Abstract
Purpose
To evaluate the association between ejaculation frequency (EF) during four stages of life and prostate cancer (PCa) according to tumor aggressiveness, PCa stage, and urinary symptomatology.Materials and methods
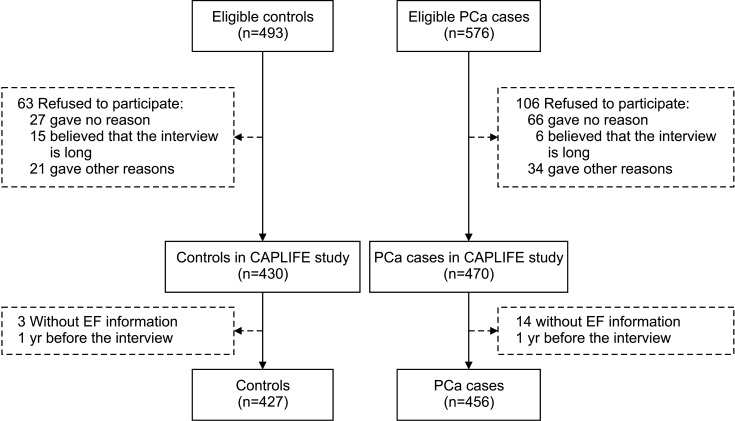
A total of 456 incident PCa cases histologically confirmed, and 427 controls aged 40-80 years from the CAPLIFE study were analyzed. This study is a population-based case-control study carried out in the south of Spain. Average EF was measured for: (1) 20s, (2) 30s, (3) 40s, and (4) one year before the interview. EF was categorized into: (1) 0-3, (2) 4, and (3) >4 ejaculations/month. Sociodemographic, lifestyle, and medical information were also collected. To estimate the association between EF and PCa, adjusted ORs (aORs) and 95% CIs were calculated by logistic regression models.Results
A year before the interview, PCa cases ejaculated less frequently than the controls. An inverse association was observed between the EF a year before and PCa, aOR=1.64 (95% CI 1.03-2.61) for men with 4 ejaculations/month, and aOR=2.38 (95% CI 1.57-3.60) for men with 0-3 ejaculations/month, compared to men with >4. The association was higher for cases with ISUP 3-5 (aOR=2.76 [95% CI 1.34-5.67] for men with 0-3 ejaculations/month) or with a locally advanced-metastatic tumor (aOR=4.70 [95% CI 1.55-14.29]). Moreover, men with moderate urinary symptoms and 0-3 ejaculations/month had the highest risk, aOR=3.83 (95% CI 1.84-7.95).Conclusions
A low EF could be associated with a higher risk of PCa, especially for cases with ISUP 3-5 or with a locally advanced-metastatic tumor.Free full text

Ejaculation Frequency and Prostate Cancer: CAPLIFE Study
Abstract
Purpose
To evaluate the association between ejaculation frequency (EF) during four stages of life and prostate cancer (PCa) according to tumor aggressiveness, PCa stage, and urinary symptomatology.
Materials and Methods
A total of 456 incident PCa cases histologically confirmed, and 427 controls aged 40–80 years from the CAPLIFE study were analyzed. This study is a population-based case-control study carried out in the south of Spain. Average EF was measured for: (1) 20s, (2) 30s, (3) 40s, and (4) one year before the interview. EF was categorized into: (1) 0–3, (2) 4, and (3) >4 ejaculations/month. Sociodemographic, lifestyle, and medical information were also collected. To estimate the association between EF and PCa, adjusted ORs (aORs) and 95% CIs were calculated by logistic regression models.
Results
A year before the interview, PCa cases ejaculated less frequently than the controls. An inverse association was observed between the EF a year before and PCa, aOR=1.64 (95% CI 1.03–2.61) for men with 4 ejaculations/month, and aOR=2.38 (95% CI 1.57–3.60) for men with 0–3 ejaculations/month, compared to men with >4. The association was higher for cases with ISUP 3–5 (aOR=2.76 [95% CI 1.34–5.67] for men with 0–3 ejaculations/month) or with a locally advanced-metastatic tumor (aOR=4.70 [95% CI 1.55–14.29]). Moreover, men with moderate urinary symptoms and 0–3 ejaculations/month had the highest risk, aOR=3.83 (95% CI 1.84–7.95).
