in the study and assigned a score, as detailed in eMethods 4 in Supplement 2. For each patient, the scores of all pairwise comparisons were summed to obtain a cumulative score, which was compared among the 3 groups using a nonparametric Mann-Whitney test.32 Effect size was reported using the probabilistic index,33 computing 95% CIs using the Newcombe method.34 Information on the primary end point was complete for the intention-to-treat analysis, whereas analyses of secondary end points were performed on a complete case basis, with no imputation of missing information.
All analyses were performed according to a predefined statistical analysis plan (detailed in the trial protocol in Supplement 1) using Stata, version 16.1 (StataCorp) and R, version 4.0.3 (R Foundation for Statistical Computing). Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from April 13, 2022, to January 3, 2023.
.05 were considered statistically significant. Data analyses were performed from April 13, 2022, to January 3, 2023.
Results
From April 14 to December 13, 2021, a total of 1142 patients were screened of whom 353 patients (mean [SD] age, 58.3 [13.1] years; 239 [67.7%] men and 114 [32.3%] women; race and ethnicity information were not collected) were included. Prerandomization CTPA detected pulmonary artery thrombosis in 13 patients, and 1 patient was deemed to be too unstable to undergo CTPA (Figure 1). Accordingly, 339 patients (mean [SD] age, 58.4 [13.0] years; 229 [67.5%] men and 110 [32.5%] women ) were randomized to receive SD-PA (116 patients; 34.2%), HD-PA (111 patients; 32.7%), and TA (112 patients, 33.0%), most with the Delta variant of SARS-CoV-2 (eFigure 2 in Supplement 2).35
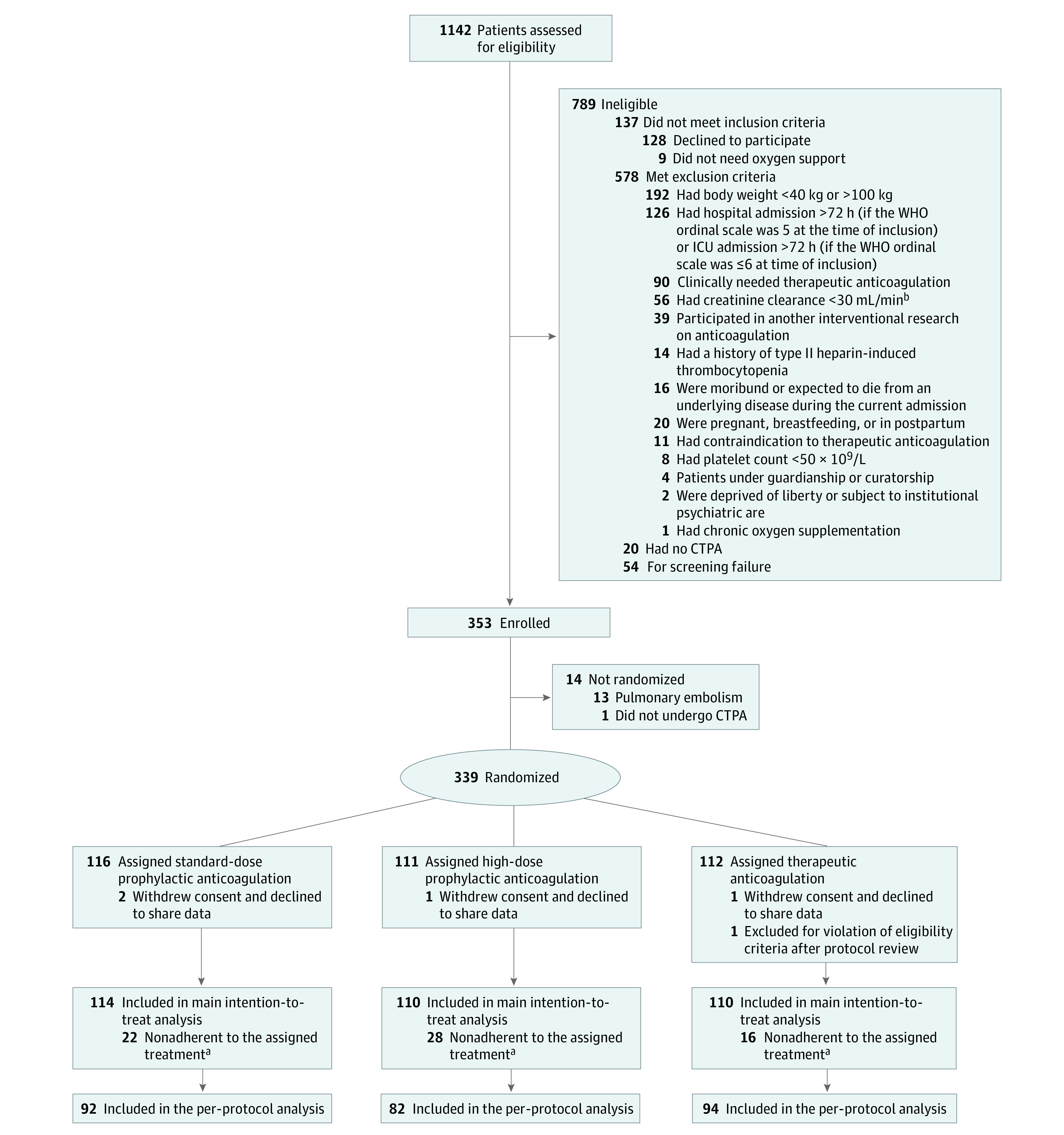
aDefined as spending less than 75% of time on assigned treatment from randomization to day 14 (or until reaching thrombosis, major bleeding, hospital discharge, or weaning of supplemental oxygen for 48 h consecutively, whichever was first).
