Abstract
Free full text

Novel Intranasal Loop Diuretic
Preclinical and first-in-human results indicate that bumetanide nasal spray could prove to be a valuable outpatient therapeutic option, which in theory offers to help selected patients avoid hospital admissions and readmissions thus potentially reducing the cost of care and improving outcomes. Corstasis plans to conduct the pivotal trial for bumetanide nasal spray in 2023.
More than 6.5 million Americans have heart failure (HF), with nearly 1 million new cases diagnosed annually. HF accounts for 1 out of every 8 deaths and costs an estimated $30.7 billion. In 2018 1,250,000 patients were discharged after a HF-related admission.1 Furthermore, the national 30-day readmission rate for HF is 23%, making it 1 of the 6 high-priority conditions included in Medicare’s Hospital Readmissions Reduction Program and value-based purchasing program.2
HF is a complex and multifaceted syndrome. Edema, congestion, and fluid overload are synonymous classic clinical features of patients presenting with HF. Oral loop diuretics such as furosemide, bumetanide, and torsemide are the cornerstone of outpatient therapy for the management of fluid volume in patients with HF. However, patients with HF frequently experience diminished response to oral diuretics, resulting in hospital admission for intravenous diuretic administration.3 Recent data suggest that gut congestion and gastrointestinal impairment are important mechanisms underlying oral diuretic resistance impacting patients suffering from fluid overload in both in- and outpatient settings.4
Oral diuretics are absorbed in the gastrointestinal tract, and any impairment of gastrointestinal motility can lead to delayed and reduced absorption of these medications. Accumulating data suggest that the need to intensify outpatient oral diuretic therapy is associated with a substantial risk of morbidity and mortality. A recent analysis of the Danish registry found intensification was common and associated with a 75% higher relative risk of 1-year mortality.5
Corstasis Therapeutics is developing bumetanide intranasal spray (RSQ -777) for the treatment of edema associated with congestive HF, hepatic, and renal disease, including the nephrotic syndrome. Intended to provide a short-term outpatient therapeutic option (≤7 days) via the nasal route of administration for patients who do not require hospitalization. Patients may include those demonstrating decreased responsiveness to oral diuretics owing to suspected gastrointestinal impairment or in whom an oral route or intravenous route of administration is not practical.
Bumetanide intranasal spray (RSQ-777) leverages the Unidose Nasal Delivery System, which is a primeless, single-use, 1-step nasal delivery system that will accurately deliver 0.5 mg bumetanide per device. To deliver therapy, the patient or caregiver simply presses a small plunger on the bottom of the device to release the drug in a single spray into the nostril, where the drug can be absorbed quickly via the nasal mucosa bypassing potentially impacted oral metabolic pathways (Figure 1A).
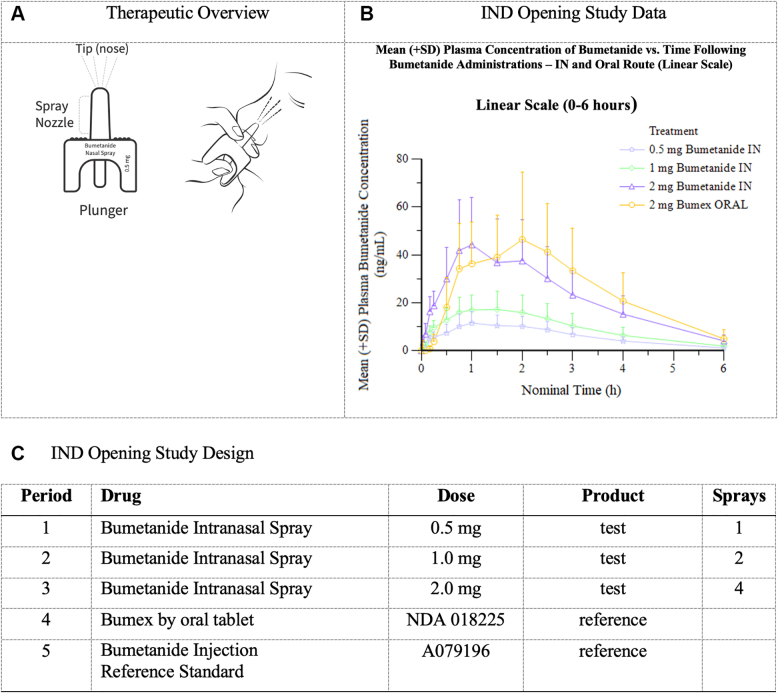
Study Overview
(A) Therapeutic overview. (B) Investigational new drug (IND) opening study data. IN = intranasal.
