Abstract
Free full text

p53 and PTEN expression evaluation with molecular evident recent criteria in laryngeal carcinoma
Abstract
The prognosis of laryngeal cancer is affected by clinicopathological factors. Because of that, an effective prognostic marker is very valuable in managing the clinical process. The p53 evaluation method, used in the literature recently, was used for the first time in laryngeal cancer. We evaluated PTEN with 2 methods with the highest significance in the literature on laryngeal cancer. All demographic and histopathological data from 140 laryngeal cancers were compared with p53 and PTEN expressions and survival. p53 staining patterns were classified as wild and mutant. PTEN expression was evaluated according to the staining intensity named PTEN1 and according to the proportion of stained cells named PTEN2. In the series, 93.6% were males, and the mean survival was 38 months. 69.3% of cases were p53 mutants. PTEN loss was found to be 85.7% and 57.9%, respectively. Tumor size and thyroid cartilage invasion for PTEN1 and age for p53 were identified as independent predictive factors (P < .01). Advanced age, total laryngectomy, and extranodal spread were independent poor prognostic factors for overall survival and the presence of subglottic involvement, perineural invasion, and extranodal spread were for disease-free survival (P < .01). This is the first study in which the new p53 classification was used in laryngeal cancer, and will contribute significantly to the literature with differences from the previous evaluation patterns. Evaluation of PTEN based on staining intensity is more appropriate compared to the percentage of stained cells.
1. Introduction
According to the data of GLOBOCAN 2020, while the newly diagnosed larynx cancer cases worldwide were 184.615, death from laryngeal cancer occurred as 99.840.[1] Smoking and drinking are among the factors that cause the development of laryngeal cancer.[2] While there is an 80% to 90% chance of cure in cases diagnosed and treated at an early stage, most patients are diagnosed at a late stage.[3] The prognosis of the disease in laryngeal cancer is affected by clinicopathological features and demographic factors.[4–6] In addition to these parameters in predicting the prognosis, the search for prognostic markers is a scorching field of study.[7–9]
TP53 mutation is the most common genetic change observed in over half of malignant tumors.[10] p53 protein is encoded by the TP53 gene located on chromosome 17. When the cell is exposed to stress that causes DNA damage, it inhibits neoplastic development by stopping the p53 cell cycle or initiating apoptosis.[11] Sequencing is not a method available in all pathology laboratories. p53 immunohistochemistry is a faster, easier and cheaper method that can be performed in the vast majority of them, so immunohistochemical (IHC) evaluation of p53 is valuable.[12]
Although p53 immunohistochemistry has been evaluated as a prognostic marker for many years, the recognition of molecular alterations of the protein and intracellular events has led to other methods of evaluating the immunohistochemistry slides. Missense mutations in the TP53 gene result in the formation of a mutant protein, and the degraded protein accumulates in the nucleus. Nonsense mutations are characterized by the complete absence of the p53 protein. C-terminal mutations disrupt the nuclear localization signal and p53 protein accumulates in the cytoplasm. Detection of these abnormal/mutant p53 expression patterns as an indicator of TP53 mutation by IHC method has started to be used as a reliable method. In recent studies, p53 IHC evaluation method, highly correlated with TP53 gene mutation results in gynecological malignancies, has been described.[13,14] However, to our knowledge, head and neck tumors were not evaluated with these recently described criteria.
PTEN is a tumor suppressor gene located on chromosome 10, and it is one of the 3 most frequently mutated tumor suppressor genes in humans, along with TP53 and Rb.[15] PTEN is a negative regulator of the PI3K pathway. The PI3K pathway is essential in many cellular processes, such as cell proliferation, motility, differentiation and survival.[16] The increase in phosphatidylinositol-3,4,5-triphosphate levels, the second messenger, after the growth hormone stimulation enables this pathway to be activated. PTEN inhibits this pathway by dephosphorylating phosphatidylinositol-3,4,5-triphosphate. Loss of PTEN can occur in the form of deletions or mutations, as well as epigenetic or posttranscriptional modifications.[17,18] PTEN IHC evaluation has been tried in many different ways in the literature. However, an evaluation method that is superior and correlates with mutation/deletion has not come to the fore. The reason for this is thought to be epigenetic mechanisms other than mutation/deletion.[18]
In this study, we evaluated the IHC expressions of the most common tumor suppressor genes in laryngeal squamous cell carcinomas (SCCs), PTEN and p53 with recently described criteria and the relationship of these results with demographic data, histopathological parameters and survival. To our knowledge, this is the first study in which the new p53 classification was used in laryngeal cancer.
2. Materials and methods
A total of 140 patients treated with laryngectomy and diagnosed with SCC between 2010 and 2020, whose blocks could be accessed, and who had follow-up and survival data, were included in the study. Age, gender, operation type, tumor localization, tumor size, tumor differentiation, thyroid cartilage invasion, cricoid cartilage invasion, surgical margin status, lymphovascular invasion, perineural invasion, lymph node metastasis, extranodal spread, stage and chemoradioteraphy information of the cases were noted. pN stage reclassified according to the new classification, including extranodal extension. Recurrence, distant metastasis, second primary status, and follow-up status of the patients were scanned from the hospital computer registry system.
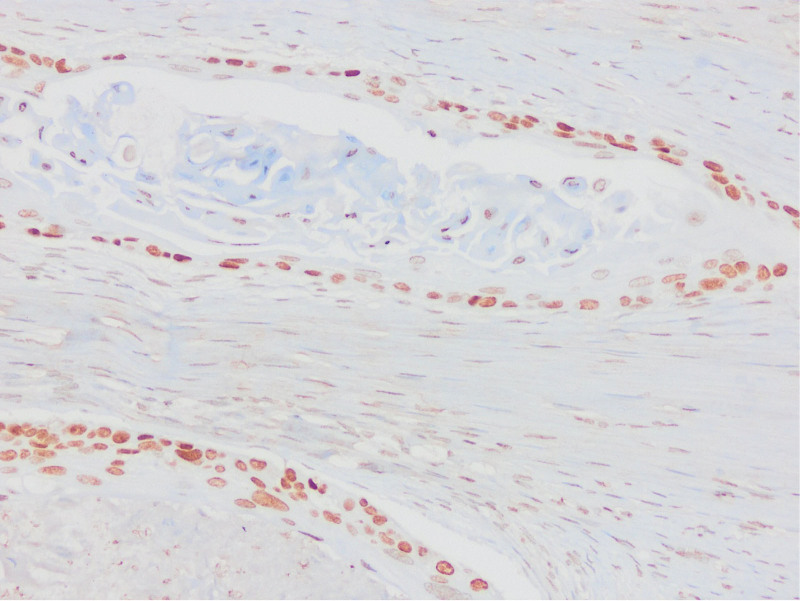
