Abstract
Free full text

Implications of tree expansion in shrubland ecosystems for two generalist avian predators
Abstract
Shrublands globally have undergone structural changes due to plant invasions, including the expansion of native trees. Removal of native conifer trees, especially juniper (Juniperus spp.), is occurring across the Great Basin of the western U.S. to support declining sagebrush (Artemisia spp.) habitats and associated wildlife species, such as greater sage-grouse (Centrocercus urophasianus). One justification for conifer removal is that it may improve survival of sagebrush-associated wildlife by reducing the abundance of avian predators. However, the relationship between conifer expansion and predator distributions has not been explicitly evaluated. Further, although structural characteristics of habitat are important for generalist predators, overall prey abundance may also affect habitat use by predators. We examined habitat use of common ravens (Corvus corax) and red-tailed hawks (Buteo jamaicensis), two generalist predators whose populations are increasing in western North America, to variation in structural characteristics and prey distributions in sagebrush habitat that has experienced conifer expansion. Structural characteristics of habitat were important predictors of habitat use for both ravens and red-tailed hawks, whereas measures of prey abundance were unimportant for both species likely because generalist predators can use a wide variety of food resources. Ravens, but not red-tailed hawks, responded positively to increasing cover of juniper and the probability of habitat use was highest (> 0.95) where juniper cover within 100 m was > 20%. Habitat use by red-tailed hawks, but not ravens, was greater near cliffs but was not associated with juniper cover. Our study suggests that the removal of conifer in similar environments may lower the probability of habitat use for ravens, a common predator with significant impacts on many prey species. Therefore, we suggest conifer removal may improve sage-grouse reproductive success and survival depending on responses to conifer removal from other predators. Our results may be reflective of similar changes in rangeland ecosystems around the world undergoing expansion of conifer and other woody vegetation. Though species identities differ from sagebrush habitats, generalist avian predators in other habitats may have similar relationships with structural resources.
Introduction
Changing habitat structure from expansion of native plants or invasion by non-native plants is a global phenomenon that has implications for fauna. Many arid, semi-arid, and Mediterranean-type ecosystems are affected by the recent expansion of woody plants attributed to land use (especially livestock grazing), fire suppression, and climate change [1, 2]. Habitat changes have significantly affected distributions of wildlife species around the world, and generalist predators in particular have benefited from novel resources in altered habitats [3, 4]. As predator/prey interactions are altered, direct and indirect effects of habitat on prey demography can interact to compound the effects of habitat change on wildlife populations [5]. For example, expansion or invasion of plants can directly reduce resources such as food or shelter for a species while indirectly increasing predation risk by subsidizing predator populations [6–9]. Given the impacts that altered vegetation structure can have on wildlife populations, understanding the effects of these alterations on the predator community is vital for conserving imperiled prey populations.
Habitat structure is an important feature that can influence habitat use for taxa such as avian predators [10–13]. For these species, structural resources such as trees, cliffs, or utility poles may provide nesting substrates or protection from predation [14, 15]. Structural resources may also facilitate hunting strategies, either as concealment for ambush hunters or as perches for visual hunters [16–18]. For example, tree planting in Israel has led to population declines for an endangered lizard, in part because avian predators spend more time hunting in areas with vertical perches [19, 20]. In the Mediterranean, expansion of conifer in shrublands has been shown to lead to reductions in songbird nest success due to the increased presence of a generalist corvid species [21]. For avian predators that may utilize a wide variety of prey items, structural resources may be a primary factor influencing habitat use [22].
Although prey abundance is an integral part of habitat selection theory, it has rarely been incorporated into studies of habitat use by avian predators (but see [11]). As a proxy for prey abundance, studies often evaluate the effect of physical landscape features under the assumption that these structures influence the distribution of prey as well as hunting efficiency for avian predators (e.g., [15, 23]). However, studies that have directly tested the effect of prey abundance on habitat use by avian predatorshave found either that an interaction between habitat structure and prey abundance affect habitat use [24–26], or that habitat use is influenced by habitat structure alone (e.g., [11, 27]). Understanding the relative effects of both structural and prey resources on habitat use by avian predators may improve our understanding of predator response to landscape change in ecosystems experiencing the broad-scale expansion of woody plants.
The sagebrush steppe ecosystem in the western United States has received much recent conservation focus due to concern over the status of the greater sage-grouse (Centrocercus urophasianus, hereafter “sage-grouse”) and other species associated with sagebrush habitat. Sage-grouse populations are broadly distributed but have declined range-wide [28, 29]. Since Euro-American settlement of the western United States, portions of the sagebrush (Artemisia spp.) biome have experienced an expansion of conifer trees, mostly pinyon pine (Pinus spp.) and juniper (Juniperus spp.), particularly in the Great Basin [30, 31]. Conifer expansion is a regional threat to sage-grouse because population declines have been attributed in part to the expansion of conifers into sagebrush habitat that was previously treeless [32]. Anecdotal evidence suggests that conifer expansion may affect predator-prey dynamics because survival rates for sage-grouse that use habitat featuring conifers are lower than those for sage-grouse in areas with no conifers [33]. However, few studies have directly tested the effect of conifer expansion on habitat use by avian predators, or predators in general (but see [34, 35]).
In recent years, increased abundance in sagebrush habitat of common ravens (Corvus corax) and red-tailed hawk (Buteo jamaicensis), two highly generalist avian predators (hereafter “avian predators”) of sage-grouse and other sagebrush-associated wildlife, have been attributed to human development [23, 36]. Ravens are a common predator of sage-grouse nests, and the removal of ravens has increased recruitment of sage-grouse [37, 38]. To our knowledge, no study has explicitly focused on the relationship between conifer expansion and associated habitat use by avian predators of sagebrush-associated species.
Given the negative effects of conifer expansion on sage-grouse populations, multiple conifer removal projects have been initiated in sagebrush habitats across the Great Basin [39]. One such project, the Bruneau-Owyhee Sage Habitat (BOSH) project, will remove juniper across ~250,000 ha [40]. Policies that have been recently enacted by the federal government will facilitate additional conifer removal in the Great Basin by minimizing environmental review requirements for projects [41]. However, critical information gaps exist concerning wildlife responses to conifer removal in sagebrush habitat, especially responses of predators. If structural resources are the primary influence of habitat use for avian predators, then conifer removal may reduce the presence of avian predators and associated predation risk for prey. Conversely, if prey distributions are the primary influence of habitat use for avian predators, then potential increases in prey abundance following conifer removal may increase habitat use by avian predators in some sagebrush habitats. For example, the overall density of small mammals is lower in conifer woodlands than sagebrush habitat [42, 43]. If avian predators are insensitive to changes in habitat structure, costly efforts to remove conifer may be ineffective. Information about the primary factors that influence habitat use for avian predators in landscapes that have experienced conifer expansion may therefore help guide habitat restoration efforts for sagebrush-dependent wildlife.
To inform future restoration efforts, we tested the effects of habitat structure and prey distributions on habitat use for avian predators within a conifer removal area. Our goal for this study was to provide a first step towards explicitly evaluating the relationship between conifer expansion and removal and avian predators. We had two main objectives: 1) evaluate the relationship between habitat structures and habitat use by avian predators in sagebrush-juniper habitat, and 2) test for an effect of abundance of prey resources on habitat use by avian predators. We tested three hypotheses: 1.) structural resources are the primary influence on habitat use for avian predators, 2.) prey distributions are the primary influence on habitat use for avian predators, and 3.) there are additive or interactive effects between structural resources and prey distribution on habitat use by avian predators. We predicted that areas with greater conifer cover would exhibit an increased probability of habitat use by avian predators, but that prey distributions would further influence predator habitat use. Consequently, we also expected conifer removal to decrease habitat use by avian predators in the year following restoration.
Methods
Study site
Removal of juniper occurred in southwest Idaho, USA in the northern Great Basin (Fig 1) and began in August 2019. The study area is composed primarily of big sagebrush (Artemisia tridentata) and low sagebrush (Artemisia arbuscula) interspersed with one conifer species, western juniper (Juniperus occidentalis; hereafter, juniper). In areas classified as < 10% conifer cover at our study site, juniper has an average height of 2.7 m ± SD 2.1 and an average stem density of 19 trees/ha ± SD 25. In areas classified as >20% conifer cover at our study site, junipers have an average height of 3.6 m ± SD 2.3 and an average stem density of 198 trees/ha ± SD 193, but individual trees could reach heights of 12 m, and areal coverage of juniper can approach 60% [44]. Cheatgrass (Bromus tectorum) has invaded much of the study area, especially at lower elevations.
Location of avian predator surveys in the Owyhee Mountains of southwest Idaho, 2017–2020.
The climate of the study area is typified by hot summers and cold, snowy winters with an average of ~ 35 cm of precipitation annually [40]. Elevation ranges from 1,250 − 1,920 m and topography is varied with low-lying riparian areas interspersed with open sagebrush tablelands and rocky ridgelines. Before European settlement, juniper was most likely limited to rocky outcrops in small portions of the study area, presumably by historical fire regimes [45]. Since European settlement, juniper has expanded into sagebrush communities forming a gradient of tree cover across the landscape, and areas of dense juniper are currently found along ridgetops and in drainages [31]. Lesser, more scattered juniper cover typifies open sagebrush flats. Cattle grazing is ubiquitous across the study site, occurring at lower elevations during April-May and moving to higher elevations as summer progresses.