bTo convert creatinine clearance to mL/s/m2, multiply by 0.0167.
CTPA refers computed tomography with pulmonary angiogram; ICU, intensive care unit; and WHO, World Health Organization.
Of the 339 patients randomized, 5 patients were removed from the main analysis for withdrawal of consent (n =
= 4) or no longer requiring oxygen support (n
4) or no longer requiring oxygen support (n =
= 1); the remaining 334 patients (mean [SD] age, 58.3 [13.0] years; 226 [67.7%] men and 108 [32.3%] women) composed the intention-to-treat population (Table 1). The median (IQR) time from hospital admission to randomization was 2 (1-3) days. At randomization, 299 (89.5%) of the enrolled patients were in the ICU, and 203 (60.8%) patients were on high-flow nasal oxygen (Table 1). Baseline characteristics, including Sepsis-related Organ Failure Assessment score,36 showed no clinically relevant difference among the 3 groups (Table 1; eTable 1 in Supplement 2).
1); the remaining 334 patients (mean [SD] age, 58.3 [13.0] years; 226 [67.7%] men and 108 [32.3%] women) composed the intention-to-treat population (Table 1). The median (IQR) time from hospital admission to randomization was 2 (1-3) days. At randomization, 299 (89.5%) of the enrolled patients were in the ICU, and 203 (60.8%) patients were on high-flow nasal oxygen (Table 1). Baseline characteristics, including Sepsis-related Organ Failure Assessment score,36 showed no clinically relevant difference among the 3 groups (Table 1; eTable 1 in Supplement 2).
Table 1.
| Characteristic | Study group, No. (%) | ||
|---|---|---|---|
| SD-PA | HD-PA | TA | |
| Participants, No. | 114 | 110 | 110 |
| Age, median (IQR), y | 57 (50-67) | 58 (49-68) | 60 (53-70) |
| Men | 71 (65) | 74 (62) | 84 (76) |
| Women | 43 (38) | 26 (24) | 39 (35) |
| Body weight, median (IQR), kg | 82 (74-90) | 80 (74-90) | 80 (70-89) |
| BMI, median (IQR) | 27 (25-31) | 28 (25-30) | 28 (25-31) |
| Symptom duration, median (IQR), d | |||
| Before hospitalization | 8 (6-10) | 7 (6-9) [n = = 109] 109] | 8 (5-10) |
| Before randomization | 10 (8-12) | 10 (8-13) | 9 (7-12) |
| Comorbidities | |||
| Hypertension | 29 (25) | 34 (31) | 42 (38) |
| Diabetes | 19 (17) | 16 (14) | 26 (24) |
| COPD | 4 (3) | 4 (4) | 4 (4) |
| Coronary artery disease | 4 (3) | 2 (2) | 8 (7) |
| Chronic heart failure | 1 (<1) | 2 (2) | 0 |
| Chronic kidney disease | 5 (4) | 2 (2) | 0 |
| Cirrhosis | 1 (<1) | 1 (<1) | 0 |
| History of venous thrombotic event | 2 (2) | 1 (<1) | 2 (2) |
| History of arterial thrombotic event | 2 (2) | 1 (<1) | 2 (2) |
| History of substantial bleeding | 0 | 1 (<1) | 0 |
| Active cancer | 10 (9) | 4 (4) | 11 (10) |
| SOFA, median (IQR) a | 3 (3-4) | 4 (3-5) | 4 (3-4) |
| Laboratory parameters | |||
| PaO2/FiO2 ratio | |||
| No. | 93 | 95 | 91 |
| Median (IQR) | 115 (82-158) | 112 (77-159) | 107 (75-154) |
| Bilirubin, μmol/L | |||
| No. | 95 | 94 | 84 |
| Median (IQR) | 8 (6-12) | 8 (7-12) | 9 (6-12) |
| Platelet count, 109/L | |||
| No. | 106 | 102 | 105 |
| Median (IQR) | 237 (189-311) | 239 (186-329) | 241 (205-298) |