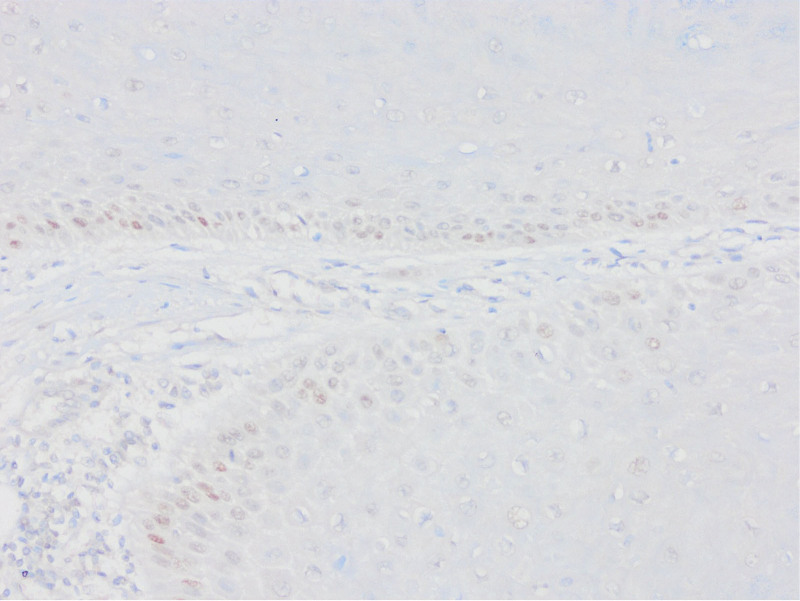
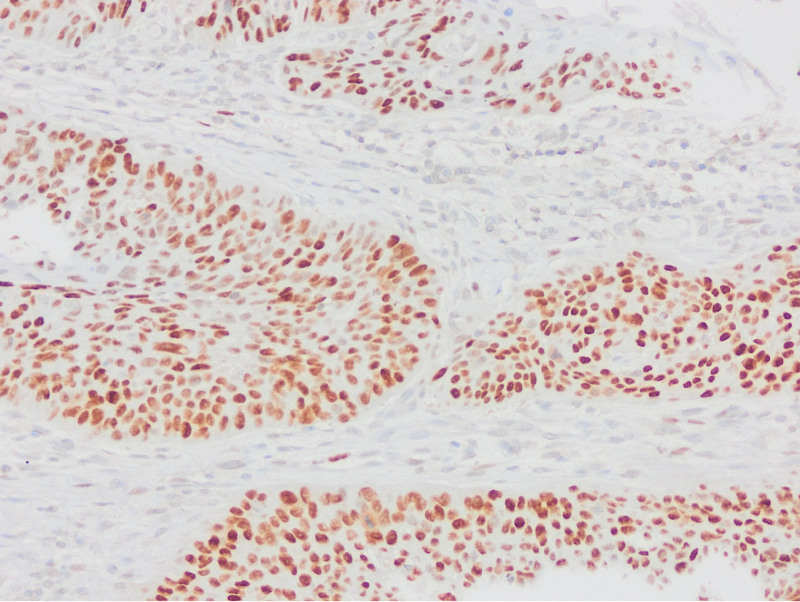
Appropriate block selection was made from all cases, and sections were taken on lysine slides. IHC staining of p53 (Clone: D07; Ventana Medical Systems, Tucson) and PTEN (Clone: A2B1; Santa Cruz Biotechnology, CA) was applied in the Ventana automatic staining device. Stromal and inflammatory cells were used as internal controls. p53 staining patterns were evaluated in 6 categories, 2 of which were wild type and 4 were mutant. Wild type (normal) staining (A.Scattered: Heterogeneous nuclear staining in the basal and/or parabasal layer, B.Mid-epithelial: Staining in the middle of the epithelium, where the basal layer is preserved); Mutant type (abnormal) staining (A.Basal overexpression: Strong nuclear staining that persists in >80% cells, B.Basal/parabasal expression: Basal and parabasal staining in >80% cells, C.Null: Absence of staining, D.Cytoplasmic: cytoplasmic staining with accompanying nuclear staining).
PTEN assessment was performed in 2 ways, mostly used in the literature. The initial evaluation, named PTEN1, was divided into weak or no staining compared to epithelial-stromal cells and more intense staining than epithelial-stromal cells. The second evaluation, PTEN2 was divided into less than 10% or no staining of cells and more than 10% of cells staining. In both assessments, the first group was considered to have PTEN loss, and the second group was considered to have no PTEN loss.
Normality analysis of numerical variables was done by Kolmogorov–Smirnov test. Normally distributed data were analyzed with the independent sample t test or one-way Anova test, non-normally distributed data with Mann–Whitney U and Kruskal–Wallis tests, and categorical data with Pearson Chi-square or Fisher’s exact test. Logistic regression analysis was performed on the statistically significant data. In the study, the median values of overall survival and disease-free survival were calculated and the most significant time was found by the survival analysis. Survival analyzes were performed using the Kaplan–Meier log rank method. The oncological risks of the variables determined as a result of this were determined by the Cox regression (proportional) risk model. A P < .05 was considered statistically significant in statistical analysis. Statistical analyzes were performed using the SPSS 20.00 (IBM SPSS- 30 day Free trial) program.
3. Results
Of the cases, 131 (93.6%) were male and 9 (6.4%) were female. The age range ranged from 41 to 83, with a mean of 60.9 ±
± 9.2 and a median of 61. While total laryngectomy was performed in 100 (71.4%) cases, the remaining cases were partial laryngectomy. Of the cases, 38 (27.2%) were supraglottic, 50 (35.7%) glottic, 3 (2.1%) subglottic, and 49 (35%) transglottic. Subglottic involvement was present in 56.4% of the tumors. Tumor size ranged from 0.6 to 7.5
9.2 and a median of 61. While total laryngectomy was performed in 100 (71.4%) cases, the remaining cases were partial laryngectomy. Of the cases, 38 (27.2%) were supraglottic, 50 (35.7%) glottic, 3 (2.1%) subglottic, and 49 (35%) transglottic. Subglottic involvement was present in 56.4% of the tumors. Tumor size ranged from 0.6 to 7.5 cm, with a mean of 3.1
cm, with a mean of 3.1 ±
± 1.3
1.3 cm and a median of 3
cm and a median of 3 cm. Eighty-one (57.9%) cases consisted of tumors ranging from 0 to 3
cm. Eighty-one (57.9%) cases consisted of tumors ranging from 0 to 3 cm. Of the tumors in the series, 23 (16.4%) were well-differentiated, 95 (67.9%) moderately, and 22 (15.7%) poorly differentiated tumors. Thyroid cartilage invasion was detected in 50 (35.7%) cases, and cricoid cartilage invasion in 17 (12.1%) cases. The surgical margin was positive in 37 (26.4%) cases. Eleven (7.9%) cases had lymphovascular invasion and 16 (11.4%) had perineural invasion. Lymph node dissection was performed in 126 cases, and the number of dissected lymph nodes ranged from 10 to 119, with a mean of 48.5
cm. Of the tumors in the series, 23 (16.4%) were well-differentiated, 95 (67.9%) moderately, and 22 (15.7%) poorly differentiated tumors. Thyroid cartilage invasion was detected in 50 (35.7%) cases, and cricoid cartilage invasion in 17 (12.1%) cases. The surgical margin was positive in 37 (26.4%) cases. Eleven (7.9%) cases had lymphovascular invasion and 16 (11.4%) had perineural invasion. Lymph node dissection was performed in 126 cases, and the number of dissected lymph nodes ranged from 10 to 119, with a mean of 48.5 ±
± 21.1 and a median of 44.5. Metastasis was detected in 53 (42%) of 126 patients who underwent lymph node dissection. The extranodal spread was detected in 26 (49.1%) of 53 patients with lymph node metastasis. While 17 (32.1%) of the patients with lymph node metastases had single lymph node metastases, multiple lymph node metastases were observed in 36 (67.9%) cases. Eight (5.8%) of the cases were stage I, 14 (10%) were stage II, 34 (24.2%) were stage III and 84 (60%) were stage IV, and 84.3% of the cases were at an advanced stage. The number of patients who received preoperative radiotherapy was 10 (7.1%). Of the patients who received postoperative adjuvant therapy, 32 received only RT and 70 received RT/CT. Cisplatin-based chemotherapy was applied to the cases.
21.1 and a median of 44.5. Metastasis was detected in 53 (42%) of 126 patients who underwent lymph node dissection. The extranodal spread was detected in 26 (49.1%) of 53 patients with lymph node metastasis. While 17 (32.1%) of the patients with lymph node metastases had single lymph node metastases, multiple lymph node metastases were observed in 36 (67.9%) cases. Eight (5.8%) of the cases were stage I, 14 (10%) were stage II, 34 (24.2%) were stage III and 84 (60%) were stage IV, and 84.3% of the cases were at an advanced stage. The number of patients who received preoperative radiotherapy was 10 (7.1%). Of the patients who received postoperative adjuvant therapy, 32 received only RT and 70 received RT/CT. Cisplatin-based chemotherapy was applied to the cases.
