Abstract
Background
Estrogens regulate disparate female physiological processes, thus ensuring reproduction. Altered estrogen levels and signaling have been associated with increased risks of pregnancy failure and complications, including hypertensive disorders and low birthweight babies. However, the role of estrogens in the periconceptional period and early pregnancy is still understudied.Objective and rationale
This review aims to summarize the current evidence on the role of maternal estrogens during the periconceptional period and the first trimester of pregnancies conceived naturally and following ART. Detailed molecular mechanisms and related clinical impacts are extensively described.Search methods
Data for this narrative review were independently identified by seven researchers on Pubmed and Embase databases. The following keywords were selected: 'estrogens' OR 'estrogen level(s)' OR 'serum estradiol' OR 'estradiol/estrogen concentration', AND 'early pregnancy' OR 'first trimester of pregnancy' OR 'preconceptional period' OR 'ART' OR 'In Vitro Fertilization (IVF)' OR 'Embryo Transfer' OR 'Frozen Embryo Transfer' OR 'oocyte donation' OR 'egg donation' OR 'miscarriage' OR 'pregnancy outcome' OR 'endometrium'.Outcomes
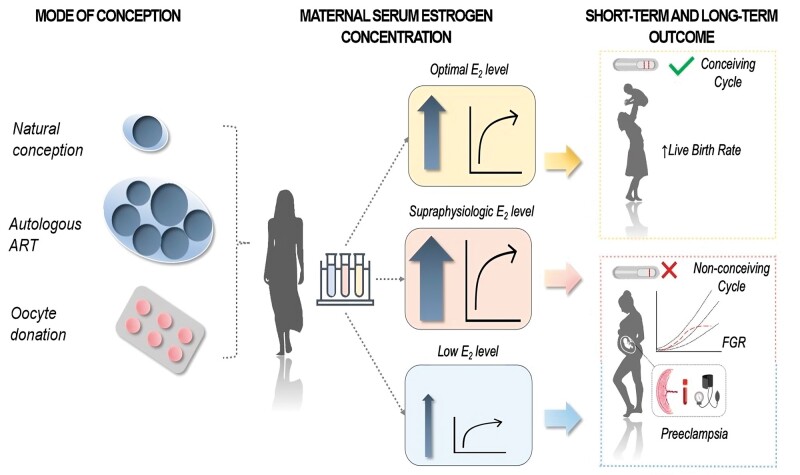
During the periconceptional period (defined here as the critical time window starting 1 month before conception), estrogens play a crucial role in endometrial receptivity, through the activation of paracrine/autocrine signaling. A derailed estrogenic milieu within this period seems to be detrimental both in natural and ART-conceived pregnancies. Low estrogen levels are associated with non-conception cycles in natural pregnancies. On the other hand, excessive supraphysiologic estrogen concentrations at time of the LH peak correlate with lower live birth rates and higher risks of pregnancy complications. In early pregnancy, estrogen plays a massive role in placentation mainly by modulating angiogenic factor expression-and in the development of an immune-tolerant uterine micro-environment by remodeling the function of uterine natural killer and T-helper cells. Lower estrogen levels are thought to trigger abnormal placentation in naturally conceived pregnancies, whereas an estrogen excess seems to worsen pregnancy development and outcomes.Wider implications
Most current evidence available endorses a relation between periconceptional and first trimester estrogen levels and pregnancy outcomes, further depicting an optimal concentration range to optimize pregnancy success. However, how estrogens co-operate with other factors in order to maintain a fine balance between local tolerance towards the developing fetus and immune responses to pathogens remains elusive. Further studies are highly warranted, also aiming to identify the determinants of estrogen response and biomarkers for personalized estrogen administration regimens in ART.Free full text

The pathophysiological role of estrogens in the initial stages of pregnancy: molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester
Abstract
BACKGROUND
Estrogens regulate disparate female physiological processes, thus ensuring reproduction. Altered estrogen levels and signaling have been associated with increased risks of pregnancy failure and complications, including hypertensive disorders and low birthweight babies. However, the role of estrogens in the periconceptional period and early pregnancy is still understudied.
