Abstract
Free full text

The effect of climate change on avian offspring production: A global meta-analysis
Significance
Numerous studies have shown that climate change has altered avian timing of breeding. However, little is known about climate-driven changes in offspring production. We collected long-term breeding data on 201 populations of 104 bird species (N = 745,962 clutches) from all continents, between 1970 and 2019, to assess temporal changes in annual offspring production by female breeders in relation to changes in local temperatures and species’ life history traits. Overall, offspring production declined over time, but responses of different populations to rising temperatures were diverse. Our analyses suggest that negative effects of rising temperatures on offspring production will mainly affect migratory and larger-bodied species, whereas smaller-bodied sedentary species may benefit from warmer climate.
Abstract
Climate change affects timing of reproduction in many bird species, but few studies have investigated its influence on annual reproductive output. Here, we assess changes in the annual production of young by female breeders in 201 populations of 104 bird species (N = 745,962 clutches) covering all continents between 1970 and 2019. Overall, average offspring production has declined in recent decades, but considerable differences were found among species and populations. A total of 56.7% of populations showed a declining trend in offspring production (significant in 17.4%), whereas 43.3% exhibited an increase (significant in 10.4%). The results show that climatic changes affect offspring production through compounded effects on ecological and life history traits of species. Migratory and larger-bodied species experienced reduced offspring production with increasing temperatures during the chick-rearing period, whereas smaller-bodied, sedentary species tended to produce more offspring. Likewise, multi-brooded species showed increased breeding success with increasing temperatures, whereas rising temperatures were unrelated to reproductive success in single-brooded species. Our study suggests that rapid declines in size of bird populations reported by many studies from different parts of the world are driven only to a small degree by changes in the production of young.
Global temperatures have been rising significantly during the 20th and 21st centuries (1). Higher temperatures and changes in precipitation patterns have resulted in shifts of climatic zones, altering the conditions that animals experience on their breeding grounds, their wintering grounds, and during migration (2). Such climate changes have multiple effects on populations of diverse organisms, including birds (3, 4).
Most studies analyzing the effects of climate change on birds have focused on changes in timing of migration and breeding. In many cases, these studies have found that migratory species arrive earlier on their breeding grounds, and that many birds start to lay their eggs earlier in the season in response to higher temperatures (4–6). This is not surprising given that many species of birds exhibit phenotypic plasticity and breed earlier during warm springs (7, 8).
Less well explored, and more difficult to predict, are consequences of climate-driven advancements in laying dates (9, 10). In particular, few studies have investigated the production of offspring in avian populations in relation to climate change. Work that has been conducted indicates that responses vary enormously. Some show advanced laying dates, but other breeding parameters, including offspring production, are unchanged (8, 11). Other studies have found a decline in the production of young (10, 12). Such a decline may be a result of mismatch between time of peak food availability and time of maximum energetic requirements of the offspring (13, 14), but also more frequent adverse weather and weather extremes (10, 15, 16), higher predation (17, 18), or a decline in food (19). However, it is also possible that some species may benefit from breeding earlier as temperatures increase. Several studies have found increased fledgling production in warmer breeding seasons (20–22), or after warm winters (23).
We used a meta-analysis to assess changes in offspring production in avian populations worldwide over the last 50 y (Fig. 1 and SI Appendix, Table S1), a period over which global temperatures have risen by about 1 °C (1). We controlled for possible effects of phylogeny (24), life history of a species (9), migratory habits (25), latitude (26), direct human impacts (27), and changes in local temperature and precipitation as components of climate (15, 20). Our analyses complement prior studies that have investigated climate-driven changes in timing of egg-laying and related breeding parameters (26, 28, 29).
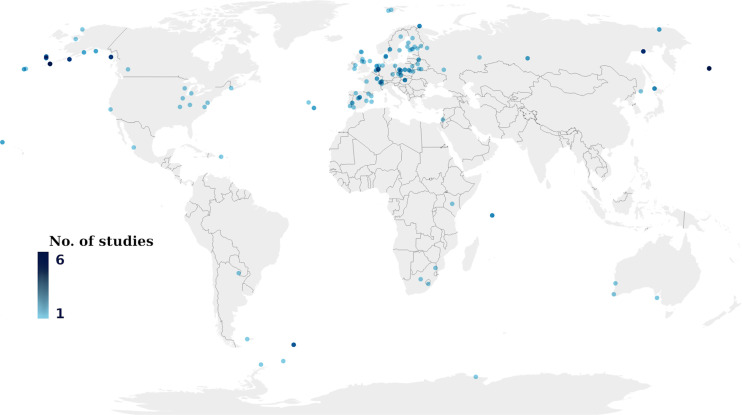
Distribution of sampling study areas around the world. Overlapping study areas result in darker dots.
