Abstract
Background
Dysglycemias have been associated with worse prognosis in critically ill patients with COVID-19, but data on the association of dysglycemia with COVID-19 in comparison with other forms of severe acute respiratory syndrome are lacking. This study aimed to compare the occurrence of different glycemic abnormalities in patients with severe acute respiratory syndrome and COVID-19 admitted to intensive care units versus glycemic abnormalities in patients with severe acute respiratory syndrome from other causes, to evaluate the adjusted attributable risk associated with COVID-19 and dysglycemia and to assess the influence of these dysglycemias on mortality.Methods
We conducted a retrospective cohort of consecutive patients with severe acute respiratory syndrome and suspected COVID-19 hospitalized in intensive care units between March 11 and September 13, 2020, across eight hospitals in Curitiba-Brazil. The primary outcome was the influence of COVID-19 on the variation of the following parameters of dysglycemia: highest glucose level at admission, mean and highest glucose levels during ICU stay, mean glucose variability, percentage of days with hyperglycemia, and hypoglycemia during ICU stay. The secondary outcome was the influence of COVID-19 and each of the six parameters of dysglycemia on hospital mortality within 30 days from ICU admission.Results
The sample consisted of 841 patients, of whom 703 with and 138 without COVID-19. Comparing patients with and without COVID-19, those with COVID-19 had significantly higher glucose peaks at admission (165 mg/dL vs. 146 mg/dL; p = 0.002) and during ICU stay (242 mg/dL vs. 187md/dL; p < 0.001); higher mean daily glucose (149.7 mg/dL vs. 132.6 mg/dL; p < 0.001); higher percentage of days with hyperglycemia during ICU stay (42.9% vs. 11.1%; p < 0.001); and greater mean glucose variability (28.1 mg/dL vs. 25.0 mg/dL; p = 0.013). However, these associations were no longer statistically significant after adjustment for Acute Physiology and Chronic Health Evaluation II scores, Sequential Organ Failure Assessment scores, and C-reactive protein level, corticosteroid use and nosocomial infection. Dysglycemia and COVID-19 were each independent risk factors for mortality. The occurrence of hypoglycemia (< 70 mg/dL) during ICU stay was not associated with COVID-19.Conclusion
Patients with severe acute respiratory syndrome due to COVID-19 had higher mortality and more frequent dysglycemia than patients with severe acute respiratory syndrome due to other causes. However, this association did not seem to be directly related to the SARS-CoV-2 infection.Free full text

Dysglycemias in patients admitted to ICUs with severe acute respiratory syndrome due to COVID-19 versus other causes - a cohort study
Abstract
Background
Dysglycemias have been associated with worse prognosis in critically ill patients with COVID-19, but data on the association of dysglycemia with COVID-19 in comparison with other forms of severe acute respiratory syndrome are lacking. This study aimed to compare the occurrence of different glycemic abnormalities in patients with severe acute respiratory syndrome and COVID-19 admitted to intensive care units versus glycemic abnormalities in patients with severe acute respiratory syndrome from other causes, to evaluate the adjusted attributable risk associated with COVID-19 and dysglycemia and to assess the influence of these dysglycemias on mortality.
Methods
We conducted a retrospective cohort of consecutive patients with severe acute respiratory syndrome and suspected COVID-19 hospitalized in intensive care units between March 11 and September 13, 2020, across eight hospitals in Curitiba-Brazil. The primary outcome was the influence of COVID-19 on the variation of the following parameters of dysglycemia: highest glucose level at admission, mean and highest glucose levels during ICU stay, mean glucose variability, percentage of days with hyperglycemia, and hypoglycemia during ICU stay. The secondary outcome was the influence of COVID-19 and each of the six parameters of dysglycemia on hospital mortality within 30 days from ICU admission.
Results
The sample consisted of 841 patients, of whom 703 with and 138 without COVID-19. Comparing patients with and without COVID-19, those with COVID-19 had significantly higher glucose peaks at admission (165 mg/dL vs. 146 mg/dL; p =
= 0.002) and during ICU stay (242 mg/dL vs. 187md/dL; p
0.002) and during ICU stay (242 mg/dL vs. 187md/dL; p <
< 0.001); higher mean daily glucose (149.7 mg/dL vs. 132.6 mg/dL; p
0.001); higher mean daily glucose (149.7 mg/dL vs. 132.6 mg/dL; p <
< 0.001); higher percentage of days with hyperglycemia during ICU stay (42.9% vs. 11.1%; p
0.001); higher percentage of days with hyperglycemia during ICU stay (42.9% vs. 11.1%; p <
< 0.001); and greater mean glucose variability (28.1 mg/dL vs. 25.0 mg/dL; p
0.001); and greater mean glucose variability (28.1 mg/dL vs. 25.0 mg/dL; p =
= 0.013). However, these associations were no longer statistically significant after adjustment for Acute Physiology and Chronic Health Evaluation II scores, Sequential Organ Failure Assessment scores, and C-reactive protein level, corticosteroid use and nosocomial infection. Dysglycemia and COVID-19 were each independent risk factors for mortality. The occurrence of hypoglycemia (<
0.013). However, these associations were no longer statistically significant after adjustment for Acute Physiology and Chronic Health Evaluation II scores, Sequential Organ Failure Assessment scores, and C-reactive protein level, corticosteroid use and nosocomial infection. Dysglycemia and COVID-19 were each independent risk factors for mortality. The occurrence of hypoglycemia (< 70 mg/dL) during ICU stay was not associated with COVID-19.
70 mg/dL) during ICU stay was not associated with COVID-19.
Conclusion
Patients with severe acute respiratory syndrome due to COVID-19 had higher mortality and more frequent dysglycemia than patients with severe acute respiratory syndrome due to other causes. However, this association did not seem to be directly related to the SARS-CoV-2 infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-023-02439-y.
Background
In critically ill patients, the occurrence of glycemic abnormalities (dysglycemias) such as hyperglycemia, glycemic variability, and increased time out of target range (70 to 140 mg/dL) are associated with increased mortality in patients with and without diabetes [1–5]. Elevations in blood glucose levels are common in patients with severe acute respiratory syndrome (SARS) caused by SARS-CoV-2 infection (i.e., COVID-19) [6] and, even in individuals without diabetes, are associated with worse prognosis, longer stay in the intensive care unit (ICU), more frequent requirement of mechanical ventilation, and higher risk of hospital mortality [7–11]. Still, the literature assessing the association of dysglycemias with severe COVID-19 against other forms of SARS and associated outcomes remains scarce [12].
Based on these considerations, the primary aim of this cohort study was to compare the occurrence of different glycemic abnormalities in patients admitted to ICU with SARS and COVID-19 versus glycemic abnormalities in patients with SARS from other causes, to evaluate the adjusted attributable risk associated with COVID-19 and dysglycemia. The secondary aim was to determine the influence of these glycemic abnormalities on mortality. By comparing the progression of dysglycemia in patients with SARS due to COVID-19 against other causes, we sought to identify whether the dysglycemia would be specifically associated with the SARS-CoV-2 infection itself or merely related to the infection severity. We hope the findings will help guide research and care of future patients.
Methods
This retrospective cohort study included consecutive patients with SARS and suspected COVID-19 admitted to ICUs across eight hospitals in the city of Curitiba (Brazil) between March 11 and September 13, 2020. During the study period, the combined maximum number of beds for patients with COVID-19 in these ICUs was 225. Of these, 124 were dedicated to patients covered by the Brazilian Unified Health System (SUS), 71 were for patients covered by health insurance plans or those paying their hospital bills, and 30 beds were for any patient regardless of health insurance coverage.
The study was approved by the local ethics committee of the Instituto de Neurologia de Curitiba under protocol number 2.899.188 on September 17, 2018 (ID: CAAE 98099918.2.0000.5227; project title: Epidemiological analysis of patients hospitalized in intensive care units in Curitiba-Paraná). The requirement for informed consent was waived by the same committee, given the noninterventional design of the study and the fact that the data were collected from clinical records and without contact with the participants and the procedures performed in this study were part of routine care. All research procedures were conducted in accordance with the ethical standards of the institutional committee on human experimentation and with the Helsinki Declaration of 1975 revised in 2013. The STROBE guidelines were used to ensure the reporting of this study.
