Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) has become less common in Germany in recent years. In this paper, we report data from the MRSA module of the Hospital Infection Surveillance System (Krankenhaus-Infektionen- Surveillance-System, KISS) for the years 2006-2021. We also describe the association of MRSA rates with the frequency of patient screening for MRSA and discuss the findings.Methods
Participation in the MRSA KISS module is voluntary. Once a year, the participating hospitals submit structural data, information on cases in which MRSA was detected (both colonizations and infections; both detected on admission and nosocomially acquired), and the number of nasal swabs taken for the detection of MRSA to the German National Reference Center for the Surveillance of Nosocomial Infections. Statistical analyses were performed with R software.Results
The number of hospitals participating in the MRSA module rose from 110 in 2006 to 525 in 2021. From 2006 onward, the overall MRSA prevalence in German hospitals increased, reaching a maximum of 1.04 cases per 100 patients in 2012. The prevalence on admission fell by 44% from 0.96 in 2016 to 0.54 in 2021. The incidence density of nosocomial MRSA fell by an average of 12% per year, from 0.27 per 1000 patient-days in 2006 to 0.06 in 2021, while MRSA screening frequency increased sevenfold by 2021. The nosocomial incidence density was stable, independently of the screening frequency.Conclusion
MRSA rates in German hospitals fell markedly from 2006 to 2021, reflecting a general trend. The incidence density was no higher in hospitals with a low or moderate screening frequency than in those with a high one. Thus, a targeted, riskadapted MRSA screening strategy on hospital admission can be recommended.Free full text

Screening for Methicillin-Resistant Staphylococcus aureus
Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) has become less common in Germany in recent years. In this paper, we report data from the MRSA module of the Hospital Infection Surveillance System (Krankenhaus-Infektionen-Surveillance-System, KISS) for the years 2006–2021. We also describe the association of MRSA rates with the frequency of patient screening for MRSA and discuss the findings.
Methods
Participation in the MRSA KISS module is voluntary. Once a year, the participating hospitals submit structural data, information on cases in which MRSA was detected (both colonizations and infections; both detected on admission and nosocomially acquired), and the number of nasal swabs taken for the detection of MRSA to the German National Reference Center for the Surveillance of Nosocomial Infections. Statistical analyses were performed with R software.
Results
The number of hospitals participating in the MRSA module rose from 110 in 2006 to 525 in 2021. From 2006 onward, the overall MRSA prevalence in German hospitals increased, reaching a maximum of 1.04 cases per 100 patients in 2012. The prevalence on admission fell by 44% from 0.96 in 2016 to 0.54 in 2021. The incidence density of nosocomial MRSA fell by an average of 12% per year, from 0.27 per 1000 patient-days in 2006 to 0.06 in 2021, while MRSA screening frequency increased sevenfold by 2021. The nosocomial incidence density was stable, independently of the screening frequency.
Conclusion
MRSA rates in German hospitals fell markedly from 2006 to 2021, reflecting a general trend. The incidence density was no higher in hospitals with a low or moderate screening frequency than in those with a high one. Thus, a targeted, risk-adapted MRSA screening strategy on hospital admission can be recommended.
Infections caused by methicillin-resistant Staphylococcus aureus (MRSA) continue to be a global challenge. Given the high morbidity and mortality associated with infections with S. aureus, including MRSA, the World Health Organization (WHO) considers research and development of new antibiotics to treat these pathogens a high priority (1). In Germany, section 23 of the Protection against Infection Act (Infektionsschutzgesetz, IfSG) requires hospitals to record specified nosocomial infections and multidrug-resistant organisms (MDROs), such as MRSA (2). Furthermore, direct detection of MRSA in blood cultures or cerebrospinal fluid (CSF) is reportable according to section 7 of the IfSG. In 2020, 1126 cases of invasive MRSA infections were reported to the Robert Koch Institute, which corresponds to an incidence of 1.4 per 100 000 population. In 2012, 4456 cases had been reported (incidence 5.4 per 100 000 population). Thus, the number of cases decreased statistically significantly by a factor of almost 4 within seven years (3, 4). Not only the number of cases, but also the proportion of MRSA in S. aureus isolates from clinical materials is steadily declining. A study on nosocomial infections in German intensive care units (ICUs) as part of the Hospital Infection Surveillance System (KISS, Krankenhaus-Infektionen-Surveillance-System) showed that the rate of MRSA in nosocomial infections caused by S. aureus fell from 33% to 20% in 10 years (5). This trend is also noted in the member countries of the European Union (EU). For example, in the EU, the proportion of MRSA in S. aureus isolates from blood cultures or cerebrospinal fluid declined from 19% in 2016 to 17% in 2020—a statistically significant decrease (6).