Based on earlier research (28, 30), we hypothesized that multi-brooded species would increase the annual number of fledged young, because they are able to take advantage of protracted food resource availability (13), whereas single-brooded species would fledge fewer young per year, because they may have problems synchronizing timing of breeding with peak food availability (12, 25). Because larger-bodied species usually adapt more slowly to environmental changes due to their slower “pace of life” (31), we anticipated that declining trends in annual offspring production would be observed more often in larger-bodied than in smaller-bodied species. Finally, we predicted that declines in offspring production would be observed less often in sedentary species than in migrants, because the latter are more likely to be constrained in their phenological response to climate change (32, 33).
Results
Overall Trends.
Changes in offspring production were normally distributed and varied between −0.171 and 0.196 SDs per year from the long-term averages (Fig. 2). The funnel plot was symmetrical (Kendall’s rank test: tau = 0.002, P = 0.97), and trim-and-fill analysis indicated no directional bias in the sample of studies included in the analyses.
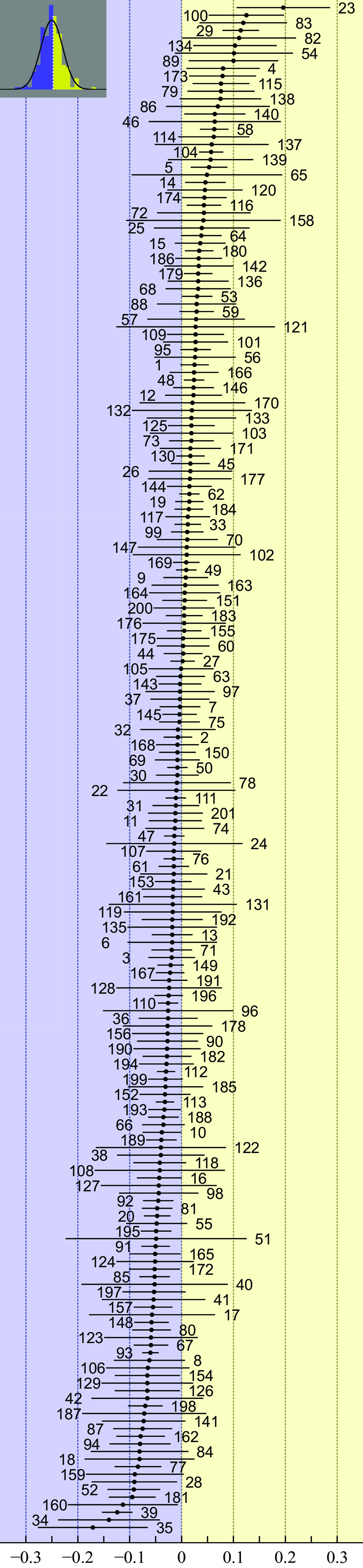
Change (±95% confidence limits) in mean number of offspring per female, measured in SD per year, in 201 populations of 104 species. Results were ranked from most negative to most positive. Numbers on the graph refer to population identifiers in SI Appendix, Table S1. The inset shows the frequency distribution of the effect sizes with a normal curve overlay.
In 114 of 201 (56.7%) populations, offspring production decreased over time; 17.4% of these did so significantly. Conversely, in 87 populations (43.3%), offspring production increased over time, 10.4% significantly. The grand effect size was slightly, but significantly, less than zero, suggesting an overall reduction in offspring production (Table 1). An analysis of spatial autocorrelation of population effect sizes yielded a Moran I of −0.003 (95% bootstrap percentile interval: −0.110 to 0.107), which was close to the value of −0.005 expected under the null hypothesis of no effect, indicating that directional changes in offspring production were not spatially clustered across geographical regions. The analyses of other life history and demographic traits indicated that nest success decreased and egg-laying started progressively earlier. Changes in clutch size and changes in duration of egg-laying periods were nonsignificant (Table 1).
Table 1.
Phylogenetically corrected grand effect sizes and their 95% bootstrap percentile confidence intervals of annual changes in demographic and life history traits of bird species around the world over the period 1970 to 2019
| Trait | Grand ES | 95% c.l. |
|---|---|---|
| Changes in offspring production (SDs), N = 104 species | −0.012 | −0.019, −0.005 |
| Changes in clutch size (SDs), N = 63 | 0.006 | −0.001, 0.012 |
| Changes in nest success (%p), N = 100 | −0.026 | −0.054, −0.008 |
| Changes in date of first egg (days), N = 104 | −0.066 | −0.093, −0.039 |
| Changes in duration of laying period (days), N = 104 | 0.009 | −0.058, 0.066 |
Grand effect sizes with 95% confidence intervals not covering a value of zero are in bold.
Correlates of Offspring Production.
