Abstract
Background
Blood platelets are known to play a role in the development of atherosclerotic disease, thrombi, and hemostasis. Investigation of blood platelet transcriptome could provide evidence of disorders that increase vulnerability to cardiovascular disease. However, research on the molecular insights of platelet activation in patients with periodontitis and patients with periodontitis and type 2 diabetes mellitus (DM) is still lacking.Objectives
In this study, we analyzed expression in blood platelets from patients with periodontitis and patients with concurrent periodontitis and DM to examine the transcriptomic profile of platelets induced by periodontitis and the modifying effects of DM.Methods
We obtained the transcriptional profiles of blood platelets from 11 healthy donors, 10 patients with periodontitis, and 6 patients with periodontitis and DM using single-cell RNA sequencing. The biological processes and coexpressed modules of transcriptionally altered genes were further explored.Results
Both the patients with periodontitis and DM and those with periodontitis without DM showed higher levels of platelet activation and coagulation signals than the healthy individuals. Platelets from the patients with periodontitis had higher expression levels of genes for RHO GTPase effectors, whereas platelets from the patients with periodontitis and DM demonstrated higher expression of genes involved in oxidative phosphorylation and cellular responses to stress than those from the controls. However, compared with the patients with only periodontitis, those with periodontitis and DM presented a lower expression level of genes for hemostasis and platelet receptors.Conclusion
These results suggest that periodontitis contributes to establishment of blood coagulation via platelet dysregulation, whereas the comorbidities of patients with periodontitis and DM impair the components of platelets, thus preventing normal functions.Free full text

Single-cell analysis of platelets from patients with periodontitis and diabetes
Abstract
Background
Blood platelets are known to play a role in the development of atherosclerotic disease, thrombi, and hemostasis. Investigation of blood platelet transcriptome could provide evidence of disorders that increase vulnerability to cardiovascular disease. However, research on the molecular insights of platelet activation in patients with periodontitis and patients with periodontitis and type 2 diabetes mellitus (DM) is still lacking.
Objectives
In this study, we analyzed expression in blood platelets from patients with periodontitis and patients with concurrent periodontitis and DM to examine the transcriptomic profile of platelets induced by periodontitis and the modifying effects of DM.
Methods
We obtained the transcriptional profiles of blood platelets from 11 healthy donors, 10 patients with periodontitis, and 6 patients with periodontitis and DM using single-cell RNA sequencing. The biological processes and coexpressed modules of transcriptionally altered genes were further explored.
Results
Both the patients with periodontitis and DM and those with periodontitis without DM showed higher levels of platelet activation and coagulation signals than the healthy individuals. Platelets from the patients with periodontitis had higher expression levels of genes for RHO GTPase effectors, whereas platelets from the patients with periodontitis and DM demonstrated higher expression of genes involved in oxidative phosphorylation and cellular responses to stress than those from the controls. However, compared with the patients with only periodontitis, those with periodontitis and DM presented a lower expression level of genes for hemostasis and platelet receptors.
Conclusion
These results suggest that periodontitis contributes to establishment of blood coagulation via platelet dysregulation, whereas the comorbidities of patients with periodontitis and DM impair the components of platelets, thus preventing normal functions.
1. Introduction
Blood platelets are major components in the formation of thrombi and hemostasis, and platelet aggregation is a key event in the development and progression of many atherosclerotic diseases, including angina pectoris, myocardial infarction, and chronic ischemic heart disease. [1] These diseases are fatal, with sudden events that limit blood flow through the formation of intraluminal thrombi in ruptured coronary atherosclerotic plaques. Accordingly, thrombotic risk assessment is important, and platelet count and activity are routinely analyzed under various conditions. However, the validity of platelet indices, such as platelet size, shape, and aggregation, is limited, and they cannot reflect complicated signaling during platelet activation and interaction with other vascular and inflammatory cells. [2] Knowledge of transcripts in platelets can provide useful insights into serious diseases, in that, transcriptomics offers a snapshot of the landscape of RNA. [3] Studies on platelet transcriptome profiling are limited and generally based on the definition of platelets as anucleate cells. Platelet transcriptomes in various diseases have been investigated following the identification that platelets can regulate transcripts or protein expression and that activated platelets release various cytokines, which play a role in platelet aggregation and thrombin production. Studies have previously reported that alterations in platelet transcriptome occur in various diseases such as coronary events, atrial fibrillation [4], and systemic lupus erythematosus. [[5], [6], [7]] Therefore, information on the expression of platelet genes involved in coagulation or hemostasis-related functions may suggest a possible correlation between periodontitis and cardiovascular diseases.