OBJECTIVE AND RATIONALE
This review aims to summarize the current evidence on the role of maternal estrogens during the periconceptional period and the first trimester of pregnancies conceived naturally and following ART. Detailed molecular mechanisms and related clinical impacts are extensively described.
SEARCH METHODS
Data for this narrative review were independently identified by seven researchers on Pubmed and Embase databases. The following keywords were selected: ‘estrogens’ OR ‘estrogen level(s)’ OR ‘serum estradiol’ OR ‘estradiol/estrogen concentration’, AND ‘early pregnancy’ OR ‘first trimester of pregnancy’ OR ‘preconceptional period’ OR ‘ART’ OR ‘In Vitro Fertilization (IVF)’ OR ‘Embryo Transfer’ OR ‘Frozen Embryo Transfer’ OR ‘oocyte donation’ OR ‘egg donation’ OR ‘miscarriage’ OR ‘pregnancy outcome’ OR ‘endometrium’.
OUTCOMES
During the periconceptional period (defined here as the critical time window starting 1 month before conception), estrogens play a crucial role in endometrial receptivity, through the activation of paracrine/autocrine signaling. A derailed estrogenic milieu within this period seems to be detrimental both in natural and ART-conceived pregnancies. Low estrogen levels are associated with non-conception cycles in natural pregnancies. On the other hand, excessive supraphysiologic estrogen concentrations at time of the LH peak correlate with lower live birth rates and higher risks of pregnancy complications. In early pregnancy, estrogen plays a massive role in placentation mainly by modulating angiogenic factor expression—and in the development of an immune-tolerant uterine micro-environment by remodeling the function of uterine natural killer and T-helper cells. Lower estrogen levels are thought to trigger abnormal placentation in naturally conceived pregnancies, whereas an estrogen excess seems to worsen pregnancy development and outcomes.
month before conception), estrogens play a crucial role in endometrial receptivity, through the activation of paracrine/autocrine signaling. A derailed estrogenic milieu within this period seems to be detrimental both in natural and ART-conceived pregnancies. Low estrogen levels are associated with non-conception cycles in natural pregnancies. On the other hand, excessive supraphysiologic estrogen concentrations at time of the LH peak correlate with lower live birth rates and higher risks of pregnancy complications. In early pregnancy, estrogen plays a massive role in placentation mainly by modulating angiogenic factor expression—and in the development of an immune-tolerant uterine micro-environment by remodeling the function of uterine natural killer and T-helper cells. Lower estrogen levels are thought to trigger abnormal placentation in naturally conceived pregnancies, whereas an estrogen excess seems to worsen pregnancy development and outcomes.
WIDER IMPLICATIONS
Most current evidence available endorses a relation between periconceptional and first trimester estrogen levels and pregnancy outcomes, further depicting an optimal concentration range to optimize pregnancy success. However, how estrogens co-operate with other factors in order to maintain a fine balance between local tolerance towards the developing fetus and immune responses to pathogens remains elusive. Further studies are highly warranted, also aiming to identify the determinants of estrogen response and biomarkers for personalized estrogen administration regimens in ART.
Graphical abstract
Introduction
Medical practice sometimes relies on assumptions or anecdotical evidence stemming from limited scientific proof that may ultimately result in inadequate interventions or therapies in clinical settings. We believe that, in view of being one of the most important biological factors throughout a woman’s reproductive life and beyond, estrogens deserve more attention in the study of the early events surrounding reproduction, and especially with respect to ART.
Estrogens are a heterogeneous group of steroid compounds that regulate disparate female physiological processes and ensure reproduction. Sex hormone concentrations are strictly dependent on the woman age and hormonal stage: estradiol (E2) predominates throughout life, estriol (E3) mainly during pregnancy, and estrone (E1) in the postmenopausal period (Orzołek et al., 2022). E2, the main product of the ovarian follicles, is the most abundant and potent estrogen, acting as a key mediator of the pathophysiological changes of the female reproductive tract.