We conducted univariate meta-regressions to assess the effects of life history and ecological characteristics of populations and their environments on changes in annual offspring production (Fig. 3). Changes in offspring production were positively associated with changes in clutch size and nest success. We also found a negative relationship between temporal changes in offspring production and body mass, with relatively larger species performing worse. None of the remaining life history traits produced a significant beta coefficient. The effect of nesting in nest-boxes was marginally positively significant. Ecological factors, including absolute latitude, environment type, protection status of the study area, and a Human Footprint Index (HFI), were not associated with changes in offspring production. Similarly, long-term trends in temperature and precipitation at different periods of the breeding season did not explain variation in changes in offspring production. In particular, changes in offspring production in relation to changing rainfall were consistently negligible.
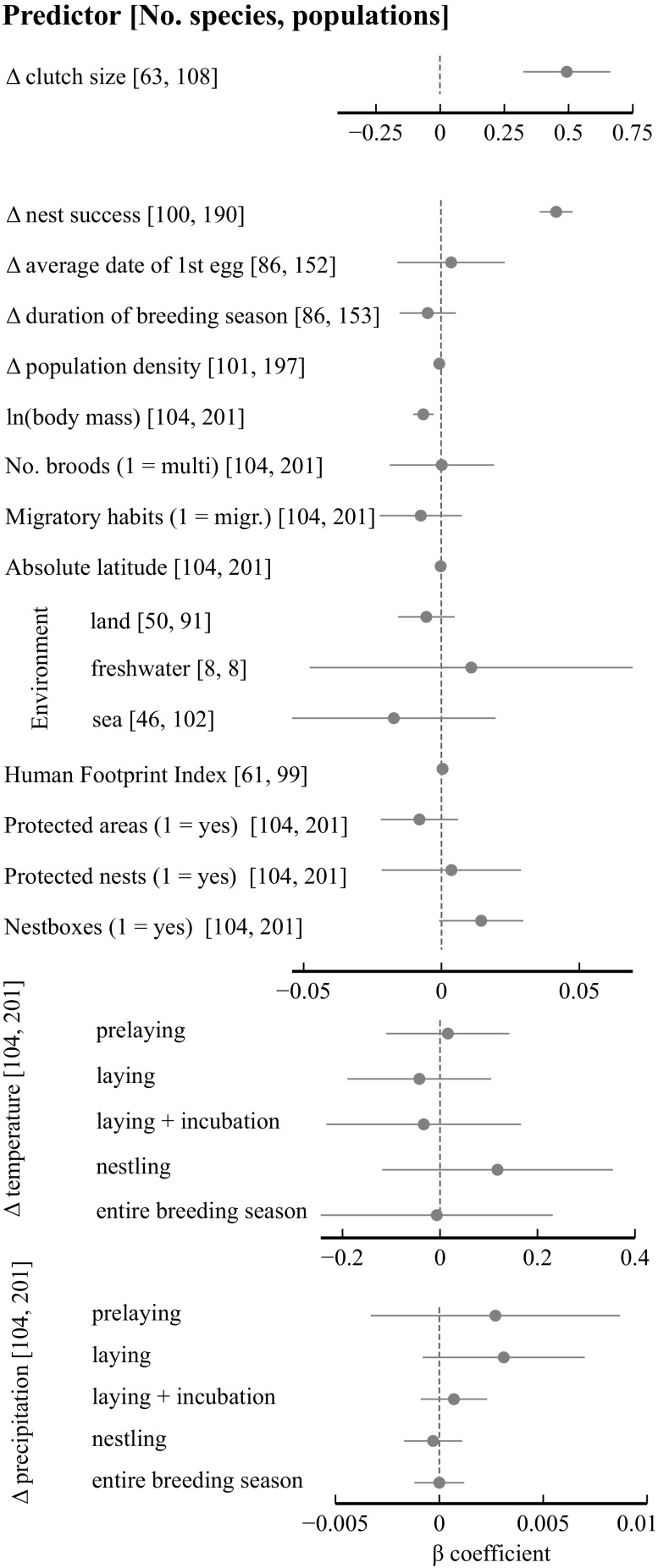
Beta coefficients (±95% confidence limits) of univariate meta-regressions in which standardized change in mean annual number of offspring per female was predicted by life history, demographic, ecological, and climatic variables. In all models, phylogenetic correlations along with identifiers of populations and species were included as random effects. Predictors expressed as a rate of change per year are shown as Δ. Note the different scales on each part of the plot.
Interactive Effects on Offspring Production and Nest Success.
In the next step, we used multivariate generalized linear mixed models (GLMMs) to model changes in offspring production and its two proxies: changes in clutch size and changes in nest success. In all models, we used the same sets of random effects (population, species, and phylogeny), and fixed predictor variables: log body mass, number of broods in a breeding season, migratory habits, and temporal change in local ambient temperatures. We considered two types of models: one that included all possible interactions between predictors and a simpler model including only two-way interactions of change in ambient temperature with the remaining fixed factors. For rate of change in local ambient temperature, we sequentially substituted measures taken in five phenological periods (enumerated in Fig. 3). Models were compared using Akaike’s information criterion (AIC) (SI Appendix, Table S2). Models with only two-way interactions outperformed the model with all possible interactions. With respect to temperatures, temperature trends in the nestling period produced the best model of changes in offspring production. Temperature trends in the prelaying period yielded the best model of changes in nest success, and temperature trends in laying period yielded the best model of changes in clutch size. Raw data and interactions between predictors in the model of changes in offspring production and model of changes in nest success are visualized in Fig. 4.