The BOSH project will eventually remove juniper cover classified as < 20% (at a 2-ha scale) from habitat formerly dominated by sagebrush across a ~250,000-ha landscape to support sage-grouse and other sagebrush-obligate species. Our sampling encompassed ~30,000 ha, and in the fall of 2019 15,000 ha of juniper was hand-cut within our study site. Juniper was cut using chainsaws and scattered so that no debris was higher than one meter.
Raven and raptor counts
We conducted repeated-visit surveys for three years before and one year after juniper removal began. We surveyed 800-m transects (n = 37) to assess the effects of habitat structure on the probability of habitat use by avian predators within our study site. We surveyed each transect three times per year with at least two weeks between visits. We conducted surveys between May 1st and July 15th each year. We selected locations for survey transects using random points in a GIS stratified by category of juniper cover (1–10%, 10–20%, and >20%) and location with respect to treatment plans for juniper removal (Fig 1). Eighteen of 37 transects were within areas where juniper would be removed. Surveys consisted of walking transects with three stationary, 10-minute observation periods placed at the beginning, middle, and end of each transect [23, 46]. Observers recorded any avian predators seen or heard while walking between or while at stationary observation points. We recorded birds if they were perched, calling, or flying/circling within the transect sample area. Each survey lasted ~ 45 minutes in total. Along each survey transect, the amount of time spent at stationary survey locations was consistent, but walking surveys between stationary survey locations varied based on terrain. We limited our data to observations of birds within 500 m of the observer to allow for more precise estimates of the effect of habitat on the probability of use and because this allowed us to assume that birds were influenced by the habitat surrounding the survey transect. We used laser range finders to estimate distances for birds detected visually and by sound. A single observer conducted each survey and three observers conducted 98% of surveys over four years. We conducted surveys before 10 am local time and never during steady rain or when estimated wind speeds exceeded 10 kph.
Prey abundance
To test the effect of prey abundance on habitat use by avian predators, we estimated abundance, density, or presence for important prey groups. We considered the following measures for groups that are known to be common prey of both ravens and red-tailed hawks: densities of the most common species of small mammals including deer mouse (Peromyscus maniculatus) and Great Basin pocket mouse (Perognathus parvus); the relative abundance of songbirds; and ground squirrel presence/absence. Belding’s ground squirrels (Urocitellus beldingi), which occur in large semi-colonial populations, are the most common ground squirrel at our study site.
To estimate the density of small mammals, we deployed 740 traps at five of the avian-predator transects in 2017 and 896 traps at the same five avian-predator transects in 2018 and 2019. We selected trapping locations for small mammals that represented a gradient of juniper cover, cheatgrass cover, and shrub structure (see [44]). In 2017, we used one trap array that consisted of 148 traps at the center of the avian predator transect. In 2018 and 2019, we used three trap arrays of 64 traps each at the center and ends of avian predator transects. We trapped small mammals over nine days broken up into four- and five-day sessions one month apart.
To estimate songbird abundance, we conducted 1,269 point-count surveys over four years along our avian-predator transects. Each survey transect for avian predators had three point counts stations placed at either end and in the middle of the survey transect. We surveyed songbirds concurrent with avian-predator surveys. We conducted 10-minute point counts three times per year at each avian-predator transect. We limited songbird observations to within 100 m of the point count station. We conducted songbird surveys from sunrise to 10 am and never during steady rain or winds stronger than 10 kph.
We noted the presence or absence of ground squirrels within 100 m of a survey transect because ground squirrels are an important food resource for red-tailed hawks. Ground squirrel presence is therefore treated as a categorical predictor variable.
Prey measures
We used measures of individual prey groups as predictor variables to assess the importance of prey for habitat use by avian predators. We estimated the density of small mammals in response to habitat characteristics at transect locations using a spatially explicit capture-recapture design (see [44]). We predicted small mammal density using the top-ranked models for both deer mice and pocket mice for sites where we did not sample mammals. We used vegetation measurements taken at each raptor-raven survey transect to predict small mammal density. The top model used to predict deer mouse density included a quadratic term for juniper cover within 100 m of the survey transect. The top model used to predict pocket mouse density included a quadratic term for mean sagebrush cover. We recorded the presence of ground squirrel colonies on survey transects using a categorical presence/absence approach. Ground squirrel presence on a survey transect was consistent across years, so each survey transect received a single classification for ground squirrel presence.
To calculate an index of songbird abundance, we pooled observations of all individuals and species and estimated the mean relative abundance of songbirds at the three points per transect. We did not adjust songbird observations for detection probability because differences in detectability among species would bias our abundance estimates, and previous studies examining the influence of songbird abundance on habitat use by avian predators have also used unadjusted relative mean counts [11].
Habitat characteristics
We focused our sampling and analysis on natural and topographic features that may influence habitat use by avian predators. We also included anthropogenic landscape features in our data collection (e.g., cabins, roads) because the effects of human subsidies such as artificial vertical structures, roads, and food are well-established as characteristics that influence the distribution of raptors and ravens. However, our study site was relatively free of human infrastructure compared to similar studies of habitat use by avian predators. (e.g., [15, 23]).
We classified juniper cover across our study site with imagery collected at a 1-m scale from the National Agriculture Imagery Program (NAIP). To identify juniper in the image, we conducted a supervised image classification in ArcGis [47]. Next, we manually corrected any misclassifications for each of our transects using visual inspection and ground truthing. We then calculated the area of juniper cover within 100, 250, 500, 750, 1000, 1500, 2000, 2500, and 3000 m of our survey transects. These distances span a range of reported movements during the breeding season for ravens (mean movement 570 m from nest, 6.6 km2 nest territory) and 40 km2 core use area for non-breeding individuals [15, 23, 48] and breeding red-tailed hawks (
Occupancy models
To assess the effect of habitat characteristics and juniper removal on habitat use by avian predators, we used Bayesian multi-season occupancy models [49]. Multi-season occupancy models [50, 51] are an extension of single-season occupancy models that allow for the estimation of changes in occurrence probability between seasons (in this case, years) through the estimation of parameters of extinction and colonization probability. Multi-season occupancy models assume that occupancy of a survey location is closed during each year but may change between years. If there is the possibility that the closure assumption is violated, as often happens for highly mobile species, the occupancy estimator may instead be considered the probability of habitat use [50]. We used the auto-logistic formulation of the multi-season occupancy model [49] to allow for inference on the effects of habitat covariates on overall occupancy probability as opposed to a decomposition of occupancy into colonization and extinction parameters. Use of the auto-logistic formulation is suitable for a limited sample size of unique sites and puts the inference focus on occupancy probability for each site for each year, as opposed to colonization and extinction probability [51].
To account for the imperfect detection of avian predators, we tested covariates that can affect the probability of detection using leave-one-out cross-validation [52]. We allowed detection to vary by year for all models and tested the effect of a terrain roughness index (TRI) and time of year on detection. We did not include an observer effect because a single observer (ACY) conducted 344 of 409 avian predator surveys (85%). Because we were interested in testing the effect of juniper cover on habitat use by avian predators, we did not include juniper cover as a covariate on detection in our occupancy models. We assumed that juniper cover would not lower our ability to detect large, loud species such as ravens. However, to validate this assumption we tested an exploratory model that included juniper cover within 100 m of the transect as a covariate on detection and with no covariates on habitat use. This exploratory model was not included in our set of candidate models. We standardized all predictor values and used normally distributed, non-informative priors for all covariate parameters (mean = 0, precision = 0.01).
Variable and model selection
Uncertainty about the spatial scale at which habitat features may influence ecosystem processes is common for ecological studies. As a result, researchers are often interested in testing the effect of a habitat covariate at several spatial scales. However, the inclusion of all habitat covariates at all potentially relevant scales can lead to models that are difficult to interpret [53]. We reduced the number of spatial scales for variables included in our models following the screening procedure recommended by Stevens and Conway [53]. We fit univariate multi-season occupancy models for each scale (100 m– 3,000 m) of juniper cover that we quantified. We also tested other juniper metrics at these scales, including clustering and proportion of landcover comprised of three cover categories (<10% cover, 10–20% cover, and >20% cover). We estimated clustering using a K-means nearest neighbor analysis in ArcGIS. We estimated proportion of juniper cover categories by categorizing 30 m2 pixels based on our juniper cover classification. We then compared the predictive power of each model in the set with leave-one-out cross-validation. Preliminary results from our model set testing the effect of different spatial scales of juniper measurement on habitat use suggested that ravens respond most strongly to the proportion of >20% juniper cover category habitat within 100 m of a transect. (β = 2.83, 90% Crl 0.41, 6.16; 99% posterior direction). However, because we were also interested in the relationship between all categories of juniper expansion and habitat use by ravens, we used continuous percent cover within 100 m of a survey transect, which was also a competitive model, as a covariate in our occupancy model set (β = 1.32, 90% Crl 0.13, 3.02; 99% posterior direction, Table A1 in S1 File).