Numerous epidemiologic and interventional studies have indicated that periodontitis is potentially associated with cardiovascular diseases. [[8], [9], [10]] Considering the correlation among periodontitis, atherosclerotic disease, and platelet activity, periodontitis may play a role in the progression of atherothrombotic diseases by activating platelet function. Many studies have previously identified increased platelet counts and indices in patients with periodontitis, supporting the idea that periodontitis may be a contributing factor in platelet activation. However, because previous studies have examined only a few platelet activation–related genes that can impact platelet physiology, they could not provide a thorough understanding of the role of periodontitis in platelet activation. [[8], [9], [10], [11]]
Moreover, periodontitis increases the susceptibility of patients to several diseases, with type 2 diabetes mellitus (DM) being 1 such example. [12] The relationship between the 2 diseases is bidirectional, which implies that the presence of 1 condition enhances the appearance of the other. [13,14] Patients with DM show worse periodontal status, with a deeper periodontal pocket and higher attachment loss; additionally, DM elevates the risk of development of periodontitis by 34%. [15] Moreover, periodontitis increases the susceptibility of patients to diabetes by 3 folds, and periodontal treatment improves glycemic control in patients with DM. Because the 2 diseases are highly correlated, it is crucial to understand and compare platelet activity between patients with periodontitis and those with periodontitis and DM to prevent the development of another disease caused by platelets and offer novel treatment strategies.
Herein, we performed single-cell RNA sequencing (scRNA-seq) to characterize, for the first time, the transcriptome of isolated peripheral blood platelets from patients with periodontitis and those with periodontitis and DM (PDDM). We identified the platelet transcriptome to gain insights into the effects of complications of periodontitis and DM on genetic profiling of platelets from patients with periodontitis and further reveal the factors responsible for this alteration.
2. Methods
2.1. Study participants and criteria
All individuals who participated in this study were informed both verbally and using a written statement about the use of their peripheral blood samples. The participants subsequently signed an informed consent form that was approved by the Institutional Review Board of Pusan National University Dental Hospital (IRB No. PNUDH-2020-001). Based on the medical history obtained through a written questionnaire and interview, systemically healthy people with or without periodontitis and patients with periodontitis and DM were selected as research subjects. The presence of periodontitis was evaluated by a periodontal specialist following the guidelines of the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions. [16] Only patients with moderate periodontitis were recruited in this study. In addition, to clearly define the effect of DM on platelets, the hemoglobin A1C (HbA1C) levels of patients with PDDM, who had already been diagnosed at an internal medicine clinic, were repeatedly measured before the study and confirmed using both HbA1C and periodontal evaluations. Healthy periodontal tissue was characterized by a pocket depth of <3 mm and no signs of clinical inflammation, such as redness, swelling, or bleeding, on probing. The exclusion criteria were as follows: systemic disease other than DM, periodontal treatment within the last 6 months, and antibiotic prescription within the last 6 weeks.
2.2. Collection and isolation of peripheral blood mononuclear cells (PBMCs)
Venous blood was obtained using routine venipuncture and collected in plastic tubes containing EDTA. PBMCs containing platelets were isolated within 30 minutes of collection using SepMate (Stemcell Technologies) according to the manufacturer’s instructions. In brief, a density-gradient medium was added to the insert, with an equal volume of blood samples diluted with phosphate-buffered saline and 2% fetal bovine serum added to the SepMate tube. The tubes were then centrifuged at 1200 g for 10 minutes at room temperature. The top layers were poured into new tubes and washed twice with phosphate-buffered saline containing 2% fetal bovine serum. The tubes were then centrifuged at 120 g for 10 minutes at room temperature. The collected PBMCs, including platelets, were frozen and stored at −80 °C prior to use.
2.3. Preprocessing of scRNA-seq
Libraries for scRNA-seq were produced using the chromium controller according to the 10× chromium Next GEM Single Cell 3’ v3.1 protocol. The complementary DNA library was magnified and sequenced on the Illumina HiSeq platform following cell suspension mixing and loading on Single Cell 3’ v3.1 gel beads. The 10× Genomics Cell Ranger, version 5.0.1, was used to process single-cell gene expression, and the command “cellranger mkfastq” was used to create FASTQ files. From these FASTQ files, a count matrix was generated for each sample by mapping to the human genome reference GrCh38. The count matrix consisted of gene expression by barcodes for each cell present in the sample.
2.4. Quality control and filtering the dataset
We used Seurat (version 4.1.1) to merge and filter the resulting scRNA-seq lists and DoubletFinder to identify and eliminate doublet cells for all samples. [17,18] Cells with <500 or >15,000 unique molecular identifiers and over 15% of mitochondrial reads were removed. Cells containing 250 to 5000 genes were preserved. The filtered dataset was normalized using SCTransform, and the samples were integrated using the SelectIntegrationFeatures, PrepSCTIntegration, FindIntegrationAnchors, and IntegrateData functions.
2.5. Platelet identification
Principal component analysis and t-stochastic neighbor embedding were performed to reduce the dimension and improve visualization. Nearest neighbors were computed on 20 principal components, and cells were grouped using the FindClusters function, which identifies 37 populations. Marker genes for each population were detected using the DEsingle package to match the criteria of a false discovery rate–adjusted P of <.01. [19] Platelet clusters displaying high expression levels of the canonical markers proplatelet basic protein (PPBP), glycoprotein IX platelet (GP9), platelet factor 4 (PF4), integrin subunit alpha 2b (ITGA2B), integrin subunit beta 3 (ITGB3), and glycoprotein Ib platelet subunit alpha (GP1BA) were investigated for further analysis.