| Creatinine, μmol/L | |||
| No. | 108 | 104 | 101 |
| Median (IQR) | 68 (58-84) | 72 (59-86) | 64 (56-84) |
| Prothrombin level | |||
| No. | 91 | 91 | 89 |
| Median (IQR) | 92 (82-100) | 87 (80-96) | 88 (78-96) |
| D-dimer, median (IQR), ng/mLb | 1020 (700-1725) | 1043 (760-2000) | 1194 (710-1730) |
| >3000 ng/mLb | 14 (12) | 11 (10) | 12 (11) |
| Affected lung parenchyma, %c | |||
| No. | 113 | 110 | 109 |
| Median (IQR) | 50 (50-75) | 50 (40-75) | 50 (30-70) |
| Medication | |||
| Glucocorticoid | 103 (90) | 105 (95) | 100 (91) |
| Hydroxychloroquine | 0 | 1 (<1) | 1 (<1) |
| Remdesivir | 1 (<1) | 0 | 1 (<1) |
| Tocilizumab | 29 (25) | 27 (24) | 28 (25) |
| ICU admission | 101 (89) | 101 (92) | 97 (88) |
| Respiratory support | |||
| Mask/nasal catheter | 28 (25) | 23 (21) | 26 (24) |
| High-flow oxygen | 68 (60) | 67 (61) | 68 (62) |
| Noninvasive mechanical ventilation | 8 (7) | 8 (7) | 6 (5) |
| Invasive mechanical ventilation | 10 (9) | 12 (11) | 10 (9) |
| Vasopressor | 5 (4) | 4 (4) | 2 (2) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; CTPA, chest computed tomography with pulmonary angiogram; HD-PA, high-dose prophylactic anticoagulation; ICU, intensive care unit; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; SD-PA, standard-dose prophylactic anticoagulation; SOFA, Sepsis-related Organ Failure Assessment; TA, therapeutic anticoagulation.
The first anticoagulation dose was administered per protocol in more than 90% of patients in all 3 groups (eTable 2 in Supplement 2 provides details on heparin intake). Patient compliance was high (≥75%) in all groups (P =
= .22; eTable 2 in Supplement 2).
.22; eTable 2 in Supplement 2).
Primary Efficacy Outcome and Its Individual Components
In the intention-to-treat population, the ranked composite primary outcome through day 28 was not significantly different among the groups. The HD-PA and SD-PA groups had similar probability of a more favorable outcome; this was also true for the TA group compared with the SD-PA group, and for the TA group compared with the HD-PA group (Table 2; eTable 6 in Supplement 2). The results of the individual components of the primary outcome were consistent with the primary analysis (Table 2).
Table 2.
| Outcome | No. (%) | HD-PA vs SD-PA | TA vs SD-PA | TA vs HD-PA | |||||
|---|---|---|---|---|---|---|---|---|---|
SD-PA (n = = 114) 114) | HD-PA (n = = 110) 110) | TA (n = = 110) 110) | Absolute difference (95% CI) | P value | Absolute difference (95% CI) | P value | Absolute difference (95% CI) | P value | |
| Primary outcome | |||||||||
| Probability of a more favorable outcome, effect size (95% CI), %a | NAb | NAb | NAb | 47.3 (39.9 to 54.8) | .48 | 50.9 (43.4 to 58.3) | .82 | 53.5 (45.8 to 60.9) | .37 |
| Efficacy outcomes | |||||||||
| Composite thrombotic eventc | 23 (20)d | 6 (5) | 6 (5) | −14.8 (−6.2 to −23.2) | .001 | −14.7 (−6.2 to −23.2) | .001 | 0.0 (6.0 to −6.0) | > .99 .99 |
| Venous thrombosis | 20 (18) | 5 (5) | 4 (4) | −13.0 (−5.0 to −21.0) | .002 | −13.9 (−6.1 to −21.7) | .001 | −0.9 (4.3 to −6.1) | > .99 .99 |