The median follow-up was 38 months (1–126 months). The overall survival rate of the cases was 74.7%, the disease-free survival rate was 78.7%. Recurrence was detected in 17 (12.1%) cases and distant metastasis in 14 (10%) cases. Distant metastasis localizations were lung in 12 (85.8%), brain in 1 (7.1%) and bone in 1 (7.1%) case. There was another primary malignancy in 19 (13.5%) cases. Lung cancer in 9 (47.2%) cases, papillary thyroid carcinoma in 3 (15.7%) cases, oropharyngeal carcinoma in 2 (10.5%) cases, and bladder cancer, chronic lymphocytic leukemia, skin SCC, SCC and nasopharyngeal carcinoma in the remaining 5 cases were observed. Of 140 patients, 42 (30%) died and 98 (70%) were still alive.
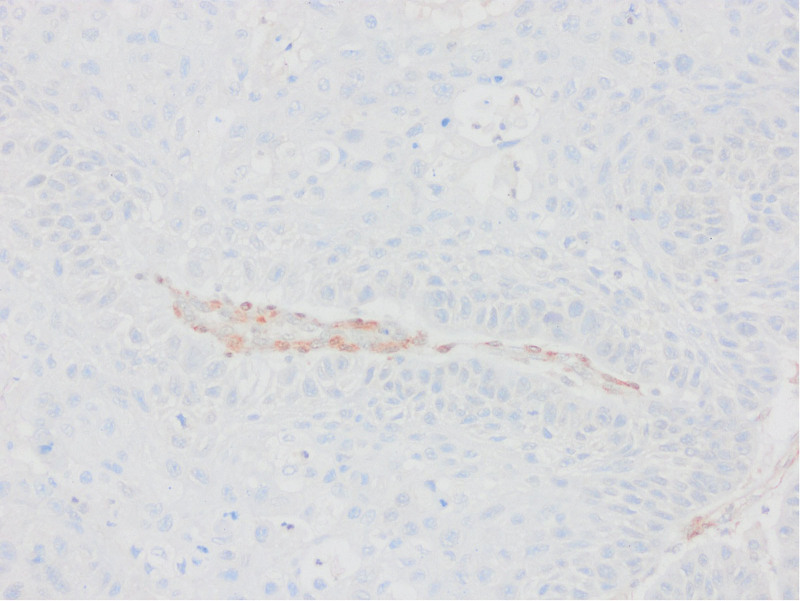
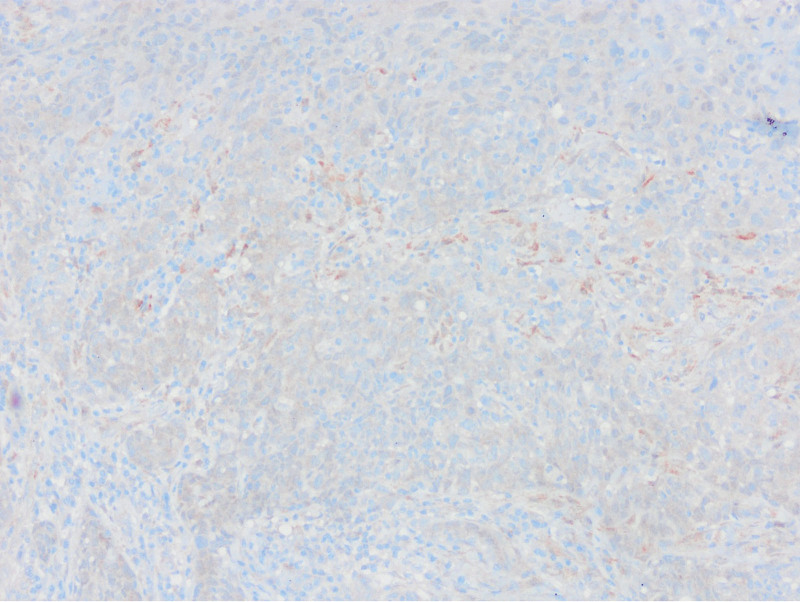
As a result of p53 IHC evaluation, 97 (69.3%) cases were found as mutants (Figs. (Figs.11–5). The most frequent pattern was the null type (44.3%) followed by the basal/parabasal type (39.2%). The ages of p53 mutant cases were higher (P = .04). p53 mutant cases constituted 81.1% of the cases with lymph node metastasis (P = .01) and 84.6% of the cases with extranodal spread (P = .01). According to 2 different methods, PTEN1 and PTEN2, PTEN loss were found to be 85.7% and 57.9%, respectively (Figs. (Figs.66–10). In PTEN1, tumors showing loss of PTEN had a smaller tumor size (3 vs 3.7 cm) (P = .04) and more thyroid cartilage invasion (39.2% vs 15%) (P = .04) (Tables (Tables11 and and2).2). In PTEN2 evaluation any statistically significant parameter was found.
cm) (P = .04) and more thyroid cartilage invasion (39.2% vs 15%) (P = .04) (Tables (Tables11 and and2).2). In PTEN2 evaluation any statistically significant parameter was found.
Table 1
The evaluation of p53 and PTEN.
| p53 | Mutant p53 staining | n (%) | Wild p53 staining | n (%) |
|---|---|---|---|---|
| Basal | 11 (11.3) | Scattered | 43 (30.7) | |
| Basal/parabasal | 38 (39.2) | Mid-epithelial | 0 | |
| Null | 43 (44.3) | |||
| Cytoplasmic | 5 (5.1) | |||
| Total | 97 (69.3) | Total | 43 (30.7) | |
| PTEN | PTEN loss | n (%) | No PTEN loss | n (%) |
| PTEN 1 | Low expression compared to the surrounding epithelium and stroma or no staining | 120 (85.7) | Equal or high staining compared to the surrounding epithelium and stroma | 20 (14.3) |
| PTEN 2 | Staining in <10% cells or no staining | 81 (57.9) | Staining ≥10% cells | 59 (42.1) |
Table 2
Relationship between demographic and histopathological parameters and the staining patterns of p53 and PTEN.
| p53 (n ya da mean) | PTEN1 (n ya da mean) | PTEN2 (n ya da mean) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild | Mutant | P | No PTEN loss | PTEN loss | P | No PTEN loss | PTEN loss | P | |
| Gender | |||||||||
 Male Male | 39 (90.7%) | 92 (94.8%) | .45 | 19 (95%) | 112 (93.3%) | .99 | 57 (96.6%) | 74 (91.4%) | .21 |
 Female Female | 4 (9.3%) | 5 (5.2%) | 1 (5%) | 8 (6.7%) | 2 (3.4%) | 7 (8.6%) | |||
| Age | 58.5 | 62 | .04 | 62 | 60.7 | .58 | 60.9 | 60.9 | .99 |
| Age | |||||||||
 0–61 0–61 | 29 (67.4%) | 47 (48.5%) | .03 | 11 (55%) | 65 (54.2%) | .99 | 33 (55.9%) | 43 (53.1%) | .73 |
 >61 >61 | 14 (32.6%) | 50 (51.5%) | 9 (45%) | 55 (45.8%) | 26 (44.1%) | 38 (46.9%) | |||
| Operation type | |||||||||
 Partial Partial | 13 (30.2%) | 27 (27.8%) | .77 | 5 (25%) | 35 (29.2%) | .70 | 20 (33.9%) | 20 (24.7%) | .23 |
 Total Total | 30 (69.8%) | 70 (72.2%) | 15 (75%) | 85 (70.8%) | 39 (66.1%) | 61 (75.3%) | |||
| Adjuvan therapy | |||||||||
 No No | 12 (27.9%) | 26 (26.8%) | .44 | 6 (30%) | 32 (26.7%) | .58 | 21 (35.6%) | 17 (21%) | .11 |
 Only RT Only RT | 7 (16.3%) | 25 (25.8%) | 6 (30%) | 26 (21.7%) | 10 (16.9%) | 22 (27.2%) | |||
 RT/KT RT/KT | 24 (55.8%) | 46 (47.4%) | 8 (40%) | 62 (51.7%) | 28 (47.5%) | 42 (51.9%) | |||
| Tumor localization | |||||||||
 Supraglottic Supraglottic | 14 (32.6%) | 24 (24.7%) | .22 | 6 (30%) | 32 (26.7%) | .57 | 17 (28.8%) | 21 (25.9%) | .78 |
 Glottic Glottic | 11 (25.6%) | 39 (40.2%) | 5 (25%) | 45 (37.5%) | 21 (35.6%) | 29 (35.8%) | |||
 Subglottic Subglottic | 2 (4.7%) | 1 (1%) | 0 | 3 (2.5%) | 2 (3.4%) | 1 (1.2%) | |||
 Transglottic Transglottic | 16 (37.2%) | 33 (34%) | 9 (45%) | 40 (33.3%) | 19 (32.2%) | 30 (37%) | |||
| Subglottic involvement | |||||||||
 No No | 18 (41.9%) | 43 (44.3%) | .85 | 9 (45%) | 52 (43.3%) | .99 | 29 (49.2%) | 32 (39.5%) | .25 |
 Yes Yes | 25 (58.1%) | 54 (55.7%) | 11 (55%) | 68 (56.7%) | 30 (50.8%) | 49 (60.5%) | |||
| Tumor size | 3.2 | 3.1 | .75 | 3.7 | 3.05 | .04 | 3.2 | 3.1 | .62 |
| Tumor size (cm) | |||||||||
 0–3 0–3 | 22 (51.2%) | 59 (60.8%) | .35 | 8 (40%) | 73 (60.8%) | .08 | 33 (55.9%) | 48 (59.3%) | .69 |
 >3 >3 | 21 (48.8%) | 38 (39.2%) | 12 (60%) | 47 (39.2%) | 26 (44.1%) | 33 (40.7%) | |||