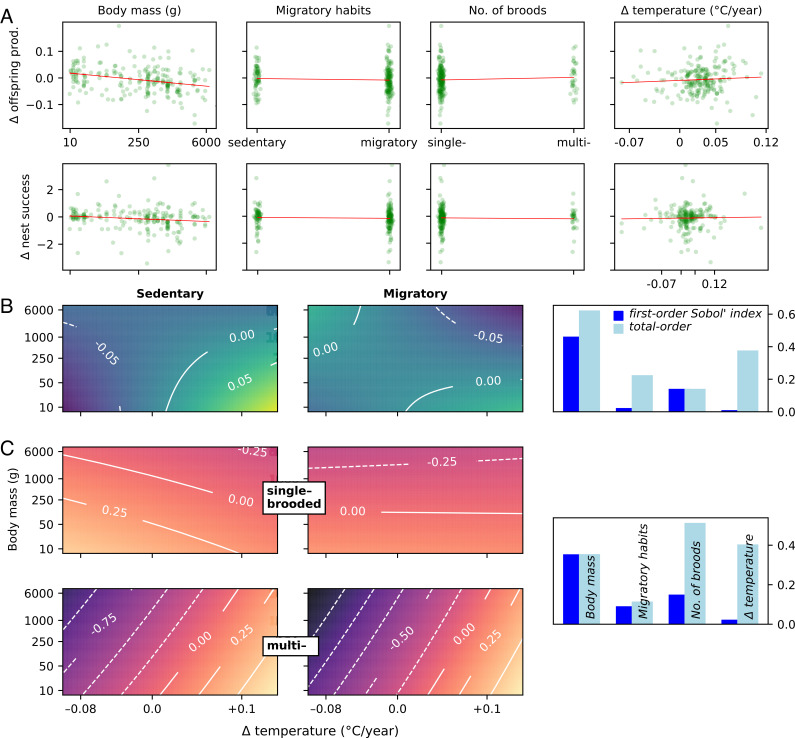
Trends in offspring production and nest success. Some redundant axis labels have been pruned to reduce visual clutter. (A) Raw data of change in the number of offspring female−1 season−1 in standard deviations per year and change in nest success (percentage points per year) regressed on life history traits and changes in local temperatures. Temperatures refer to the nestling period (upper plot) and prelaying period (lower plot). (B) Heatmaps visualizing the interactive effect of body mass and changes in local temperatures on the predicted change in mean number of offspring per female, expressed in SD per year, and represented by contours and colors. The bar graphs show the results of a sensitivity analysis. (C) Heatmap of predicted change in nest success, expressed in percentage points per year, with the results of a sensitivity analysis. The models in B and C are described in Table 2.
The final model of changes in offspring production (left section of Table 2 and Fig. 4B) revealed that increasing ambient temperatures during the chick-rearing period were associated with increased offspring production in small and/or sedentary birds, whereas most migratory birds (except for the smallest ones) experienced declines in offspring production in warming climates. Increasing offspring production tended to correlate with nesting in nest-boxes (Fig. 3). Therefore, we added nest-boxes as a fixed factor to test its effect in combination with other predictors. It was insignificant (beta = −0.003, 95% c.l.: −0.022 to 0.016), and the resulting model (SI Appendix, Table S3) had ΔAIC = 6.2, indicating a poorer fit.
Table 2.
Final meta-regression models of annual changes in fitness-related traits
| Term | Changes in offspring production [104, 201] | Changes in nest success[100, 190] | Changes in clutch size[63, 108] | |||
|---|---|---|---|---|---|---|
| Estimate | 95% c. l. | Estimate | 95% c. l. | Estimate | 95% c. l. | |
| Intercept | 0.002 | −0.043, 0.047 | 0.567 | 0.130, 1.003 | 0.065 | 0.015, 0.115 |
| Ln (body mass) | −0.003 | −0.009, 0.003 | −0.080 | −0.139, −0.020 | −0.011 | −0.017, −0.004 |
| Migratory habits (sedentary vs. migratory) | 0.012 | −0.010, 0.034 | −0.191 | −0.374, −0.009 | −0.019 | −0.045, 0.007 |
| Number of broods (single- vs. multi-brooded) | −0.021 | −0.055, 0.014 | −0.457 | −0.695, −0.219 | 0.001 | −0.031, 0.033 |
| Change in temperature* | 1.260 | 0.254, 2.267 | −1.768 | −7.110, 3.574 | −0.348 | −0.981, 0.285 |
| Ln(body mass) × change in temp. | −0.139 | −0.271, −0.007 | 0.078 | −0.749, 0.905 | 0.037 | −0.051, 0.125 |
| Migratory habits × change in temp. | −0.620 | −1.174, −0.066 | 1.363 | −1.226, 3.951 | 0.324 | −0.101, 0.749 |
| Number of broods × change in temp. | 0.006 | −0.727, 0.739 | 7.381 | 3.069, 11.693 | −0.340 | −0.828, 0.149 |
In all models, phylogenetic correlations between species and identifiers of populations and species were included as random effects. Beta coefficients whose 95% confidence limits do not include zero are shown in bold. The number of species and populations is in square brackets. Models of changes in offspring production and nesting success are visualized in Fig. 4.