Corstasis has conducted 2 pharmacokinetic absorption studies of Bumetanide Intranasal Spray formulations in rabbits and dogs to generate proof-of concept data and to evaluate the pharmacokinetics and pharmacodynamics of intranasal bumetanide administration. Corstasis has further conducted 28-day toxicity studies of bumetanide intranasal spray using intranasal instillation in and with a 14-day recovery phase.
Corstasis completed RQ-777-01, a phase I, investigational new drug–opening, open-label, 5-period, 1-sequence, cross-over study to evaluate the safety, tolerability, and pharmacokinetic parameters of bumetanide from bumetanide intranasal spray. Sixteen participants were enrolled in the study, which was conducted in Orange County, California, in October of 2022. The study treatments can be found in Figure 1C.
Intranasal bumetanide was safe and generally well-tolerated with no overt signs of nasal irritation across the study population. Following intranasal or oral administrations, bumetanide was absorbed with a median time to maximum plasma concentration (tmax) ranging from 1.02 to 1.50 hours post dose. Plasma bumetanide concentration remained steady between 1.0 and 2.0 hours post dose, followed by a slow decline. Increases in bumetanide exposure seemed to be dose proportional over the 0.5- to 2.0-mg dose range after intranasal administration. Furthermore, bumetanide exposure after a 2.0-mg intranasal administration was similar to a 1.0-mg intravenous or a 2.0-mg oral dose. For intranasal vs oral administration, particularly in the 2.0 mg intranasal group, the intranasal tmax did occur sooner compared to oral, although differences in tmax were not statistically significant. There is no significant difference in the maximum concentration and area under the curve 0-last between the intranasal route and the oral route at the 2.0-mg dose level (Figure 1B). An exploratory observation of the study demonstrated similar urine output for the intranasal arms as compared with the oral and intravenous cohorts.
Footnotes
This work was presented at the 2023 CRF THT Shark Tank; March 20-22, 2023; Boston, Massachusetts.
Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2023-shark-tank.
Financial support for this research letter provided by Corstasis Therapeutics Inc. Mr Esque is an Executive with Corstasis Therapeutics. Dr Adler is a member of the Scientific Advisory Board, Corstasis Therapeutics. Dr Bensimhon is a member of the Scientific Advisory Board, Corstasis Therapeutics.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
Articles from JACC: Basic to Translational Science are provided here courtesy of Elsevier
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/146486894
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Estimation of absorption rate of alpha-human atrial natriuretic peptide from the plasma profile and diuretic effect after intranasal administration to rats.
Biopharm Drug Dispos, 22(4):137-146, 01 May 2001
Cited by: 3 articles | PMID: 11745916
Diuretic response and effects of diuretic omission in ambulatory heart failure patients on chronic low-dose loop diuretic therapy.
Eur J Heart Fail, 23(7):1110-1119, 15 Mar 2021
Cited by: 8 articles | PMID: 33641220
Decrease in loop diuretic treatment from 2005 to 2014 in Swedish real-life patients with chronic heart failure.
Eur J Clin Pharmacol, 75(2):247-254, 15 Oct 2018
Cited by: 5 articles | PMID: 30318559 | PMCID: PMC6348069
Management of loop diuretic resistance in the intensive care unit.
Am J Health Syst Pharm, 66(18):1635-1640, 01 Sep 2009
Cited by: 14 articles | PMID: 19729568
Review