While the role of estrogens during pregnancy has been extensively described in late pregnancy and parturition, few human studies have investigated their function during the periconceptional period and the first trimester of pregnancy. Furthermore, maternal sex steroid concentrations during pregnancy have been associated with health risks in both the mother and the offspring later in life, mainly predicting the risk of steroid-sensitive cancers in the mother, and atopic, cancer and neurodevelopmental abnormalities in children (Toriola et al., 2011). This further highlights the urgency to fill the knowledge gap on physiological estrogen concentrations in early pregnancy, as well as the feto-maternal determinants of the hormonal milieu. Such knowledge would be of relevance in light of the increasing rates of autologous and heterologous ART pregnancies, currently accounting for around 3% of livebirths in industrialized countries (Wyns et al., 2022). ART include IVF-embryo transfer (IVF/ET and frozen embryo transfer (FET)). These techniques apply to both autologous and heterologous gametes. IVF/ET is the most common technique and involves three steps: oocyte collection from the ovary, its fertilization with semen in vitro, and the subsequent transfer of the embryo into the uterus. To increase the success of the procedure, multiple oocytes are obtained through controlled ovarian stimulation with parenteral gonadotropins administration. Different stimulation protocols are available, based on patients’ characteristics and experience of the physician. Once follicles reach at least 18 mm in diameter at the ultrasound evaluation, a trigger is administered to mimic the LH peak and induce ovulation. After 36
mm in diameter at the ultrasound evaluation, a trigger is administered to mimic the LH peak and induce ovulation. After 36 h, transvaginal ultrasound-guided needle aspiration is performed. Oocyte fertilization happens in vitro: in case of male infertility, ICSI may be used to overcome the problem. The last step of the procedure is the ET, where one or more embryos, suspended in a drop of culture medium, are placed into the uterine cavity. Supernumerary embryos are generally cryopreserved and transferred in subsequent cycles, if pregnancy is not achieved on the first attempt (Goldberg et al., 2007; Sallam and Rizk, 2012). Among heterologous techniques, oocyte or embryo donation from young donors represents a standard and effective treatment for age-related infertility or premature ovarian failure. Oocyte donation (OD) involves egg retrieval from the donor, insemination with semen of the recipient’s partner, and in vitro culture and transfer of cleaved embryos into the recipient’s uterus. The stimulation of the oocyte donor is similar to the standard IVF protocol. ET in the recipient may be performed both in a natural cycle, if the woman has an adequate ovarian function, or to a prepared endometrium. Endometrial preparation is achieved by replacing endogenous steroid with E2 administered both orally (4–6
h, transvaginal ultrasound-guided needle aspiration is performed. Oocyte fertilization happens in vitro: in case of male infertility, ICSI may be used to overcome the problem. The last step of the procedure is the ET, where one or more embryos, suspended in a drop of culture medium, are placed into the uterine cavity. Supernumerary embryos are generally cryopreserved and transferred in subsequent cycles, if pregnancy is not achieved on the first attempt (Goldberg et al., 2007; Sallam and Rizk, 2012). Among heterologous techniques, oocyte or embryo donation from young donors represents a standard and effective treatment for age-related infertility or premature ovarian failure. Oocyte donation (OD) involves egg retrieval from the donor, insemination with semen of the recipient’s partner, and in vitro culture and transfer of cleaved embryos into the recipient’s uterus. The stimulation of the oocyte donor is similar to the standard IVF protocol. ET in the recipient may be performed both in a natural cycle, if the woman has an adequate ovarian function, or to a prepared endometrium. Endometrial preparation is achieved by replacing endogenous steroid with E2 administered both orally (4–6 mg/day) or transdermally (50–100
mg/day) or transdermally (50–100 mg every 72
mg every 72 h). When endometrial thickness reaches at least 7
h). When endometrial thickness reaches at least 7 mm, ET may be performed (Barbieri et al., 2010; Melnick and Rosenwaks, 2018; Kaser et al., 2019).
mm, ET may be performed (Barbieri et al., 2010; Melnick and Rosenwaks, 2018; Kaser et al., 2019).