*At the laying period in the model of clutch size, prelaying period in the model of nest success, and nestling period in the model of offspring production.
The final model of changes in nest success (middle section of Table 2 and Fig. 4C) suggested that the proportion of fledged nests declined in larger-bodied species. In single-brooded species, nest success was independent of climate changes. In multi-brooded bird species, nest success improved with increasing temperatures.
The final model of changes in clutch size (right section of Table 2) did not include significant interactions between predictor variables. The only important predictor was body mass, with large species producing smaller clutches.
The results of a sensitivity analysis (bar graphs in Fig. 4) showed that body mass alone captured around 40% of the uncertainty in trends of offspring production and nest success. Body mass also explained 60% of the uncertainty in the model of changes in clutch size. The other predictors had minor first-order effects. In particular, first-order sensitivity indices of changes in local temperatures were negligibly small in all final models. However, coupled effects of climate variability and life history traits were influential.
Discussion
Our meta-analysis shows that overall production of young in bird populations has been declining over the past 50 y across the globe and across species. This effect is significant even though it appears small: The decline is about 0.01 standard deviations per year in long-term mean offspring production. Over the course of decades, however, this can lead to a large reduction in the number of produced young. We also found substantial differences among sampled populations: 56.7% showed a declining trend in offspring production, and 43.3% of populations tended to produce more offspring. Generally, bird species whose offspring production declined were relatively large and migratory, whereas species whose production increased were small bodied and sedentary. This is a rough generalization, as some species do not fit this pattern. Additionally, in 5 of 35 species (14.3%) represented by more than one population, we found significant, but opposite trends in offspring production. This large variation suggests that reasons for changes in offspring production reflect, at least to some extent, some differences in local conditions. However, the change in offspring production was not associated with protection status of study areas or with anthropogenic transformation of their habitats.
Body mass, both as a stand-alone predictor and in relation to climate change, was the most important correlate of temporal changes in clutch size and offspring production. Our model suggests that larger species are more vulnerable to declines in offspring production and nest success (Fig. 4A), and that body mass exceeding 1 kg for sedentary species, and 50 g for migratory species, is associated with negative trends in offspring production for climate warming at a rate of 0.1 °C per year (Fig. 4B). Larger-bodied species may be slower in responding to changing environmental and climatic conditions due to their lower fecundity, extended maturation, and longer generation time (34, 35). Recent analyses suggest that selection favoring smaller body size under warming condition operates at the within-species, as well as among-species, level (36).
Sensitivity analysis of the final models indicates that climate change does not correlate with avian offspring production directly, but through complex interactions with their life history and ecological traits. Thus, climate variability is an influential factor when coupled with migratory habits and number of broods raised in the breeding season.
Our results suggest that nonmigratory species, especially smaller ones, are usually able to adjust to changes in local conditions and may benefit from climate warming (20, 37, 38), whereas migratory species, with the exception of the smallest, may suffer (25). This corroborates the phenology-mismatch hypothesis, which proposes that a lack of correlation between rate of warming in breeding and wintering areas causes a phenological mismatch on breeding grounds, resulting in population declines (32).
Previous research has provided evidence that an increase in local temperatures (both mean temperatures and extremes) may be correlated with changes in nest success (16, 17, 20, 22), a pattern found only for multi-brooded species in our analysis (Fig. 4C). As we did not have information on causes of nest losses, we cannot assess whether this reflects changes in predation pressure or some other factor. Multi-brooded birds often experience selection pressure for early breeding (39). A warming climate may be beneficial for such species, because earlier development of vegetation allows for better nest concealment, thereby reducing the risk of nest depredation and/or resulting in more food at the beginning of the season (13, 22). Nevertheless, nest-site selection may involve trade-offs between reduced risk of detection versus enhanced escape opportunities and information about approaching predators (40), which may result in selection for intermediate nest concealment (41, 42).