Once we determined the spatial scale and measurement of juniper that best predicted habitat-use probability for ravens and red-tailed hawks, we constructed a model set that included combinations of habitat features in addition to juniper that may influence habitat use for avian predators (Table 1). We then ran each model for at least 300,000 iterations using JAGS called from R [54]. We assessed model convergence using
Table 1
| Detection | Habitat Structure Models | Prey Models |
|---|---|---|
| Null | Null | Structure model (sm) |
| Time of year | Juniper Cover | Small mammals |
| Terrain roughness index | Distance to cliff | Songbirds |
| Distance to water | Ground squirrels | |
| Distance to stream | Small mammals + sm | |
| Distance to road | Songbirds + sm | |
| Distance to human dwelling | Ground squirrels + sm | |
| Distance to cliff + juniper | Small mammals*sm | |
| Distance to cliff + distance to stream | Songbirds*sm | |
| Distance to road + juniper | All prey groups additive | |
| Distance to water + juniper | ||
| Distance to human dwelling + juniper | ||
| Small mammal density | ||
| Juniper Removal |
Permits and ethics statement
Field work for this study was carried out under Idaho Fish and Game research permit 161213 (T.N.J.). Sampling of small mammals was approved by the Institutional Animal Care and Use Committee of the University of Idaho (protocol #2017–14).
Results
Effect of structure on habitat use
We completed 409 avian predator surveys on the 29 transects sampled from 2017 to 2020 and eight additional transects sampled from 2018 to 2020. In 2017, we completed three surveys at 21 transects, two surveys at 10 transects, and one survey at two transects (n = 76 surveys). In 2018–2020, we completed three surveys at each of 37 transects (n = 111 surveys/year). Mean juniper cover within 100 m of all survey transects (n = 37) was 7.5% (8.1% SD) before juniper removal and 5.2% (8.4% SD) after removal, representing an average reduction in juniper cover of 30.6% per 10 ha. At transects where juniper was removed in fall 2019, average juniper cover within 100 m of treated transects decreased from 4% (4% SD) to 0.009% (0.02% SD), representing an average reduction in juniper cover of 99% per 10 ha (Table 2).
Table 2
| Pre-removal 2017 | Pre-removal 2018 | Pre-removal 2019 | Post-removal 2020 | |
|---|---|---|---|---|
| Transects sampled | 30 | 37 | 37 | 37 |
| Surveys completed | 76 | 111 | 111 | 111 |
| Ravens | ||||
| # Observations1 | 35 | 236 | 205 | 161 |
| % of surveys 2 | 32% | 64% | 57% | 43% |
| % of transects3 | 50% | 89% | 91% | 70% |
| Red-tailed hawks | ||||
| # detections | 17 | 36 | 22 | 20 |
| % of surveys | 18% | 21% | 24% | 16% |
| % of transects | 40% | 45% | 40% | 37% |
| Transect juniper cover category (100 m) | ||||
| 0% | 5 | 5 | 6 | 17 |
| 1–10% | 16 | 21 | 20 | 12 |
| 10–20% | 5 | 6 | 6 | 3 |
| > 20% | 4 | 5 | 5 | 5 |
1 Total observations per year. 2 Percentage of surveys where a species was detected. 3 Unadjusted percentage of transects where a species was detected.
We observed interannual variability in the number and spatial distribution of detections for both avian predator species (Table 2). Raven habitat use was positively influenced by percent cover of juniper and proximity to water (Fig 2A, Table A2 in S1 File). As juniper cover increased within 100 m of a transect, the probability of habitat use for ravens increased (β = 1.74, 90% Crl 0.32, 3.76; 99.5% posterior direction), and the highest probability of habitat use for ravens was areas with > 20% juniper cover. Credible intervals for the effect of distance to water on habitat use by ravens overlapped zero (β = -0.65, 90% Crl -1.40, 0.03), but most of the posterior distribution for distance to water indicated that the probability habitat use by ravens declined as distance to water increased (95% posterior direction). The average probability of habitat use for ravens declined by 55% in the year following juniper removal (2019 = 0.77, SE 0.05, 2020 = 0.34, SE 0.06, Table 3). Posterior predictive checks and visual inspections did not indicate a lack of fit or lack of convergence for the most predictive model (Freeman-Tukey p value = 0.35,
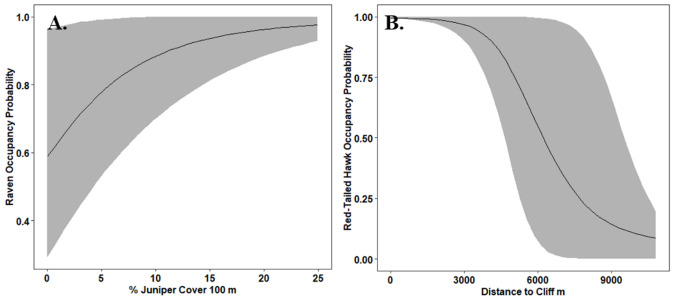
Probability of habitat use for A.) common ravens (Corvus corax) and B.) red-tailed hawks (Buteo jamaicensis) in southwest Idaho, 2017–2020.
Table 3
| Parameter | Pre-removal 2017 | Pre-removal 2018 | Pre-removal 2019 | Post-removal 2020 | ||||
|---|---|---|---|---|---|---|---|---|
| Est. | SE | Est. | SE | Est. | SE | Est. | SE | |
| 1 ψ | 0.82 | 0.05 | 0.69 | 0.07 | 0.77 | 0.06 | 0.34 | 0.06 |
| 2 p | 0.43 | 0.01 | 0.45 | 0.01 | 0.58 | 0.01 | 0.58 | 0.01 |
| 3 n.occ | 28.21 | 0.93 | 36.16 | 0.37 | 37.74 | 0.84 | 30.00 | 0.53 |
1 average probability of habitat use, 2 mean detection probability, 3 estimated total number of used transects
Probability of habitat use by red-tailed hawks was most strongly influenced by distance to a cliff, but differences in estimates of expected log predictive density (ELPD) suggest that this top model was only marginally more predictive than the null model. Probability of habitat use for red-tailed hawks declined as distance to cliff increased (β = -4.09, 90% Crl -7.57, -1.08; 97.3% posterior direction; Fig 2B, Table A4 in S1 File). Bayesian p-values suggest overdispersion in the models. We did not detect a statistically significant change in habitat use estimates for red-tailed hawks following juniper removal (Table 4). Red-tailed hawks responded most strongly to the clustering index of trees at a scale of 500 m, responding positively to more dispersed juniper. However, confidence intervals overlapped zero (β = 1.72, 90% Crl—4.82, 8.02; 77% posterior direction, Table A5 in S1 File). We did not identify important predictors for detection probability for red-tailed hawks (Table A6 in S1 File). Percent juniper cover did not have a strong effect on detection probability for red-tailed hawks (β = -0.15, 90% Crl -0.39, 0.09), which ranged from 0.18 − 0.24 over four years (90% Crl 0.10, 0.32).
Table 4
| Parameter | Pre-removal 2017 | Pre-removal 2018 | Pre-removal 2019 | Post-removal 2020 | ||||
|---|---|---|---|---|---|---|---|---|
| Est. | SE | Est. | SE | Est. | SE | Est | SE | |
| 1 ψ | 0.50 | 0.07 | 0.85 | 0.05 | 0.94 | 0.03 | 0.99 | 0.00 |
| 2 p | 0.18 | 0.01 | 0.24 | 0.01 | 0.23 | 0.01 | 0.16 | 0.00 |
| 3n.occ | 31.70 | 0.45 | 31.98 | 0.24 | 32.02 | 0.29 | 37.73 | 0.12 |
1 average occupancy probability, 2 mean detection probability, 3 estimated total number of occupied transects
Effect of prey on habitat use
Estimates of prey abundance alone did not influence habitat use for either ravens or red-tailed hawks. For ravens, a model that included the relative abundance of songbirds along with juniper cover ranked higher than the top-ranked model that included habitat structure only. However, as songbird abundance increased the probability of habitat use by ravens decreased (β = -0.80, 90% Crl -1.73, 0.12; Table A7 in S1 File) suggesting that ravens are less likely to use habitat with a higher abundance of songbirds. For red-tailed hawks, models including songbirds and small mammals as predictors of habitat use were ranked higher than distance to cliff alone (Table A8 in S1 File). However, increased songbird abundance was associated with a lower probability of habitat use for red-tailed hawks (β = -4.88, 90% Crl -9.17, -0.54), and credible intervals for the effect of small mammal density on habitat use by hawks widely crossed zero.
Discussion
Our results provide evidence that structures such as trees and cliffs have a stronger influence on habitat use by generalist avian predators than the abundance of some of their most common prey resources. Higher juniper cover and proximity to cliffs increased the probability of habitat use by ravens and red-tailed hawks, respectively, while we found no effects of individual prey resources on habitat use by either species. Further, we found no evidence of an interaction between habitat structure and prey resources on habitat use by avian predators. For generalist predators such as ravens and red-tailed hawks, which have a high degree of diet plasticity, our results shed light on how habitat structure can influence habitat use whereas the abundance of any individual prey type may not. For example, in areas near agricultural fields, grains can make up the majority of raven diets [61], while in more natural types of vegetation cover, small mammals and songbirds can constitute the majority of raven diets [62]. Near roads, carrion is often consumed by both red-tailed hawks and ravens [26, 63]. Red-tailed hawks are also adaptable, with diet composition varying by region and prey abundance [64], and passerine birds and medium and small mammals including deer mice can be an important part of red-tailed hawk diets. Therefore, habitat structures that increase the probability of habitat use by generalist predators may increase the risk of predation for a wide range of prey species.