The study included patients older than 18 years admitted to the ICUs with SARS due to strongly suspected or confirmed COVID-19, who had available result of a real-time polymerase chain reaction (RT-PCR) test for detection of SARS-CoV-2 collected by nasopharyngeal swab. Patients were classified according to suspicion through the set of clinical and radiological criteria for screening of COVID-19, which was routinely used in the study institutions during the pandemic period. They were considered to have SARS with strongly suspected COVID-19 when two of the following set of clinical-radiological criteria were present: (A) at least one flu-like symptom, i.e., cough, runny nose, fever, or sore throat; (B) at least two items from the modified quick SOFA scale (systolic blood pressure <
< 100 mmHg, respiratory rate
100 mmHg, respiratory rate >
> 22 bpm, decreased level of consciousness with Glasgow
22 bpm, decreased level of consciousness with Glasgow <
< 15, and oxygen pulse saturation
15, and oxygen pulse saturation <
< 93%); and (C) chest computed tomography (CT) scans with images suggestive of COVID-19 (ground-glass opacity and peripheral lesions distributed across both lungs) obtained in the first 48 h after admission [13, 14]. We excluded patients without complete medical records of daily follow-up during the ICU stay and those without records of venous or capillary glucose values on at least 40% of the ICU days.
93%); and (C) chest computed tomography (CT) scans with images suggestive of COVID-19 (ground-glass opacity and peripheral lesions distributed across both lungs) obtained in the first 48 h after admission [13, 14]. We excluded patients without complete medical records of daily follow-up during the ICU stay and those without records of venous or capillary glucose values on at least 40% of the ICU days.
An electronic database was created in REDCap (Vanderbilt University, Nashville, TN, USA) for daily and systematic recording and sharing of the data collecting form among the group researchers. The data, collected from all study patients, included demographic characteristics, medical history, and daily clinical and laboratory information related to the first 30 days in the ICU or until the outcome of discharge or death, whichever occurred first.
The highest and lowest daily values of capillary and venous blood glucose were used to calculate the parameters of glucose abnormalities during ICU as follows: highest glucose level on admission, corresponding to the highest glucose value recorded in the first 24 h in the ICU; highest glucose level during ICU stay, corresponding to the highest glucose level recorded during the entire ICU stay; mean glucose level during ICU stay, calculated daily and individually from the mean glucose level of each day in the ICU, subsequently added up and divided by the number of days in which glucose was recorded during ICU stay; mean glucose variability during ICU stay, as reflected by mean of daily glycemic coefficient of variation (CV) during ICU stay; the percentage of days with hyperglycemia (≥ 180 mg/dL), calculated as the number of days with any glycemic value
180 mg/dL), calculated as the number of days with any glycemic value ≥
≥ 180 mg/dL divided by the number of ICU days in which glycemia was recorded and multiplied by 100; and the occurrence of hypoglycemia (<
180 mg/dL divided by the number of ICU days in which glycemia was recorded and multiplied by 100; and the occurrence of hypoglycemia (< 70 mg/dL) during ICU stay, which represents the number of patients who had at least one glycemic value
70 mg/dL) during ICU stay, which represents the number of patients who had at least one glycemic value <
< 70 mg/dL during the ICU stay period.
70 mg/dL during the ICU stay period.
Glucose variability was calculated from capillary blood samples analyzed with a glucometer, while all other parameters of glucose abnormalities were measured in capillary or venous blood samples. Although glucose values are on average 10.19% higher when measured in capillary compared with venous blood, the proportions of patients with glucose values measured in venous blood were not different between groups.
Glucose control
In the present study, hyperglycemia was defined as blood glucose level >
> 180 mg/dl and hypoglycemia as blood glucose value
180 mg/dl and hypoglycemia as blood glucose value <
< 70 mg/dl. According to the treatment protocol followed by all hospitals involved in the study, intravenous insulin infusion was initiated after the first measurement of blood glucose higher than 180 mg/dl. Then, the following measurements were performed every 2 h, and insulin infusion was adjusted according to the new value found, as follow: insulin drip was stopped when blood glucose was <
70 mg/dl. According to the treatment protocol followed by all hospitals involved in the study, intravenous insulin infusion was initiated after the first measurement of blood glucose higher than 180 mg/dl. Then, the following measurements were performed every 2 h, and insulin infusion was adjusted according to the new value found, as follow: insulin drip was stopped when blood glucose was < 80 mg/dl, it was decreased by 2UI/ml/h if it was between 81 and 110, between 111 and 180 no change of rate was made, and if the blood glucose was >
80 mg/dl, it was decreased by 2UI/ml/h if it was between 81 and 110, between 111 and 180 no change of rate was made, and if the blood glucose was > 180, insulin drip was increased by 2UI/ml/h.
180, insulin drip was increased by 2UI/ml/h.
The patients were divided into a group with a diagnosis of COVID-19 confirmed by RT-PCR (COVID-19 group) and another group in whom this diagnosis was refuted (non-COVID-19 group). To mitigate the bias of false-negative test results, the non-COVID-19 group included either patients who presented more than one negative RT-PCR result or with only one negative RT-PCR result, if the patient had another cause most likely to explain the diagnosis of SARS than SARS-CoV-2 infection. The diagnosis of patients who comprised the non-COVID-19 group are described in the Results section.
We evaluated the influence of COVID-19 on the variation of the six parameters of glycemic abnormalities described as the primary outcome. As the secondary outcome, we evaluated the influence of COVID-19 and each of the six parameters of glycemic abnormalities on hospital mortality within 30 days of ICU admission.
Following recommendations for observational studies in critically ill patients [15], we determined a priori the confounding factors of the relationship between the dysglycemias and the outcomes, based on the probability of association of these confounders both with COVID-19 and with the parameters of glycemic abnormality [16]. Thus, we considered as possible confounders or modifiers of the effect of COVID-19 on the outcomes the following variables: sex; age; prior history of diabetes [9, 17]; presumed obesity; Charlson Comorbidity Index; Acute Physiology and Chronic Health Evaluation (APACHE) II scores, Sequential Organ Failure Assessment (SOFA) scores [18], and C-reactive protein (CRP) values in the first 24 h in the ICU and the mean values and scores during the entire ICU stay [19, 20]; presence of nosocomial infection (presence of bacterial infection after 48 h in the ICU and antibiotic therapy that continued for at least 72 h) [21]; and use of corticosteroids, defined as the use of corticosteroids on admission or started within 24 h, of admission and maintained during hospitalization, initially hydrocortisone 200 mg daily. After the preliminary results of the RECOVERY study were released, dexamethasone 6 mg daily was the standard corticosteroid therapy [22, 23]. Such variables were also considered as possible confounders or modifiers of the independent effect of COVID-19 and glycemic abnormalities on mortality.
Statistical analysis
Categorical variables are presented as frequencies and percentages. The normality of the distribution was verified visually using box plots, skewness and kurtosis assessments, and the Kolmogorov-Smirnov test. Quantitative variables with normal distribution are presented as means and standard deviations, and those with non-normal distribution as medians and interquartile ranges. Frequencies were compared using the chi-square test or Fisher’s exact test, as appropriate. Quantitative variables were compared between groups using Student’s t test for independent samples when the data were normally distributed or the nonparametric Mann-Whitney test when the data were not normally distributed.
We fitted univariate and multivariate generalized linear models with log(n)-transformed data and linear scale response using Wald parameter estimates to assess the explanatory potential of COVID-19, sex, diabetes history, presumed obesity, Charlson Comorbidity Index, age, APACHE II scores, SOFA scores, CRP levels, corticosteroid use, and presence of nosocomial infection for each of the hyperglycemic parameters: highest glucose value at admission; highest glucose value during ICU stay; mean glucose level during ICU stay, and mean glucose variability during ICU stay. The same models were used without log(n)-transformed data to evaluate the glycemic abnormality “percentage of days with hyperglycemia.“ The significance of each model’s variables was evaluated using the Wald test. The results of the log-linear models are presented as exponentiated linear regression coefficients (eCoef.) and 95% confidence intervals (95% CIs), while the results of the linear models are presented as linear regression coefficients (Coef.) and 95% CIs.