| DVT (including CVC-related) | 7 (6) | 2 (2) | 1 (1) | −4.3 (0.7 to −9.4) | .17 | −5.2 (−0.5 to −10.0) | .07 | −0.9 (2.2 to −4.0) | > .99 .99 |
| Pulmonary artery thrombosis | 15 (13) | 3 (3) | 3 (3) | −10.4 (−3.5 to −17.3) | .006 | −10.4 (−3.5 to −17.3) | .006 | 0.0 (4.3 to −4.3) | > .99 .99 |
| Arterial thrombosis | 5 (4) | 1 (1) | 2 (2) | −3.5 (0.7 to −7.6) | .21 | −2.6 (1.9 to −7.1) | .45 | 0.9 (4.0 to −2.2) | > .99 .99 |
| All-cause death | 16 (14) | 13 (12) | 14 (13) | −2.2 (6.6 to −11.0) | .69 | −1.3 (7.6 to −10.2) | .85 | 0.9 (9.6 to −7.8) | > .99 .99 |
| Time to clinical improvement, de | 8 (5-13) | 9 (6-14) | 8 (5-14) | 0.0 (2.0 to −1.0) | .81 | −1.0 (1.0 to −2.0) | .52 | −1.0 (1.0 to −2.0) | .37 |
| Supplemental oxygen-free, d | 18 (0-23) | 16 (0-22) | 18 (0-23) | 0.0 (1.0 to −2.0) | .38 | 0.0 (1.0 to −1.0) | .71 | 0.0 (2.0 to −1.0) | .65 |
| Ventilator-free, d | 28 (15-28) | 28 (14-28) | 28 (11-28) | 0 | .82 | 0 | .76 | 0 | .94 |
| Vasopressor-free, d | 28 (26-28) | 28 (27-28) | 28 (26-28) | 0 | .98 | 0 | .89 | 0 | .87 |
| Sepsis-induced coagulopathy score at d 7f | |||||||||
| No. | 38 | 36 | 41 | NA | NA | NA | NA | NA | NA |
| Median (IQR) | 2 (2-2) | 2 (2-2) | 2 (2-3) | 0 | .64 | 0 | 1.1 | 0 | .26 |
| D-dimer at d 7, ng/mLg | |||||||||
| No. | 58 | 58 | 56 | NA | NA | NA | NA | NA | NA |
| Median (IQR) | 1898 (1140-5229) | 1981 (994-5220) | 1645 (825-3244) | −180.0 (510.0 to −935.0) | .68 | −583.0 (40.0 to −1327.0) | .06 | −410.0 (190.0 to −1150.0) | .18 |
| ICU stay, d | 15 (9-22) | 17 (11-30) | 14 (9-27) | 2.0 (5.0 to −1.0) | .19 | 0.0 (3.0 to −3.0) | .75 | −2.0 (1.0 to −5.0) | .24 |
| Hospital stay, d | 14 (9-22) | 16 (10-28) | 14 (8-24) | 2.0 (4.0 to −1.0) | .18 | 0.0 (2.0 to −3.0) | .87 | −2.0 (0.0 to −5.0) | .09 |
| EQ-5D-5L index value, d 90h | |||||||||
| No. | 60 | 71 | 70 | NA | NA | NA | NA | NA | NA |
| Median (IQR) | 1 (1-1) | 1 (1-1) | 1 (1-1) | 0 | .68 | 0 | .42 | 0 | .48 |
| EQ-5D-5L score at d 90i | |||||||||
| No. | 57 | 69 | 64 | NA | NA | NA | NA | NA | NA |
| Median (IQR) | 80 (70-90) | 80 (70-90) | 80 (60-90) | 0.0 (5.0 to −5.0) | .82 | −5.0 (0.0 to −10.0) | .19 | −5.0 (0.0 to −10.0) | .16 |
| All-cause death at d 90 | 22 (19) | 22 (20) | 18 (17) | 0.7 (11.1 to −9.7) | > .99 .99 | −2.6 (7.5 to −12.7) | .73 | −3.3 (6.9 to −13.6) | .60 |
| Safety outcomes | |||||||||
| Major bleedingj | 3 (3) | 4 (4) | 4 (4) | 1.0 (5.6 to −3.6) | .72 | 1.0 (5.6 to −3.6) | .72 | 0.0 (4.9 to −4.9) | > .99 .99 |
| Efficacy and safety outcomes | |||||||||
| Net clinical outcomek | 34 (30) | 18 (16) | 22 (20) | −13.5 (−2.6 to −24.3) | .02 | −9.8 (1.4 to −21.1) | .12 | 3.6 (13.8 to −6.5) | .60 |
Abbreviations: CVC, central venous catheter; DVT, deep vein thrombosis; EQ-5D-5L, Euroqol 5-dimension 5-level; HD-PA, high-dose prophylactic anticoagulation; ISTH, International Society on Thrombosis and Hemostasis; SD-PA, standard-dose prophylactic anticoagulation; SOFA, Sepsis-related Organ Failure Assessment; TA, therapeutic anticoagulation.