The aim of our study was to describe the developments in MRSA epidemiology in hospitals in Germany during the period from 2006 to 2021, to elucidate MRSA detection numbers in association with screening frequency and to critically evaluate the impact of hospital admission screening on the decline in the number of MRSA cases based on our findings. We also formulate recommendations for an adequate screening strategy.
Methods
Since 1997, the German national Hospital Infection Surveillance System (KISS) has served as a procedure to monitor nosocomial infections in Germany (7). Within the KISS framework, the MRSA-KISS module was established as a pathogen surveillance component in 2006 (8, 9). As with the other KISS modules, participation in the MRSA component is voluntary. This module can be used for collecting data on multidrug-resistant organisms, as required by section 23 of the German Protection against Infection Act (IfSG). The MRSA module collects information on MRSA detection among hospitalized patients for the entire hospital; rehabilitation hospitals are also eligible to participate in MRSA surveillance. Data collection and transmission to the National Reference Center (NRZ, Nationales Referenzzentrum) for Surveillance of Nosocomial Infections is performed via the online platform “webKess“ (9, 10).
The definitions used for data collection are as follows (8, 9): A patient in whom MRSA is detected in screening swabs (colonization) or isolated from clinical material (colonization or infection) is considered an MRSA case. Hospital cases, not patients with MRSA are counted, meaning that if a patient in whom MRSA was detected is readmitted after discharge from hospital, they are considered a new case. With regard to hospital cases overall (with and without detection of MRSA), we will use the term patients in the following for ease of understanding; however, the hospital admissions of the patients are counted (9). Each case of MRSA is only once considered for the hospital, meaning that if the patient is transferred to another department of the same hospital, the case is not counted again.
The number of MRSA hospital days is calculated from the day of diagnosis or detection of MRSA (date of swab or specimen collection) or from the day of admission, if MRSA had already been detected before hospital admission (9):
until the day of discharge of the MRSA case (the day of discharge is counted)
until the patient has no detectable MRSA colonization – as determined by the participating hospitals—for example, after collection of three MRSA-negative screening specimens on three consecutive days.
Participating hospitals are asked to submit once a year the following data on MRSA cases as well as hospital structural and process parameters:
Number of patients admitted to the hospital per calendar years (number of cases)
Patient days for a calendar year
Rehabilitation facility available (yes/no)
Number of nasal swabs (adjusted for patients yes/no, i.e. only one swab is counted per patient; a nasal swab is considered a screening swab)
MRSA cases present on admission (MRSA known at the time of admission or MRSA isolated from screening swab or material collected within the first two days after admission)
Nosocomial MRSA cases (MRSA isolated from swab or material collected from day three, i.e. later than day two, after admission)
MRSA hospital days (assessment as defined above)
Based on the data submitted to the National Reference Center for the Surveillance of Nosocomial Infections (NRZ), the following measures are calculated:
Overall prevalence (overall number of MRSA cases per 100 patients)
Admission prevalence (number of MRSA cases present on admission per 100 patients)
Incidence of nosocomial MRSA cases (number of nosocomial MRSA cases per 100 patients)
Incidence density of nosocomial MRSA cases (number of nosocomial MRSA cases per 1000 patient days)
Mean MRSA disease burden in the hospital (number of MRSA patient days per 100 patient days)
Screening frequency (number of nasal swabs per 100 patient admissions; only one swab test during the hospital stay counts per patient)
These data are then analyzed at the German National Reference Center (NRZ); subsequently, the participating hospitals are provided with their own figures as well as the reference data.