The generalized linear models with binary logistic distribution using hybrid parameter estimation and fixed value scale were used to estimate the explanatory potential of COVID-19, sex, diabetes history, presumed obesity, Charlson Comorbidity Index, age, APACHE II scores, SOFA scores, CRP levels, corticosteroid use, presence of nosocomial infection and occurrence of hypoglycemia (< 70 mg/dL) during ICU stay, with results presented as Odds ratio (OR), 95% CI, and statistical significance of the Wald test.
70 mg/dL) during ICU stay, with results presented as Odds ratio (OR), 95% CI, and statistical significance of the Wald test.
Variables with significance less than 0.200 in the Wald test in the univariate analysis were included in the multivariate analysis. In cases where both diabetes history and the Charlson Comorbidity Index met this criterion, the Charlson Comorbidity Index was not included in the multivariable model given that diabetes is contained in the index result and therefore should not be adjusted in the same multivariable model. Using the same adjusted models mentioned above, we also performed an association analysis of COVID-19 with glycemic abnormalities variables stratified by use of corticosteroids during ICU stay for groups using corticosteroids against those not using corticosteroids. Generalized linear models with binary logistic distribution using hybrid parameter estimation and fixed value scale were used to estimate the explanatory potential of each of the parameters representing glucose abnormality and the variables COVID-19, sex, history of diabetes, presumed obesity, Charlson Comorbidity Index, age, APACHE II scores, SOFA scores, CRP levels, use of corticosteroids, and presence of nosocomial infection on mortality during ICU stay up to 30 days from admission. Odds ratio (OR), 95% CI, and statistical significance of the Wald test in the univariate model are presented for COVID-19, while for the other variables, the results of crude models that had COVID-19 as a covariate are presented. The ORs and 95% CIs of COVID-19 and each of the six variables of glycemic abnormality for mortality were adjusted, using multiple models, for the potential confounders established a priori. A p value <
< 0.05 was considered to indicate statistical significance.
0.05 was considered to indicate statistical significance.
The quality of the adjustments of the multiple models, given by their explanatory potential, was evaluated by the Bayesian information criterion (BIC). The statistical significance level was set at 5%, and data were analyzed using the statistical software IBM SPSS Statistics, version 28.0 (IBM SPSS Inc., Chicago, IL, USA). Missing data were not imputed.
Results
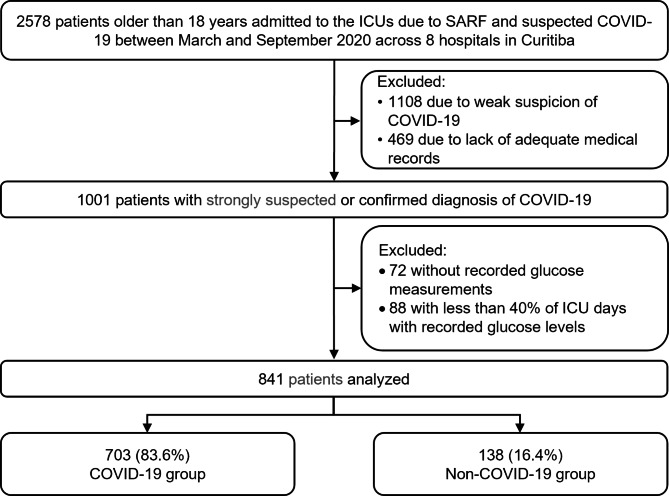
From March to September 2020, a total of 2578 patients older than 18 years were admitted to the participating ICUs with SARS due to strongly suspected or confirmed COVID-19. We excluded 1108 patients not meeting the inclusion criteria of strongly suspected COVID-19, 469 patients due to the absence of medical records of daily ICU follow-up, 72 without records of glucose measurements, and 88 with glucose values recorded in less than 40% of the days in the ICU. Thus, we analyzed data from 841 patients, of whom 83.6% (703) comprised the COVID-19 group and 16.4% (138) comprised the non-COVID-19 group (Fig. 1).
The non-COVID-19 group (n =
= 138) included the following diagnosis established to explain the diagnosis of acute respiratory failure: 54.3% had pneumonia; 18.1% cardiovascular diseases; 15.2% exacerbated chronic pneumopathies (asthma, COPD or pulmonary fibrosis); 3.6% sepsis of extrapulmonary etiology; 2.4% lung cancer; 2.2% neurological diseases; 1.5% pulmonary thromboembolism; 1.5% pneumonitis; 1.5% metabolic decompensation.
138) included the following diagnosis established to explain the diagnosis of acute respiratory failure: 54.3% had pneumonia; 18.1% cardiovascular diseases; 15.2% exacerbated chronic pneumopathies (asthma, COPD or pulmonary fibrosis); 3.6% sepsis of extrapulmonary etiology; 2.4% lung cancer; 2.2% neurological diseases; 1.5% pulmonary thromboembolism; 1.5% pneumonitis; 1.5% metabolic decompensation.
Table 1 presents the characteristics and outcomes of the entire cohort and each group between admission and 30 days of ICU stay, including the results of the six parameters of glycemic abnormalities analyzed. Patients with COVID-19 had significantly higher peaks of glucose values at admission and during ICU stay, higher mean daily glucose values, higher percentage of days with hyperglycemia during ICU stay, and greater mean daily glucose variability. However, there was no significant difference in the proportion of patients with an episode of hypoglycemia (< 70 mg/dL) during the ICU stay.
70 mg/dL) during the ICU stay.