2.6. Identification of differentially expressed genes (DEGs) and gene enrichment analysis
Platelets were extracted, and DEGs were analyzed using the DEsingle package. [19] We subsequently compared the PD and PDDM group with the healthy group and the PDDM group with PD group. DEGs with an absolute log2 fold change of >0.3, false discovery rate–adjusted P of <.05, and expression of at least 10% platelets in the comparison group were considered significant. Using focused DEGs, functional analyses, including gene overrepresentation analysis carrying gene ontology biological processes and functional enrichment analysis, were performed and visualized using the Metascape gene enrichment analysis tool (https://metascape.org) and Cytoscape (version 3.9.1). [20,21] Terms with a P of <.01 and a minimum gene count of 3 were selected and exhibited.
2.7. Coexpression module analysis and disease ontology analysis
To analyze the system-level functionality of genes, we established gene coexpression modules using CEMiTool. [22] Correlation-based associations between genes with an absolute Pearson correlation coefficient of >0.8 were assigned. Modules containing <30 genes were eliminated. Each module describes which biological pathways are activated by coregulated genes under specific conditions. For each coexpression module, we performed an overrepresentation analysis and disease ontology analyses using Disease Ontology Semantic and Enrichment analysis (DOSE). [23]
2.8. Platelet-related function scoring
To inspect the activity of platelet-associated functions according to the condition, we searched and collected gene sets from the AmiGO2 (http://amigo.geneontology.org/amigo) database, which is based on gene ontology. From the gene sets, we calculated the activity of each term using AddModuleScore, which computes the score per cell with 100 randomly selected control genes from the Seurat package.
2.9. Comparing expression level of platelet receptor
To focus on platelet receptors, we compared the expression level of the subunits of major receptors based on previous literature. [24,25] The statistical significance of gene expression for platelet receptors between the 2 groups was determined using the 2-sided Wilcoxon rank sum test with a P value threshold of .05.
3. Results
3.1. Periodontitis significantly alters key regulators of disease progression in circulating platelets
After quality control, we isolated a population with high expression levels of the canonical platelet markers PPBP, GP9, PF4, ITGA2B, ITGB3, and GP1BA and confirmed their dissociation from white blood cells (Figure 1A and Supplementary Figure 1). A total of 894 platelets with 23,492 genes were retained and visualized using uniform manifold approximation and projection (Figure 1B). The characteristics of 27 participants are presented in Supplementary Table 1. The periodontal parameters, erythrocyte sedimentation rate, C-reactive protein levels, and HbA1C levels of the healthy control group were within the normal range. The erythrocyte sedimentation rate and C-reactive protein levels were substantially high in both patients with periodontitis and those with PDDM, whereas the HbA1C levels were high only in patients with PDDM, indicating that all the patients enrolled in this study matched the disease criteria (Figure 1C). It is worth noting that all patients with PDDM in this cohort had a body mass index of ≤25 kg/m2, indicating that they are nonobese, which is the demographic feature of the majority of Asian patients with DM (Supplementary Table 1). [26] We performed DEG analysis on platelet RNA, and hierarchical clustering of genes with altered transcripts showed distinct grouping in the healthy controls, patients with periodontitis, and patients with PDDM, suggesting that periodontitis and DM shifted the gene expression of platelets (Figure 1D). We identified 180 DEGs (104 upregulated genes and 76 downregulated genes) between the healthy donors and patients with periodontitis, 267 DEGs which were significant (142 upregulated and 125 downregulated genes) in the patients with PDDM compared to the healthy controls (Supplementary Table 2). Periodontitis has been confirmed to significantly affect the transcriptional landscape of platelets. An increase in the expression of genes, such as WFDC1, TUBB2A, and HBG2, was prominent in the platelets of the patients with periodontitis, although the patients with PDDM showed more significant DEGs than those with periodontitis and the healthy controls, suggesting the effect of DM (Figure 1E and Supplementary Figure 2).

Periodontitis (PD) alters the transcriptional profiles of circulating platelets. (A) Feature plot of the canonical platelet marker genes PPBP, GP9, PF4, ITGA2B, ITGB3, and GP1BA. (B) t-stochastic neighbor embedding plots for platelets. The left panel demonstrates integrated platelets, whereas the right panel illustrates platelets separated under 3 conditions: healthy, PD, and PD with type 2 diabetes mellitus (PDDM). (C) Box plot of the clinical variables erythrocyte sedimentation rate, C-reactive protein, and hemoglobin A1C. (D) Hierarchical clustering of status-specific genes in platelets. A list of genes distinctly segregates the healthy, PD, and PDDM groups. (E) Volcano plots depicting log2 fold change and false discovery rate–adjusted P value. The panels compare PD with healthy, PDDM with healthy, and PDDM with PD. Differentially expressed genes (DEGs) with a log2 fold change of >0.3 are shown in orange, DEGs with an adjusted P of <.05 are shown in yellow, and DEGs with both are in blue. CRP, C-reactive protein; DM, diabetes mellitus; ESR, erythrocyte sedimentation rate; HbA1C, hemoglobin A1C; ns, nonsignificant; PD, periodontitis; PDDM, periodontitis with diabetes mellitus; t-SNE, t-stochastic neighbor embedding.  P ≤ .05;
P ≤ .05; 
 P ≤ .01;
P ≤ .01; 

 P ≤ .001; P ≥ .05 (ns).
P ≤ .001; P ≥ .05 (ns).