Conclusions
A low EF could be associated with a higher risk of PCa, especially for cases with ISUP 3–5 or with a locally advanced-metastatic tumor.
INTRODUCTION
The etiology of prostate cancer (PCa) remains largely unknown [1] despite being a tumor with a high incidence, and which is increasing in recent years [2]. Only non-modifiable risk factors such as age, family history, and ethnicity have been established for PCa [1,3,4]. Other factors related to life habits have been proposed such as diet, physical activity, or occupational exposure, but their individual role in the etiology of PCa remains unclear [5,6].
Among the carcinogenic agents with limited evidence recognized by the International Agency for Research on Cancer (IARC) are androgenic steroids. Its side effects include erectile dysfunction [7]. Hence, sexual behavior may be another of the habits related to PCa. The number of sexual partners, age at first intercourse [8,9], and ejaculation frequency (EF) have been some of the main characteristics studied in relation to PCa [9,10,11,12], yielding contradictory results. While two cohort studies propose the existence of a possible inverse association between EF and PCa risk [11,12], a case-control study proposes a possible risk association with the number of orgasms [9]. In this sense, a 2019 systematic review of the European Association of Urology Section of Oncological Urology (ESOU) concluded that well-conducted longitudinal studies are required to assess whether suggested associations between sexual behavior, including EF, and PCa are “real or spurious” [13].
In addition, urinary symptoms, tumor aggressiveness and stage of PCa may play a fundamental role in the association between EF and PCa. Recently, it has been suggested that urinary symptoms in patients with benign prostatic hyperplasia could be associated with erectile dysfunction [14], a factor that could condition the EF. For this reason, we decided to study this relationship using different stages of life. To our knowledge, no study has evaluated the association between EF and PCa based on urinary symptoms at the moment of the diagnosis. Furthermore, regarding tumor aggressiveness and stage of PCa, few studies have considered it, and their results do not point in the same direction [10,11,12].
Given the increase in the incidence of PCa, the need for longitudinal studies as recognized by the ESOU, and the lack of consideration of urinary symptoms, tumor aggressiveness, and stage in the association between EF and PCa, this study aimed to evaluate the association between the average EF in different stages of the life and PCa, according to the tumor aggressiveness, stage of PCa, and urinary symptomatology.
MATERIALS AND METHODS
1. Study design and participants
CAPLIFE study is a case-control study carried out at two main University Hospitals in Granada (Spain): Virgen de las Nieves and Clínico San Cecilio Hospitals and their catchment areas. The recruitment period was from May 2017 to September 2020. The main characteristics of the CAPLIFE study have been described elsewhere [15,16].
The selection criteria for cases and controls were: (1) 40 to 80 years and (2) residence in the coverage area of the reference hospitals for at least 6 months prior to recruitment. Additionally, PCa cases were newly diagnosed with histological confirmation before receiving treatment (International Classification of Diseases and Related Health Problems 10th Revision [ICD-10]: C61) [17].
Incident PCa cases were selected and invited to participate in the urology services of the participating hospitals. For it, the listings of Pathological Anatomy Service were used. The controls were selected randomly from the general population through the lists assigned to practitioners at primary health centers.
2. Ethics statement
The protocol of this study was approved by the Ethics Committee for Biomedical Research of Andalusia in March 2017. Informed consent was confirmed by it. Participants were fully informed about the study and voluntarily signed a written consent before their inclusion. Confidentiality of data was secured, removing personal identifiers in the dataset.
3. Information sources and data collection
Information was collected through face-to-face interviews using a structured computerized questionnaire and participants’ medical history. For the cases, the time between the PCa diagnosis and the interview never exceeded three months.