For the period 2006–2021, we report the screening frequency in addition to these measures of MRSA epidemiology. For our data evaluation, we divided the screening frequency into four groups (categories/quartiles) of equal size, based on the frequency of nasal swabs performed. By analyzing overall MRSA prevalence and MRSA incidence density in the various categories, we were able to assess possible associations between epidemiological measures and screening frequency. Differences between categories were tested for significance using the Kruskal-Wallis test. For both overall MRSA prevalence and nosocomial MRSA incidence density, separate regression models were run to describe the temporal trend. Data analysis was performed using the open-source R statistical software (11).
We extrapolated the results of the MRSA surveillance data to all hospitals in Germany to estimate the overall burden of MRSA case in 2019 (the last calendar year before the start of the SARS-coronavirus 2 pandemic). German national hospital statistics data were used for this calculation (12)
Results
Since participation in KISS is voluntary, the number of reporting hospitals has fluctuated from year to year. Following the start of the MRSA module in 2006, 110 hospitals participated in this component. Since then, the number of participating hospitals has increased significantly, reaching a peak of 623 hospitals in 2018 which is about one third of all German hospitals. In 2021, 525 hospitals participated in the MRSA module.
Table 1 shows the development of MRSA epidemiology in German hospitals during the 16-year period from 2006 to 2021. In the pre-pandemic year 2019, the number of patient days included in the MRSA module corresponded to 41% of all patient days in German hospitals (the total number of patient days in 2019 was 139 million). On the assumption of an equal distribution of MRSA patient days in hospitals, the total number of MRSA patient days in Germany in 2019 was approximately 1.7 million, accounting for 1.2% of all annual patient days in German hospitals.
In 2012, overall MRSA prevalence peaked with 1.04 cases per 100 patients, remaining for several years on this level before starting to continuously and significantly decrease in 2017 to 0.72 (-32%) and 0.54 (-50%) in 2019 and 2021, respectively (p<0.001). Admission prevalence and mean daily MRSA disease burden also followed this trend. The incidence of nosocomial MRSA cases declined steadily from 0.26 per 100 patients in 2006 to 0.04 per 100 patients in 2021. Nosocomial incidence density decreased by an average of 12% per year and was 0.06/1000 patient days in 2021 (table 1). Figure 1 provides an overview of the development of MRSA epidemiology in hospitals and the frequency of screening (nasal swabs) for the period from 2006 to 2021.
Table 1
| Year | Number (n) of hospitals | Patient hospital days, total (n) | MRSA patient days (n) | MRSA cases (n) | Nosocomial MRSA cases (n) | MRSA cases present on admission (n) | Patients, total (n) | Prevalence (MRSA cases/ 100 pts) | Admission prevalence (MRSA cases poa/100 pts) | Nosocomial incidence (noso. MRSA cases/100 pts) | Nosocomial incidence density (noso. MRSA cases/1000 patient days [pds]) | Mean MRSA disease burden (MRSA pds/total pds*100 | Screening frequency (nasal swabs/100 pts) |
| 2006 | 110 | 14 717 772 | 202  917 917 | 13 171 | 3 978 978 | 9 193 193 | 1 516 516 083 083 | 0.87 | 0.61 | 0.26 | 0.27 | 1.38 | 5.78 |
| 2007 | 148 | 18 161 161 181 181 | 299  968 968 | 19 267 | 5 068 | 14 068 068 | 2 197 197 826 826 | 0.88 | 0.64 | 0.23 | 0.28 | 1.65 | 6.34 |