3.2. Enhancement of platelet activation and coagulation-associated signals in the circulating platelets of patients with periodontitis
Next, we focused on the functional role of the transcriptional changes in circulating platelets associated with patients with periodontitis and those with PDDM. We identified the biological network of DEGs by performing gene set analysis such as Gene Ontology. We observed that platelets of the patients with periodontitis were mostly involved in “RHO GTPase effectors,” “aerobic glycolysis,” and “hemostasis,” which included platelet activation and aggregation (Figure 2A–E). This indicates that periodontitis may contribute to the progression of atherosclerotic thrombi by altering the expression of platelet RNAs and modulating the biological activity of platelets. In contrast, positive regulation of response to external stimuli and positive regulation of cell migration were downregulated, suggesting decreased immunologic protection function in patients with periodontitis (Supplementary Figure 2). Next, the patients with PDDM displayed prominent modification in functional pathways by upregulated genes compared with the healthy controls. The functions, including hemostasis, oxidative phosphorylation, and cellular response to stress, were elevated, whereas the immune effector process and response to bacterium were diminished in the patients with PDDM (Figure 3A–E and Supplementary Figure 3). We subsequently investigated the effects of DM on the function of platelets in periodontitis and observed increased immune defense response based on adaptive immunity (Figure 4A–D). However, “platelet activation, signaling and aggregation” and “response to wounding” were decreased in the PDDM group compared with that in the periodontitis-only group, implying that dysregulated biochemical factors suppress or delay wound healing (Supplementary Figure 4).
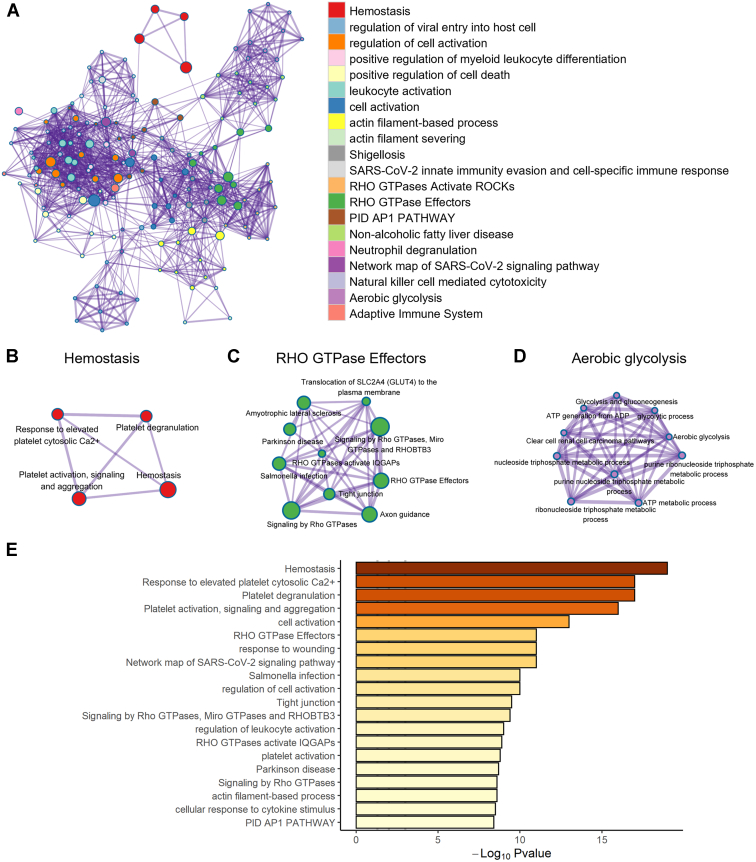
Platelets are more strongly activated in patients with periodontitis than in healthy donors. (A) Functional enrichment analysis of upregulated RNAs in patients with periodontitis compared with that in healthy participants. The enriched network of functional terms for (B) hemostasis, (C) RHO GTPase effectors, and (D) aerobic glycolysis is displayed sequentially. (E) The bar plot displays the gene ontology pathways for upregulated RNAs of circulating platelets in patients with periodontitis. ATP, adenosine triphosphate.