Table 1
Characteristics of the overall cohort and the COVID-19 and non-COVID-19 groups
| Variables | Overall cohort (n = = 841) 841) | COVID-19 group (n = = 703) 703) | Non-COVID-19 group (n = = 138) 138) | p value |
|---|---|---|---|---|
| Baseline | ||||
| Age, years | 61 ± ± 16.6 16.6 | 60.6 ± ± 15.8 15.8 | 63.5 ± ± 19.7 19.7 | 0.052† |
| Male sex | 371 (44.1) | 302 (43) | 69 (50) | 0.134* |
| Comorbidities | ||||
| Charlson Comorbidity Index | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.001# |
| History of diabetes | 259 (30.8) | 222 (31.6) | 37 (26.8) | 0.313* |
| Hypertension | 436 (51.8) | 369 (52.5) | 67 (48.6) | 0.403* |
| Cardiopathy | 156 (18.5) | 121 (17.2) | 32 (25.4) | 0.031* |
| Chronic obstructive pulmonary disease | 105 (12.5) | 69 (9.8) | 36 (26.1) | < 0.001* 0.001* |
| Renal disease | 50 (5.9) | 44 (6.4) | 4 (4.3) | 0.554* |
| Peripheral vascular diseases | 20 (2.4) | 18 (2.6) | 2 (1.4) | 0.758* |
| Cerebrovascular diseases | 40 (4.8) | 28 (4.0) | 12 (8.7) | 0.027* |
| Chronic neurologic diseases | 34 (4.0) | 23 (3.3) | 11 (8.0) | 0.017* |
| Cognitive impairment or dementia | 31 (3.7) | 17 (2.4) | 14 (10.1) | < 0.001* 0.001* |
| Rheumatologic or connective tissue disease | 28 (3.3) | 26 (3.7) | 2 (1.4) | 0.295* |
| Chronic liver disease | 10 (1.2) | 7 (1.0) | 3 (2.2) | 0.217* |
| Cancer | 33 (3.9) | 27 (3.8) | 6 (4.3) | 0.810* |
| HIV/AIDS | 11 (1.3) | 9 (1.3) | 2 (1.4) | 0.699* |
| Presumed obesity | 250 (29.7) | 226 (32.1) | 24 (17.4) | < 0.001* 0.001* |
| ICU admission status | ||||
| Time from symptom onset to ICU admission, daysa | 7 (4–9) | 7 (5–10) | 4 (2–7) | < 0.001# 0.001# |
| APACHE II score in the first 24 h in ICU | 13 (8–19) | 13 (8–20) | 13 (8–18) | 0.707# |
| SOFA score in the first 24 h in ICU | 4 (2–6) | 3 (2–6) | 4 (2–6) | 0.350# |
| CRP level in the first 24 h in the ICUb | 115 (57.1–177) | 23 (68.9–184) | 43.1 (15–124) | < 0.001# 0.001# |
| VAD at admission | 144 (17.1) | 119 (16.9) | 25 (18.1) | 0.712* |
| IMV at admission | 232 (27.6) | 191 (27.2) | 41 (29.7) | 0.534* |
| Related to glycemic abnormalities in the ICU | ||||
| Highest glucose value at ICU admissionc (mg/dL) | 161(123–224) | 165 (124–235) | 146 (118–187) | 0.002 # |
| Highest glucose value during ICU stay (mg/dL) | 233 (167–372) | 242 (172–383) | 187 (142–289) | < 0.001# 0.001# |
| Mean glucose value during ICU stay (mg/dL) | 145.9 (121.5–196.8) | 149.7 (122.9–201) | 132.6 (111.6–171.6) | < 0.001# 0.001# |
| Mean glucose variability (CV %) during ICU stayd | 27.4 (20.2–39.9) | 28.1 (20.4–41.1) | 25.0 (18.7–34.5) | 0.013# |
% of days with hyperglycemia (≥ 180 mg/dL) 180 mg/dL) | 33.3 (0–82.4) | 42.9 (0–85.7) | 11.1 (0–66.7) | < 0.001# 0.001# |
Occurrence of hypoglycemia (< 70 mg/dL) during ICU stay 70 mg/dL) during ICU stay | 218 (25.9) | 184 (26.2) | 34 (24.6) | 0.751* |
| Related to progression within 30 days of ICU stay | ||||
| Nosocomial infection | 169 (20.1) | 151 (21.5) | 18 (13) | 0.027* |
| Mean SOFA score during ICU stay | 4.3 (2.3–7) | 4.4 (2.3–7.3) | 4.1 (2.2–5.9) | 0.033# |
| Mean CRP level during ICU stayb | 118.7 (65.3–178.7) | 126.5 (73.9–182.7) | 66.4 (24–144.6) | < 0.001# 0.001# |
| Use of VAD during ICU stay | 423 (50.3) | 370 (52.6) | 53 (38.4) | 0.003* |
| Use of IMV during ICU stay | 445 (52.9) | 385 (54.8) | 60 (43.5) | 0.016* |
| Lowest PaO2/FiO2 ratio during ICU stay | < 0.001‡ 0.001‡ | |||
 > > 300 300 | 72 (8.6) | 54 (7.7) | 18 (13) | |
 ≤ ≤ 300 300 | 164 (19.5)) | 120 (17.1) | 44 (31.9) | |
 ≤ ≤ 200 200 | 258 (30.7) | 208 (29.6) | 50 (36.2) | |
 ≤ ≤ 100 100 | 347 (41.3) | 321 (45.7) | 26 (18.8) | |
| Use of corticosteroid during ICU stay | 551 (65.5) | 499 (71) | 52 (37.7) | < 0.001* 0.001* |
| ICU length of stay | 6 (3–12) | 6 (3–13) | 4.5 (3–10) | 0.011# |
| ICU mortality | 320 (38) | 290 (41.3) | 30 (21.7) | < 0.001* 0.001* |
Data are expressed as number (percent), mean ± standard deviation, or median (first quartile - third quartile)
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein (mg/L); VAD: vasoactive drug; IMV: invasive mechanical ventilation. The glucose values are shown as mg/dL; hrs: hours; CV, Coefficient of variation
Missing data: a105 in the overall cohort, 84 in the COVID-19 group, and 21 in the non-COVID-19 group; b4 in the overall cohort, 4 in the COVID-19 group, and none in the non-COVID-19 group; c4 in the overall cohort, 3 in the COVID-19 group, and 1 in the non-COVID-19 group; d76 in the overall cohort, 49 in the COVID-19 group, and 27 in the non-COVID-19 group
*Fisher’s exact test
†Student’s t test for independent samples
#Nonparametric Mann-Whitney test
‡Chi-square test
p < 0.05 indicates statistical significance
The characteristics of patients from each participating hospital are presented separately in Additional File 1: Table S1.
The COVID-19 group had a higher percentage of patients with presumed obesity (as determined by the attending physician), higher CRP values through ICU stay, higher mean SOFA score during ICU stay, higher incidence of nosocomial infection, and need for vasoactive drug and invasive mechanical ventilation. Considering the first 30 days of ICU stay, patients with COVID-19 stayed longer in the ICU and had a higher mortality rate in the period (Table 1).
Given differences between the groups regarding characteristics potentially influencing the causal relationship of COVID-19 and glucose abnormalities, the influence of COVID-19 on each of five of the continuous variables representing glucose abnormality at admission and during ICU stay were evaluated separately and adjusted for sex, history of diabetes, presumed obesity, age, APACHE II score, SOFA score, CRP level, presence of nosocomial infection, and use of corticosteroid. The results of the unadjusted and adjusted models are presented in Table 2.
Table 2
Influence of COVID-19 on each of the parameters of glycemic abnormality unadjusted and adjusted for the variables sex, history of diabetes, presumed obesity, age, APACHE II scores, SOFA scores, CRP levels, and corticosteroid use
| Factors associated with highest glucose level on admission | n$ | e Coef. (95% CI) unadjusted* | p value§ | n$$ | e Coef. (95% CI) adjusted** | p value§ |
|---|---|---|---|---|---|---|
| COVID-19 a | 837 | 1.144 (1.054–1.241) | 0.001 | 833 | 1.034 (0.959–1.115) | 0.380 |
| Male sex b | 837 | 0.939 (0.884–0.999) | 0.046 | 0.981 (0.93–1.035) | 0.489 | |
| History of diabetes c | 837 | 1.518 (1.43–1.611) | < 0.001 0.001 | 1.479 (1.395–1.568) | 0.000 | |
| Presumed obesity d | 837 | 1.084 (1.015–1.159) | 0.017 | 1.032 (0.973–1.094) | 0.293 | |
| Charlson Comorbidity Index | 837 | 1.036 (1.013–1.059) | 0,002 | - | - | |
| Age | 837 | 1.002 (1–1.003) | 0.080 | 0.997 (0.996–0.999) | 0.003 | |
| APACHE II scores | 837 | 1.012 (1.009–1.015) | < 0.001 0.001 | 1.008 (1.005–1.012) | 0.000 | |
| SOFA scores in the first 24 h | 837 | 1.034 (1.023–1.046) | < 0.001 0.001 | 1.017 (1.005–1.028) | 0.005 | |