| Differentiation | |||||||||
 İyi İyi | 9 (20.9%) | 14 (14.4%) | .29 | 2 (10%) | 21 (17.5%) | .68 | 8 (13.6%) | 15 (18.5%) | .72 |
 Orta Orta | 30 (69.8%) | 65 (67%) | 15 (75%) | 80 (66.7%) | 41 (69.5%) | 54 (66.7%) | |||
 Kötü Kötü | 4 (9.3%) | 18 (18.6%) | 3 (15%) | 19 (15.8%) | 10 (16.9%) | 12 (14.8%) | |||
| Thyroid cartilage invasion | |||||||||
 No No | 28 (65.1%) | 62 (63.9%) | .99 | 17 (85%) | 73 (60.8%) | .04 | 42 (71.2%) | 48 (59.3%) | .14 |
 Yes Yes | 15 (34.9%) | 35 (36.1) | 3 (15%) | 47 (39.2%) | 17 (28.8%) | 33 (40.7%) | |||
| Cricoid cartilage invasion | |||||||||
 No No | 38 (88.4%) | 85 (87.6%) | .99 | 18 (90%) | 105 (87.5%) | .99 | 51 (86.4%) | 72 (88.9%) | .66 |
 Yes Yes | 5 (11.6%) | 12 (12.4%) | 2 (10%) | 15 (12.5%) | 8 (13.6%) | 9 (11.1%) | |||
| Lenfovascular invasion | |||||||||
 No No | 41 (95.3%) | 88 (90.7%) | .50 | 19 (95%) | 110 (91.7%) | .60 | 52 (88.1%) | 77 (95.1%) | .13 |
 Yes Yes | 2 (4.7%) | 9 (9.3%) | 1 (5%) | 10 (8.3%) | 7 (11.9%) | 4 (4.9%) | |||
| Perineural invasion | |||||||||
 No No | 38 (88.4%) | 86 (88.7%) | .99 | 19 (95%) | 105 (87.5%) | .32 | 53 (89.8%) | 71 (87.7%) | .68 |
 Yes Yes | 5 (11.6%) | 11 (11.3%) | 1 (5%) | 15 (12.5%) | 6 (10.2%) | 10 (12.3%) | |||
| Surgical margin | |||||||||
 Negative Negative | 34 (79.1%) | 69 (71.1%) | .40 | 12 (60%) | 91 (75.8%) | .13 | 41 (69.5%) | 62 (76.5%) | .35 |
 Positive Positive | 9 (20.9%) | 28 (28.9%) | 8 (40%) | 29 (24.2%) | 18 (30.5%) | 19 (23.5%) | |||
| Lymph node metastasis | |||||||||
 No No | 33 (76.7%) | 54 (55.7%) | .01 | 9 (45%) | 78 (65%) | .08 | 32 (54.2%) | 55 (67.9%) | .10 |
 Yes Yes | 10 (23.3%) | 43 (44.3%) | 11 (55%) | 42 (35%) | 27 (45.8%) | 26 (32.1%) | |||
| Number of lymph node metastases | |||||||||
 Single Single | 5 (50%) | 12 (27.9%) | .17 | 2 (18.2%) | 15 (32.1%) | .26 | 8 (29.6%) | 9 (34.6%) | .69 |
 Multipl Multipl | 5 (50%) | 31 (72.1%) | 9 (81.8%) | 27 (67.9%) | 19 (70.4%) | 17 (65.4%) | |||
| Extranodal spread | |||||||||
 No No | 39 (90.7%) | 75 (77.3%) | .04 | 14 (70%) | 100 (83.3%) | .13 | 48 (81.4%) | 66 (81.5%) | .57 |
 Yes Yes | 4 (9.3%) | 22 (22.7%) | 6 (30%) | 20 (16.7%) | 11 (18.6%) | 15 (18.5%) | |||
| Recurrence | |||||||||
 No No | 37 (86%) | 37 (86%) | .78 | 17 (85%) | 106 (88.3%) | .67 | 54 (91.5%) | 69 (85.2%) | .25 |
 Yes Yes | 6 (14%) | 11 (11.3%) | 3 (15%) | 14 (11.7%) | 5 (8.5%) | 12 (14.8%) | |||
| Distant metastasis | |||||||||
 No No | 38 (88.4%) | 88 (90.7%) | .76 | 18 (90%) | 108 (90%) | .99 | 54 (91.5%) | 72 (88.9%) | .77 |
 Yes Yes | 5 (11.6%) | 9 (9.3%) | 2 (10%) | 12 (10%) | 5 (8.5%) | 9 (11.1%) | |||
| pT | |||||||||
 T1 T1 | 3 (7%) | 5 (5.2%) | .49 | 2 (10%) | 6 (5%) | .45 | 6 (10.2%) | 2 (2.5%) | .31 |
 T2 T2 | 8 (18.6%) | 10 (10.3%) | 3 (15%) | 15 (12.5%) | 7 (11.9%) | 11 (13.6%) | |||
 T3 T3 | 12 (27.9%) | 35 (36.1%) | 8 (40%) | 39 (32.5%) | 19 (32.2%) | 28 (34.6%) | |||
 T4 T4 | 20 (46.5%) | 47 (48.5%) | 7 (35%) | 60 (50%) | 27 (45.8%) | 40 (49.4%) | |||
| pN | |||||||||
 N0 N0 | 33 (76.7%) | 54 (55.7%) | .10 | 9 (45%) | 78 (65%) | .27 | 32 (54.2%) | 55 (67.9%) | .06 |
 N1 N1 | 4 (9.3%) | 11 (11.3 %) | 2 (10%) | 13 (10.8%) | 7 (11.9%) | 8 (9.9%) | |||
 N2 N2 | 2 (4.7%) | 11 (11.3%) | 3 (15%) | 10 (8.3%) | 10 (16.9%) | 3 (3.7%) | |||
 N3 N3 | 4 (9.3%) | 21 (21.6%) | 6 (30%) | 19 (15.8%) | 10 (16.9%) | 15 (18.5%) | |||
| Stage | |||||||||
 I I | 3 (7%) | 5 (5.2%) | .65 | 2 (10%) | 6 (5%) | .54 | 6 (10.2%) | 2 (2.5%) | .14 |
 II II | 6 (14%) | 8 (8.2%) | 3 (15%) | 11 (9.2%) | 7 (11.9%) | 7 (8.6%) | |||
 III III | 11 (25.6%) | 23 (23.7%) | 3 (15%) | 31 (25.8%) | 11 (18.6%) | 23 (28.4%) | |||
 IV IV | 23 (53.5%) | 61 (62.9%) | 12 (60%) | 72 (60%) | 35 (59.3%) | 49 (60.5%) | |||
| Stage category | |||||||||
 Early stage Early stage | 9 (20.9%) | 13 (13.4%) | .22 | 5 (25%) | 17 (14.2%) | .31 | 13 (22%) | 11 (13.6%) | .19 |
 Late stage Late stage | 34 (79.1%) | 84 (86.6%) | 15 (75%) | 103 (85.8%) | 46 (78%) | 70 (86.4%) | |||
| Overall survival | |||||||||
 Alive Alive | 32 (74.4%) | 66 (68%) | .44 | 15 (75%) | 83 (69.2%) | .59 | 43 (72.9%) | 55 (67.9%) | .52 |
 Exitus Exitus | 11 (25.6%) | 31 (32%) | 5 (25%) | 37 (30.8%) | 16 (27.1%) | 26 (32.1%) | |||
| Disease free survival | |||||||||
 No recurrence or metastasis No recurrence or metastasis | 35 (81.4%) | 77 (79.4%) | .78 | 15 (75%) | 97 (80.8%) | .54 | 50 (84.7%) | 62 (76.5%) | .23 |
 Recurrence or metastasis Recurrence or metastasis | 8 (18.6%) | 20 (20.6%) | 5 (25%) | 23 (19.2%) | 9 (15.3%) | 19 (23.5%) | |||
| p53 | |||||||||
 Wild Wild | 2 (10%) | 41 (34.2%) | .03 | 15 (25.4%) | 28 (34.6%) | .24 | |||
 Mutant Mutant | 18 (90%) | 79 (65.8%) | 44 (74.6%) | 53 (65.4%) | |||||
| Total | 43 | 97 | 20 | 120 | 59 | 81 | |||
P values that are statistically significant are shown in bold.