1) EF
EF was collected by interviewers trained in the 4 stages of life of the participants: (1) the 20s; (2) 30s; (3) 40s; and (4) one year prior to the interview (for cases one year before the diagnosis and for the controls one year before to interview). The 8 response categories were: (1) never; (2) less than once a month; (3) between 1 and 3 times a month; (4) once a week; (5) between 2 and 3 times a week; (6) between 4 and 6 times a week; (7) daily; (8) does not know. Using these 8 categories, the final classification was built based on a previously used one [9], as follows: (1) 0–3 ejaculations/month; (2) 4 ejaculations/month; and (3) >4 ejaculations/month.
2) Clinical characteristics of PCa cases
Urinary symptomatology. The International Prostate Symptom Score (IPSS), validated in the Spanish population, was selected to evaluate the urinary symptomatology of PCa cases [18]. It is based on the answers to seven questions, which referred to the month before the interview. Each question was scored from 0 to 5. Total score ranged from 0 to 35 (a higher score is indicative of a greater presence of urinary symptoms). Cases were classified as: (1) without (0 points), (2) mild (1–7 points), (3) moderate (8–19 points), and (4) severe urinary symptoms (20–35 points).
Tumor aggressiveness. The International Society of Urological Pathology (ISUP) was used to classify the tumors as ISUP 1–2 and ISUP 3–5 [19].
Staging of PCa. The tumors were classified as localized, locally advanced, and metastatic PCa.
3) Sociodemographic, lifestyles data, and personal antecedents
Information was also collected on sociodemographic data (age, education, and marital status), lifestyle (smoking status, physical activity, sedentary behavior, alcohol consumption, and energy intake), and personal antecedents (first-degree family history of PCa, diagnosis of diabetes mellitus, and vasectomy). In addition, information on weight and height was obtained, calculating the body mass index (BMI).
Physical activity and sedentary behavior were collected through the International Physical Activity Questionnaire Short Form (IPAQ-SF). This questionnaire is validated for the Spanish population and referred to the week prior to the interview [20].
For alcohol consumption and energy intake, we only consider participants with plausible energy intakes (≤800 kcal/d and ≥4000 kcal/d) [21].
4. Statistical analysis
Characteristics of the PCa cases and controls were examined using the median and interquartile range (IQR) for continuous variables and percentages for categorical variables. The differences between groups were analyzed using Mann–Whitney U test for continuous variables and chi-squared test for categorical variables. In addition, the characteristics of cases and controls were compared according to the level of EF though Kruskal–Wallis or chi-squared tests.
Multivariable logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between EF and PCa risk. The reference group was the subjects with >4 ejaculations/month. We adjusted for the variables found in scientific literature that have been related to PCa and EF (p-value <0.20 in the crude analysis). Three logistic models were run: (1) model 1, adjusting for age, educational level, and first-degree family history of PCa; (2) model 2, additionally adjusting for height, sedentary behavior, diabetes mellitus, and vasectomy and (3) model 3, additionally adjusting for energy intake and alcohol consumption. The association between EF and PCa risk was analyzed stratifying by ISUP, stage of PCa, and urinary symptomatology.
All statistical tests were two-sided and statistical significance was set at p<0.05. Statistical analyses were performed using the statistical program Stata v.15 (Stata Corp., College Station, TX, USA).
RESULTS
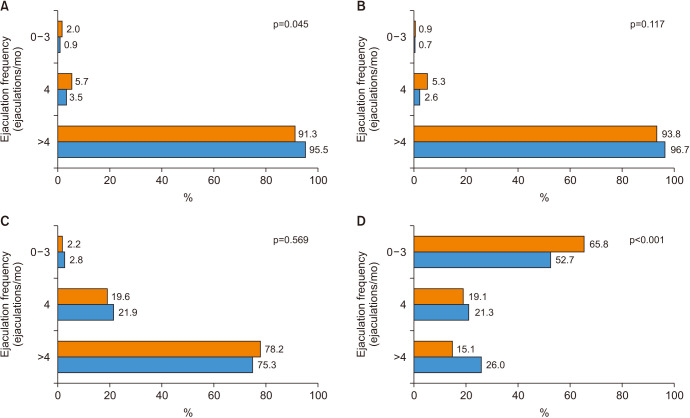
A total of 456 PCa cases and 427 controls had complete information about EF one year before the interview (Fig. 1). The average EF in the 4 stages of life is shown in Fig. 2. Thus, the year prior to the interview, PCa cases ejaculated less frequently than the controls; 15.1% vs. 26.0% ejaculated >4 times a month (Fig. 2D).