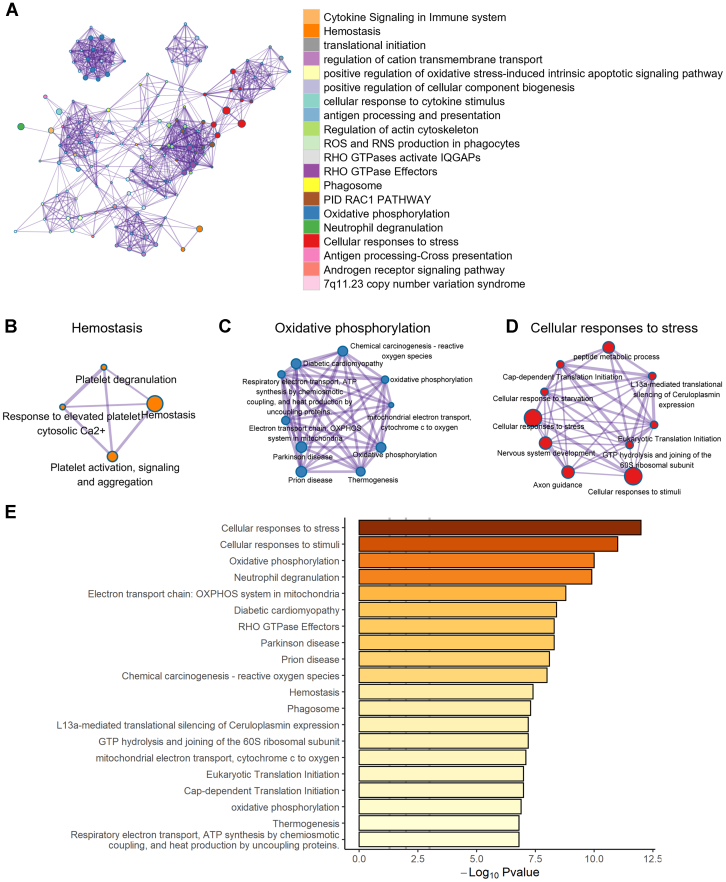
The comorbidity with periodontitis and diabetes mellitus induces platelet activation and cellular responses to stress. (A) Functional network augmented in patients with periodontitis and diabetes mellitus than in healthy individuals. The genes related to biological functions, such as (B) hemostasis, (C) oxidative phosphorylation, and (D) cellular responses to stress, were upregulated. (E) Significantly increased gene ontology pathway observed in patients with periodontitis and diabetes mellitus. GTP, guanosine triphosphate; RNS, reactive nitrogen species; ROS, reactive oxygen species.
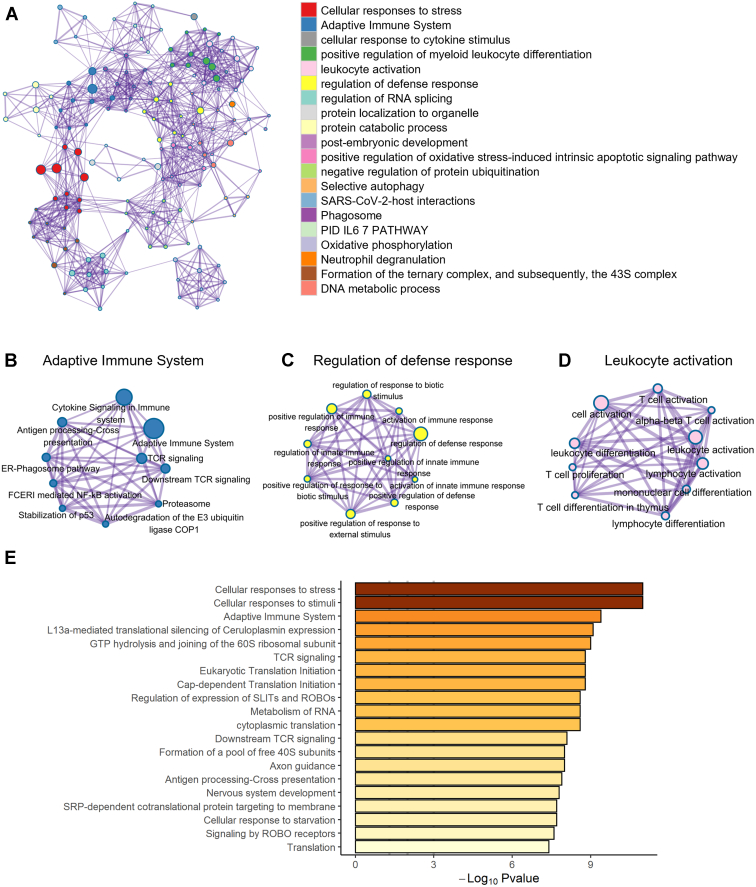
The presence of diabetes mellitus shifts the biological processes of platelets in patients with periodontitis. (A) Functional network augmented by diabetes mellitus (DM). This shows increased pathways in patients with periodontitis and DM compared with those in patients with periodontitis. As an additional response to DM, (B) adaptive immune system, (C) regulation of defense response, and (D) leukocyte activation were intensified. (E) Elevated expression of the gene ontology pathway is observed in patients with periodontitis and DM. GTP, guanosine triphosphate.
3.3. Gene modules in periodontitis are associated with platelet activation
To further investigate the biological network of transcriptional changes in the circulating platelets of patients with periodontitis and those with PDDM, we constructed coexpression modules based on DEGs. Next, we explored diseases to confirm that the correlated genes were functionally associated with previously curated lists of genes in disease pathology using DOSE. [23] We found that modules 1 and 2 were key subnetworks of circulating platelets in the patients with periodontitis, whereas modules 3 and 4 were key subnetworks of circulating platelets in those with PDDM (Figure 5A). All modules were highly correlated with adenosine triphosphate synthesis and the oxidative phosphorylation process (Figure 5B–E). Especially, the gene set from module 1 was characterized by hemostasis and platelet activation. Among various disease terms from disease enrichment analysis, patients with periodontitis showed a close association with not only gingival disease but also coagulation-related diseases such as atrioventricular block (obtained from module 1, Figure 5F). Furthermore, the patients with PDDM exhibited diseases related to artery occlusion (obtained from module 4, Figure 5G).