| CRP in the first 24 h | 833 | 1.001 (1.001–1.001) | < 0.001 0.001 | 1.000 (1.000–1.001) | 0.039 | |
| Use of corticosteroids f | 837 | 1.180 (1.108–1.257) | < 0.001 0.001 | 1.163 (1.099–1.231) | 0.000 | |
| BIC: 838.168‡ | ||||||
| Factors associated with highest glucose levels during hospitalization | n $ | e Coef. (95% CI) unadjusted* | p value § | n $$ | e Coef. (95% CI) adjusted** | p value § |
| COVID-19 a | 841 | 1.237 (1.133–1.35) | < 0.001 0.001 | 837 | 1.067 (0.995–1.145) | 0.069 |
| Male sex b | 841 | 0.959 (0.898–1.025) | 0.217 | - | - | |
| History of diabetes c | 841 | 1.583 (1.485–1.688) | < 0.001 0.001 | 1.524 (1.444–1.609) | 0.000 | |
| Presumed obesity d | 841 | 1.177 (1.096–1.264) | < 0.001 0.001 | 1.098 (1.039–1.161) | 0.001 | |
| Charlson Comorbidity Index | 841 | 1.061 (1.035–1.086) | < 0.001 0.001 | - | - | |
| Age | 841 | 1.004 (1.002–1.006) | < 0.001 0.001 | 0.999 (0.998–1.001) | 0.369 | |
| APACHE II scores | 841 | 1.014 (1.011–1.018) | < 0.001 0.001 | 1.003 (1–1.006) | 0.060 | |
| Nosocomial infection e | 841 | 1.57 (1.455–1.695) | < 0.001 0.001 | 1.376 (1.29–1.468) | 0.000 | |
| Mean SOFA during ICU stay | 841 | 1.068 (1.058–1.079) | < 0.001 0.001 | 1.045 (1.032–1.057) | 0.000 | |
| Mean CRP during ICU stay | 837 | 1.002 (1.001–1.002) | < 0.001 0.001 | 1.000 (1.000–1.000) | 0.745 | |
| Use of corticosteroids f | 837 | 1.211 (1.131–1.296) | < 0.001 0.001 | 1.172 (1.111–1.236) | 0.000 | |
| BIC: 741.262‡ | ||||||
| Factors associated with mean glycemic levels during hospitalization | n $ | e Coef. (95% CI) unadjusted* | p value § | n $$ | e Coef. (95% CI) adjusted** | p value § |
| COVID-19 a | 841 | 1.121 (1.054–1.191) | < 0.001 0.001 | 837 | 1.029 (0.977–1.084) | 0.284 |
| Male sex b | 841 | 0.977 (0.933–1.023) | 0.312 | - | - | |
| History of diabetes c | 841 | 1.432 (1.371–1.495) | < 0.001 0.001 | 1.398 (1.342–1.455) | < 0.001 0.001 | |
| Presumed obesity d | 841 | 1.123 (1.069–1.18) | < 0.001 0.001 | 1.078 (1.035–1.123) | < 0.001 0.001 | |
| Charlson Comorbidity Index | 841 | 1.044 (1.027–1.062) | < 0.001 0.001 | - | - | |
| Age | 841 | 1.003 (1.001–1.004) | < 0.001 0.001 | 0.999 (0.998–1.001) | 0.267 | |
| APACHE II | 841 | 1.009 (1.007–1.012) | < 0.001 0.001 | 1.004 (1.001–1.006) | 0.003 | |
| Nosocomial infection e | 841 | 1.185 (1.121–1.254) | < 0.001 0.001 | 1.101 (1.049–1.155) | < 0.001 0.001 | |
| Mean SOFA during ICU stay | 841 | 1.035 (1.028–1.043) | < 0.001 0.001 | 1.02 (1.011–1.029) | < 0.001 0.001 | |
| Mean CRP during ICU stay | 837 | 1.001 (1.001–1.001) | < 0.001 0.001 | 1.000 (1.000–1.000) | 0.466 | |
| Use of corticosteroids f | 837 | 1.104 (1.053–1.158) | < 0.001 0.001 | 1.089 (1.047–1.134) | < 0.001 0.001 | |
| BIC: 252.794‡ | ||||||
Factors associated with % of days with hyperglycemia (≥ 180 mg/dL) 180 mg/dL)
| n $ | Coef. (95% CI) unadjusted # | p value § | n $$ | Coef. (95% CI) adjusted ## | p value § |
| COVID-19 a | 841 | 13.528 (6.44–20.616) | < 0.001 0.001 | 837 | 3.233 (-2.813–9.28) | 0.295 |
| Male sex b | 841 | -3.805 (-9.13–1.519) | 0.161 | 0.176 (-4.178–4.53) | 0.937 | |
| History of diabetes c | 841 | 41.881 (36.895–46.867) | < 0.001 0.001 | 39.565 (34.869–44.261) | 0.000 | |
| Presumed obesity d | 841 | 9.123 (3.364–14.881) | 0.002 | 4.271 (-0.483–9.026) | 0.078 | |
| Charlson Comorbidity Index | 841 | 5.365 (3.437–7.294) | < 0.001 0.001 | - | - | |
| Age | 841 | 0.328 (0.17–0.487) | < 0.001 0.001 | -0.051 (-0.196–0.094) | 0.490 | |
| APACHE II scores | 841 | 1.036 (0.774–1.298) | < 0.001 0.001 | 0.356 (0.075–0.636) | 0.013 | |
| Nosocomial infection e | 841 | 20.309 (13.848–26.77) | < 0.001 0.001 | 12.01 (6.443–17.576) | 0.000 | |
| Mean SOFA during ICU stay | 841 | 3.965 (3.121–4.808) | < 0.001 0.001 | 2.164 (1.131–3.197) | 0.000 | |
| Mean CRP during ICU stay | 837 | 0.107 (0.074–0.139) | < 0.001 0.001 | 0.023 (-0.009–0.055) | 0.163 | |
| Use of corticosteroid f | 837 | 12.994 (7.495–18.493) | < 0.001 0.001 | 12.054 (7.443–16.665) | 0.000 | |
| BIC: 8208.738‡ | ||||||
| Factors associated with mean glucose variability (CV %) during ICU stay | n $ | Coef. (95% CI) unadjusted # | p value § | n $$ | Coef. (95% CI) adjusted ## | p value § |
| COVID-19 a | 765 | 4.243 (1.033–7.454) | 0.010 | 762 | 1.999 (-0.773–4.771) | 0.158 |
| Male sex b | 765 | -3.114 (-5.392 - -0.837) | 0.007 | -0.716 (-2.642–1.21) | 0.466 | |
| History of diabetes c | 765 | 16.237 (14.071–18.404) | < 0.001 0.001 | 15.04 (12.978–17.102) | < 0.001 0.001 | |
| Presumed obesity d | 765 | -1.634 (-4.114–0.847) | 0.202 | - | - | |
| Charlson Comorbidity Index | 765 | 3.279 (2.44–4.119) | < 0.001 0.001 | - | - | |
| Age | 765 | 0.204 (0.136–0.272) | < 0.001 0.001 | 0.011 (-0.053–0.076) | 0.730 | |
| APACHE II scores | 765 | 0.527 (0.413–0.64) | < 0.001 0.001 | 0.252 (0.127–0.377) | < 0.001 0.001 | |
| Nosocomial infection e | 765 | 4.282 (1.532–7.033) | 0.002 | 1.186 (-1.216–3.588) | 0.333 | |
| Mean SOFA during ICU stay | 765 | 1.678 (1.303–2.052) | < 0.001 0.001 | 1.012 (0.545–1.479) | < 0.001 0.001 | |
| Mean CRP during ICU stay | 762 | 0.033 (0.019–0.047) | < 0.001 0.001 | -0.003 (-0.017–0.012) | 0.722 | |
| Use of corticosteroid f | 765 | 4.197 (1.744–6.65) | < 0.001 0.001 | 4.098 (2.018–6.178) | < 0.001 0.001 | |
| BIC: 6153.797‡ |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; CRP: C-reactive protein (mg/L)
Reference categories: anon-COVID-19; bfemale sex; cabsence of history of known diabetes; dabsence of presumed obesity; eabsence of nosocomial infection; fno use of corticosteroids during ICU stay
*Exponentiated linear regression coefficient (eCoef.) and 95% confidence interval (95% IC) of the univariate log-linear regression model
**Exponentiated linear regression coefficient (eCoef.) and 95% confidence interval (95% IC) of the multivariate log-linear regression model
#Linear regression coefficient (Coef.) and 95% confidence interval (95% IC) of the univariate log-linear regression model
##Linear regression coefficient (Coef.) and 95% confidence interval (95% IC) of the multivariate log-linear regression model
$number of cases (n) included in each univariate model. $$number of cases (n) included in the multivariate model
§Wald test p value, p <
< 0.05 indicates statistical significance
0.05 indicates statistical significance
‡Bayesian information criterion (BIC) of the multivariate generalized linear model, representative of the quality of the model given its explanatory potential, with the lower the BIC, the more the model is able to explain the dependent variable