Several recent studies have provided evidence that populations of birds are severely declining on different continents (43–45). However, we found a relatively small overall decline in offspring production of avian populations across the globe. This suggests that recent population declines may reflect changes in adult and juvenile survival (10, 43, 46), and, to a lesser degree, changes in offspring production. It is also possible that habitat loss and deteriorating conditions make a higher proportion of the total population of declining bird species unable to breed, a hypothesis that remains to be tested.
Our dataset was biased in terms of the geographic location of populations, as we collected few data from the tropics and central parts of continents. Tropical regions host a large proportion of avian diversity and are experiencing rapid anthropogenic change, whereas areas inside continents usually undergo stronger climatic changes than areas affected by marine conditions (1). We therefore expect that the heterogeneity in our sample may be lower than the true global heterogeneity.
In conclusion, analyses based on 201 wild bird populations from all continents reveal that offspring production has declined during recent decades, but with considerable variation among species and populations. Species with relatively large body mass are the most vulnerable to decline in offspring production. Climate change appears to influence changes in offspring production through complex interactions with ecological and life history traits of species. Future studies should identify the reasons for nest failures to understand better the factors underlying declines in offspring production in avian populations.
Materials and Methods
Definitions.
We defined offspring production as mean annual number of fledglings produced by a breeding female in a population monitored throughout the breeding season. Mean clutch size was calculated for all clutches (first, replacements and second clutches) laid by females. We defined nest success as the proportion of successful clutches (producing at least one fledgling) out of all clutches monitored across a breeding season. Second broods were defined as those initiated after successfully fledging young from a first clutch. We classified a species’ population as single-brooded or multi-brooded depending on the presence and frequency of second broods in a population (SI Appendix, Table S1 and SI Text S1). We created three environmental categories, dividing species into land birds (not associated with water), freshwater species (breeding and foraging in inland water bodies), and seabirds. For each study population, we also calculated the temporal trend in the number of breeding females present at a study area. We divided study sites into protected and nonprotected; if >50% of a study area was under some forms of legal protection (national parks, nature reserves, etc.), we classified it as protected, otherwise as nonprotected. Similarly, we divided studies with nests protected against predators and nests nonprotected; if >50% of nests were protected (e.g., concrete nest-boxes, fences around nests), we classified the population as nest-protected. Finally, we divided studies into those using nest-boxes (if >50% of clutches were laid in nest-boxes) and those using natural nest sites (monitoring open-nesting and cavity-nesting species).
Criteria for Data Inclusion.
The criteria for a dataset to be included in our project were: 1) a minimum study period of 15 y, during which data were collected for at least 10 breeding seasons; 2) average seasonal number of breeding females was greater than 10; 3) in multi-brooded species, females were individually marked; and 4) research had to be conducted for the whole breeding season to determine accurately the fate of successive broods, and annual offspring production of each breeding female.
Data Collection.
Data were obtained from two major sources: 1) published information and 2) unpublished data from authors conducting long-term studies on the breeding biology of single species. 1) To locate information published in the current century concerning long-term changes in offspring production of avian populations, we undertook an exhaustive literature search in Google Scholar using the following key words: breeding, nesting, breeding success, offspring production, population productivity, climate change, and laying dates. Because we wanted to use standardized estimates of changes in offspring production across time (see below), we looked for publications that included original data. From published literature, we collected data on 72 bird populations representing 28 species (SI Appendix, SI Text S2). 2) Our literature search enabled us to identify researchers who potentially had long-term data on annual production of young in the bird populations they studied. Between October 2018 and April 2019, we contacted 313 authors of long-term studies asking them whether they would like to collaborate in our meta-analysis project. A total of 101 authors shared their data on 86 species and 129 populations (for details on searching process, see SI Appendix, SI Text S3). From both data sources, we collected a total of 201 datasets on 104 bird species (for problems with species number, see SI Appendix, SI Text S4) covering the histories of 745,962 clutches. The data were gathered in both hemispheres between 1970 and 2019 (Fig. 1), and different populations were sampled over a period ranging from 15 to 49 breeding seasons (median 26; interquartile range 20 to 35) (SI Appendix, Table S1). Literature sources, including descriptions of field methods used in the studies included in the analyses, can be found in SI Appendix, Table S4.
In addition to the data on offspring production, we collected data on laying dates, clutch size, nest success, breeding density, and proportion of females with second broods or replacement clutches. These data were not provided by all researchers, and not found in all literature sources.
Meteorological, Body Mass, and Migratory Data.
Meteorological data for the study areas, including mean monthly temperatures and total monthly precipitation, were provided by authors or, for published data, obtained from the National Oceanic and Atmospheric Administration (http://www.ncdc.noaa.gov/cdo-web/datatools/findstation) or Tutiempo.net (https://en.tutiempo.net/climate/europe.html). We used data from meteorological stations closest to study areas with complete records across study years. For each area, we calculated mean temperatures (oC) and total precipitation (mm) corresponding with each of the five breeding periods: 1) prelaying (a month preceding commencement of egg laying); 2) egg laying; 3) egg laying and incubation; 4) nestling period; and 5) whole breeding season (from the commencement of egg laying until the end of parental care). The information about timing and duration of these periods was obtained from main investigators or extracted from the literature (47, 48). We subsequently calculated the rate of change in mean temperature and total precipitation over the study period using linear regressions as an index of climate change intensity.