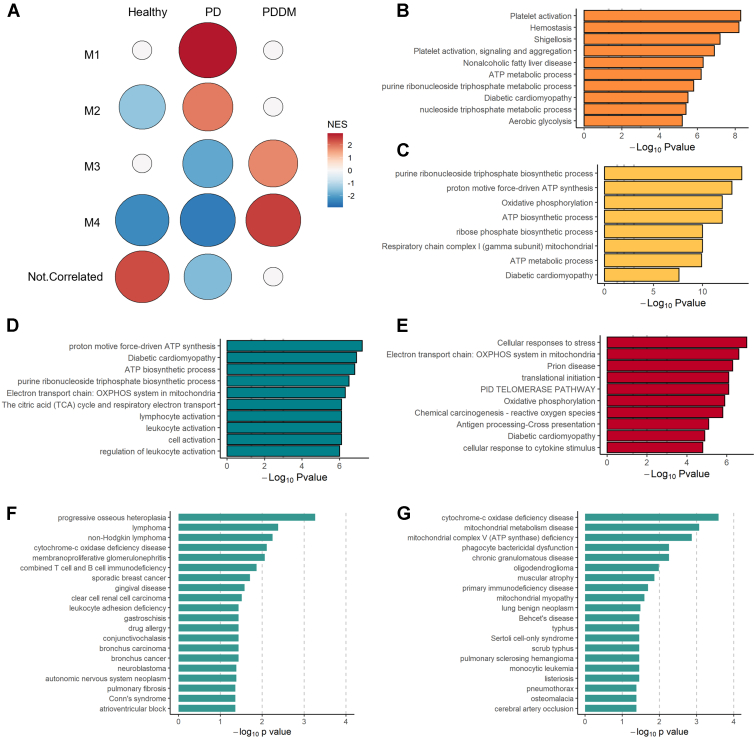
Coexpression modules generated using CEMiTool. (A) Gene module activity profiles under various conditions. The normalized enrichment score is used to assess module activity with respect to the group and is reflected in the circle size and color. Overrepresentation analysis of gene sets from modules (B) 1, (C) 2, (D) 3, and (E) 4 is described. The disease ontology of the gene sets from modules (F) 1 and (G) 4 are illustrated. ATP, adenosine triphosphate; PD, periodontitis; PDDM, periodontitis with diabetes mellitus.
A detailed analysis of specific functions showed increased platelet activation, platelet aggregation, and blood coagulation in both the patients with periodontitis and those with PDDM compared with those in the healthy controls, although the functions were reduced in the patients with PDDM compared with those in the patients with periodontitis only (Figure 6A–D).
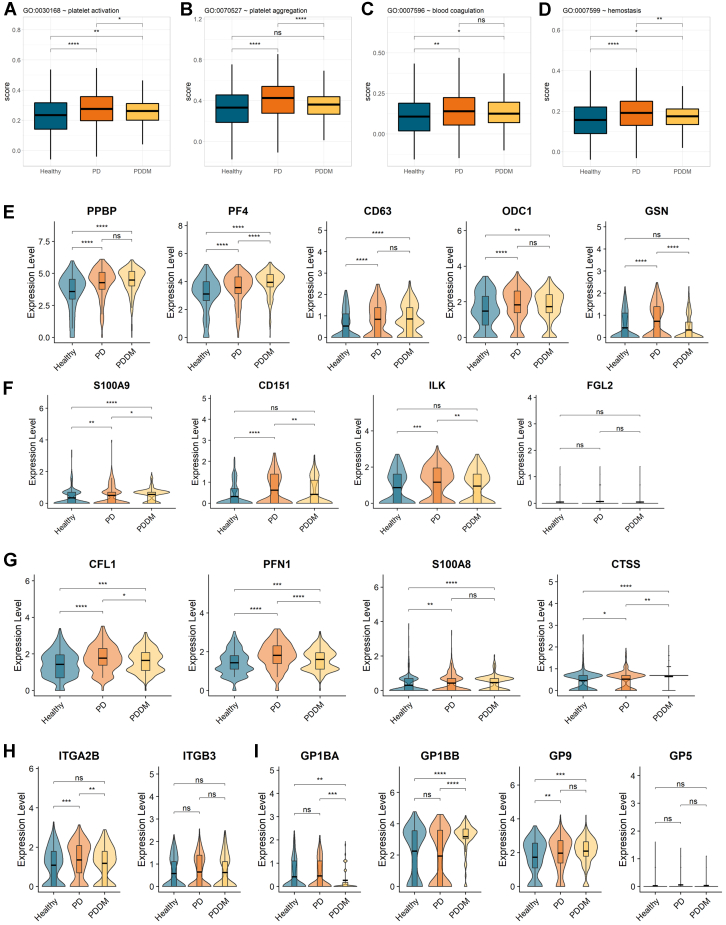
Scores of platelet-associated functions from the Gene Ontology database. Boxplot showing (A) platelet activation, (B) platelet aggregation, (C) blood coagulation, and (D) hemostasis. Violin plots of (E) platelet-activating genes, (F) expression levels of thrombosis-related genes, (G) atherosclerosis and cardiovascular marker genes. (H) Violin plots for subunits of αIIb β3 and (I) the GPIb-IX-V complex.