The distribution of the characteristics of PCa cases and controls is shown in Table 1. Compared to the controls, PCa cases were slightly older, 68.4 years (IQR 62.7–73.9) vs. 66.2 (IQR 61.2–72.1), consumed higher intakes of alcohol (16.2% vs. 10.5% consumed more than 28 g/day), and more frequently had a first-degree family history of PCa (21.8% vs. 10.5%). Cases were mostly ISUP 1–2 tumors (75.9%) and localized tumors (86.4%). In addition, the advanced stage of PCa was related to lower EF. Age, educational level, height, alcohol consumption, diabetes mellitus, and vasectomy were associated with EF a year before the interview in cases and controls (Supplement Table 1). In this way, younger, taller, with a higher educational level, who drank larger amounts of alcohol, were non-diabetic, and vasectomized men had a higher EF, for both groups. Furthermore, sedentary behavior was associated with a lower EF only in the control group.
Table 1
| Variable | Controls | PCa cases | p-valuea | |
|---|---|---|---|---|
| Total | 427 (100) | 456 (100) | ||
| Age (y) | 66.2 (61.2–72.1) | 68.4 (62.7–73.9) | 0.008 | |
| 40−54 | 41 (9.6) | 28 (6.1) | ||
| 55−69 | 234 (54.8) | 246 (54.0) | ||
| 70−80 | 152 (35.6) | 182 (39.9) | ||
| Education | 0.267 | |||
| Primary | 121 (28.3) | 141 (30.9) | ||
| Secondary | 214 (50.1) | 236 (51.8) | ||
| University | 92 (21.6) | 79 (17.3) | ||
| Marital status | 0.934 | |||
| Married | 354 (82.9) | 379 (83.1) | ||
| Not married | 73 (17.1) | 77 (16.9) | ||
| Height (cm) | 170.0 (166.0–174.0) | 170.0 (165.0–174.0) | 0.271 | |
| BMI | 0.448 | |||
| Normal weight | 75 (17.6) | 95 (20.8) | ||
| Overweight | 229 (53.6) | 231 (50.7) | ||
| Obesity | 123 (28.8) | 130 (28.5) | ||
| Smoking status | 0.677 | |||
| Never smoker | 110 (25.8) | 116 (25.4) | ||
| Former smoker | 238 (55.7) | 245 (53.7) | ||
| Current smoker | 79 (18.5) | 95 (20.8) | ||
| Sedentary behavior (h/d) | 7.0 (5.0–9.0) | 7.0 (5.0–10.0) | 0.386 | |
| Physical activity (MET-h/wk) | 24.8 (9.9–40.4) | 23.1 (6.6–35.8) | 0.123 | |
| Alcohol consumption (g/d)b | 0.052 | |||
| 0 | 71 (18.2) | 76 (18.2) | ||
| 0–28 | 279 (71.3) | 275 (65.6) | ||
| ≥28 | 41 (10.5) | 68 (16.2) | ||
| Energy intake, (kcal/d)b | 2,295.9 (1,958.0–2,820.6) | 2,424.2 (2,057.4–2,915.7) | 0.045 | |
| First-degree family history of PCa | 0.001 | |||
| No | 382 (89.5) | 356 (78.2) | ||
| Yes | 45 (10.5) | 99 (21.8) | ||
| Unknown | - | 1 | ||
| Diabetes mellitus | 0.205 | |||
| No | 326 (76.5) | 365 (80.0) | ||
| Yes | 100 (23.5) | 91 (20.0) | ||
| Unknown | 1 | - | ||
| Vasectomy | 0.769 | |||
| Yes | 388 (90.9) | 416 (91.4) | ||
| No | 39 (9.1) | 39 (8.6) | ||
| Missing | - | 1 | ||
| Urinary symptoms | ||||
| Without | - | 123 (27.0) | ||
| Mild | - | 177 (38.9) | ||
| Moderate | - | 122 (26.8) | ||
| Severe | - | 33 (7.3) | ||
| Unknown | - | 1 | ||
| ISUP | ||||
| 1–2 | - | 346 (75.9) | ||
| 3–5 | - | 110 (24.1) | ||
| Stage of PCa | ||||
| Localized | - | 394 (86.4) | ||
| Locally advanced | - | 39 (8.6) | ||
| Metastatic | - | 23 (5.0) | ||
Values are presented as number (%) or median (interquartile range).