 =
= .33).
.33).Net Clinical Outcome and Its Individual Components
The net clinical outcome was significantly reduced in the HD-PA group compared with the SD-PA group, whereas there was no significant difference between the TA and SD-PA groups, or between the HD-PA and the TA groups (Table 2; Figure 2; eFigure 4 in Supplement 2). The rate of thrombosis was significantly reduced (× 4 less) in the HD-PA and TA groups compared with the SD-PA group (Table 2). The CTPA screening for pulmonary artery thrombosis from randomization to day 28 was performed in 69 of 334 (21%) patients and the rate of positive CTPA from randomization to day 28 was significantly higher for the SD-PA group compared with the HD-PA and TA groups (eFigure 3 in Supplement 2). Most of the 21 cases of pulmonary artery thromboses were truncular or lobar (eTable 4 in Supplement 2). Major bleeding occurred in 3 (2.2%), 4 (3.6%), and 4 (3.6%) patients in the SD-PA, HD-PA, and TA groups, respectively, and included 3 fatal bleedings: 1 spinal bleeding in the HD-PA group and 1 hemoptysis and 1 gastrointestinal bleeding in the SD-PA group (eTable 5 in Supplement 2). The overlapping numbers of patients with thrombosis, major bleeding, and death of the 3 groups are presented in eFigure 4 in Supplement 2. There were no significant differences in other secondary efficacy and safety outcomes among the 3 groups, nor in all-cause death and quality of life at day 90 (Table 2).
4 less) in the HD-PA and TA groups compared with the SD-PA group (Table 2). The CTPA screening for pulmonary artery thrombosis from randomization to day 28 was performed in 69 of 334 (21%) patients and the rate of positive CTPA from randomization to day 28 was significantly higher for the SD-PA group compared with the HD-PA and TA groups (eFigure 3 in Supplement 2). Most of the 21 cases of pulmonary artery thromboses were truncular or lobar (eTable 4 in Supplement 2). Major bleeding occurred in 3 (2.2%), 4 (3.6%), and 4 (3.6%) patients in the SD-PA, HD-PA, and TA groups, respectively, and included 3 fatal bleedings: 1 spinal bleeding in the HD-PA group and 1 hemoptysis and 1 gastrointestinal bleeding in the SD-PA group (eTable 5 in Supplement 2). The overlapping numbers of patients with thrombosis, major bleeding, and death of the 3 groups are presented in eFigure 4 in Supplement 2. There were no significant differences in other secondary efficacy and safety outcomes among the 3 groups, nor in all-cause death and quality of life at day 90 (Table 2).