Effect of structure on habitat use
The establishment of trees and other woody vegetation in rangeland habitats is occurring globally as a consequence of altered fire regimes, climate change, enhanced atmospheric CO2, and livestock grazing [65, 66]. Conifer trees have expanded into shrub-steppe habitats in southern Canada [67], acacia (Acacia saligna) and pines (Pinus spp.) have become established in the fynbos shrublands of South Africa [68, 69], and pines have invaded the high Andean paramos ecosystem [70]. The establishment of trees in these systems has had negative impacts on some native wildlife associated with vegetation that existed before expansion. As the expansion of woody plants continues in other rangeland ecosystems across the globe, understanding how expansion affects distributions of both predator and prey species and subsequent predator-prey dynamics will be an important part of conserving grassland and shrubland-dependent species. Where habitat structure can be modified through the removal of expanding woody species, it will be important to assess whether there are limitations to the effectiveness of removal as a restoration technique, or if additional efforts are required. For instance, tree removal can increase cover of understory vegetation such as bunchgrasses and sagebrush shrubs [71, 72]. However, variation in soil condition, hydrologic factors, and the pre-removal dominance of conifer can limit the response of understory plants to conifer removal [73]. Further, variation in plant responses to tree removal techniques (i.e., mastication, hand cutting, etc.) may lead to the establishment of invasive annual grasses and contribute to differences in the recovery of faunal communities [74].
Effects of generalist predators are a widespread conservation concern for sensitive prey populations, and efforts to control predator populations can be difficult and controversial [75]. The conversion of sagebrush habitat to conifer woodlands has reduced and fragmented habitat for sagebrush-obligate wildlife, and increased abundances of generalist predators can compound the negative effects of habitat loss on prey species [76]. For example, conifer expansion has been linked to population declines for greater sage-grouse, and increased predator populations are suspected to play a role in these trends [32, 33]. However, evidence for an association between juniper expansion and numerical or functional responses of avian predators has been limited or speculative (e.g., [15, 23, 77]). Our results show that ravens are more likely to use sagebrush habitat experiencing conifer expansion, a relationship that has implications for the conservation of sagebrush-associated wildlife.
Subsidized populations of generalist predators can significantly impact prey populations because predation may continue even after the prey density becomes very low [78]. There are likely fewer anthropogenic subsidies at our study site than in many areas of the western U.S. given the low human density of Owyhee County, but raven populations more broadly have benefited from human development, leading to increased abundance in the Great Basin [79]. Housing density is low at our site (0.02 houses/km2), and two lightly used dirt roads run through the study site (road density is 0.08 km/km2). There are no agricultural fields within 10 km (distance from a transect to agriculture ranged from 10–43 km) and no transmission lines within 21 km (distance from a transect to a transmission line ranged from 21–55 km) of the study site. As a result, the effects of natural structures on the probability of habitat use are less likely to be confounded by the positive effects of human subsidies and structures. However, further research is needed to elucidate the relative effects of conifer woodlands and anthropogenic resources on the probability of habitat use for ravens. The addition of human structures to a landscape that also contains conifer woodlands may have an additive effect on the probability of habitat use for ravens. Ravens are ‘incidental’ hunters, consuming a wide variety of prey including small mammals, songbirds, and lizards [80]. As the amount of habitat used by ravens increases, the likelihood that ravens will incidentally prey on sage-grouse nests may also increase [36, 81].
For highly mobile species such as avian predators, violations of the assumption that animals remain in a survey location throughout a season may require that researchers consider the effects of habitat on an animal’s presence and availability to be observed (i.e., “availability”; [50, 82]). Availability is defined as the probability that an animal is present at a survey location and able to be observed. Ravens are large, conspicuous birds that are easily seen and heard over long distances. Previous studies have sometimes assumed that observer error in detecting ravens is close to zero and estimated availability of ravens in relation to habitat, often referred to as use (e.g., [13]). For example, a study of ravens in the eastern United States estimated a 0.99 probability of detecting ravens over three visits to cliff locations if those locations were in fact occupied [83]. When the objective is to estimate the effects of habitat on an observer’s ability to detect an avian predator, researchers most often test for effects of habitat features that can obscure avian predators, including trees or rough terrain. For example, O’Neil et al. [23] found support for using a ‘viewshed index’ that factored tree cover and TRI into an observer’s ability to detect ravens. We found that juniper cover and TRI positively influenced detection of ravens, indicating that ravens are spending more time in habitat featuring juniper, regardless of whether or not ravens were being obscured from the observer by juniper or landscape features. At 0.43–0.58, our estimate of detection probability for ravens is higher than other studies (e.g., 35%, [23]). Given that we truncated our detections to 500 m, a smaller area than other studies (e.g., [23, 84]), we assume that our ability to see and hear ravens was high and that our estimate of detection probability represents availability to a large degree. Therefore, the fact that both the probability of habitat use and availability are positively influenced by juniper cover provides further support for a relationship between juniper cover and habitat use by ravens. For red-tailed hawks, a relatively low detection rate in our data is likely a result of low density and home range sizes larger than the area of our survey transects. Low detection rates can bias occupancy estimates if there is unmodeled, non-random movement in and out of sampling units often associated with the breeding season or migration [50]. However, we tested a model with time of year as a covariate for detection and this model was not supported. When the closure assumption is violated but individual movements in and out of sampling units is random, the occupancy estimator is likely unbiased. In this case, occupancy estimates can be interpreted as probability of use [50].
Effect of prey on habitat use
Similar to others, our study suggests that habitat structure is relatively more important than any specific prey resource for generalist avian predators [11, 27]. In fact, ravens were less likely to use habitat with higher abundances of songbirds and densities of small mammals. Densities of small mammals at our site, largely driven by deer mice, are highest at 10% juniper cover and decline as juniper cover increases [41]. Songbird abundance and diversity also increased in areas featuring early juniper expansion because the habitat can support sagebrush, ecotone, and conifer-associated songbirds [44, 67]. Longer-term data on prey-population distributions may reveal more complex predator-prey relationships that influence habitat use by generalist avian predators. It may also be that for generalist predators, no one prey population strongly influences habitat use. Data collected on prey populations at finer scales that account for within-season population variability and estimation errors associated with predictions for small mammal density and songbird abundance may improve inference. However, given the strong relationship we observed between habitat use for ravens and juniper woodlands, a disconnect between habitat use and prey abundance may be explained by the strong influence of vertical structure on habitat use (also reported in [23]).
Management implications
Conifer removal may benefit wildlife species associated with sagebrush habitat by reducing habitat use for ravens, a common generalist predator. The relationship documented in this study between increasing tree cover in a historically tree-limited habitat and changes in habitat use for a generalist predator species has corollaries in shrubland and grassland ecosystems globally. For instance, an increased abundance of generalist predators has been shown to increase predation risk for small prey, such as in Australia where increased abundances of ravens and crows in rangelands decreased the abundance of shrub and ground-nesting songbirds [85]. Moreover, direct and indirect effects of woody-plant expansion on understory vegetation and interactions between trophic levels, respectively, may combine to alter rangeland wildlife communities and contribute to population declines of specialist species. As an example, changes to the structure of the small-mammal community can affect seed dispersal, potentially affecting vegetation structure and composition [86].
In our study, habitat use by ravens was most strongly influenced by juniper cover >20%, which is considered conifer woodland. This relationship has implications for habitat restoration efforts that focus primarily on the removal of conifer cover that is < 20% (e.g., BOSH) while allowing conifer cover >20% stands to remain intact. The relationship between habitat use by ravens and juniper cover < 20% was highly variable and far less predictive than the effect of juniper woodlands on habitat use. Sage-grouse avoid using habitat where the abundance of avian predators is high [87], and our findings suggest that riparian habitat lined with juniper and near cliffs is likely to have the highest probability of use by avian predators at our site. Given that riparian habitat is important for juvenile sage-grouse during a vulnerable life stage, managing conifer expansion in and around riparian habitat may be particularly beneficial for sage-grouse populations. However, because we did not directly examine the impact of habitat-use by avian predators on sage-grouse demographics, future research on this topic will be an important next step for managers aiming to conserve populations of prey species.
Although areas with juniper cover < 20% are likely to have retained some shrub structure required by sagebrush wildlife, the association between ravens and juniper cover >20% suggests restoration efforts that do not remove dense juniper stands may not significantly alter the avian predator community. However, this is not to suggest removal of juniper cover < 20% may not have net benefits for sagebrush wildlife, as the mean probability of habitat use for ravens was lowest in the year following removal of juniper cover and would likely represent a net reduction in avian predator density for sagebrush wildlife. Moreover, logistical considerations are also important, as a primary objective of tree removal is to prevent the continued expansion of juniper [40]. Restoration of rangeland habitat dominated by late-stage conifer development may not be as successful as restoration of earlier successional stages [73], and removal of trees from shrublands can be costly and require long-term management through regular retreatment [88]. Finally, juniper woodlands are important for many wildlife species as well, and often feature high songbird diversity [39]. Though more research is needed on the implications of leaving juniper woodlands in sagebrush habitat, the benefits of removing juniper cover < 20% for sagebrush wildlife are important considerations for the conservation of sagebrush wildlife.
Given observed yearly variation in habitat use and abundance of avian predators, long-term monitoring, especially after tree removal, is required to better assess the effect of juniper removal on avian predators. An important next step will be to assess the effect of conifer removal on predation rates for prey species. However, to our knowledge, no long-term studies of habitat use by avian predators in a sagebrush-conifer woodland habitat exist. Therefore, our study provides early and valuable information on the overall effect of juniper cover on occupancy by avian predators.
Supporting information
S1 File
Model rankings for habitat use and detection.Model rankings for the the effects of habitat structure and prey covariates on habitat use and the effect of habitat characteristics on detection of common raven (Corvus corax) and red-tailed hawk (Buteo jamaicensis) in southwest Idaho, 2017–2020.