| 2008 | 191 | 23 724809 | 416  702 702 | 25 495 495 | 5 801 801 | 19  694 694 | 3 181 181 412 412 | 0.80 | 0.62 | 0.18 | 0.24 | 1.76 | 8.17 |
| 2009 | 230 | 26 011 907 | 488  306 306 | 32 491 | 6 172 | 26  319 319 | 3 399 399 475 475 | 0.96 | 0.77 | 0.18 | 0.24 | 1.88 | 10.70 |
| 2010 | 303 | 32 230 230 693 693 | 633  078 078 | 44 411 411 | 7 151 | 37  260 260 | 4 280 280 846 846 | 1.04 | 0.87 | 0.17 | 0.22 | 1.96 | 14.60 |
| 2011 | 350 | 35 790 790 741 741 | 685 457 457 | 50 875 875 | 7 072 | 43 803 803 | 5 218 218 411 411 | 0.97 | 0.84 | 0.14 | 0.20 | 1.92 | 15.45 |
| 2012 | 400 | 40 917154 | 795  054 054 | 59 992 | 7 617 617 | 52 375 375 | 5 750 750 417 417 | 1.04 | 0.91 | 0.13 | 0.19 | 1.94 | 21.77 |
| 2013 | 459 | 46 048 614 | 873  582 582 | 67 641 641 | 7 416 | 60 225 225 | 6 622 622 224 224 | 1.02 | 0.91 | 0.11 | 0.16 | 1.90 | 25.73 |
| 2014 | 492 | 48 981 503 | 882  793 793 | 70 891 | 7 021 021 | 63 870 870 | 7 189 189 343 343 | 0.99 | 0.89 | 0.10 | 0.14 | 1.80 | 30.00 |
| 2015 | 535 | 52 169 289 | 901  926 926 | 77 479 | 6 734 | 70 745 745 | 8 031 031 532 532 | 0.96 | 0.88 | 0.08 | 0.13 | 1.73 | 33.45 |
| 2016 | 572 | 57 041 683 | 954  744 744 | 81 986 | 6  408 408 | 75 578 578 | 8 571 571 016 016 | 0.96 | 0.88 | 0.07 | 0.11 | 1.67 | 38.76 |
| 2017 | 591 | 58 314 314 151 151 | 886  016 016 | 79 869 | 5 909 909 | 73 960 960 | 8 884 884 668 668 | 0.90 | 0.83 | 0.07 | 0.10 | 1.52 | 41.14 |
| 2018 | 623 | 58 780 627 | 827  669 669 | 76 746 746 | 5 303 303 | 71 443 443 | 9 121 121 328 328 | 0.84 | 0.78 | 0.06 | 0.09 | 1.41 | 43.05 |
| 2019 | 610 | 57 413 703 703 | 683 536 536 | 64 832 | 4 221 221 | 60 611 611 | 8 964 964 243 243 | 0.72 | 0.68 | 0.05 | 0.07 | 1.19 | 43.46 |
| 2020 | 583 | 48 397 647 | 496  998 998 | 48 216 | 3  017 017 | 45 199 199 | 7 593 593 173 173 | 0.63 | 0.60 | 0.04 | 0.06 | 1.03 | 43.46 |
| 2021 | 525 | 44 882 632 | 386 778 778 | 37 715 | 2  503 503 | 35 212 212 | 6 967 967 104 104 | 0.54 | 0.51 | 0.04 | 0.06 | 0.86 | 42.52 |
n, number; pts, patients; poa, present on admission; noso., nosocomial; pds, patient days
For the further analyses, the frequency of nasal swabs per 100 hospital admissions was used as a marker for screening frequency and divided into four equally sized categories (quartiles 1–4). Since the screening frequencies varied from year to year, the categories differed between years/time periods. From 2006–2018, a steady increase in screening frequencies was observed; in 2013 a nasal swab was obtained from at least one quarter of patients for the first time; from 2019, screening frequencies have plateaued on a high level (figure 1). The analyses included the year 2021 and the period 2013–2021. The categories used are presented in Table 2.
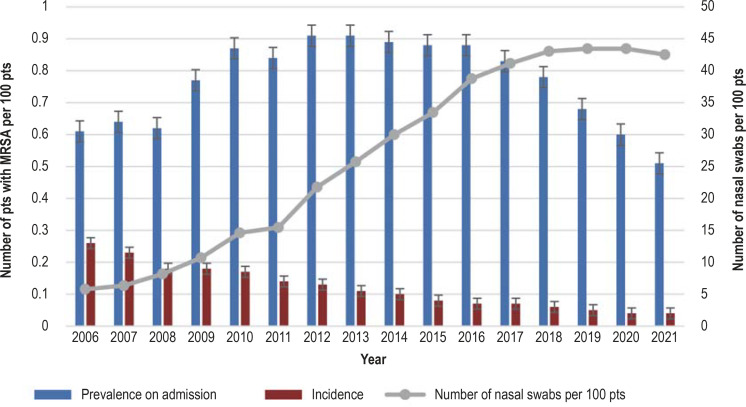
Annual MRSA prevalence on admission; nosocomial MRSA incidence and MRSA screening frequency (nasal swabs) in German hospitals in the period 2006–2021 (MRSA-KISS module)
MRSA, methicillin-resistant Staphylococcus aureus; pts, patients
In 2021, hospitals with high screening frequencies had a significantly higher MRSA admission prevalence compared to hospitals with a low screening frequency (Kruskal-Wallis test p<0.0001). However, the same observation was not made for nosocomial incidence density, as it remained stable across all screening categories (p = 0.8402) (table 3). When comparing these results with data from the 2013–2021 period, it can be seen that nosocomial incidence density was stable across all screening categories, consistent with the findings for the year 2021 alone (table 3).