Data on mean female body mass for each species were obtained from Handbook of Avian Body Masses (49). The distribution of body masses was strongly skewed; therefore, we ln-transformed it for our analyses. Migratory status (full migrant vs. sedentary/partial migrant) followed the International Union for Conservation of Nature classification (50).
HFI.
For each study area, we calculated a Human Footprint Index (HFI), which quantifies the degree to which humans have impacted the landscape (on 1 km2 grid cells) by combining measures of human population density, buildings, crop land, pasture land, night-time lights, railways, roads, and navigable waterways (27). Values of the index range from 0 to 50. HFI data were extracted from ref. 51 using QGIS software (version 3.22.11). For each study area, we calculated a mean HFI score by overlaying the HFI raster layer with a site raster layer. Because HFI data are not available for islands, we were able to calculate the index for only 114 continental bird populations.
Statistical Analysis.
Analyses were conducted using R version 4.2.2 (52) and Phylometa version 1.3 (53). They were carried out in two steps: 1) we estimated grand effect sizes for a group of variables describing changes in species’ parameters and 2) using metaregression, we looked for factors explaining heterogeneity in effect sizes (hereafter ESs) from the first step.
A list of all variables used in the analysis (including the species’ parameters) is provided in SI Appendix, Table S5. All ESs studied were slopes of linear regressions, in which response variables were regressed on time (years). Slopes are intuitively interpreted as changes per annum. Annual production of offspring per female and clutch size were standardized and expressed as the number of standard deviations from the long-term mean measured across all study years of a particular population. This allowed comparison across species whose mean offspring production and clutch sizes differed.
Because of between-study heterogeneity due to variation in local environmental conditions, life histories of study species, and research methods, we used random-effects models in all meta-analyses. Sensitivity of the model due to publication bias was assessed using funnel plots and a trim-and-fill method (54).
We controlled for a potential bias due to phylogenetic relatedness among species (SI Appendix, Table S1 and SI Text S4) using a set of 5,000 equally plausible trees, downloaded from birdtree.org (55, 56). Grand ESs of changes in species’ parameters were calculated by an iterative procedure with original phylogenetic trees. For meta-regression modeling, we built a 50% majority-rule consensus tree (56) using the R package “ape,” version 5.5 (57).
Grand ESs in Table 1 were calculated using Phylometa software. We reran 5,000 iterations, each time using a different phylogenetic tree, and a subset of ESs in question, which included only a single record for each species. If a species was represented by several local populations, one was selected at random for each iteration. Grand ESs were means across all resamples, with 95% confidence limits represented by the 2.5th and 97.5th percentiles.
Meta-regression models (Fig. 3, Table 2, and Fig. 4) were run using R package “metafor”, version 3.4 (54). Each model included the same set of random effects: species and study identification (to control for nonindependence of multiple studies of the same species) and species identity associated with phylogenetic correlations (to control for nonindependence due to common ancestry).
In analyses where changes in offspring production and nest success were regressed upon different sets of climate variables, we used Akaike’s information criterion to assess the performance of competing models (58) (SI Appendix, Table S2) and to select climate variables for final meta-regression models (Table 2).
For sensitivity analysis of the final models, we used Sobol’s global variance decomposition method (59) implemented in R package “sensobol” version 1.1.3 (60). This procedure decomposes variance into fractions (Sobol’s indices), which isolate effects of predictor variables and their interactions. Pseudo-random samples for the model’s predictions were generated using a Latin hypercube sampling design. Estimates of Sobol’s indices were based on Azzini’s algorithm, using 10,000 bootstrap iterations, which guaranteed convergence under the criterion that the 95% confidence interval around the most sensitive predictor was less than 5% of its sensitivity index. For each predictor variable, we reported first-order and total-order indices. The former measures the fraction of the output variance explained by a respective predictor alone, whereas the latter includes effects of its interactions with other predictors.
Acknowledgments
We are grateful to our collaborators who worked with us and collected field data. The list of principal collaborators and institutions is published in SI Appendix, Table S6. We thank Heather Renner for the information about the data collected by Alaska Maritime National Wildlife Refuge. We sincerely appreciate valuable comments and suggestions made by two reviewers, which helped us in improving the manuscript. This meta-analysis was financed by the grant of the Polish National Science Centre (Narodowe Centrum Nauki) (no. 2017/27/B/NZ8/00465) awarded to Lucyna Hałupka.