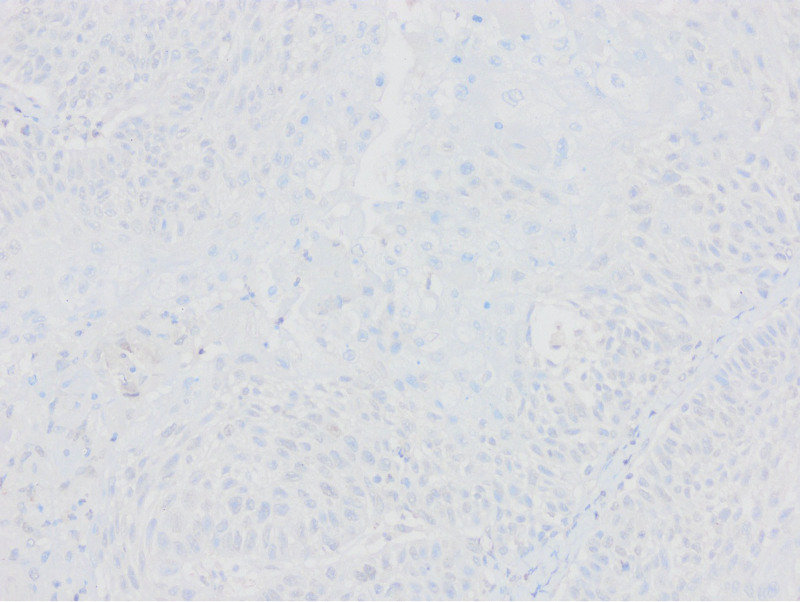
Absence of PTEN staining in tumor cells by PTEN1 and PTEN2 evaluation considered as PTEN loss (PTENX400).
Low expression in PTEN staining compared to surrounding epithelium and stroma by PTEN1 evaluation considered as PTEN loss (PTENX200).
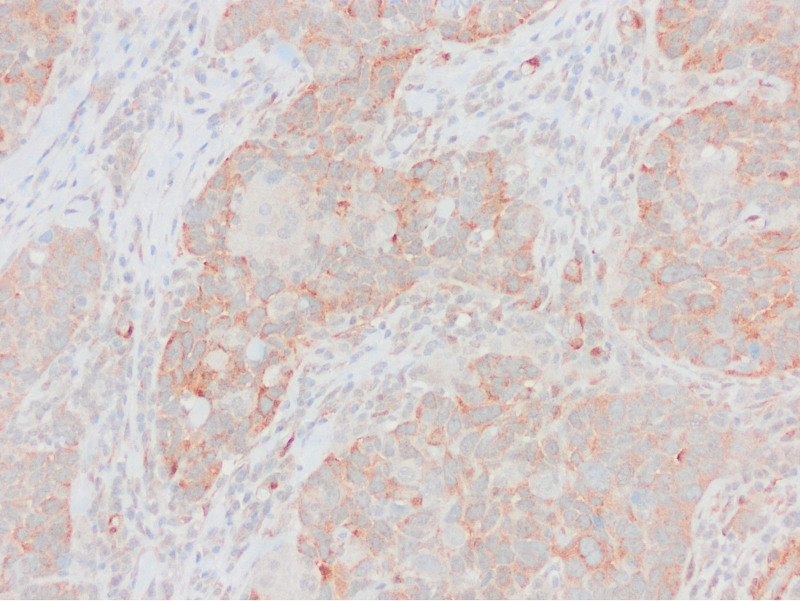
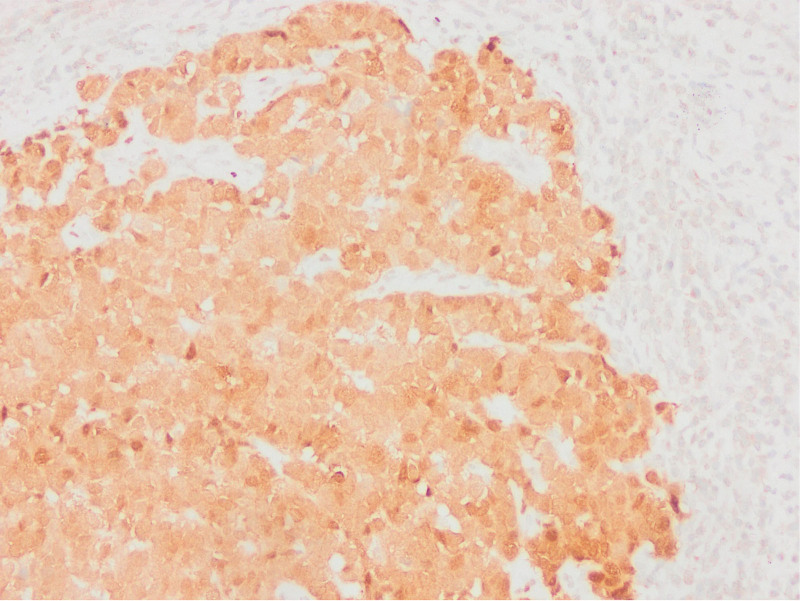
Equal or high expression in PTEN staining compared to the surrounding epithelium and stroma by PTEN1 evaluation considered as no PTEN loss (PTENX400).
Tumor size and thyroid cartilage invasion for PTEN1 and age for p53 were identified as independent predictive factors when multivariate analysis was performed. While p53 mutation was more common in elderly patients (hazard ratio [HR] 2.203, 95% confidence interval [CI] 1.021–4.754, P = .04), PTEN loss, according to PTEN1 evaluation method, was more frequent in those with smaller tumors (HR 0.547, 95% CI 0.367–0.813, P < .01) and patients with thyroid cartilage invasion (HR 7.012, 95% CI 1.662–29.590, P < .01). The multivariate analysis could not be performed for PTEN2 results because there was any statistically significant result in univariate analysis (Table (Table33).
Table 3
Multivariate analysis of p53 and PTEN expressions by PTEN1 criteria by logistic regression.
| B | HR | 95% CI | P | ||
|---|---|---|---|---|---|
| p53 (wild-mutant) | |||||
 Age Age | 0–61 etc >61 | 0.790 | 2203 | 1.021–4754 | .04 |
 Lymph node metastasis Lymph node metastasis | No etc Yes | 0.815 | 2259 | 0.814–6.269 | .11 |
 Extranodal spread Extranodal spread | No etc Yes | 0.360 | 1.434 | 0.348–5.911 | .61 |
| PTEN1 (no PTEN loss-PTEN loss) | |||||
 Tumor size Tumor size | 3.7 etc 3 | −0.604 | 0.547 | 0.367–0.813 | <.01 |
 Thyroid cartilage invasion Thyroid cartilage invasion | No etc Yes | 1948 | 7.012 | 1.662–29.590 | <.01 |
P values that are statistically significant are shown in bold.