Table 3
| Screening category (nasal swabs per 100 pts.) | Number of hospitals | Screening frequency (nasal swabs per 100 pts) | Admission prevalence (MRSA cases poa/100 pts) | Nosocomial incidence density (nosocomial MRSA cases/1000 patient days) |
| 2021 | ||||
| ≤ 24 |   119 119 | 14.33 (9.87–20.32) | 0.28 (0.19–0.41) | 0.04 (0.01–0.07) |
| 24 to ≤ 40 |   102 102 | 32.36 (27.98–37.59) | 0.38 (0.27–0.53) | 0.04 (0.02–0.07) |
| 40 to ≤ 64 |   112 112 | 48.89 (44.31–54.16) | 0.53 (0.38–0.76) | 0.04 (0.02–0.08) |
| > 64 |   115 115 | 85.67 (73.67–97.80) | 0.61 (0.45–0.89) | 0.04 (0.01–0.06) |
| Not stated |    77 77 | Not stated | 0.40 (0.26–0.72) | 0.03 (0.00–0.07) |
| All hospitals in 2021 |   525 525 | 40.36 (23.49–64.39) | 0.43 (0.29–0.66) | 0.04 (0.01–0.07) |
| 2013–2021 | ||||
| ≤ 19 | 1 062 062 | 9.88 (4.92–14.35) | 0.43 (0.27–0.71) | 0.06 (0.02–0.11) |
| 19 to ≤ 37 | 1 081 081 | 28.23 (23.31–32.48) | 0.60 (0.41–0.89) | 0.06 (0.03–0.11) |
| 37 to ≤ 58 | 1 051 051 | 46.00 (41.26–51.34) | 0.77 (0.53–1.14) | 0.06 (0.03–0.12) |
| > 58 | 1 071 071 | 81.70 (67.25–96.70) | 0.93 (0.68–1.38) | 0.07 (0.03–0.13) |
| Not stated |   725 725 | Not stated | 0.66 (0.40–1.03) | 0.07 (0.02–0.13) |
| All hospitals in 2013–2021 | 4 990 990 | 36.76 (19.03–58.18) | 0.68 (0.43–1.04) | 0.06 (0.03–0.12) |
The median and the interquartile range (lower and upper quartile) are shown. MRSA-KISS module, Germany; pts, patients
Discussion
A continuous decrease in nosocomial MRSA incidence is noted for the entire observation period and, since 2017, also a reduction in the overall and admission prevalence of MRSA (table 1). Therefore, the epidemiological trend observed in other surveillance systems for Germany and Europe can also be applied to the MRSA incidence in German hospitals (5, 6, 13). Similar to Germany, a statistically significant reduction in the incidence of invasive MRSA infections has been shown in the United States. From 2005–2013, the nosocomial incidence of bloodstream infections caused by MRSA strain USA 100 decreased from 6.1 to 0.9/100 000 population and for strain USA 300 from 1.5 to 0.6/100 000 population (14). Globally, MRSA rates in S. aureus isolates from blood or cerebrospinal fluid vary widely; in the period 2000–2016, for example, it ranged between 0% and 98% in the Asia-Pacific region (15). Figure 2 provides an overview of 2019–2020 MRSA rates for selected countries (6, 16, 17). Multiple pathogen-specific and host-specific factors as well as the heterogeneity of health care and surveillance systems worldwide have been identified as causes of variation in MRSA rates.