(DOCX)
Acknowledgments
Our thanks to S. Copeland and B. Schoberle for logistical support and to our many field technicians for their hard work. Thank you also to S. Copeland for comments on a previous version of this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding Statement
This research was supported by the Great Basin Landscape Conservation Cooperative and U.S. Fish and Wildlife Service, award # F16AC01182 (TJ), National Institute of Food and Agriculture, U.S. Department of Agriculture, McIntire Stennis project 1009779 (TJ), the Palouse Audubon Society (AY), and the University of Idaho College of Natural Resources Travel Award (AY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. https://lccnetwork.org/lcc/great-basin https://www.nifa.usda.gov/grants/programs/capacity-grants/mcintire-stennis-capacity-grant.
Data Availability
All relevant data are within the manuscript and its Supporting Information files. Data is also available at https://github.com/achristophery/Generalist-avian-predators-Young-et-al.-2023.
References
Decision Letter 0
9 Jan 2023
PONE-D-22-29377Structural resources and generalist avian predators: implications for tree expansion in shrubland ecosystemsPLOS ONE
Dear Dr. Young,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Both reviewers acknowledge that the manuscript addresses an interesting question, although they have reasonable concerns about some aspects of the manuscript. I kindly invite the authors to try to address the methodological queries raised by reviewer 1, and to try to clarify the focus of the introduction and title of the manuscript as suggested by reviewer 2.
Please submit your revised manuscript by Feb 23 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Juan Manuel Pérez-García, PhD
Academic Editor
PLOS ONE
Journal requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
2. In your Methods section, please provide additional information regarding the permits you obtained for the work. Please ensure you have included the full name of the authority that approved the field site access and, if no permits were required, a brief statement explaining why.
3. In your Data Availability statement, you have not specified where the minimal data set underlying the results described in your manuscript can be found. PLOS defines a study's minimal data set as the underlying data used to reach the conclusions drawn in the manuscript and any additional data required to replicate the reported study findings in their entirety. All PLOS journals require that the minimal data set be made fully available. For more information about our data policy, please see http://journals.plos.org/plosone/s/data-availability.
"Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories. Any potentially identifying patient information must be fully anonymized.
Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions. Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access.
We will update your Data Availability statement to reflect the information you provide in your cover letter.
4. We note that you have stated that you will provide repository information for your data at acceptance. Should your manuscript be accepted for publication, we will hold it until you provide the relevant accession numbers or DOIs necessary to access your data. If you wish to make changes to your Data Availability statement, please describe these changes in your cover letter and we will update your Data Availability statement to reflect the information you provide.
5. Please include your full ethics statement in the ‘Methods’ section of your manuscript file. In your statement, please include the full name of the IRB or ethics committee who approved or waived your study, as well as whether or not you obtained informed written or verbal consent. If consent was waived for your study, please include this information in your statement as well.
6. We note that Figure 1 in your submission contain [map/satellite] images which may be copyrighted. All PLOS content is published under the Creative Commons Attribution License (CC BY 4.0), which means that the manuscript, images, and Supporting Information files will be freely available online, and any third party is permitted to access, download, copy, distribute, and use these materials in any way, even commercially, with proper attribution. For these reasons, we cannot publish previously copyrighted maps or satellite images created using proprietary data, such as Google software (Google Maps, Street View, and Earth). For more information, see our copyright guidelines: http://journals.plos.org/plosone/s/licenses-and-copyright.
We require you to either (a) present written permission from the copyright holder to publish these figures specifically under the CC BY 4.0 license, or (b) remove the figures from your submission:
a. You may seek permission from the original copyright holder of Figure 1 to publish the content specifically under the CC BY 4.0 license.
We recommend that you contact the original copyright holder with the Content Permission Form (http://journals.plos.org/plosone/s/file?id=7c09/content-permission-form.pdf) and the following text:
“I request permission for the open-access journal PLOS ONE to publish XXX under the Creative Commons Attribution License (CCAL) CC BY 4.0 (http://creativecommons.org/licenses/by/4.0/). Please be aware that this license allows unrestricted use and distribution, even commercially, by third parties. Please reply and provide explicit written permission to publish XXX under a CC BY license and complete the attached form.”
Please upload the completed Content Permission Form or other proof of granted permissions as an "Other" file with your submission.
In the figure caption of the copyrighted figure, please include the following text: “Reprinted from [ref] under a CC BY license, with permission from [name of publisher], original copyright [original copyright year].”
b. If you are unable to obtain permission from the original copyright holder to publish these figures under the CC BY 4.0 license or if the copyright holder’s requirements are incompatible with the CC BY 4.0 license, please either i) remove the figure or ii) supply a replacement figure that complies with the CC BY 4.0 license. Please check copyright information on all replacement figures and update the figure caption with source information. If applicable, please specify in the figure caption text when a figure is similar but not identical to the original image and is therefore for illustrative purposes only.
The following resources for replacing copyrighted map figures may be helpful:
USGS National Map Viewer (public domain): http://viewer.nationalmap.gov/viewer/
The Gateway to Astronaut Photography of Earth (public domain): http://eol.jsc.nasa.gov/sseop/clickmap/
Maps at the CIA (public domain): https://www.cia.gov/library/publications/the-world-factbook/index.html and https://www.cia.gov/library/publications/cia-maps-publications/index.html
NASA Earth Observatory (public domain): http://earthobservatory.nasa.gov/
Landsat: http://landsat.visibleearth.nasa.gov/
USGS EROS (Earth Resources Observatory and Science (EROS) Center) (public domain): http://eros.usgs.gov/#
Natural Earth (public domain): http://www.naturalearthdata.com/
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: No
Reviewer #2: Partly
**********
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: No
Reviewer #2: Yes
**********
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: No
**********
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #1: Yes
Reviewer #2: Yes
**********
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: This manuscript addresses an interesting management question and is based on a relatively large dataset. However, before it can be considered ready for publication I think it requires some work, both in its format and in some of the approaches/analyses.
I suggest that you phrase the hypotheses in a non-conditional way. E.g. “structural resources ARE the primary influence…”
The methods section requires much more detail and clarity. I would say that very few paragraphs would pass the test of successfully having a reader to replicate what you did. For instance, how many visits per year and per transect were done? Please explain. Also, during what season (or date ranges) was the field work done? What was the criterion for the inclusion of flying birds?, etc.
I do not understand the sentence “We did not adjust songbird observations for detection probability because differences in detectability among species would bias our abundance estimates…”. Precisely, adjusting for detectability tries to correct for a bias that is likely affecting your data. And in your case it could not only be related to species, sex and age of individual birds, but on habitat structure as well. Besides, simply because someone published a study without including such a correction, does not make it right. Because I assume that you did not record the distance at which individuals were detected, you may not be able to conduct this correction. So I suggest that you simply acknowledge that you did not do it, and later discuss the potential implications of this decision.
Please explain in detail how did you predict the abundance of small mammals for those transects where no trapping was conducted. I see a potential problem in this approach (i.e. using vegetation structure as a predictor of rodent abundance) since, later, you analyze both vegetation and prey abundance as, supposedly independent, predictors of predator occupancy.
The explanation for the procedure to generate the prey abundance score is very confusing, and, from what I could understand, rather arbitrary. The weights used for each attribute and value need to be better justified. Why, for instance, having presence of ground squirrels in the transect is equal to it being located at 5 km from the nearest lek? A more parsimonious way of integrating these different prey data could be using biomass per unit area. Probably you would have to make some assumptions for each case, but, at least you would have a more natural way of integrating these data.
If I understand it correctly, when you state “Multi-season occupancy models assume that occupancy of a survey location is closed during each year but may change between years.”, that means that the model considers that individuals are restricted to an area similar to that of the transect (i.e. 80 hectares) during a single season (year?). Although the home range of a hawk is likely much larger than that, I do not see a big problem if only one visit is made during the season. But if there are more than one visit during a year, then the closure assumption would be violated. As I mentioned in a previous paragraph, this information is not presented, but from my reading of the results section, it becomes evident that more that one visit was conducted in a year. I do not see a clear and easy way to address this problem, but it is certainly something that needs to be dealt with and, if possible, corrected.
I wonder to what extent the lack of patterns observed for red-tailed hawks is due to the spatial scale of the analysis. If you use a sampling area that is much smaller than the species’ territory or home range, then “occupancy” loses its meaning. First, because you likely have very few individuals to sample from, and, mostly because at some point they may leave the sampling area even for a few minutes or hours and you might miss them. You discuss this issue in lines 484-505, but I cannot understand why you conclude that in your case detection probability can be considered be equivalent to “availability”.
In order to rule out a significant effect of prey density on predator occupancy, it is important that this predictor is estimated with enough accuracy. In this particular study, different prey types (all with different methodological issues) were integrated in an index whose structure is not strongly justified. In addition, it is important to consider that in many cases, even though predators are likely to be attracted to areas with high abundance of prey, their very presence/abundance may reduce the carrying capacity for the prey, making more difficult the detection of such a relationship. You should be more cautious with your conclusions regarding this topic.
Reviewer #2: Review of Young et al.
This work assesses the relationship of habitat and prey availability with the occupancy of two generalist avian predators, common raven and red-tailed hawk. The authors analysed the habitat changes developed on an area that has suffered the elimination of conifer forest in order to compare it with areas that have not been eliminated, considering it to be an area of special interest for the conservation of the greater sage-grouse. The field work conducted, the variable selection and the methodology used are appropriate and provide great value to the manuscript. However, there are some aspects, mainly in the focus of the introduction, that could be improved for the understanding the results obtained in this study.