3.4. Periodontitis exhibited higher expression of genes that are known as predictors of cardiovascular diseases and atherosclerosis
We evaluated the expression of individual genes extracted by combining the results of functional enrichment analysis, key regulators of upregulated pathways, and previous studies that reported a correlation between platelet-derived factors and atherosclerotic cardiovascular accidents. Although genes that are involved in platelet aggregation, activation, thrombus formation and stability, atherosclerosis progression, and cardiovascular accidents are not separate or mutually exclusive, we, nevertheless, categorized these genes into platelet-related functions and cardiovascular accidents depending on literature-based information and surveys. First, we compared the expression levels of genes required for platelet activation. The expression levels of platelet activation–related genes—PPBP, PF4, CD63, ODC1, and GSN—were significantly higher in both the patients with PDDM and those with only periodontitis than the healthy controls (Figure 6E). The expression levels of S100A9, CD151, ILK, and FGL2, which are thrombosis-related genes, were higher in the patients with periodontitis (Figure 6F). Numerous studies have previously evaluated potential biomarker genes for atherosclerosis and cardiovascular accidents, and we found that the expression of numerous genes, including CFL1, PFN1, S100A8, and CTSS, was significantly higher in circulating platelets from patients with periodontitis. The expression of biomarker genes was also higher in the patients with PDDM than in the healthy controls but lower in those with periodontitis only (Figure 6G).
3.5. Platelet receptors are dysregulated in comorbid conditions of periodontitis and diabetes
Considering the close association between DM and blood coagulation–associated diseases, such as atherosclerotic cardiovascular disease, the above results showing lower activation and coagulation of platelets in patients with PDDM contradicted our expectations. To examine the cause of these observations from the signal transduction mechanism, we explored the transcripts of major receptors of platelets, αIIb β3, GPIb-IX-V complex, α2 β1, SELPCLEC1B, and TBXA2R (Figure 6H, I and Supplementary Figure 5). [24,25] Noticeably, αIIb β3, which is the most abundant platelet adhesion receptor and only found on platelets, was reduced in the patients with PDDM compared with that in those with periodontitis (Figure 6H). [27] In addition, the expression of the second most prevalent platelet receptor, GPIb-IX-V complex, exhibited inconsistent fluctuations in complex subunits (Figure 6I). Particularly, GP1BA, cytoplasmic binding sites for intracellular signaling molecules, was downregulated in the patients with PDDM compared with that in the patients with periodontitis and healthy individuals. [28] The expression of both SELP and TBXA2R showed a propensity to be higher in the patients with periodontitis but lower in those with DM (Supplementary Figure 5). Therefore, these observations indicate that the coexistence of periodontitis and DM drives abnormal transcription of platelet receptors and diminish platelet activation.
4. Discussion
It has previously been reported that the RNA profiles of platelets may be altered depending on environmental stress or disease. [[29], [30], [31]] However, because periodontitis is not a serious systemic disease, it is questionable whether it would evoke significant changes in the RNA expression profiles of circulating platelets or whether these patients would maintain genetic profiles similar to those of healthy individuals. The alterations in the transcriptomes of the patients with PDDM and those with periodontitis without DM in this study, particularly the alterations in platelet-related pathways, including platelet activation, aggregation, and coagulation, indicate that patients with periodontitis induce a high risk of coagulation-related diseases. This observation reinforces the findings of previous studies that reported platelet activation in patients with periodontitis and reduced aggravated platelet activation after periodontal therapy, which only observed a few blood markers. [8,11] In addition, the unique platelet characteristics of patients with PDDM provide useful clues for the modifying effects of DM. These findings suggest the risk of periodontitis and the need for comparative research on diseases that could be accompanied by periodontitis.
The transcripts of genes associated with coagulation were upregulated in both the patients with periodontitis and those with PDDM, suggesting that coagulation cascades in the vascular system are strongly activated in individuals with periodontitis and PDDM compared with those in healthy individuals. In addition, the patients with periodontitis showed the same enriched pathways of GTPase activity, hemostasis, and platelet activation as the platelet transcriptome of 32 patients with myocardial infarction in a previous study. [32] These findings imply that periodontitis contributes to the growth of thrombi and the progression of atherosclerosis and support the correlation between periodontitis and the risk of cardiovascular events.
The significance of circulating platelets in the imminent lethal event of periodontitis is further supported by the upregulation of signature genes. The increased expression of key genes, including CFL1, PFN1, S100A8, and CTSS, which have been validated as predictive or prognostic biomarkers of cardiovascular accidents, strengthens the hypothesis of an intimate association between periodontitis and cardiovascular diseases. [[33], [34], [35], [36], [37], [38]] Although we observed higher expression of these genes in the circulating platelets of the patients with periodontitis, the markers were mostly extracted from previous studies on proteomics and RNA sequencing of lesions, serum, or plasma. [3] Considering platelet physiology and its role in the progression of cardiovascular diseases, genetic information from platelets could better reflect disease progression than that from plasma, serum, or other peripheral blood cells. The upregulation of pivotal platelet genes indicates a strong association between periodontitis and atherosclerotic diseases and suggests a possible role of periodontitis control as a method to decrease cardiovascular accidents.