Significantly fewer patients were treated in German hospitals in 2022 and 2021 compared to pre-pandemic years (12). The MRSA-KISS data also show that 8% fewer patients received care in 2021 compared to 2019 (last pre-pandemic calendar year) (table 1). Elective hospital admissions, in particular, were deferred due to the SARS-CoV-2 pandemic. By contrast, patients with underlying chronic diseases who, for example, required dialysis or devices which are permanently left in place (such as an indwelling urinary catheter), continued to be admitted. Patients receiving dialysis on a regular basis or with indwelling urinary catheter or open wounds are at a higher risk of MRSA colonization (18). Nevertheless, admission prevalence continued to decline during the pandemic years. A meta-analysis concerned with the incidence of antimicrobial resistance in hospitals worldwide showed that the COVID-19 pandemic had no impact on the incidence density of MRSA (19); in the United States, the overall case rates for MRSA bloodstream infections remained stable between 2018 and 2020 (20). These observations made internationally during the years of the pandemic are reflected in the MRSA-KISS data.
Various causes have been attributed to the generally noted decline in MRSA. Apart from a potentially reduced biological fitness of drug-resistant S. aureus strains compared to drug-sensitive S. aureus, as well as a change in how antibiotics are used (21– 23), the decline in MRSA could be due to various bundles of infection prevention and control (IPC) measures. In 1999 and 2014, the Commission for Hospital Hygiene and Infection Prevention (KRINKO, Kommission für Krankenhaushygiene und Infektionsprävention) published recommendations for MRSA-related IPC measures to be taken in hospitals and care facilities (18, 24). These recommendations include enhancing basic hygiene and hand disinfection compliance. In addition, KRINKO supports targeted admission screening for patients at risk of MRSA and recommends the preparation of an infection control plan for each hospital, taking into account the local situation and country-specific recommendations for MRSA admission screening (18).
Notwithstanding this recommendation and a steady decline in MRSA incidence, screening frequency has increased in German hospitals over time since 2006 to remain at a high level since 2019 (figure 1). One reason for this could be that targeted screening of patients with an increased risk of MRSA—for example, re-admissions with known history of MRSA or admissions or transfers from facilities with known endemic or suspected MRSA occurrence (18, 24)—is considered to be more time-consuming to implement than screening entire departments or the entire hospital. What is more, MRSA detected during hospitalization is classified as nosocomially acquired in patients who have not undergone admission screening. To address this issue, admission screening has been expanded in some hospitals. Furthermore, hospitals could use a comprehensive MRSA screening strategy for promotional purposes, as the media keeps reporting on alleged successes of universal admission screening. In these reports, however, reference is often made to the Netherlands, despite the fact that only risk-based screening is performed there (25).
Finally, it appears that there is still a widespread perception that universal admission screening is an evidence-based IPC measure. However, this view is refuted or critically discussed in various studies (26– 30). Likewise, MRSA-KISS figures reported in recent years indicate that hospitals with low or moderate screening frequencies did not see an increase in the number of nosocomial MRSA cases detected. For example, for the year 2021, the admission prevalence for a hospital was found to be significantly higher if the hospital was in the 4th quartile with respect to the frequency of admission swabs instead of the first quartile. However, the incidence density of nosocomial MRSA was found to be stable at 0.04/1000 patient days, independent of the screening frequency (table 3). Thus, the question arises whether a high screening frequency is necessary at all. Rather, IPC measures appear to be increasingly implemented successfully to prevent nosocomially acquired MRSA cases. Risk-adapted targeted MRSA admission screening can help to conserve staff capacities and laboratory resources. Therefore, such a screening strategy appears to be useful, especially in view of the stable epidemiological situation.
Limitations
The following limitations apply to the surveillance survey within the German National Nosocomial Infections Surveillance System (KISS): First, the number of hospitals participating in the MRSA-KISS module varied over time, and this may have influenced MRSA rates. The number of participating hospitals was rather stable from 2017 to 2019, but decreased during the SARS-CoV-2 pandemic.
Second, statements can only be made for participating hospitals. It remains unclear whether these can be considered representative of all hospitals in Germany. However, the number of hospitals participating in MRSA KISS surveillance is by no means insignificant—even in the first pandemic year of 2020, one-third of all approximately 1,900 hospitals participated (12).
The MRSA-KISS findings reflect the general epidemiological trend of declining MRSA incidence rates. For this reason, a stable database can be assumed despite the limitations mentioned above (6).