The authors introduce this manuscript with an focus that does not fit exactly with the context of the analyses performed and results obtained, since the introduction explains the effects of greater sage-grouse in relation to the removal of juniperus, due to the indirect effects that predators associated with juniperus forests may have. I consider that this relationship of causal effects is a more ambitious context than that which is subsequently analyzed in the models and the results obtained. I suggest focusing the introduction on what is strictly analyzed in methods, which is to address the factors that influence the occupancy of ravens and red-tailed hawks (e.g. L. 419-435). However, this can be contextualized that these results may have indirect implications for the greater sage-grouse, which has been explained in the "management implications" section.
L. 553-561. As indicated here, when more years of monitoring are available, the indirect relationship of predators on the greater sage-grouse due to juniperus management could be evaluated.
L. 105-117. Here, the authors focus this study in the sage-grouse, but the methodology is not developed in this way.
However, if the authors want to evaluate the grouse conservation problem, or the landscape management actions due to the elimination of conifers, they should specifically analyze the occupation of the grouse in relation to the % of junipers, as well as the abundance of predators, to know specifically if it is a problem of sage-grouse habitat or of predators. The juniper habitat may be removed, but the predators may still be there because they may be ecotone zones and the predators may have extensive hunting areas.
Other comments:
Title: I suggest a more concise and causal title: Implications of tree expansion in shrubland ecosystems on two generalist avian predators.
L. 33-37. I suggest to remove or summarize these sentences since I believe that it is not the main focus of this study.
L. 47. The latin name of western juniper should be previously in the abstract.
L. 53: ….”Therefor, we suggest that …” since it is not evaluated.
L.91. Please, include references about nest-trees as a resource for raptors and other predators.
L. 122-123. Rewrite this sentence if you refer to the relationship between forest expansion with predator occupancy and their indirect effects on prey, since specific effects of conifer exapansion and raptor occupancy could be for instance Jiménez-Franco et al. 2018. Plos One. Nest sites as a key resource for population persistence: A case study modelling nest occupancy under forestry practices.
L. 165: … of the study area…
L. 201-205: You shoukld justify the selected prey groups since in the introduction you only mention the sage-grouse.
L. 219: It should be the same unit that L. 197?
L. 246-253. Explain if this index is from previous work or is designed specifically for this study. Include an example of value or equation for its replication.
L. 323. Where is the results of the rest of models? Include them in Appendix.
L. 334-340. Include a table with these results.
Table 1. It should be explained if these variables are included in the same models, o different models.
L. 519: “ … generalist predators, the ravens”.
**********
6. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #1: No
Reviewer #2: No
**********
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
6 Mar 2023
Reviewer 1:
I suggest that you phrase the hypotheses in a non-conditional way. E.g. “structural resources ARE the primary influence…”
Thank you, we made this change starting on line 151-154
The methods section requires much more detail and clarity. I would say that very few paragraphs would pass the test of successfully having a reader to replicate what you did. For instance, how many visits per year and per transect were done? Please explain. Also, during what season (or date ranges) was the field work done? What was the criterion for the inclusion of flying birds?, etc.
L189-192 Thank you, we more explicitly stated that we visited each survey location three times per year as well as the dates during which surveys were completed.
L. 197-198 We added language to clarify our approach. “Birds were recorded if they were perched, calling, or flying/circling within the transect sample area”.
See Anderson 2007 for raptor survey techniques including flying individuals
I do not understand the sentence “We did not adjust songbird observations for detection probability because differences in detectability among species would bias our abundance estimates…”. Precisely, adjusting for detectability tries to correct for a bias that is likely affecting your data. And in your case it could not only be related to species, sex and age of individual birds, but on habitat structure as well. Besides, simply because someone published a study without including such a correction, does not make it right. Because I assume that you did not record the distance at which individuals were detected, you may not be able to conduct this correction. So I suggest that you simply acknowledge that you did not do it, and later discuss the potential implications of this decision.
We added the following on L531:
“Data collected on prey populations at finer scales that account for within-season population variability and estimation errors associated with predictions for small mammal density and songbird abundance may improve inference.
”Please explain in detail how did you predict the abundance of small mammals for those transects where no trapping was conducted. I see a potential problem in this approach (i.e. using vegetation structure as a predictor of rodent abundance) since, later, you analyze both vegetation and prey abundance as, supposedly independent, predictors of predator occupancy.
L 240 Thank you, we revised to more explicitly explain our prediction method.
“We predicted small mammal abundance using the top-ranked models for both deer mice and pocket mice for sites where we did not sample mammals. We used vegetation measurements taken at each raptor-raven survey transect to predict small mammal density.”
Juniper cover and prey abundance were not linearly correlated (r = 0.01 – 0.23) so we included them together in models.
The explanation for the procedure to generate the prey abundance score is very confusing, and, from what I could understand, rather arbitrary. The weights used for each attribute and value need to be better justified. Why, for instance, having presence of ground squirrels in the transect is equal to it being located at 5 km from the nearest lek? A more parsimonious way of integrating these different prey data could be using biomass per unit area. Probably you would have to make some assumptions for each case, but, at least you would have a more natural way of integrating these data.
L 347 Table 1 We agree that the prey index and models including distance to lek may require too many assumptions. Therefore, we removed models that included the prey index and distance to lek from the candidate model set. Instead of combined prey index model, we added a global prey model that included songbird abundance + small mammal density + ground squirrel occurrence as predictor variables.
If I understand it correctly, when you state “Multi-season occupancy models assume that occupancy of a survey location is closed during each year but may change between years.”, that means that the model considers that individuals are restricted to an area similar to that of the transect (i.e. 80 hectares) during a single season (year?). Although the home range of a hawk is likely much larger than that, I do not see a big problem if only one visit is made during the season. But if there are more than one visit during a year, then the closure assumption would be violated. As I mentioned in a previous paragraph, this information is not presented, but from my reading of the results section, it becomes evident that more that one visit was conducted in a year. I do not see a clear and easy way to address this problem, but it is certainly something that needs to be dealt with and, if possible, corrected.
See revisions L 512-520: The reviewer is correct that the closure assumption may be violated, especially for red-tailed hawks. However, violations of the closure assumption are common for highly mobile species leading to a reinterpretation of the detection parameter as a combination of errors resulting from both the observer’s ability to observe the animal (observer error) and the availability of the animal to be seen (Gould et al. 2019). When the closure assumption is violated, it is more appropriate to interpret occupancy model estimates as “habitat use” (Mackenzie et al. 2018). If individuals randomly move in and out of a sample unit, MacKenzie et al. assert that the occupancy estimator is likely unbiased (p. 147). If individual movements are not random (as can happen during the breeding season or migration), covariates used to predict an effect on detection can limit this bias (MacKenzie et al. 2018, p. 148). We tested for an effect of time of year on detection for both species but this model was not supported. Moreover, a single visit to a transect within a season would preclude detection estimates within the mark-recapture occupancy framework (MacKenzie et al. 2009).
There is strong support for the utility of occupancy models even when violations of the closure assumption occur (MacKenzie et al. 2009, Latif et al. 2016)
I wonder to what extent the lack of patterns observed for red-tailed hawks is due to the spatial scale of the analysis. If you use a sampling area that is much smaller than the species’ territory or home range, then “occupancy” loses its meaning. First, because you likely have very few individuals to sample from, and, mostly because at some point they may leave the sampling area even for a few minutes or hours and you might miss them. You discuss this issue in lines 484-505, but I cannot understand why you conclude that in your case detection probability can be considered be equivalent to “availability”.
See response above.
L 512-520 “For red-tailed hawks, a relatively low detection rate in our data is likely a result of low density and home range sizes larger than the area of our survey transects. Low detection rates can bias occupancy estimates if there is unmodeled, non-random movement in and out of sampling units often associated with the breeding season or migration [48]. However, we tested a model with time of year as a covariate for detection and this model was not supported. When the closure assumption is violated but individual movements in and out of sampling units is random, the occupancy estimator is likely unbiased. In this case, occupancy estimates can be interpreted as probability of use [48]”.
”In order to rule out a significant effect of prey density on predator occupancy, it is important that this predictor is estimated with enough accuracy. In this particular study, different prey types (all with different methodological issues) were integrated in an index whose structure is not strongly justified. In addition, it is important to consider that in many cases, even though predators are likely to be attracted to areas with high abundance of prey, their very presence/abundance may reduce the carrying capacity for the prey, making more difficult the detection of such a relationship. You should be more cautious with your conclusions regarding this topic.
Thank you, we removed the prey index from our model set and added the following on L531-534
“Data collected on prey populations at finer scales that account for within-season population variability and estimation errors associated with predictions for small mammal density and songbird abundance may improve inference.
Reviewer 2:
The authors introduce this manuscript with an focus that does not fit exactly with the context of the analyses performed and results obtained, since the introduction explains the effects of greater sage-grouse in relation to the removal of juniperus, due to the indirect effects that predators associated with juniperus forests may have. I consider that this relationship of causal effects is a more ambitious context than that which is subsequently analyzed in the models and the results obtained. I suggest focusing the introduction on what is strictly analyzed in methods, which is to address the factors that influence the occupancy of ravens and red-tailed hawks (e.g. L. 419-435). However, this can be contextualized that these results may have indirect implications for the greater sage-grouse, which has been explained in the "management implications" section.