BMI: body mass index, EF: ejaculation frequency, ISUP: International Society of Urological Pathology, PCa: prostate cancer.
aKruskal–Wallis test or chi-squared test were used to calculate the differences between EF categories in PCa cases and controls.
bDietary information was available for a total of 391 controls and 419 PCa cases.
The association between EF one year before the interview and PCa risk is presented in Table 2. A low EF was associated with a higher risk of PCa. Specifically, those subjects with 4 ejaculations/month presented an adjusted OR (aOR)=1.64 (95% CI 1.03–2.61) in the fully adjusted model, while participants with 0–3 ejaculations/month had an aOR=2.38 (95% CI 1.57–3.60), compared to subjects with >4 ejaculations/month. An additional analysis was performed where subjects who never ejaculated were separated from those who reported ejaculating 1–3 times per month. Those subjects who never ejaculated had the highest risk of developing PCa, aOR=3.71 (95% CI 2.05–6.71). A risk trend is observed for the decade of the 20s, but no clear trend could be observed for EF in the 30s and 40s and PCa risk (Supplement Table 2).
Table 2
| EF (ejaculations/mo) | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| >4 | Reference | Reference | Reference |
| 4 | 1.46 (0.94–2.24) | 1.46 (0.94–2.26) | 1.64 (1.03–2.61) |
| 0–3 | 2.01 (1.38–2.94) | 2.04 (1.39–3.00) | 2.38 (1.57–3.60) |
| p-trend | 0.001 | 0.001 | 0.001 |
Values are presented as adjusted odds ratio (95% confidence interval).
EF: ejaculation frequency, PCa: prostate cancer.
Model 1: adjusted for age, educational level, and first-degree family history of PCa.
Model 2: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, and vasectomy.
Model 3: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, vasectomy, energy intake, and alcohol consumption.
Table 3 shows the association between EF one year before the interview and PCa according to ISUP and stage. It seems the higher the ISUP and stage, the higher the PCa risk, although this association is observed for all the types of PCa cases. That is, those men with 0–3 ejaculations/month had a 129% higher risk of presenting an ISUP 1–2 tumor (95% CI 1.47–3.57) and a 176% higher risk of a ISUP 3–5 tumor (95% CI 1.34–5.67) in the fully adjusted model. Regarding stage of PCa, a low EF was especially associated with locally advanced-metastatic tumors, aOR=4.70 (95% CI 1.55–14.29). In addition, men with moderate urinary symptoms and 0–3 ejaculations/month were those with the highest risk of developing PCa, aOR=3.83 (95% CI 1.84–7.95) (Table 4).