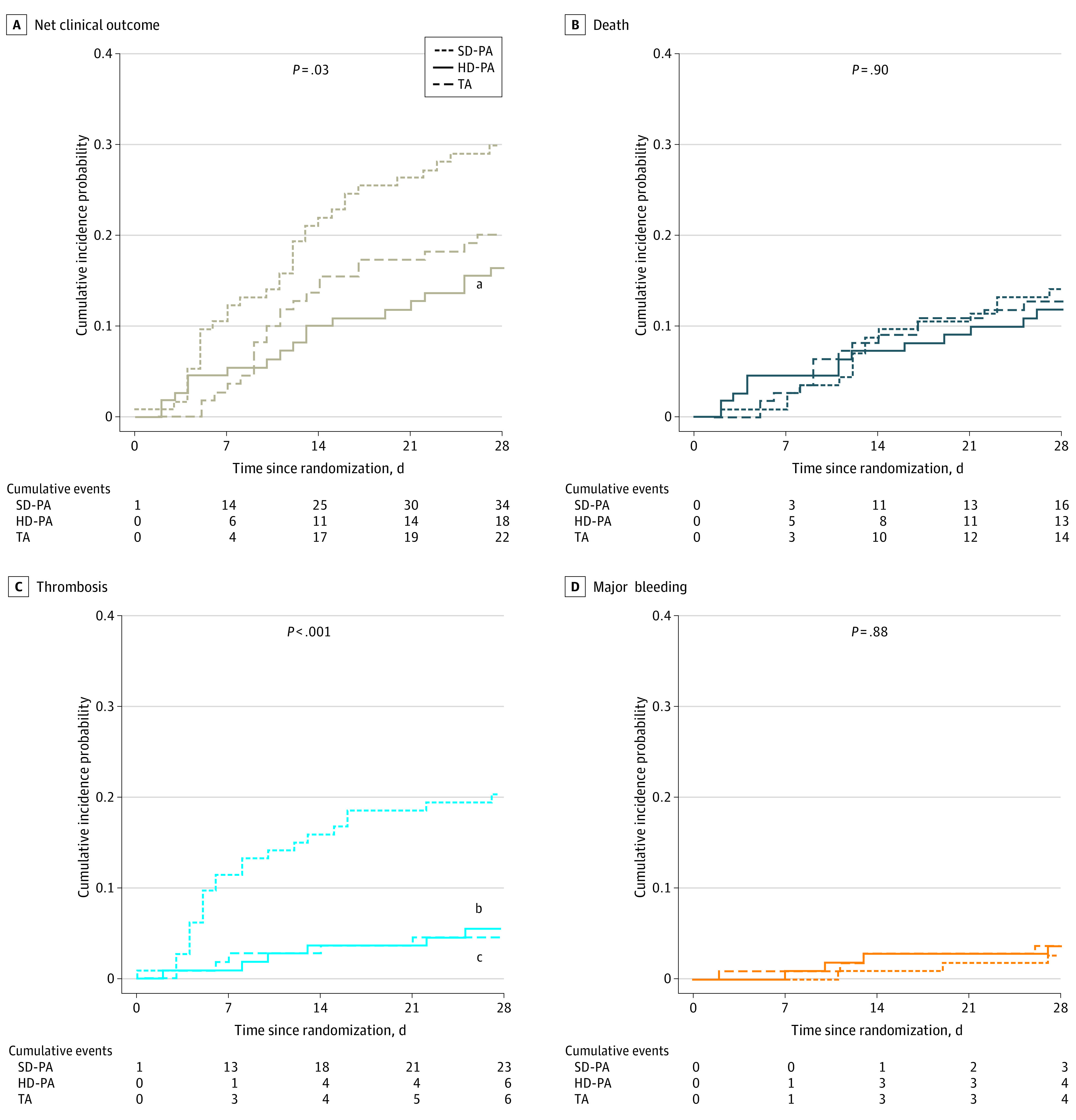
A, Cumulative net clinical outcome curves, including any composite thrombotic event, major bleeding, and all-cause death. B, Cumulative mortality curves. C, Cumulative thrombosis curves. D, Cumulative major bleeding curves. P-values shown in the graphs are for global comparison of cumulative incidence curves using Gray test. HD-PA refers to high-dose prophylactic anticoagulation; SD-PA, standard-dose prophylactic anticoagulation; and TA therapeutic anticoagulation.
aP =
= .01 for comparison of HD-PA vs SD-PA.
.01 for comparison of HD-PA vs SD-PA.
bP <
< .001 for comparison of HD-PA vs SD-PA.
.001 for comparison of HD-PA vs SD-PA.
cP <
< .001 for comparison of TA vs SD-PA.
.001 for comparison of TA vs SD-PA.
Subgroup and Sensitivity Analyses
Results of the primary outcome were consistent across all prespecified subgroups, in patients hospitalized in the ICU at randomization (eTable 6 in Supplement 2), and when considering home nasal oxygen after hospital discharge, ie, classifying those patients as 4 in the 7-category ordinal scale27 (eTable 7 in Supplement 2). The reduction in net clinical outcome obtained with HD-PA use compared with SD-PA use was also consistent in patients who were in the ICU at randomization (eTable 8 in Supplement 2). Findings of the per-protocol analysis were similar to those of the intention-to-treat analysis (eTable 9 in Supplement 2).
Discussion
In this trial using separate groups to compare 3 regimens of anticoagulation therapy in patients with hypoxemic COVID-19 pneumonia (90% of critically ill patients) and with no macrovascular thrombosis at inclusion, a 14-day course of HD-PA or TA did not improve the primary hierarchical criterion (death and time to clinical improvement) more than SD-PA. However, HD-PA use provided the best net benefit, driven by a 4-fold reduction in de novo thrombosis (mostly, pulmonary artery) compared with SD-PA use, with no increase in major bleeding; and TA use did not provide additional benefit compared with HD-PA use.