Thank you for this helpful suggestion, and we agree that we do not address the effect of juniper removal on sage-grouse. However, we maintain that sage-grouse management is a primary driver for the funding for this work, and thus of primary interest for the management agencies who will use this work for inference (BLM 2018).
L92-96 To clarify and broaden the context for our work, we added citations highlighting the effects of afforestation on predator-prey relationships in other parts of the world (Hawelena at al. 2006, 2010, Ben-David et al. 2019).
L. 557-565. As indicated here, when more years of monitoring are available, the indirect relationship of predators on the greater sage-grouse due to juniperus management could be evaluated.
We agree that further research directly assessing the effect of conifer removal on predator-prey relationships is needed.
We added L146-147 “Our goal for this study was to provide a “first step” towards explicitly evaluating the relationship between conifer expansion and removal and avian predators.”
L. 105-117. Here, the authors focus this study in the sage-grouse, but the methodology is not developed in this way.
However, if the authors want to evaluate the grouse conservation problem, or the landscape management actions due to the elimination of conifers, they should specifically analyze the occupation of the grouse in relation to the % of junipers, as well as the abundance of predators, to know specifically if it is a problem of sage-grouse habitat or of predators. The juniper habitat may be removed, but the predators may still be there because they may be ecotone zones and the predators may have extensive hunting areas.
Thank you for this helpful suggestion. On L 578-581 we added the following:
“An important next step will be to assess the effect of conifer removal on predation rates for prey species.”
Though we agree that directly linking predator occupancy to sage grouse demographics and habitat use is an important next step, this type of analysis is beyond the scope of our study question and these data. Our goal for this study was to first examine the prediction that conifer expansion influences habitat use by avian predators. See Coates et al. 2020 and BLM 2018 for examples of connecting conifer expansion, increased abundance of avian predators, and concern for sage-grouse population status. The goal of our study was to be among the first to test the assumption that habitat use by avian predators is influenced by conifer expansion.
Title: I suggest a more concise and causal title: Implications of tree expansion in shrubland ecosystems on two generalist avian predators.
Thank you, we have made this change.
L. 33-37. I suggest to remove or summarize these sentences since I believe that it is not the main focus of this study.
Thank you for this suggestion. We have retained this text because it is a primary justification for conifer removal in our study system and helps readers understand the motivation for our study (BLM 2018)
L. 47. The latin name of western juniper should be previously in the abstract.
Thank you for this suggestion. Western juniper is not the only species at our study site, so it seems incorrect to only mention that species. However, we agree that it is important to mention the latin name and so we have added the generic name of junipers to the second sentence of the abstract.
L. 53: ….”Therefor, we suggest that …” since it is not evaluated.
Thank you, we made the suggested revision at line 51.
L.91. Please, include references about nest-trees as a resource for raptors and other predators.
L 90.We added the following additional reference:
Bosakowski et al. 1996
L. 122-123. Rewrite this sentence if you refer to the relationship between forest expansion with predator occupancy and their indirect effects on prey, since specific effects of conifer exapansion and raptor occupancy could be for instance Jiménez-Franco et al. 2018. Plos One. Nest sites as a key resource for population persistence: A case study modelling nest occupancy under forestry practices.
L 125-127 We clarified this sentence:
To our knowledge, no study has explicitly focused on the relationship between conifer expansion and associated habitat use by avian predators of sagebrush-associated species. L. 165: … of the study area…
We are unclear what the reviewer is suggesting.
L. 201-205: You should justify the selected prey groups since in the introduction you only mention the sage-grouse.
We added the following language explaining that these groups are common prey species for both ravens and red-tailed hawks in lines L 212-216:
“We considered the following measures for groups that are known to be common prey of both ravens and red-tailed hawks.”
L. 219: It should be the same unit that L. 197?
L 204 Thank you, we made this change.
L. 246-253. Explain if this index is from previous work or is designed specifically for this study. Include an example of value or equation for its replication.
We removed this combined prey index model from our model set per earlier comments.
L. 323. Where is the results of the rest of models? Include them in Appendix.
Thank you, we have included the complete model rankings in Appendix A
L. 334-340. Include a table with these results.
As suggested we have included all model rankings and p-values in Appendix A.
Table 1. It should be explained if these variables are included in the same models, o different models.
Table 1 L. 347 We revised the language in the table caption to clarify that these are the models we tested. It now reads:
“Models used to test effects of habitat features on detection and probability of habitat use for common ravens (Corvus corax) and red-tailed hawks (Buteo jamaicensis) in southwest Idaho, 2017-2020.
L. 519: “ … generalist predators, the ravens”.
Thank you, we made this change on L 539:
“Conifer removal may benefit wildlife species associated with sagebrush habitat by reducing the occupancy of ravens, a common generalist predator.”
Literature cited
Andersen D.E. Survey techniques. Raptor research and management techniques. 2007:89-100.
BLM, 2018. Bruneau-Owyhee Sage-grouse Habitat Project (BOSH). DOI-BLM-ID-B000-2014-0002-EIS
Bosakowski, T., R.D. Ramsey, and D.G. Smith. 1996. Habitat and spatial relationships of nesting Swainson's Hawks (Buteo swainsoni) and Red-tailed Hawks (B. jamaicensis) in northern Utah. The Great Basin Naturalist, 56:341-347.
Bui T.V., J.M. Marzluff, B. Bedrosian. 2010. Common raven activity in relation to land use in western Wyoming: implications for greater sage-grouse reproductive success. The Condor, 112:65-78.
Coates P.S., S.T. O'Neil, B.E. Brussee, M.A. Ricca, P.J. Jackson, J.B. Dinkins, K.B. Howe, A.M. Moser, L.J. Foster, D.J. Delehanty. 2010. Broad-scale impacts of an invasive native predator on a sensitive native prey species within the shifting avian community of the North American Great Basin. Biological Conservation, 243:108409.
Conover M.R., A.J. Roberts. 2017. Predators, predator removal, and sage‐grouse: A review. The Journal of wildlife management, 81:7-15.
Gould, M.J., W.R. Gould, J.W. Cain III, and G.W. Roemer. 2019. Validating the performance of occupancy models for estimating habitat use and predicting the distribution of highly-mobile species: A case study using the American black bear. Biological Conservation, 234:28-36.
Harju, S. M., P.S Coates, S.J. Dettenmeier, J.B. Dinkins, P.J. Jackson, M.J. Chenaille. 2021. Estimating trends of common raven populations in North America, 1966-2018. Human-Wildlife Interactions, 15:248-269.
Hawlena D, D. Saltz, Z. Abramsky, A. Bouskila. 2010. Ecological trap for desert lizards caused by anthropogenic changes in habitat structure that favor predator activity. Conservation Biology, 24:803-9.
Latif, Q. S., M.M. Ellis, and C.L. Amundson. 2016. A broader definition of occupancy: comment on Hayes and Monfils. Journal of Wildlife Management, 80:192–194.
MacKenzie, D.I., J.D. Nichols, M.E. Seamans, and R.J. Gutiérrez. 2009. Modeling species occurrence dynamics with multiple states and imperfect detection. Ecology 90:833.
MacKenzie D.I., J.D. Nichols, J.A. Royle, K.H. Pollock, L. Bailey, J.E. Hines. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier; 2017 Nov 17.
O'Neil ST, P.S. Coates, B.E. Brussee, P.J. Jackson, K.B. Howe, A.M. Moser, L.J. Foster, D.J. Delehanty. 2018. Broad‐scale occurrence of a subsidized avian predator: Reducing impacts of ravens on sage‐grouse and other sensitive prey. Journal of Applied Ecology. 55:2641-52.
Attachment
Submitted filename: Reponse to Reviewers.docx
Decision Letter 1
28 Apr 2023
PONE-D-22-29377R1Implications of tree expansion in shrubland ecosystems on two generalist avian predators .PLOS ONE
Dear Dr. Young,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
We are sorry to be late with your MS decision, but we were unable to contact one of the previous reviewers, so we had to invite a new reviewer.
Despite this, both reviewers are very positive and have only shown minor concerns that are easily addressed by you.
Please submit your revised manuscript by Jun 12 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
We look forward to receiving your revised manuscript.
Kind regards,
Juan Manuel Pérez-García, PhD
Academic Editor
PLOS ONE
Journal Requirements:
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #2: All comments have been addressed
Reviewer #3: (No Response)
**********
2. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #2: Yes
Reviewer #3: Yes
**********
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #2: Yes
Reviewer #3: Yes
**********
4. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #2: No
Reviewer #3: Yes
**********
5. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
Reviewer #2: Yes
Reviewer #3: Yes
**********
6. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #2: The manuscript is clear and easy to foollow, it is now appropiate for publication. I have included only a couple of minor comments related to editing and to the replicability of this work in terms of its statistical analyses.
Line 170. Replace project by study area: "The climate of the study area is ...".
I suggest including a JAGS code in an appendix for the replicability of the work in other contexts. I believe that this information will add value to the manuscript, as it is free software that is being included in an increasing way of scientific articles and open source journals.
Reviewer #3: This is a well-written paper that uses a robust dataset to evaluate the implications of tree expansion over two generalist predator presence in a sagebrush ecosystem. Clearly a lot of work went into data collection, which includes predator species and his prey across a sagebrush steppe. The study has strong potential to be useful for management and conservation, but in my opinion there are some issues that need to be resolved before this paper is published.