Table 3
| EF (ejaculations/mo) | Model | Model 2 | Model 3 | |
|---|---|---|---|---|
| ISUP 1–2 | ||||
| >4 | Reference | |||
| 4 | 1.45 (0.92–2.30) | 1.46 (0.92–2.33) | 1.63 (0.99–2.68) | |
| 0–3 | 1.89 (1.26–2.83) | 1.96 (1.30–2.96) | 2.29 (1.47–3.57) | |
| p-trend | 0.002 | 0.001 | 0.001 | |
| ISUP 3–5 | ||||
| >4 | Reference | |||
| 4 | 1.47 (0.67–3.26) | 1.53 (0.68–3.43) | 1.58 (0.68–3.65) | |
| 0–3 | 2.53 (1.28–5.00) | 2.46 (1.24–4.91) | 2.76 (1.34–5.67) | |
| p-trend | 0.004 | 0.006 | 0.003 | |
| Localized PCa | ||||
| >4 | Reference | |||
| 4 | 1.42 (0.91–2.21) | 1.43 (0.92–2.24) | 1.59 (0.99–2.57) | |
| 0–3 | 1.78 (1.21–2.63) | 1.83 (1.23–2.71) | 2.17 (1.42–3.33) | |
| p-trend | 0.004 | 0.003 | 0.001 | |
| Locally advanced-metastatic PCa | ||||
| >4 | Reference | |||
| 4 | 2.03 (0.57–7.24) | 2.04 (0.57–7.37) | 1.92 (0.52–7.06) | |
| 0–3 | 5.61 (1.89–16.65) | 5.21 (1.74–15.62) | 4.70 (1.55–14.29) | |
| p-trend | 0.001 | 0.001 | 0.002 | |
Values are presented as adjusted odds ratio (95% confidence interval).
EF: ejaculation frequency, ISUP: International Society of Urological Pathology, PCa: prostate cancer.
Model 1: adjusted for age, educational level, and first-degree family history of PCa.
Model 2: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, and vasectomy.
Model 3: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, vasectomy, energy intake and alcohol consumption.
Table 4
| Urinary symptomatology | EF (ejaculations/mo) | Model 1 | Model | Model 3 |
|---|---|---|---|---|
| Without | >4 | Reference | ||
| 4 | 1.29 (0.66–2.54) | 1.33 (0.67–2.64) | 1.41 (0.68–2.93) | |
| 0–3 | 1.67 (0.93–3.01) | 1.69 (0.93–3.06) | 2.09 (1.10–3.97) | |
| p–trend | 0.075 | 0.079 | 0.018 | |
| Mild | >4 | Reference | ||
| 4 | 1.16 (0.65–2.06) | 1.16 (0.64–2.08) | 1.26 (0.66–2.39) | |
| 0–3 | 1.62 (0.98–2.67) | 1.65 (1.00–2.74) | 1.93 (1.11–3.36) | |
| p–trend | 0.045 | 0.037 | 0.013 | |
| Moderate | >4 | Reference | ||
| 4 | 2.27 (1.08–4.78) | 2.37 (1.12–5.02) | 2.62 (1.18–5.80) | |
| 0–3 | 3.13 (1.60–6.11) | 3.43 (1.73–6.78) | 3.83 (1.84–7.95) | |
| p–trend | 0.001 | 0.001 | 0.001 | |
| Severe | >4 | Reference | ||
| 4 | 2.33 (0.56–9.71) | 2.39 (0.56–10.12) | 2.42 (0.57–10.26) | |
| 0–3 | 3.26 (0.91–11.76) | 3.19 (0.87–11.61) | 3.09 (0.84–11.39) | |
| p-trend | 0.065 | 0.077 | 0.090 | |
Values are presented as adjusted odds ratio (95% confidence interval).
EF: ejaculation frequency, PCa: prostate cancer.
Model 1: adjusted for age, educational level, and first-degree family history of PCa.
Model 2: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, and vasectomy.
Model 3: adjusted for age, educational level, first-degree family history of PCa, height, sedentary behavior, diabetes mellitus, vasectomy, energy intake and alcohol consumption.
DISCUSSION
To our knowledge, it is the first study to evaluate the association between EF and the risk of PCa, considering tumor aggressiveness, stage, and urinary symptomatology. An association was observed between a low EF one year before the interview and PCa risk, especially for ISUP 3–5 and locally advanced-metastatic tumor. Moreover, low EF was more strongly associated with PCa risk in patients with moderate urinary symptoms. A similar association was observed for the EF during the 20s. However, the homogeneity for EF at 30 and 40s prevented us from analyzing this association at these stages of life.