CI = confidence interval, HR = hazard ratio.
Subglottic involvement, tumor size larger than 3 cm, thyroid cartilage invasion, cricoid cartilage invasion, lymph node metastases and extranodal spread were important parameters in predicting pT stage and were statistically significant (P < .05).
cm, thyroid cartilage invasion, cricoid cartilage invasion, lymph node metastases and extranodal spread were important parameters in predicting pT stage and were statistically significant (P < .05).
Subglottic involvement, tumor size larger than 3 cm, thyroid cartilage invasion, lymphovascular invasion, extranodal spread, and mutant type p53 status were important parameters in predicting lymph node metastasis and were statistically significant (P < .05).
cm, thyroid cartilage invasion, lymphovascular invasion, extranodal spread, and mutant type p53 status were important parameters in predicting lymph node metastasis and were statistically significant (P < .05).
Subglottic involvement, tumor size larger than 3 cm, thyroid cartilage invasion, lymph node metastases, the number of lymph node metastases, extranodal spread and pT stage were important parameters in predicting pN stage and were statistically significant (P < .05).
cm, thyroid cartilage invasion, lymph node metastases, the number of lymph node metastases, extranodal spread and pT stage were important parameters in predicting pN stage and were statistically significant (P < .05).
In univariate analyses comparing demographic and histopathological parameters with overall survival, age > 61 years, total laryngectomy operation, subglottic involvement, thyroid cartilage invasion, surgical margin positivity, presence of lymph node metastases, multipl metastatic lymph nodes, extranodal spread, recurrence and distant metastases, pT and stage were associated with worse survival and statistically significant (P < .05) (Table (Table4).4). Age, operation type, subglottic involvement, surgical margin, lymph node metastasis, extranodal spread and pT stage parameters that are significant in univariate analysis for overall survival were included in the multivariate analysis. Advanced age, having had a total laryngectomy, and the presence of extranodal spread were independent poor prognostic factors for overall survival (Table (Table55).
Table 4
Univariate analysis of risk factors associated with overall and disease-free survival.
| Overall survival | P | Disease free survival | P | |
|---|---|---|---|---|
| Gender | ||||
 Male Male | 73.6% | .19 | 79.9% | .42 |
 Female Female | 88.9% | 64.8% | ||
| Age | ||||
 0–61 0–61 | 79.5% | <.01 | 80.9% | .58 |
 >61 >61 | 68.9% | 76% | ||
| Operation type | ||||
 Partial Partial | 91.5% | <.01 | 86.3% | .07 |
 Total Total | 67.9% | 75.4% | ||
| Adjuvan therapy | ||||
 No No | 72.9% | .88 | 84.3% | .28 |
 Only RT Only RT | 82.6% | 83.2% | ||
 RT/KT RT/KT | 71.6% | 73.5% | ||
| Tumor localization | ||||
 Supraglottik Supraglottik | 86.4% | .11 | 85.7% | .31 |
 Glottik Glottik | 77% | 82.8% | ||
 Subglottik Subglottik | 66.7% | 66.7% | ||
 Transglottik Transglottik | 63.6% | 69.3% | ||
| Subglottic involvement | ||||
 No No | 81.8% | .04 | 87.3% | .02 |
 Yes Yes | 69.1% | 71.5% | ||
| Tumor size (cm) | ||||
 0–3 0–3 | 79.4% | .27 | 81.4% | .33 |
 >3 >3 | 67.9% | 75.2% | ||
| Differentiation | ||||
 İyi İyi | 79.9% | .77 | 86.5% | .54 |
 Orta Orta | 75.6% | 76.9% | ||
 Kötü Kötü | 64.9% | 76.7% | ||
| Thyroid cartilage invasion | ||||
 No No | 84.3% | <.01 | 85.1% | .01 |
 Yes Yes | 56.1% | 66.1% | ||
| Cricoid cartilage invasion | ||||
 No No | 76.5% | .11 | 79.4% | .89 |
 Yes Yes | 62.5% | 78.6% | ||
| Lenfovascular invasion | ||||
 No No | 75.4% | .52 | 79.1% | .61 |
 Yes Yes | 62.5% | 71.4% | ||
| Perineural invasion | ||||
 No No | 75.9% | .47 | 81.5% | .05 |
 Yes Yes | 66.7% | 59.3% | ||
| Surgical margin | ||||
 Negative Negative | 77.5% | .04 | 82.6% | .06 |
 Positive Positive | 67.4% | 68.1% | ||
| Lymph node metastasis | ||||
 No No | 78.6% | .05 | 84.8% | .01 |
 Yes Yes | 67.3% | 68% | ||
| Number of lymph node metastases | ||||
 Single Single | 92.9% | <.01 | 72.7% | .40 |
 Multipl Multipl | 55.9% | 65.9% | ||
| Extranodal spread | ||||
 No No | 78.9% | <.01 | 82.3% | .03 |
 Yes Yes | 54.6% | 62.1% | ||
| Recurrence | ||||
 No No | 83.6% | <.01 | 90.2% | <.01 |
 Yes Yes | 17.6% | 5.9% | ||
| Distant metastasis | ||||
 No No | 77.1% | <.01 | 88.2% | <.01 |
 Yes Yes | 55.1% | 7.1% | ||
| Pt | ||||
 T1 T1 | 100% | <.01 | .08 | |
 T2 T2 | 81.6% | 82.2% | ||
 T3 T3 | 85.9% | 85.6% | ||
 T4 T4 | 61.9% | 69.8% | ||
| Pn | ||||
 N0 N0 | 78.6% | .08 | 84.8% | .11 |
 N1 N1 | 75% | 66.6% | ||
 N2 N2 | 60.3% | 71.2% | ||
 N3 N3 | 50% | 50% | ||
| Stage | ||||
 I I | 100% | <.01 | 0 | <.01 |
 II II | 88% | 92.3% | ||
 III III | 84% | 93.8% | ||
 IV IV | 65% | 67.3% | ||
| Stage IV | ||||
 IVa IVa | 66.7% | <.01 | 82.4% | <.01 |
 IVb IVb | – | – | ||
 IVc IVc | 55.1% | 1% | ||
| Stage category | ||||
 Early stage Early stage | 92% | .01 | 95% | .02 |
 Late stage Late stage | 79.5% | 75.3% | ||
| p53 | ||||
 Wild Wild | 74.5% | .38 | 79.3% | .79 |
 Mutant Mutant | 74.7% | 78.6% | ||
| PTEN1 | ||||
 No PTEN loss No PTEN loss | 83.6% | .49 | 83.6% | .82 |
 PTEN loss PTEN loss | 73.1% | 77.9% | ||
| PTEN2 | ||||
 No PTEN loss No PTEN loss | 76.7% | .77 | 83.2% | .40 |
 PTEN loss PTEN loss | 73.4% | 76% | ||
P values that are statistically significant are shown in bold and italic.
Table 5
Multivariate analysis of risk factors associated with overall survival.
| Overall survival | 95% CI | P | |||
|---|---|---|---|---|---|
| Cox regression analysis | |||||
| B | HR | ||||
| Age | 0–61 etc >61 | 0.798 | 2.471 | 1.182–4.175 | .01 |
| Operation type | Partial etc total | 1.145 | 3.142 | 1.198–8.236 | .02 |
| Subglottic involvement | No etc Yes | 0.270 | 1.310 | 0.633–2.713 | .45 |
| Surgical margin | Negative etc Positive | 0.521 | 1.683 | 0.854–3.316 | .13 |
| Lymph node metastases | No etc Yes | 0.284 | 0.753 | 0.290–1.955 | .55 |
| Extranodal spread | No etc Yes | 0.905 | 2.471 | 1.245–4.907 | .01 |
| pT | T1 etc T2 | 0.697 | 2.008 | 0.213–18.949 | .54 |
| T1 etc T3 | 0.101 | 1.107 | 0.113–10.824 | .93 | |
| T1 etc T4 | 0.979 | 2.663 | 0.277–25.623 | .39 | |
P values that are statistically significant are shown in bold and italic.