Microvascular Thrombosis and Organ Failure
Escalating anticoagulation dose did not improve survival or disease resolution. These findings support those of previous RCTs conducted in critically ill patients. An international multiplatform RCT found that heparin TA use did not improve the primary outcome of organ support–free days at day 21 compared with mixed PA (SD-PA/HD-PA, per local practice) use .17 In the INSPIRATION trial,11 HD-PA use did not improve the primary composite outcome of thrombosis, indication for extracorporeal membrane oxygenation, or mortality within 30 days compared with SD-PA use. Similarly, a recent meta-analysis of 3220 noncritically ill patients reported that TA use compared with mixed PA13,14,15 (SD-PA/HD-PA per local practice, BMI, or creatinine clearance) or SD-PA use, had no significant effect on all-cause death or progression to invasive mechanical ventilation.37 Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated.
De Novo Macrovascular Thrombosis
The 20% rate of macrovascular thrombosis in the SD-PA group, of whom 90% were patients in the ICU, is consistent with that of previous observational studies of critically ill patients treated with SD-PA.3,4 Our results suggest that (1) HD-PA use, given its 4-fold reduction thrombosis risk compared with SD-PA use, is the lowest effective dose to prevent de novo macrovascular thrombosis, and (2) TA use does not outperform HD-PA use in lowering thrombotic risk. Our results concur with those of a multiplatform trial17 and the COVID-PACT trial19 of critically ill patients, and with the meta-analysis of Ena and Valls8 that showed that TA reduced macrovascular thrombosis. Of note, 4 of 5 RCTs included in the meta-analysis enrolled patients with high D-dimer levels.14,15,37,38 However, 2 recent RCTs in critically ill patients reported no benefit of HD-PA use to prevent macrovascular thrombosis.11,12 The very low incidence of thrombosis in those studies’ control groups, possibly because screening for thrombosis using CTPA was rarely performed11 (4% of patients vs 21% in our trial), may explain the discrepancies.
Bleeding and Net Benefit
The rate of major bleeding in our study was low irrespective of the anticoagulation dose regimen, and similar to that reported in previously published RCTs.11,12,13,14,15,16,17 To minimize the bleeding risk, we did not include patients with severe kidney failure (entangled as an independent risk factor for bleeding in critically ill patients)39 or at the extreme ends of body weight (<40 kg or >100 kg, for whom low-molecular-weight heparin dosage has not been assessed). Although the limited sample size does not allow for definitive conclusions regarding the bleeding profile of the 3 anticoagulation regimens, our study provides additional evidence for the safety of escalating anticoagulant dose to prevent macrovascular thrombosis in patients with a low baseline bleeding risk. Compared with SD-PA, HD-PA provided a significantly better net benefit (lower net clinical outcome for thrombosis, major bleeding, or death).
Strengths and Limitations
The strengths of our study were its design—a comparison of 3 distinct anticoagulation strategies to explore the lowest effective dose—the systematic exclusion of macrothrombosis at randomization, and the selection of a population with a low risk of bleeding.
Our study had several limitations. First, anticoagulation assignment was open-label given the overburdened health system during the COVID-19 pandemic. Performance bias cannot be denied because time to clinical improvement can be subjective. Likewise, detection bias cannot be denied because potential events (especially incidental thromboses) were less likely to be investigated in patients treated with TA or HD-PA rather than SD-PA. However, the risk of detection bias regarding thrombosis is low because the rate of positive CTPA was significantly higher in the SD-PA group than in the HD-PA and TA groups, whereas the rate of negative CTPA was similar in the 3 groups. Second, a relatively small number of patients were randomized, based on assumptions and power calculations for the primary hierarchical outcome. Therefore, the study was not specifically powered to detect effect sizes regarding prespecified secondary outcomes, including clinical net benefit, which may have limited power and enlarged confidence intervals. Third, the rate of pulmonary artery thrombosis detected on prerandomization CTPA was lower than expected. This discrepancy may be explained by the disease time course because the prerandomization CTPA was typically performed early and the likelihood of positive CTPA increases with disease duration.40,41,42 Fourth, most patients had the Delta variant of SARS-CoV-2, in contrast with previous reports. This discrepancy may explain the lower-than-expected mortality rate during follow-up, which likely affected the outcomes assessment. Fifth, all of the study patients (admitted to ICU or not) were oxygen-treated, which could make the comparison with other RCTs, which were strictly limited to critically ill or noncritically ill patients, difficult. However, our study results are consistent regarding the patients admitted to ICU and they represented 90% of our sample. Sixth, only 10% of patients were hospitalized in regular wards, and we did not include patients with extremely high or low body weight or kidney failure, which limits the generalizability of the results to all COVID-19 inpatients. Finally, we did not quantify microvascular thrombosis on CTPA. Given the huge workload during the COVID-19 pandemic, D-dimer levels and sepsis-induced coagulopathy scores on day 7 were seldomly collected and therefore hindered the analysis of those data.