COVID-19, history of diabetes, presumed obesity, age, APACHE II scores, use of corticosteroid, presence of nosocomial infection, SOFA scores, CRP values in the first 24 h, and mean values during ICU stay were each individually associated with the highest glucose level at admission, mean and highest glucose levels during ICU stay, mean glucose variability, and percentage of days with hyperglycemia, as shown in Table 2. However, the relationship between COVID-19 and these five parameters of glycemic abnormalities was no longer statistically significant after adjustment for confounders. On the other hand, APACHE II score, SOFA score, use of corticosteroids, and presence of nosocomial infection remained associated with these parameters representing glycemic abnormality, even after adjustments in the multivariate models (Table 2). The COVID-19 was not associated to the occurrence of hypoglycemia (> 70 mg/dL) during ICU stay nor in univariate analysis, nor after adjustments in the multivariate model (Additional file: Table S2)
70 mg/dL) during ICU stay nor in univariate analysis, nor after adjustments in the multivariate model (Additional file: Table S2)
The lack of association of COVID-19 with the six parameters of glycemic abnormalities was also confirmed in models restricted for use and no use of corticosteroids and adjusted for sex, history of diabetes, presumed obesity, age, APACHE II score, SOFA score, and CRP level (Table 3)
Table 3
Significance of the dysglycemias variables comparison between COVID-19 and non-COVID-19, restricted by corticosteroids’ use or no use
| Variables | Use of corticosteroids | No use of corticosteroids |
|---|---|---|
| p value (COVID-19 vs. non-COVID-19) | p value (COVID-19 vs. non-COVID-19) | |
| Highest glucose level at ICU admission | 0.157* | 0.967* |
| Highest glucose level during ICU stay | 0.151# | 0.234# |
| Mean glucose level during ICU stay | 0.203# | 0.741# |
| Mean glucose variability (CV %) during ICU stay | 0.793† | 0.078† |
% of days with hyperglycemia (≥ 180 mg/dL) 180 mg/dL) | 0.713§ | 0.277§ |
*Wald test p value of the multivariate log-linear regression models adjusted for sex, history of diabetes, presumed obesity, age, APACHE II scores, SOFA scores in the first 24 h, and CRP levels in the first 24 h
#Wald test p value of the multivariate log-linear regression models adjusted for history of diabetes, presumed obesity, age, APACHE II scores, Nosocomial infection, Mean SOFA during ICU stay, Mean CRP during ICU stay
†Wald test p value of the multivariate linear regression models adjusted for sex, history of diabetes, age, APACHE II scores, Nosocomial infection, Mean SOFA during ICU stay, Mean CRP during ICU stay
§Wald test p value of the multivariate linear regression models adjusted for sex, history of diabetes, presumed obesity, age, APACHE II scores, Nosocomial infection, Mean SOFA during ICU stay, Mean CRP during ICU stay
In the univariate analysis, patients with SARS due to causes other than COVID-19 compared with those with SARS due to COVID-19 were 1.5 times more likely to die (OR 2.528, 95% CI 1.642–3.892, p <
< 0.001). The six parameters of glycemic abnormalities emerged as risk factors for ICU mortality within 30 days when adjusted for COVID-19 alone. Similarly, after adjustment for COVID-19, the odds of death increased with higher APACHE II scores, SOFA scores, CRP values, age, prior history of diabetes, and development of nosocomial infection (Table 4)
0.001). The six parameters of glycemic abnormalities emerged as risk factors for ICU mortality within 30 days when adjusted for COVID-19 alone. Similarly, after adjustment for COVID-19, the odds of death increased with higher APACHE II scores, SOFA scores, CRP values, age, prior history of diabetes, and development of nosocomial infection (Table 4)
Table 4
Risk factors for 30 days ICU mortality of patients admitted for SARS with or without COVID-19
| Factors | Mortality in the overall cohort | OR (95% CI) | p value§ | ||
| n † | Results # | ||||
| Diagnosis | Non-COVID-19 | 30 | 30 (21.7) | Ref. | |
| COVID-19 | 290 | 290 (41.3) | 2.528 (1.642–3.892)* | < 0.001 0.001 | |
| Sex | Female | 371 | 144 (38.8) | Ref. | |
| Male | 470 | 176 (37.4) | 0.911 (0.686–1.211)** | 0.522 | |
| History of diabetes | No | 582 | 207 (35.6) | Ref. | |
| Yes | 259 | 113 (43.6) | 1.377 (1.018–1.861)** | 0.038 | |
| Presumed obesity | No | 591 | 232 (39.3) | Ref. | |
| Yes | 250 | 88 (35.2) | 0.770 (0.563–1.052)** | 0.101 | |
| Charlson Comorbidity Index | No death | 521 | 1 (0–1) | ||
| Death | 320 | 1 (0–2) | 1.300 (1.167–1.447) | < 0.001 0.001 | |
| Age | No death | 521 | 57.6 ± ± 16.7 16.7 | ||
| Death | 320 | 66.7 ± ± 14.7 14.7 | 1.041 (1.030–1.051)** | < 0.001 0.001 | |
| APACHE II in the first 24 h | No death | 521 | 10 (6–15) | ||
| Death | 320 | 19 (12–27) | 1.123 (1.101–1.146)** | < 0.001 0.001 | |
| SOFA scores in the first 24 h | No death | 521 | 3 (2–4) | ||
| Death | 320 | 6 (3–8) | 1.524 (1.423–1.633)** | < 0.001 0.001 | |
| CRP levels in the first 24 h | No death | 518 | 91.6 (39–156) | ||
| Death | 319 | 142 (88–219) | 1.006 (1.004–1.008)** | < 0.001 0.001 | |
| Highest glucose level at admission(mg/dL) | No death | 520 | 150.5 (118–199.5) | ||
| Death | 317 | 180 (136–290) | 1.005 (1.004–1.007)** | < 0.001 0.001 | |
| Highest glucose level during ICU stay (mg/dL) | No death | 521 | 196 (156–294) | ||
| Death | 320 | 335 (209.5–453.5) | 1.006 (1.005–1.007)** | < 0.001 0.001 | |
| Mean glucose level during ICU stay (mg/dL) | No death | 521 | 133.6 (115.5–166.3) | ||
| Death | 320 | 178.7 (138.5–226.9) | 1.012 (1.009–1.015)** | < 0.001 0.001 | |
| Mean glucose variability (CV %) during ICU stay | No death | 469 | 23.9 (18.2–33.1) | ||
| Death | 296 | 35.5 (25.0–46.8) | 1.041 (1.030–1.052) | < 0.001 0.001 | |
| % of days with hyperglycemia | No death | 521 | 17.6 (0–60) | ||
| Death | 320 | 66.7 (28.6–93.3) | 1.018 (1.014–1.022)** | < 0.001 0.001 | |
Occurrence of hypoglycemia (< 70 mg/dL) during ICU stay 70 mg/dL) during ICU stay | No | 623 | 197 (31.6) | Ref. | |
| Yes | 218 | 123 (56.4) | 2.848 (2.065–3.927) | < 0.001 0.001 | |
| Nosocomial infection | No | 672 | 211 (31.4) | Ref. | |
| Yes | 169 | 109 (64.5) | 3.841 (2.685–5.493)** | < 0.001 0.001 | |
| Mean SOFA score during ICU stay | No death | 521 | 2.8 (2–4.1) | ||
| Death | 320 | 7.6 (6–9.2) | 2.786 (2.432–3.192)** | < 0.001 0.001 | |
| Mean CRP level during ICU stay | No death | 518 | 83.2 (43.9–135.9) | ||
| Death | 319 | 175.7 (126.9–224.9) | 1.018 (1.015–1.020)** | < 0.001 0.001 | |
| Use of corticosteroids during ICU stay | No | 290 | 95 (32.8) | Ref. | |
| Yes | 551 | 225 (40.8) | 1.215 (0.891–1.655)** | 0.218 | |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein (mg/L)
†Total number of cases in the row
#Data are expressed as number (percent), mean ±
± standard deviation, or median (first quartile - third quartile)
standard deviation, or median (first quartile - third quartile)
*Odds ratio and 95% confidence interval of the generalized linear model with univariate binary logistic distribution for mortality during ICU stay up to 30 days
**Odds ratio and 95% confidence interval of the generalized linear model with binary logistic distribution for mortality during ICU stay up to 30 days, adjusted for the presence of COVID-19 (crude model)
§Wald test p value, p <
< 0.05 indicates statistical significance
0.05 indicates statistical significance