CI = confidence interval, HR = hazard ratio.
In univariate analyzes comparing disease-free survival, subglottic involvement, thyroid cartilage invasion, perineural invasion, presence of lymph node metastasis, extranodal spread, recurrence, and distant metastases and stage indicated poor prognosis and were statistically significant (P < .05) (Table (Table4).4). Subglottic involvement, thyroid cartilage invasion, perineural invasion, lymph node metastases, extranodal spread and stage parameters that are significant in univariate analysis for disease-free survival were included in the multivariate analysis. The presence of subglottic involvement, perineural invasion, and extranodal spread were independent poor prognostic factors for disease-free survival (Table (Table66).
Table 6
Multivariate analysis of risk factors associated with disease-free survival.
| Disease free survival | 95% CI | P | |||
|---|---|---|---|---|---|
| Cox regression analysis | |||||
| B | HR | ||||
| Subglottic involvement | No etc Yes | 0.868 | 2.383 | 1.039–5.465 | .04 |
| Thyroid cartilage invasion | No etc Yes | 0.439 | 1.551 | 0.691–3.478 | .28 |
| Perineural invasion | No etc Yes | 0.855 | 2.351 | 1.001–5.878 | .05 |
| Lymph node metastasis | No etc Yes | 0.021 | 0.979 | 0.345–2.781 | .96 |
| Extranodal spread | No etc Yes | 0.855 | 2.352 | 1.019–5.430 | .04 |
| Stage | Early etc Late | 1.389 | 4.009 | 0.511–31.484 | .18 |
P values that are statistically significant are shown in bold and italic.
CI = confidence interval, HR = hazard ratio.
Overall survival rates were 74.7% and 74.5% in p53 mutant and wild-type cases, respectively. In cases with or without PTEN loss, it was found as 73.1% and 83.6% in PTEN1 evaluation and 73.4% and 76.7% in PTEN2 evaluation. Disease-free survival rates were 78.6% and 79.3% in p53 mutant and wild-type cases, respectively. In cases with or without PTEN loss, it was determined as 77.9% and 83.6% in PTEN1 evaluation and 76% and 83.2% in PTEN2 evaluation. No statistically significant results were obtained between p53 and PTEN and overall or disease-free survival (Table (Table44).
4. Discussion
Larynx and oral cavity carcinomas are among the most frequent head and neck carcinomas and the 6th most common cancer globally. They are usually diagnosed at an advanced stage and therefore their prognosis is not very promising.[19,20] In order to predict the prognosis and clinical course of the patient in laryngeal carcinomas, many protein-expression studies are carried out, and no marker is currently in routine practice.[7–9]
The earliest and most common mutation in SCCs is the p53 gene mutation.[21,22] It is reported in the literature that TP53 mutation develops at a rate of 50% to 80%,[23] and according to TCGA data, TP53 mutation is the most frequently mutated cancer gene in laryngeal carcinoma with a rate of 87.4%.[24,25]
p53 IHC examination was routinely used to differentiate dysplasia or invasive tumor, and overexpression was interpreted as an abnormal p53 gene status. However, due to the studies, new scoring systems were developed due to the incompatibility of p53 sequencing data with histopathological and clinicopathological parameters. The IHC evaluation of p53 in 6 categories, specially studied in gynecological studies, revealed promising results and increased the contribution of immunohistochemistry use to clinical practice.[13,14,21,26,27] To our knowledge this is the first series applying these recently developed criteria in larynx carcinomas.
Various classification schemes have been used for p53 assessments in head and neck group publications. Karpathipou et al[21] evaluated p53 IHC expression in 3 categories in their study on 120 head and neck SCCs. Overexpression: strong nuclear staining in more than 50% of the cells, negative: no staining in the tumor when there is staining in the surrounding normal tissue, normal: weak staining in less than 50% of the cells. Overexpression was reported in 66.7% of the cases, negative staining in 20.8%, and normal staining in 12.5%. In the series with 87.5% abnormal staining, this finding was statistically significant with smoking, pT and keratinized tumor type. No significant difference was found between p53 status and overall and disease-free survival. Wang et al,[28] in their study with head and neck SCC, including 85 cases, evaluated p53 expression as low (staining in <25% of tumor cells) and high (staining in ≥25% of cells). Higher p53 expression was associated with worse clinical outcome and disease-free survival, and these findings were statistically significant. Plath et al,[29] in their study on 313 patients with oropharyngeal carcinoma, classified the p53 evaluation as Wang et al did and found high expression in 33.5% of the patients. While the relationship of expression with 5-year overall survival was statistically significant in both univariate and multivariate analyzes, there is no relationship between disease-free survival. Mielcarek-Kuchta et al[30] was assessed p53 on a 5-point grading system (0, lack of reaction; +<1%; 1+, 1–9%; 2+, 10–40%; 3+, 50–100%). In their series consisting of 73 laryngeal SCC cases, they could not find a significant difference between p53 expression and any parameter. Kara et al,[31] in their study with p53 expression in 43 laryngeal SCCs, considered nuclear staining positive in more than 10% of the cells and reported p53 mutations 44.4% and 56% for supraglottic and subglottic tumors. They could not find a statistically significant result between p53 mutation and important clinical parameters.
The 6-point evaluation method used in gynecological pathology recently is quite successful. Tessier-Cloutier et al[13] performed p53 IHC evaluation with 6 categories in their study on 61 vulvar SCC and 42 vulvar in situ lesions and compared them with sequencing data. They found 95% agreement in vulvar SCC and 93% in vulvar in situ lesion between immunohistochemistry and sequencing data. In this study, 62.2% of the cases of vulvar SCCs showed abnormal p53 staining and 37.8% showed normal staining pattern. Of the abnormal stainings, 71% were parabasal/diffuse, 15.7% were null type, 7.8% were basal, and 5.2% were cytoplasmic staining; 86.9% of normal stainings were reported as scattered type and 13.1% as midepithelial type staining. Kortekaas et al[14] evaluated the p53 IHC analysis with a similar algorithm and compared them with the sequencing results in their study in a series of 59 cases of vulvar SCC. They found the sensitivity of the results of p53 IHC findings to be 95.3%, the specificity as 100%, and the accuracy as 96.6%. They stated that p53 immunohistochemistry assessment is a very usable and highly accurate method. Kortekaas et al, in their study of 59 cases of vulvar SCC, approximately 60% of the cases considered mutant as immunohistochemistry showed parabasal/diffuse staining, followed by null type, basal and cytoplasmic staining in order of frequency. In our series, 43 (30.7%) of p53 mutant cases showed null type, 38 (27.1%) parabasal, 11 (7.9%) basal and 5 (3.6%) cytoplasmic staining. As in other studies, null and parabasal staining were in the first 2 lines. All wild type cases showed scattered type staining. Although there were no sequence data, this entire staining pattern was highly relevant to the literature. All these studies show that IHC evaluation of p53 in 6 categories can be used instead of detecting TP53 mutation.
PTEN mutations have been detected in many tumors, mainly breast, endometrium, prostate and thyroid.[32–35] As in many cancers, a decrease in PTEN expression has been shown in laryngeal cancer, mainly due to mutation or deletion in the PTEN gene.[19] According to TCGA data, PTEN mutation in laryngeal carcinoma was reported at a rate of 3.6%.[25] PTEN IHC evaluation in laryngeal cancers has been performed with very different algorithms.