Similar to the findings in previous studies, we observed a risk association between a low EF one year before the interview and PCa risk. The risk association was even greater for those who had never ejaculated in the previous year. In this sense, two previous cohort studies, carried out by Leitzmann et al [12] in an American professional health population and Rider et al [11] in US general population, yield similar results. However, the magnitude of the association differs slightly between studies, possibly due to the different categorization of the EF and the chosen reference category. Specifically, these two previous studies opt for a higher number of categories using an intermediate category as reference, from 4 to 7 ejaculations/month. Our initial hypothesis was that a higher frequency of ejaculation less PCa risk. However, considering the manuscript of Nair-Shalliker et al. we decided to work with only 3 categories, mainly due to our sample size and the low number of participants in the following categories: (1) between 2 and 3 times a week, (2) between 4 and 6 times a week, and (3) daily. Hence, we decided to use >4 ejaculations/month as the reference category. Regarding the previous decades, we observed an inverse trend between EF in the 20s and 30s and PCa risk. No association was observed for the decade of the 40s. This may be due to various reasons: (1) EF maintained after the age of 40 is the one that influences the risk of PCa; or (2) there was an association for the decades of the 20s, 30s, and 40s, but we were not able to find it given the sample size and that most of the subjects reported a high EF (>4 ejaculations/month), which makes to find possible differences difficult.
In this sense, various biological mechanisms have been proposed to explain this possible association: (1) Prostate stagnation hypothesis, which implies the prostatic accumulation of potentially carcinogenic secretions that could create a tumorigenesis inflammatory microenvironment [22,23]. A higher EF could decrease prostate stagnation and, therefore, the risk of PCa; (2) Development of prostatic intraluminal crystalloids, which leads to mechanical trauma, chronic inflammation, and the development of fibrosis [22]. A higher EF may reduce their development, and they have been associated with an increased the risk of occurrence of prostatic adenocarcinoma [24]; and (3) Central sympathetic nervous system, a higher EF may be linked to lowering of psychological tension and central sympathetic nervous system suppression, which could reduce stimulation of prostate epithelial cell division [25,26]. Therefore, there are pathophysiological mechanisms that support the observed association.
However, PCa is not a homogeneous pathology. The factors associated with mildly aggressive PCa and aggressive PCa may be different, hence the need to consider aggressiveness and stage at the time of first diagnosis. When the association between EF and PCa risk was analyzed according to ISUP and stage, we found that low EF was especially associated with highly aggressive and advanced tumors. In this sense, in a case-control study developed with an Australian population including only aggressive PCa cases, a protective association was suggested for men in their 30s with ≥14 vs. <7 ejaculations/week (aOR=0.55 [95% CI 0.34–0.88]), but found no association for the 20s or 40s [10]. We observed similar findings for the 30s (aOR=5.53 [95% CI 1.05–29.29] for men with 0–3 vs. >4 ejaculations/month) and the 20s (aOR=3.34 [95% CI 0.44–25.53]). Furthermore, as also found by Papa et al [10], we are not able to identify an association for the 40s. In contrast to our results, the two cohort studies mentioned previously indicated that the greatest risk was presented for the low-risk or organ-confined tumors [11,12]. Rider et al [11] and Leitzmann et al [12] used a different classification for measuring tumor aggressiveness and stage. In fact, Papa et al [10] recognizes it as a limitation.
Another of our findings was that the strongest association between EF and PCa was in subjects with moderate and severe urinary symptoms. To our knowledge, urinary symptomatology has not been considered in previous studies, so we cannot compare our results with those of previous studies. Some possible explanations of this greater magnitude found for cases with moderate and severe symptomatology could be: (1) an attempt to avoid ejaculation during the previous one year among those with greater urinary symptoms, and (2) those men with greater urinary symptoms have a higher risk of erectile dysfunction and premature ejaculation [27,28]. In this way, it could be that those men with greater symptoms present erectile dysfunction and premature ejaculation more frequently, which leads them to a lower frequency of ejaculation and, as a consequence, to greater risk of PCa.