The effects of DM on platelet transcriptome are controversial, and no consensus has yet been reached. Various prior studies have reported hyperactivity of platelets observed in patients with DM [[39], [40], [41]], although more recent studies found no significant effect of DM on platelet transcriptome, including in the expression of coagulation and thrombosis-related genes. [[42], [43], [44]] Our study compared the activity of platelets and the expression level of platelet receptors to inspect the influence of DM on periodontitis. We noticed that the patients with PDDM showed increased platelet activation compared with the healthy individuals but with a decreased degree compared with the patients with periodontitis. The major platelet receptors also demonstrated this characteristic. This indicates that when DM is accompanied by periodontitis, it may impair the expression of platelet receptors, whereas platelet coagulation remains activated. Taken together, the possibility of receptor-mediated platelet dysregulation should be considered to inspect the intervention impact of DM on periodontitis.
Nevertheless, this study has several limitations. First, there are several risk factors that can enhance the prevalence of DM, for example, smoking, sex, age, obesity, or ethnicity. [45,46] In our cohort, the PDDM group consisted of nonobese individuals. Although we focused on the effects of DM in nonobese individuals without any underlying diseases, an unexpected impact of other risk factors may have existed. In addition, it should be noted that blood platelets were identified using the transcriptional level of a typical platelet marker, not by sorting but through cell surface proteins. Although messenger RNA levels reflect protein levels to some extent, validation at the protein level is needed to verify the results. [47,48] Because these findings were obtained at the transcriptome level, it is important to note that the described result may reflect the characteristics of reticulated platelets with a relatively high messenger RNA content. Furthermore, there was lack of information on traditional platelet analysis, including number, size, and aggregation. Finally, it is necessary to verify whether altered genetic profiles of platelets are specific to periodontitis or whether chronic persistent systemic inflammation due to periodontitis could evoke these changes.
To the best of our knowledge, this is the first study to analyze the transcriptome of platelets in peripheral blood from patients with periodontitis with type 2 DM and those with periodontitis without type 2 DM by characterizing single-cell transcriptional changes. A novel aspect of this study is the comparison of platelet function between patients with periodontitis and those with DM, which suggested direct evidence to explain the changes in the platelets of patients, which supports previous knowledge.
Acknowledgments
The authors would like to thank all members of ROKIT GENOMICS for their helpful comments and discussions.
Funding
This study was supported by the National Research Foundation of Korea (NRF-2020R1A2C1005203 and NRF-2018R1A5A2023879), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (HI22C1377), funded by the Ministry of Health & Welfare, Republic of Korea, and Korea Research Environment Open Network (KREONET).
Ethics statement
All individuals who participated in this study were informed both verbally and using a written statement about the use of their peripheral blood samples. The participants subsequently signed an informed consent form that was approved by the Institutional Review Board of Pusan National University Dental Hospital (IRB No. PNUDH-2020-001).
Author contributions
H.L., Y.H.K., and H.R.P. designed and supervised the study. J.-Y.J. and H.R.P. obtained patient consent for study publication and sample collection. H.L. and Y.H.K. performed computational analyses. H.L., J.-Y.J., J.K., Y.Y., Y.H.K., and H.R.P. discussed the results and wrote the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Handling Editor: Dr Henri Spronk
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100099
Supplementary material
References
Articles from Research and Practice in Thrombosis and Haemostasis are provided here courtesy of Elsevier
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.rpth.2023.100099
Read article for free, from open access legal sources, via Unpaywall:
http://www.rpthjournal.org/article/S2475037923000687/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/146073727
Article citations
Periodontitis impacts on thrombotic diseases: from clinical aspect to future therapeutic approaches.
Int J Oral Sci, 16(1):58, 15 Oct 2024
Cited by: 0 articles | PMID: 39402049 | PMCID: PMC11473739
Review Free full text in Europe PMC
Single-cell transcriptomic landscape reveals the role of intermediate monocytes in aneurysmal subarachnoid hemorrhage.
Front Cell Dev Biol, 12:1401573, 10 Sep 2024
Cited by: 0 articles | PMID: 39318997 | PMCID: PMC11420033
Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics.
Int J Mol Sci, 25(11):5941, 29 May 2024
Cited by: 0 articles | PMID: 38892129 | PMCID: PMC11173046
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Decreased phosphorylation of platelet vasodilator-stimulated phosphoprotein in periodontitis--a role of periodontal pathogens.
Thromb Res, 128(2):155-160, 24 Mar 2011
Cited by: 7 articles | PMID: 21435699
Diabetic Rats Present High Mean Platelet Count in the Presence of Oral Infections.
Braz Dent J, 28(5):548-551, 01 Sep 2017
Cited by: 6 articles | PMID: 29215677
Platelet Indices be a New Biomarker for Periodontal Disease.
Contemp Clin Dent, 12(3):289-293, 01 Jul 2021
Cited by: 2 articles | PMID: 34759687 | PMCID: PMC8525805
Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond.
Crit Rev Clin Lab Sci, 53(6):409-430, 22 Jul 2016
Cited by: 133 articles | PMID: 27282765
Review
Funding
Funders who supported this work.
Korea Health Industry Development Institute (1)
Grant ID: HI22C1377
Korea Research Environment Open Network
Ministry of Health and Welfare
National Research Foundation of Korea
Pusan National University (1)
Grant ID: PNUDH-2020-001