In the multivariate explanatory models of mortality, the parameters of glycemic abnormalities: highest glucose level at admission, mean and highest glucose levels during ICU stay, glucose variability, and percentage of days with hyperglycemia, remained independent risk factors for mortality even after adjustment for variables related to mortality, including COVID-19, history of diabetes, nosocomial infection, age, APACHE II score, SOFA score, and CRP level. In this same context, COVID-19 also remained an independent risk factor for mortality (Table 5). The occurrence of hypoglycemia (< 70 mg/dL) during ICU stay was no longer statistically significant in relation to mortality when adjusted for confounders (Additional file: Table S3)
70 mg/dL) during ICU stay was no longer statistically significant in relation to mortality when adjusted for confounders (Additional file: Table S3)
Table 5
Adjusted models explaining mortality within 30 days in the ICU
| Multivariate explanatory models of mortality in the overall cohort | n# | OR (95% CI) adjusted* | p value § | BIC ‡ |
|---|---|---|---|---|
| Highest glucose level at admission | 833 | 1.004 (1.002–1.006) | < 0.001 0.001 | 847.026 |
| COVID-19 (ref: non-COVID-19) | 2.990 (1.767–5.060) | < 0.001 0.001 | ||
| History of diabetes (ref: no history) | 0.658 (0.435–0.995) | 0.048 | ||
| Age | 1.026 (1.013–1.038) | < 0.001 0.001 | ||
| APACHE II score | 1.057 (1.032–1.082) | < 0.001 0.001 | ||
| SOFA score in the first 24 h | 1.299 (1.201–1.405) | < 0.001 0.001 | ||
| CRP level in the first 24 h | 1.004 (1.002–1.006) | < 0.001 0.001 | ||
| Highest glucose level during ICU stay | 837 | 1.003 (1.000–1.005) | 0.021 | 519.168 |
| COVID-19 (ref: non-COVID-19) | 2.655 (1.363–5.17) | 0.004 | ||
| History of diabetes (ref: no history) | 0.816 (0.458–1.457) | 0.493 | ||
| Age | 1.020 (1.003–1.037) | 0.023 | ||
| APACHE II score | 0.997 (0.966–1.028) | 0.847 | ||
| Nosocomial infection (ref: no infection) | 0.976 (0.558–1.707) | 0.933 | ||
| Mean SOFA score | 2.327 (1.993–2.717) | < 0.001 0.001 | ||
| Mean CRP level | 1.010 (1.006–1.013) | < 0.001 0.001 | ||
| Mean glucose level during ICU stay | 837 | 1.011 (1.006–1.016) | < 0.001 0.001 | 502.875 |
| COVID-19 (ref: non-COVID-19) | 2.665 (1.336–5.315) | 0.005 | ||
| History of diabetes (ref: no history) | 0.566 (0.312–1.029) | 0.062 | ||
| Age | 1.024 (1.006–1.041) | 0.009 | ||
| APACHE II score | 0.993 (0.962–1.026) | 0.684 | ||
| Nosocomial infection (ref: absent) | 1.080 (0.630–1.851) | 0.779 | ||
| Mean SOFA score | 2.348 (2.014–2.737) | < 0.001 0.001 | ||
| Mean CRP level | 1.01 (1.006–1.014) | < 0.001 0.001 | ||
% of days with hyperglycemia (≥ 180 mg/dL) 180 mg/dL) | 837 | 1.017 (1.010–1.025) | < 0.001 0.001 | 504.526 |
| COVID-19 (ref: non-COVID-19) | 2.350 (1.192–4.633) | 0.014 | ||
| History of diabetes (ref: no history) | 0.555 (0.302–1.019) | 0.058 | ||
| Age | 1.020 (1.003–1.038) | 0.020 | ||
| APACHE II score | 0.993 (0.962–1.025) | 0.669 | ||
| Nosocomial infection (Ref: absent) | 1.010 (0.588–1.734) | 0.971 | ||
| Mean SOFA score | 2.370 (2.032–2.765) | < 0.001 0.001 | ||
| Mean CRP level | 1.010 (1.006–1.014) | < 0.001 0.001 | ||
| Mean glucose variability (CV %) during ICU stay | 762 | 1.032 (1.013–1.051) | 0.001 | 476.005 |
| COVID-19 (ref: non-COVID-19) | 3.126 (1.503–6.502) | 0.002 | ||
| History of diabetes (ref: no history) | 0.651 (0.347–1.222) | 0.181 | ||
| Age | 1.022 (1.004–1.04) | 0.015 | ||
| APACHE II score | 0.984 (0.951–1.017) | 0.329 | ||
| Nosocomial infection (ref: no infection) | 1.235 (0.716–2.13) | 0.449 | ||
| Mean SOFA score | 2.444 (2.079–2.873) | 0.000 | ||
| Mean CRP level | 1.010 (1.006–1.014) | 0.000 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein (mg/L)
#Case numbers included in the model
* Odds ratio (OR) and 95% confidence interval (95% CI) for mortality within 30 days in the ICU, generalized linear models with multivariate binary logistic regression
§Wald test p value, with values <
< 0.05 indicating statistical significance
0.05 indicating statistical significance
‡Bayesian information criterion (BIC) of the multivariate generalized linear model representing the quality of the model given its explanatory potential; the lower the BIC, the more the model is able to explain the dependent variable
The crude relationship between mean glucose level during ICU stay intervals and mortality within 30 days in the ICU is as show in Additional file 1: Figure S1. An U-shaped relationship was identified between mean glucose and mortality in the SARS without COVID-19 cohort, while in the SARS due to COVID-19 cohort the mortality does appears to increase in a linear fashion with mean glucose levels (Additional file 1: Figure S1)
Discussion
The findings of the study demonstrated that in a population of patients admitted to the ICU with SARS and suspected COVID-19, those in whom the diagnosis of COVID-19 was confirmed, compared with those in whom this diagnosis was refuted, had higher glycemic load, represented by higher glucose level at admission, higher glucose level and higher mean glucose level during ICU stay, mean glucose variability, and higher percentage of days with hyperglycemia during ICU stay. However, this association between COVID-19 and glycemic abnormalities was no longer statistically significant after being adjusted for confounding factors. On the other hand, APACHE II scores, SOFA scores, use of corticosteroids, and presence of nosocomial infection remained associated with the parameters representing dysglycemia, which is consistent with findings in critically ill patients [1]. The development of dysglycemia, independent of the patient’s preadmission glucose level, has been frequently reported in hospitalized patients with severe COVID-19 and is associated with worse outcomes [6, 11]. Many studies have analyzed the relationship between hyperglycemia and severe COVID-19, but no study has evaluated hyperglycemia and other glycemic abnormalities in patients with SARS due to COVID-19 compared with contemporary controls. Our retrospective cohort of patients admitted to the ICU addressed this issue specifically. The objective of our study was to assess whether dysglycemias were directly associated with COVID-19 or if they were more common in these patients because they were more seriously ill. Another objective was to evaluate whether COVID-19 and dysglycemias were each independently related to mortality in these patients.
Observational studies have shown associations of severe hyperglycemia and increased glucose variability with poor outcomes in patients with COVID-19 [8, 24], although the causality of these associations remains uncertain since hyperglycemia and insulin resistance are closely related to disease severity [25]. The hypothesis that COVID-19 is specifically related to hyperglycemia and recent-onset diabetes [6] has even led to a search for effects directly mediated by the virus on the function and survival of insulin-producing beta cells and insulin resistance [26].