Snietura et al performed IHC studies on 147 cases in their series of 279 cases, of which 158 located in the larynx. In their evaluation with the semi-quantification method used in many studies in the literature, they accepted lower staining or no staining as low expression compared to the surrounding epithelium and stroma, and high PTEN expression if staining with equal or more intensity.[34,36–41] Low PTEN expression was detected in 59.9% of all cases and in 62.2% of laryngeal carcinomas. They stated that low PTEN expression is associated with an aggressive clinical course.[42] Mastronicolos et al[43] examined the IHC expression of PTEN in 50 cases of laryngeal SCC. They considered 56% cases with lower staining and no staining than the surrounding normal epithelium as PTEN loss and found a more aggressive course in these cases. PTEN loss was significantly higher in glottic tumors and moderately-poorly differentiated tumors. In this study, the evaluation called PTEN1 was performed as in the study of Snietura et al and Mastronicolos et al, as many other studies in the literature. In cases with PTEN loss, smaller tumor size and thyroid cartilage invasion was more common and significant. PTEN loss was higher in glottic tumors, but it was not statistically significant. According to TCGA data, most of the cases with thyroid cartilage invasion were at advanced stage, but this data was not statistically significant.[25]
Bruine de Bruin et al[44] scored PTEN expression as negative, weak positive, positive and strong positive in 52 cases of supraglottic laryngeal carcinoma and accepted a value of >7.5% as high staining. They found high expression in 25% of the cases. Recurrence was higher in cases with high expression and in cases with lymph node metastasis, and this was statistically significant. In multivariate analyzes, both parameters were determined as independent prognostic factors. In our study, in contrast to the study of Bruine de Bruin et al, recurrence was found at a higher rate in cases with PTEN loss, but it was not statistically significant. While it was stated in many studies in the literature that PTEN loss progressed with a worse prognosis, it was concluded that Bruine de Bruin et al found the opposite result, which may be because they took the cutoff value as 7.5%.
Malm et al[45] examined the differences between smokers and nonsmokers in their study of 521 laryngeal carcinoma cases and performed IHC studies on only 34 cases (14 nonsmokers, 20 smokers). They found loss of expression in 43% of nonsmokers and 45% of smokers, and they did not detect a relationship between PTEN expression and smoking. A cutoff value for loss of expression was not specified in the study in which the number of cases whose PTEN expression was evaluated was small.
Anderson et al[46] reported that lymphovascular invasion and histological grade were the most important parameters in predicting lymph node metastasis in 2033 laryngeal cancer cases. In our study, subglottic involvement, tumor size larger than 3 cm, and invasion of the thyroid cartilage were important parameters. Brandstorp-Boesen et al,[47] in their study of 1616 laryngeal cancer, showed a very good prognosis in early stage tumors; stated that the prognosis is very poor in advanced age, and tumors with supraglottic involvement. In our study, the prognosis was worse in cases with advanced age, but differently, cases with subglottic involvement had a worse prognosis. In our series, thyroid cartilage invasion, positive surgical margins and extranodal extension were poor prognostic criteria. In their study of 998 laryngeal cancers, Zhu et al[48] reported 3-year overall and disease-free survival as 84.3% and 78.8% in well-moderately differentiated tumors, and 70.6% and 63.6% in poorly differentiated tumors, respectively. They stated that differentiation is an independent prognostic factor in predicting prognosis. It has been reported that supraglottic and subglottic tumors, advanced cases, extranodal spread and lymphovascular invasion have a worse clinical course. In this study, no significant correlation was found between differentiation and supraglottic tumors in predicting prognosis and lymph node metastasis. In contrast, subglottic involvement was found to be associated with a worse prognosis. Zhu et al,[49] in their study including 1272 cases diagnosed with laryngeal SCC, found that both overall and disease-free survival was lower in the presence of perineural invasion. In this study, a significant decrease in survival was observed in the presence of perineural invasion, but this finding was statistically significant only in disease-free survival.
cm, and invasion of the thyroid cartilage were important parameters. Brandstorp-Boesen et al,[47] in their study of 1616 laryngeal cancer, showed a very good prognosis in early stage tumors; stated that the prognosis is very poor in advanced age, and tumors with supraglottic involvement. In our study, the prognosis was worse in cases with advanced age, but differently, cases with subglottic involvement had a worse prognosis. In our series, thyroid cartilage invasion, positive surgical margins and extranodal extension were poor prognostic criteria. In their study of 998 laryngeal cancers, Zhu et al[48] reported 3-year overall and disease-free survival as 84.3% and 78.8% in well-moderately differentiated tumors, and 70.6% and 63.6% in poorly differentiated tumors, respectively. They stated that differentiation is an independent prognostic factor in predicting prognosis. It has been reported that supraglottic and subglottic tumors, advanced cases, extranodal spread and lymphovascular invasion have a worse clinical course. In this study, no significant correlation was found between differentiation and supraglottic tumors in predicting prognosis and lymph node metastasis. In contrast, subglottic involvement was found to be associated with a worse prognosis. Zhu et al,[49] in their study including 1272 cases diagnosed with laryngeal SCC, found that both overall and disease-free survival was lower in the presence of perineural invasion. In this study, a significant decrease in survival was observed in the presence of perineural invasion, but this finding was statistically significant only in disease-free survival.
5. Conclusions
While the literature on head and neck SCCs is mainly related to oral cavity-oropharyngeal carcinomas, there are more limited studies on larynx and p53 or PTEN. We believe that this study, which is the first study in which the new p53 classification, which has been extensively studied especially in gynecological malignancies and has proven to be highly correlated with sequencing data, was used in laryngeal cancer, will contribute significantly to the literature. In this study, in which the PTEN IHC results, which were evaluated with very different algorithms in the literature, were evaluated by 2 different methods, it was concluded that the evaluation according to the staining intensity rather than the percentage of staining is a more reliable algorithm in evaluating the loss of protein expression. Our p53 and PTEN evaluation results are correlated with known poor prognostic factors, making the classification of expression results more correlated. Although the survival rates were worse in the groups evaluated as p53 mutant and PTEN loss, it was not statistically significant. It was concluded that the classifications applied for both IHC markers are convenient and appropriate compared with the literature. They should be used in studies involving more cases with survival data for more meaningful results.
Author contributions
Conceptualization: Ayca Tan, Sulen Sarioglu.
Data curation: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Formal analysis: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Funding acquisition: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Investigation: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Methodology: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Project administration: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Resources: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Software: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Supervision: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Validation: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Visualization: Ayca Tan, Gorkem Eskiizmir, Ugur Kamiloglu, Sulen Sarioglu.
Writing – original draft: Ayca Tan, Sulen Sarioglu.
Writing – review & editing: Ayca Tan, Sulen Sarioglu.
Abbreviations:
- IHC
- immunohistochemical
- SCC
- squamous cell carcinoma
Dokuz Eylul University Ethics Committee approval was obtained for the study (No: 2021/13-03, Date: 19.4.2021). The study was conducted in line with the principles of the Helsinki Declaration
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Tan A, Eskiizmir G, Kamiloglu U, Sarioglu S. p53 and PTEN expression evaluation with molecular evident recent criteria in laryngeal carcinoma. Medicine 2023;102:19(e33676).
References
Articles from Medicine are provided here courtesy of Wolters Kluwer Health
Citations & impact
Impact metrics
Article citations
The Prognostic Role of Perineural Invasion for Survival in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis.
Cancers (Basel), 16(14):2514, 11 Jul 2024
Cited by: 0 articles | PMID: 39061154 | PMCID: PMC11274576
Review Free full text in Europe PMC
Role of Human Papilloma Virus 16/18 In Laryngeal Carcinoma with Correlation to the Expression of Cyclin D1, p53, p16 and EGFR.
Pak J Med Sci, 39(6):1768-1773, 01 Nov 2023
Cited by: 1 article | PMID: 37936777 | PMCID: PMC10626090
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Expression of PTEN protein in patients with laryngeal squamous cell carcinoma.
Auris Nasus Larynx, 34(4):481-486, 01 May 2007
Cited by: 12 articles | PMID: 17475427
Divergence of P53, PTEN, PI3K, Akt and mTOR expression in tonsillar cancer.
Head Neck, 37(5):636-643, 03 Apr 2014
Cited by: 10 articles | PMID: 24616007
[Expression of p-STAT3 in laryngeal squamous carcinoma and its correlation with PTEN].
Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 27(18):981-985, 01 Sep 2013
Cited by: 1 article | PMID: 24459921
Over-expression of p53 and c-erbB-2 oncoproteins in laryngeal carcinoma.
Eur Arch Otorhinolaryngol, 258(7):329-335, 01 Sep 2001
Cited by: 14 articles | PMID: 11699821
Review

 a,b,
*
a,b,
*