Conclusions
The results of the ANTICOVID RCT indicate that neither HD-PA nor TA use improved the primary hierarchical outcomes—death and time to clinical improvement—compared with SD-PA use among patients with hypoxemic COVID-19 pneumonia and with no initial macrovascular thrombosis. However, HD-PA use compared with SD-PA use was associated with a better net clinical benefit that was driven by a significantly lower rate of thrombosis and with a low risk of major bleeding. There was no additional benefit of TA use compared with HD-PA use. The results of the main analysis were consistent with the findings in the subgroup of critically ill patients, which represented 90% of our study population.
Notes
Supplement 2.
eAppendix. Investigators and Committees
eMethods 1. Study Design, Interventions and Products
eMethods 2. Eligibility Criteria
eMethods 3. Definitions of Outcome Events
eMethods 4. Statistical Aspects
eFigure 1. Study Design
eFigure 2. Proportion of Variant of Concern in France during the Study Period of Inclusion
eFigure 3. Computed Tomography with Pulmonary Angiogram Screening for Thrombosis Performed from Randomization to Day 28
eFigure 4. Net Clinical Outcome
eTable 1. Baseline Characteristics and Medication
eTable 2. Compliance with Study Protocol
eTable 3. Secondary Efficacy and Safety Outcomes
eTable 4. Anatomical Localization of Adjudicated Pulmonary Artery Thrombosis
eTable 5. Description of Major Bleeding Events at Day 28 as per ISTH Definition
eTable 6. Intention-to-treat and Subgroup Analysis of the Primary Outcome
eTable 7. Day 28 Outcomes taking onto account home nasal oxygen after hospital discharge
eTable 8. Day 28 Secondary Outcomes of the Intensive Care Unit Subgroup
eTable 9. Day 28 Outcomes in the Per-Protocol Cohort
eReferences
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamainternmed.2023.0456
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2802821/jamainternal_labb_2023_oi_230012_1679077031.66451.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/144174070
Article citations
Alteplase in COVID-19 severe hypoxemic respiratory failure: the TRISTARDS multicenter randomized trial.
Ann Intensive Care, 14(1):170, 10 Nov 2024
Cited by: 0 articles | PMID: 39522090 | PMCID: PMC11551089
The COVID-19 thrombus: distinguishing pathological, mechanistic, and phenotypic features and management.
J Thromb Thrombolysis, 23 Aug 2024
Cited by: 0 articles | PMID: 39179952
Review
Appropriate thromboprophylaxis strategy for COVID-19 patients on dosage, antiplatelet therapy, outpatient, and postdischarge prophylaxis: a meta-analysis of randomized controlled trials.
Int J Surg, 110(6):3910-3922, 01 Jun 2024
Cited by: 1 article | PMID: 38549227
Vascular Alterations Following COVID-19 Infection: A Comprehensive Literature Review.
Life (Basel), 14(5):545, 24 Apr 2024
Cited by: 0 articles | PMID: 38792566 | PMCID: PMC11122535
Review Free full text in Europe PMC
Efficacy and Safety of Intensified vs Standard Prophylactic Anticoagulation Therapy in Patients Hospitalized With Coronavirus Disease 2019: Updated Systematic Review and Meta-analysis.
Open Forum Infect Dis, 10(11):ofad506, 10 Oct 2023
Cited by: 0 articles | PMID: 37953813 | PMCID: PMC10633781
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04808882
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial.
JAMA, 325(16):1620-1630, 01 Apr 2021
Cited by: 399 articles | PMID: 33734299 | PMCID: PMC7974835
Comparison of standard prophylactic, intermediate prophylactic and therapeutic anticoagulation in patients with severe COVID-19: protocol for the ANTICOVID multicentre, parallel-group, open-label, randomised controlled trial.
BMJ Open, 12(4):e059383, 26 Apr 2022
Cited by: 1 article | PMID: 35473740 | PMCID: PMC9044512
Cov-hep study: heparin in standard anticoagulation based on citrate for continuous veno-venous hemodialysis in patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial.
Trials, 21(1):920, 11 Nov 2020
Cited by: 4 articles | PMID: 33176886 | PMCID: PMC7656196
Efficacy and safety of heparin full-dose anticoagulation in hospitalized non-critically ill COVID-19 patients: a meta-analysis of multicenter randomized controlled trials.
J Thromb Thrombolysis, 54(3):420-430, 03 Aug 2022
Cited by: 13 articles | PMID: 35922578 | PMCID: PMC9362611
Review Free full text in Europe PMC
 1
,
2
,
3
1
,
2
,
3