Our study comparing patients with SARS due to COVID-19 against patients with SARS due to other causes suggests that the dysglycemia in patients with COVID-19 was not directly associated with the SARS-CoV-2 infection as previously proposed [27], but is due to greater insulin resistance, probably secondary to higher levels of counterregulatory hormones and cytokines and the use of medications, although beta cell dysfunction secondary to prolonged hyperglycemia, inflammation, and/or low beta-cell reserve cannot be ruled out [25].
Compared with patients who do not meet diabetes or uncontrolled hyperglycemia criteria, patients with theses abnormalities are significantly older [9, 17], sometimes with a higher percentage of male [9]. Both daily SOFA and APACHE II have been considered mortality predictors in critically ill COVID-19 patients [18]. Steroids have been known for increasing blood sugar by increasing the hepatic gluconeogenesis or production of glucose from the liver and by enhancing the effect of counter regulatory hormones, among other mechanisms [21, 23]. Elevations in the levels of inflammatory cytokines as seen in COVID-19 further worsen insulin resistance [19], and levels of inflammatory markers and markers of COVID-19 severity, including C-reactive protein, have been found to be higher in patients with newly diagnosed diabetes, compared to those with pre-existing DM [20].
Regarding the relationship between dysglycemia and mortality, our study showed that the same parameters representing glycemic load and related to COVID-19 are independent risk factors for mortality in up to 30 days in the ICU, even when adjusted for factors also related to mortality. Hyperglycemia has been linked to a higher risk of mortality and, together with cardiac injury and use of high doses of corticosteroids [10], is considered an independent predictor of mortality in hospitalized patients with COVID-19 [28], which has been confirmed in the present study. Other retrospective studies in patients hospitalized with COVID-19 have also shown associations between hyperglycemia and worse prognosis [7, 29] independent of pre-existing diabetes [8], and even higher risk of mortality in patients with COVID-19 without diabetes compared with those with diabetes. The finding that the variables related to glycemic load and length of ICU stay remained associated with mortality suggests that effective methods to detect and treat dysglycemia may reduce the impact of these glucose abnormalities in patients with COVID-19 and improve their outcome [30, 31].
Due to the observational nature of the study, not all data were available for all patients, and we were unable to control for all factors possibly interfering with glucose levels, including the daily amount of insulin units used to correct hyperglycemia. However, all included patients received the same standard capillary blood glucose correction protocol, with a blood glucose target <
< 140 mg/dL. Also, since critically ill patients may present with hemodynamic failure, the agreement between venous and capillary glucose levels may be lower than expected. But even though we considered capillary and venous glucose levels indistinctively, the differences in dysglycemia between groups could not be attributed only to this fact. Other limitations of our study include the information about presumed obesity, which was subjectively determined by the attending physician, and the fact that the absence of a history of diabetes does not exclude the possibility of undiagnosed diabetes. As an important strength, the findings of our study, with a longitudinal follow-up of a large number of patients with SARS, offer potentially useful information about the influence of COVID-19 on glucose levels in this population and its independent impact on mortality.
140 mg/dL. Also, since critically ill patients may present with hemodynamic failure, the agreement between venous and capillary glucose levels may be lower than expected. But even though we considered capillary and venous glucose levels indistinctively, the differences in dysglycemia between groups could not be attributed only to this fact. Other limitations of our study include the information about presumed obesity, which was subjectively determined by the attending physician, and the fact that the absence of a history of diabetes does not exclude the possibility of undiagnosed diabetes. As an important strength, the findings of our study, with a longitudinal follow-up of a large number of patients with SARS, offer potentially useful information about the influence of COVID-19 on glucose levels in this population and its independent impact on mortality.
Conclusion
In conclusion, our study demonstrated that patients with SARS and COVID-19 have more frequent dysglycemia and higher mortality than patients with SARS due to other causes. However, the dysglycemias were not independently associated with COVID-19, but rather related to disease severity, inflammatory markers and the use of glucocorticoids.
Acknowledgements
We thank all the participating centers for the support and interventions in our study during this challenging period for everyone. We thank Miguel Morita Fernandes da Silva and Verônica Barros and the research team for all the support and collaboration.
Abbreviations
| ICU | Intensive care unit |
| ICUs | Intensive care units |
| SARS | Severe acute respiratory syndrome |
| SUS | Brazilian Unified Health System |
| RT-PCR | Real-time polymerase chain reaction |
| CT | Computed tomography |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| SOFA | Sequential Organ Failure Assessment |
| CRP | C-reactive protein (mg/L) |
| eCoef. | Exponentiated linear regression coefficients |
| Coef. | Regression coefficients |
| OR | Odds ratio |
| BIC | Bayesian information criterion |
| VAD | Vasoactive drug |
| IMV | Invasive mechanical ventilation |
Author Contribution
Conceptualization: RRR and ARN; data curation, RSB, MO and ARN; formal analysis, RSB, MO, MMJ; investigation, RRR; methodology, ARN, RSB, ACKN and MCO; project administration, RRR and ARN; supervision, RRR and ARN; visualization, RRR, ARN, RSB, ACKN and MCO; writing—original draft preparation, RRR, RSB, ACKN, MCO; writing—review and editing, RRR, ARN, RSB, ACKN, MMJ, and MCO. All authors have approved the submitted version and agreed to be personally accountable its own contributions and ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
No funding was received.
Data Availability
The dataset supporting the conclusions of this article is available in the Zenodo.org repository, with the link 10.5281/zenodo.6959376.
Declarations
The study was approved by the local ethics committee of the Instituto de Neurologia de Curitiba under protocol number 2.899.188 on September 17, 2018 (ID: CAAE 98099918.2.0000.5227; project title: Epidemiological analysis of patients hospitalized in intensive care units in Curitiba-Paraná). The requirement for informed consent was waived by the same committee, given the noninterventional design of the study and the fact that the data were collected from clinical records and without contact with the participants and the procedures performed in this study were part of routine care. All research procedures were conducted in accordance with the ethical standards of the institutional committee on human experimentation and the Declaration of Helsinki of 1975, as revised in 2013.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from BMC Pulmonary Medicine are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s12890-023-02439-y
Read article for free, from open access legal sources, via Unpaywall:
https://bmcpulmmed.biomedcentral.com/counter/pdf/10.1186/s12890-023-02439-y
Citations & impact
Impact metrics
Citations of article over time
Article citations
Assessing glycemic variability in critically ill patients: A prospective cohort study comparing insulin infusion therapy with insulin sliding scale.
Sci Rep, 14(1):10128, 02 May 2024
Cited by: 1 article | PMID: 38698018 | PMCID: PMC11066101
Diabetes Mellitus, Energy Metabolism, and COVID-19.
Endocr Rev, 45(2):281-308, 01 Mar 2024
Cited by: 8 articles | PMID: 37934800 | PMCID: PMC10911957
Review Free full text in Europe PMC
Hyperglycemia and glucose variability are associated with worse survival in mechanically ventilated COVID-19 patients: the prospective Maastricht Intensive Care Covid Cohort.
Diabetol Metab Syndr, 15(1):253, 06 Dec 2023
Cited by: 0 articles | PMID: 38057908 | PMCID: PMC10698889
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Association between intensive care unit-acquired dysglycemia and in-hospital mortality.
Crit Care Med, 40(12):3180-3188, 01 Dec 2012
Cited by: 73 articles | PMID: 22971590
Association Between Achieving Inpatient Glycemic Control and Clinical Outcomes in Hospitalized Patients With COVID-19: A Multicenter, Retrospective Hospital-Based Analysis.
Diabetes Care, 44(2):578-585, 15 Dec 2020
Cited by: 53 articles | PMID: 33323475 | PMCID: PMC7818335
Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study.
Rev Esp Anestesiol Reanim (Engl Ed), 67(8):425-437, 13 Jul 2020
Cited by: 79 articles | PMID: 32800622 | PMCID: PMC7357496
Specific and Non-specific Aspects and Future Challenges of ICU Care Among COVID-19 Patients with Obesity: A Narrative Review.
Curr Obes Rep, 13(3):545-563, 04 Apr 2024
Cited by: 0 articles | PMID: 38573465
Review

 1,2,6
1,2,6