Conclusion
A significant decrease in MRSA cases in German hospitals has been observed for the period 2006–2021. The incidence density of nosocomial MRSA is not associated with screening frequency. This means that the probability of nosocomial transmission of MRSA is not increased in hospitals with a low number of nasal swabs per 100 patients. Given the lack of a preventive effect and the resource consumption associated with a high screening frequency, a targeted screening strategy focused on patients at increased risk of MRSA should be preferred over universal MRSA admission screening.
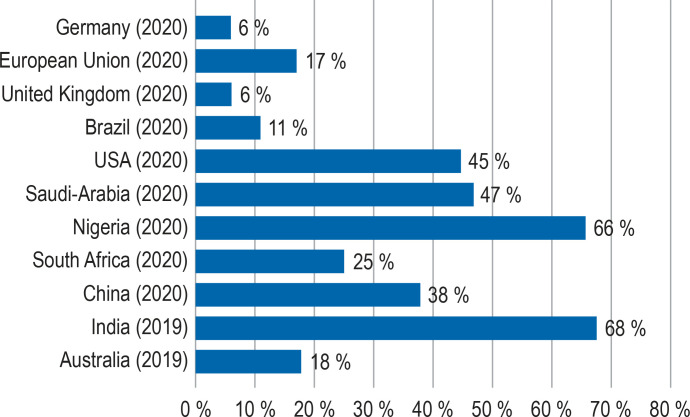
MRSA rate (in percent) in S. aureus isolates from clinical material (blood or CSF) in different countries/regions worldwide. The data are from the years 2019–2020 (6, 16, 17).
Table 2
| 2013–2021 | 2021 | |
| Quartile 1 | ≤ 19 | ≤ 24 |
| Quartile 2 | 19 to ≤ 37 | 24 to ≤ 40 |
| Quartile 3 | 37 to ≤ 58 | 40 to ≤ 64 |
| Quartile 4 | > 58 | > 64 |
Number of nasal swabs in the respective quartile, each per 100 patients admitted; MRSA-KISS module, Germany
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
Articles from Deutsches Ärzteblatt International are provided here courtesy of Deutscher Arzte-Verlag GmbH
Full text links
Read article at publisher's site: https://doi.org/10.3238/arztebl.m2023.0117
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10481939
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/148536108
Article citations
The genetic relationship between human and pet isolates: a core genome multilocus sequence analysis of multidrug-resistant bacteria.
Antimicrob Resist Infect Control, 13(1):107, 20 Sep 2024
Cited by: 1 article | PMID: 39304920 | PMCID: PMC11416027
In Reply.
Dtsch Arztebl Int, 120(44):756, 01 Nov 2023
Cited by: 0 articles | PMID: 38014442 | PMCID: PMC10722491
A Preventive "Number Needed to Screen" Would Have Been More Relevant.
Dtsch Arztebl Int, 120(44):756, 01 Nov 2023
Cited by: 0 articles | PMID: 38014441 | PMCID: PMC10722493
Risk-Adapted Screening for Methicillin-Resistant Staphylococcus aureus.
Dtsch Arztebl Int, 120(26):445-446, 01 Jun 2023
Cited by: 0 articles | PMID: 37594462 | PMCID: PMC10481943
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antibiotics: MRSA prevention measures in German hospitals: results of a survey among hospitals, performed as part of the MRSA-KISS module.
Dtsch Arztebl Int, 107(37):631-637, 17 Sep 2010
Cited by: 5 articles | PMID: 20959890 | PMCID: PMC2956199
Development of a surveillance system for methicillin-resistant Staphylococcus aureus in German hospitals.
Infect Control Hosp Epidemiol, 28(4):446-452, 15 Mar 2007
Cited by: 21 articles | PMID: 17385151
Screening and control of methicillin-resistant Staphylococcus aureus in 186 intensive care units: different situations and individual solutions.
Crit Care, 15(6):R285, 25 Nov 2011
Cited by: 3 articles | PMID: 22118016 | PMCID: PMC3388634
Methicillin-resistant Staphylococcus aureus: source control and surveillance organization.
Clin Microbiol Infect, 15 Suppl 7:31-38, 01 Dec 2009
Cited by: 19 articles | PMID: 19951332
Review