Author contributions
L.H. and K.H. designed research; L.H. and M. Czuchra conducted literature search and collected meteorological data; L.H., P. Adamík, P. Albert, W.J.A., D.A., A.V.A., V.B., J. Bańbura, M. Bańbura, E. Barba, R.T.B., P.H.B., E. Belskii, P. B., M. Bolton, E.K.B., J. Bried, L. Brouwer, M. Bukacińska, D.B., L. Bulluk, K.F.C., I.C., M. Charter, A.C, R.C., D.C.D., F.d.L., A.S.D.G., V.C.D., H.D., M.J.D., T.E., L.M.E., Y.O.E., J.A.F., S.I.G., E.Y.G., M.G., M.P.H., J.P.H., P.K., J.K., W.D.K., H.K., M.K.-A., E.K., I.K., M.K., S.K., B.D.K., X.L., M.P.L., A.L., A. Margalida, A. Marzal, A. Millon, A.P.M., V.C.N., J.T.N., A.N., B.O., D.O, M.Ö., R.A.P., H. Pietiäinen, V.P., J. Porkert, J. Potti, H. Pöysä, T.P., J. Prop, P.Q., J.A.R., P.-A.R., R.N.R., A.R., D.R.R, I.E.S., D.A.S., M.S., J.C.S., F.S., T.S., D.V.S., J.S., P.M.T., M.T., J.Tolvanen, J. Török, M.v.d.P., L.V., M.E.V., D.F.W., N.T.W., J.W., K.W., A.G.W., A.W., D.W., M. Z. performed research and contributed the data; Z.J. calculated Human Footprint Indices; K.H. analysed the data; L.H. and K.H. wrote the manuscript, and all authors edited the manuscript. Valuable scientific contributions throughout the writing and editing process were done by D.A., J. Tolvanen, A. Millon, W.D.K., P.B., M.G., M.S., H. Pöysä, M.Ö., M.T., M.v.d.P., D.A.S., and A. Margalida.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Although PNAS asks authors to adhere to United Nations naming conventions for maps (https://www.un.org/geospatial/mapsgeo), our policy is to publish maps as provided by the authors.
Data, Materials, and Software Availability
All data and R scripts relevant to the article are available at Figshare (61). Other study data are included in the article and/or SI Appendix, Tables S1–S3.
Supporting Information
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/147134556
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.2208389120
Article citations
Rural reality contradicts the ethnographic literature-a nationwide survey on folk beliefs and people's affection for the stork in Poland.
J Ethnobiol Ethnomed, 20(1):51, 14 May 2024
Cited by: 0 articles | PMID: 38745225 | PMCID: PMC11094895
Will a changing climate affect hatching success in cavity-nesting birds: A case study with Eastern Bluebirds (Sialia sialis)?
Sci Prog, 107(2):368504241245222, 01 Apr 2024
Cited by: 0 articles | PMID: 38745552 | PMCID: PMC11097714
Avian Haemosporidian Infection in Wildlife Rehabilitation Centres of Portugal: Causes, Consequences, and Genetic Diversity.
Animals (Basel), 14(8):1216, 18 Apr 2024
Cited by: 0 articles | PMID: 38672371 | PMCID: PMC11047687
Effect of heat stress on the hypothalamic expression profile of water homeostasis-associated genes in low- and high-water efficient chicken lines.
Physiol Rep, 12(5):e15972, 01 Mar 2024
Cited by: 2 articles | PMID: 38467563 | PMCID: PMC10927601
Factors affecting fledglings survival in urban population of European blackbirds in Szczecin (NW Poland).
Sci Rep, 13(1):18723, 31 Oct 2023
Cited by: 0 articles | PMID: 37907582 | PMCID: PMC10618181
Go to all (7) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Data Citations
- (1 citation) DOI - 10.7927/H46T0JQ4
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The effect of climate change on the duration of avian breeding seasons: a meta-analysis.
Proc Biol Sci, 284(1867):20171710, 01 Nov 2017
Cited by: 20 articles | PMID: 29167360 | PMCID: PMC5719171
Climate change affects the duration of the reproductive season in birds.
J Anim Ecol, 79(4):777-784, 01 Mar 2010
Cited by: 33 articles | PMID: 20202013
Fitness consequences of longer breeding seasons of a migratory passerine under changing climatic conditions.
J Anim Ecol, 90(7):1655-1665, 04 May 2021
Cited by: 6 articles | PMID: 33724451 | PMCID: PMC8360183
Patterns and drivers of intraspecific variation in avian life history along elevational gradients: a meta-analysis.
Biol Rev Camb Philos Soc, 91(2):469-482, 12 Mar 2015
Cited by: 21 articles | PMID: 25765584
Review
Funding
Funders who supported this work.
Narodowe Centrum Nauki (1)
Grant ID: 2017/27/B/NZ8/00465
Natural Environment Research Council (1)
Grant ID: bas0100035

 a
,
1
a
,
1