Some limitations may affect the results found. The sample size should be highlighted, and is most notable in analyses stratified by ISUP, stage, and urinary symptomatology, which could lead to a lack of statistical power to detect significant associations. Moreover, most of the cases and controls included in our study declare an EF >4 ejaculations/month; making it difficult to analyze this factor. Second, we cannot rule out a recall bias, especially for the EF at 20s, 30s, and 40s. This bias affects both the cases and controls groups, and therefore it would be a non-differential bias, biasing the estimates obtained toward the null. Third, although we adjusted for a range of potential confounders, such as vasectomy or the diagnosis of diabetes mellitus, we cannot rule out the absence of confounding by other exposures/agents related to EF and PCa. Information about sexually transmitted infections was not collected, which could increase the risk of PCa [29,30].
Our study also has several advantages, and the following should be highlighted: (1) to our knowledge, it is the first study to evaluate the association between EF and the risk of PCa considering ISUP, stage, and urinary symptomatology; (2) detailed information about EF was collected, including both from sexual intercourse and masturbation, in different stages of the adult life (during the 20s, 30s, 40s, and the previous year); (3) most PCa cases (97.0%) and controls (99.3%) had complete EF information one year before the interview.
CONCLUSIONS
In this Spanish population-based case-control study, an association was observed between a low EF one year before the interview and PCa risk, especially for ISUP 3–5 and locally advanced-metastatic tumor. In addition, the strongest associations between low EF and PCa risk were found in subjects with moderate urinary symptoms. A similar association was observed for the 20s. Nevertheless, more prospective studies would be necessary to confirm the results obtained.
Acknowledgements
We thank all the subjects who participated in the study and all CAPLIFE, and Ingrid de Ruiter, MBChB, PhD, for editorial support during the preparation of the manuscript draft.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This research was funded by Regional Ministry of Health and Families of Andalusia/Consejería de Salud y Familias, Junta de Andalucía (PI-0514-2016).
Author Contribution:
Conceptualization: ROR, JJJM.
Data curation: MLL.
Formal analysis: MLL, ROR, RBR, MRB.
Funding acquisition: ROR.
Investigation: MLL, ROR, RBR, JJJM.
Methodology: ROR, JJJM.
Project administration: ROR.
Resources: ROR, JJJM.
Supervision: ROR, JJJM, AJP, FVA, HMCB.
Writing – original draft: MLL, ROR, RBR, JJJM.
Writing – review & editing: MLL, ROR, RBR, AJP, FVA, HMCB, MRB, JJJM.
Data Sharing Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220216.
Characteristics of controls and PCa cases according to EF a year before the interview in the CAPLIFE study
Association between EF in different decades of life and PCa risk in CAPLIFE study
References
Articles from The World Journal of Men's Health are provided here courtesy of Korean Society for Sexual Medicine and Andrology
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/149780287
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.5534/wjmh.220216
Article citations
Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study.
Nutrients, 16(6):836, 14 Mar 2024
Cited by: 0 articles | PMID: 38542747 | PMCID: PMC10975556
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Salivary Melatonin Rhythm and Prostate Cancer: CAPLIFE Study.
J Urol, 207(3):565-572, 25 Oct 2021
Cited by: 4 articles | PMID: 34694161
Dietary Patterns and Prostate Cancer: CAPLIFE Study.
Cancers (Basel), 14(14):3475, 17 Jul 2022
Cited by: 4 articles | PMID: 35884536 | PMCID: PMC9316982
Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up.
Eur Urol, 70(6):974-982, 28 Mar 2016
Cited by: 31 articles | PMID: 27033442 | PMCID: PMC5040619
Prostate-Specific Antigen-Based Screening for Prostate Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force
Agency for Healthcare Research and Quality (US), Rockville (MD), 08 Aug 2018
Cited by: 0 articles | PMID: 30085502
ReviewBooks & documents Free full text in Europe PMC
Funding
Funders who supported this work.
Regional Ministry of Health and Families of Andalusia/Consejería de Salud y Familias, Junta de Andalucía (1)
Grant ID: PI-0514-2016

 2,3,4
2,3,4