The authors discuss potential implications of their study for the management and conservation of Sage-grouse but the data and results support this indirectly. Although data on the abundance of the potential prey of two generalist predators were used, there are no data on the abundance or presence of the Sage-grouse. In the same way, the authors argue that the abundance of prey does not influence the habitat use by the two species of predators, but only a part of the potential prey was studied. I suggest caution with the conclusions drawn from the results. Is it possible to predators are responding to another prey species present in the area?
Detailed comments
Introduction
Overall, the introduction is very well written and does a great job of setting the study.
L 96-97 delete space before “For”
You mentioned “For generalist predators that may utilize a wide variety of prey items, structural resources may be an important factor influencing habitat use.” But this also happens with forest-dwelling species and specialists. See Martínez-Hesterkamp S, Rebollo S, Pérez-Camacho L, García-Salgado G, Fernández-Pereira JM (2018) Assessing the ability of novel ecosystems to support animal wildlife through analysis of diurnal raptor territoriality.PLoSONE13(10):e0205799
Also, could you include a reference supporting those lines.
In the title and abstract you mentioned the term generalist predators but in the introduction only referred to avian predators, except in L. 96-97. This may be confused for readers. I suggest being consistently with one term for predators. In the objectives L. 148-155 you use avian predators not generalist predators. I suggest the use of generalist predators in the manuscript.
Methods
Please include references in the section of Raven and raptors counts.
L 198 How much time did you spend in stationary counts? This was equal in all transects? Please describe.
L 215 Please mention the ground squirrel species
L225-226 The transects for songbirds was an extension of 1.200 m, you mentioned that transects for avian predators had 800 m with 3 points counts within the transect in L 194-195. This it is not clear.
L 233-235 “Belding’s ground squirrels (Urocitellus beldingi), which occur in large semi-colonial populations, are the most common ground squirrel at our study site.” This could be used in the first paragraph of prey abundance. L. 214-216.
Discussion
L 555-560 I suggest being more cautious with these lines, the relationship between the study and the presence of the sage-grouse is not clear.
**********
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Reviewer #2: No
Reviewer #3: No
**********
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif. Please note that Supporting Information files do not need this step.
Author response to Decision Letter 1
10 May 2023
Reviewer #2: The manuscript is clear and easy to foollow, it is now appropiate for publication. I have included only a couple of minor comments related to editing and to the replicability of this work in terms of its statistical analyses.
Line 170. Replace project by study area: "The climate of the study area is ...".
Thank you, we made this change on line 172
I suggest including a JAGS code in an appendix for the replicability of the work in other contexts. I believe that this information will add value to the manuscript, as it is free software that is being included in an increasing way of scientific articles and open source journals.
Thank you, we have included JAGS model code in the data submitted to PLOS one during the resubmission process and this code will be available freely.
Reviewer #3: This is a well-written paper that uses a robust dataset to evaluate the implications of tree expansion over two generalist predator presence in a sagebrush ecosystem. Clearly a lot of work went into data collection, which includes predator species and his prey across a sagebrush steppe. The study has strong potential to be useful for management and conservation, but in my opinion there are some issues that need to be resolved before this paper is published.
The authors discuss potential implications of their study for the management and conservation of Sage-grouse but the data and results support this indirectly. Although data on the abundance of the potential prey of two generalist predators were used, there are no data on the abundance or presence of the Sage-grouse. In the same way, the authors argue that the abundance of prey does not influence the habitat use by the two species of predators, but only a part of the potential prey was studied. I suggest caution with the conclusions drawn from the results. Is it possible to predators are responding to another prey species present in the area?
Thank you for helpful comments, we appreciate your time and effort. We agree that our examination of the effect of prey distributions on predator occupancy may be limited by our ability to include all potential prey and that the effects of avian predators on sage-grouse demographics at our study site is unknown. However, because the removal of juniper at our study site was largely prompted by concerns over sage-grouse population conservation, we want to present our findings in the context of potential changes to the predator community for this species and highlight directions for future research. We added language in the discussion to this effect.
L 562. However, because we did not directly examine the impact of habitat-use by avian predators on sage-grouse demographics, future research on this topic will be an important next step for managers aiming to conserve populations of prey species.
Detailed comments
Introduction
Overall, the introduction is very well written and does a great job of setting the study.
L 96-97 delete space before “For”
Thank you, we corrected this formatting.
You mentioned “For generalist predators that may utilize a wide variety of prey items, structural resources may be an important factor influencing habitat use.” But this also happens with forest-dwelling species and specialists. See Martínez-Hesterkamp S, Rebollo S, Pérez-Camacho L, García-Salgado G, Fernández-Pereira JM (2018) Assessing the ability of novel ecosystems to support animal wildlife through analysis of diurnal raptor territoriality.PLoSONE13(10):e0205799
Also, could you include a reference supporting those lines.
Thank you, we adjusted the language of this sentence to be more general and added the following citation
L 96. “For avian predators that may utilize a wide variety of prey items, structural resources may be a primary factor influencing habitat use [22].”
Greenwald DN, Crocker-Bedford DC, Broberg L, Suckling KF, Tibbitts T. A review of northern goshawk habitat selection in the home range and implications for forest management in the western United States. Wildlife Society Bulletin. 2005;33: 120–148.
In the title and abstract you mentioned the term generalist predators but in the introduction only referred to avian predators, except in L. 96-97. This may be confused for readers. I suggest being consistently with one term for predators. In the objectives L. 148-155 you use avian predators not generalist predators. I suggest the use of generalist predators in the manuscript.
Thank you, we added language on L. 122 to indicate that when we refer to avian predators throughout the rest of the manuscript we are referring to our two generalist avian predator study species.
In recent years, increased abundance in sagebrush habitat of common ravens (Corvus corax) and red-tailed hawk (Buteo jamaicensis), two highly generalist avian predators (hereafter “avian predators”)
Methods
Please include references in the section of Raven and raptors counts.
Thank you, we added the following reference
L198. 46. Andersen, D.E. Survey techniques. In: Raptor research and management techniques. 2007. pp. 89-100.
L 198 How much time did you spend in stationary counts? This was equal in all transects? Please describe.
Thank you, we clarified these methods..
L. 195 10-minute observation periods
L199 – 200. Along each survey transect, the amount of time spent at stationary survey locations was consistent, but walking surveys between stationary survey locations varied based on terrain.
L 215 Please mention the ground squirrel species
Thank you, we moved our description of the ground squirrel species at our site from line 233 to line 216.
L225-226 The transects for songbirds was an extension of 1.200 m, you mentioned that transects for avian predators had 800 m with 3 points counts within the transect in L 194-195. This it is not clear.
Thank you, we clarified this sentence describing the placement of songbird survey stations along the avian predator survey transect:
L 227-229. Each survey transect for avian predators had three point counts stations placed at either end and in the middle of the survey transect.
L 233-235 “Belding’s ground squirrels (Urocitellus beldingi), which occur in large semi-colonial populations, are the most common ground squirrel at our study site.” This could be used in the first paragraph of prey abundance. L. 214-216.
See above
Discussion
L 555-560 I suggest being more cautious with these lines, the relationship between the study and the presence of the sage-grouse is not clear.
L. 562-564 Thank you, we added the following language about the limits for inference that our study provides.
“However, because we did not directly examine the impact of habitat-use by avian predators on sage-grouse demographics, future research on this topic will be an important next step for managers aiming to conserve populations of prey species.”
Attachment
Submitted filename: Response to Reviewers.docx
Decision Letter 2
17 May 2023
Implications of tree expansion in shrubland ecosystems for two generalist avian predators .
PONE-D-22-29377R2
Dear Dr. Young,
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua.
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno.
Kind regards,
Juan Manuel Pérez-García, PhD
Academic Editor
PLOS ONE
Acceptance letter
23 May 2023
PONE-D-22-29377R2
Implications of tree expansion in shrubland ecosystems for two generalist avian predators.
Dear Dr. Young:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno.
If we can help with anything else, please email us at gro.solp@enosolp.
Thank you for submitting your work to PLOS ONE and supporting open access.
Kind regards,
PLOS ONE Editorial Office Staff
on behalf of
Dr. Juan Manuel Pérez-García
Academic Editor
PLOS ONE
Articles from PLOS ONE are provided here courtesy of PLOS
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/149515620
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Living on the edge: Predicting songbird response to management and environmental changes across an ecotone.
Ecol Evol, 13(11):e10648, 14 Nov 2023
Cited by: 1 article | PMID: 38020705 | PMCID: PMC10646169
Western Juniper Management: Assessing Strategies for Improving Greater Sage-grouse Habitat and Rangeland Productivity.
Environ Manage, 56(3):675-683, 10 May 2015
Cited by: 3 articles | PMID: 25957623 | PMCID: PMC4527980
Better living through conifer removal: A demographic analysis of sage-grouse vital rates.
PLoS One, 12(3):e0174347, 23 Mar 2017
Cited by: 3 articles | PMID: 28333995 | PMCID: PMC5363946
Common juniper, an overlooked conifer with high invasion potential in protected areas of Patagonia.
Sci Rep, 13(1):9818, 17 Jun 2023
Cited by: 0 articles | PMID: 37330618 | PMCID: PMC10276858
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Great Basin Landscape Conservation Cooperative and U.S. Fish and Wildlife Service (1)
Grant ID: F16AC01182
Palouse Audubon Society
U.S. Department of Agriculture, McIntire Stennis project (1)
Grant ID: 1009779

 #
1
,*
#
1
,* 