Therefore, pharmacological manipulation of the female sex hormone regulation system has long been leveraged in ART, in order to mimic menstrual cycle hormone fluctuations in the setting of IVF/ET protocols. However, the lack of high quality randomized controlled trials has hampered the definition of standardized protocols to ensure ART success and no definitive consensus has been reached among the scientific community to date (Mackens et al., 2017; Glujovsky et al., 2020).
Today the treatment with exogenous estrogens in heterologous and autologous reproduction is not supported by sufficient scientific evidence and appears empirical at best. Our hypothesis is that women seeking pregnancy with ART would benefit from a personalized regimen of endometrial stimulation as a crucial step to maximize reproductive success. This review aims to summarize the current understanding of the role of estrogens in the physiopathology of the periconceptional period (here defined as the critical time window starting 1 month before conception) and early pregnancy, detailing the underlying molecular mechanisms affecting the endometrium before and after embryo implantation, as well as during the first trimester.
month before conception) and early pregnancy, detailing the underlying molecular mechanisms affecting the endometrium before and after embryo implantation, as well as during the first trimester.
Methods
Data for this narrative review were identified by seven researchers (A.Z., A.I., F.P., C.S., C.F., M.B., and V.S.) on PubMed and Embase databases from June 2021 to October 2022. The search strategy included the following keywords: ‘estrogens’ OR ‘estrogen level(s)’ OR ‘serum estradiol’ OR ‘estradiol concentration’, AND ‘early pregnancy’ OR ‘first trimester of pregnancy’ OR ‘periconceptional period’ OR ‘Assisted Reproduction technology (ART)’ OR ‘In Vitro Fertilization (IVF)’ OR ‘Embryo Transfer’ OR ‘Frozen Embryo Transfer’ OR ‘oocyte donation’ OR ‘egg donation’ OR ‘miscarriage’ OR ‘pregnancy outcome’ OR ‘endometrium’. English language and original data studies, regardless the sample size, were taken into consideration.
Estrogens and their receptors
There are three major types of estrogens in women: E1, E2, and E3. E2 is the most potent estrogen and, along with progesterone, is a master regulator of the changes occurring during the menstrual cycle, whereas E3 becomes the primary estrogen during late pregnancy (Cui et al., 2013). In women, all these estrogen types are synthesized from androgens, namely testosterone and androstenedione, by the enzyme aromatase in the ovaries, or the corpus luteum upon ovulation. Although other organs may produce estrogens, and minor estrogen types that do not require aromatase do exist, they will not be covered in this review.
Unlike other mammals, the primates’ placenta becomes the primary source of estrogens during pregnancy (Pepe et al., 2018). However, the primate placenta does not express the cytochrome P450 17A1 (steroid 17-alpha-hydroxylase/17,20 lyase) which participates in corticoid and androgen biosynthesis. Therefore, the placenta cannot convert c21-steroids (pregnenolone and progesterone) into estrogen precursors c19-steroids (dehydroepiandrosterone—DHEA—and androstenedione), leading to the placental dependence on estrogen precursors from maternal and fetal adrenal glands. As a result of extensive 16-hydroxylation of c19-steroids within the fetus, large quantities of E3 are produced by the placenta during human pregnancy. Likewise, the synthesis of a fourth estrogen, estetrol (E4), takes place in the fetus and its concentration is much higher in the fetal than the maternal circulation. While the production and preferential excretion of E3 and E4 was proposed to protect the fetus from the effects of the more potent E2, their function remains to be clarified and this review will focus mainly on E2.
Two receptors account for estrogens’ activity in the endometrium, namely estrogen receptor (ER) 1 (ERα) and 2 (ERβ), whose tissue distribution differs across multiple organs (Heldring et al., 2007). ERα is primarily expressed in the uterus, and to a lesser extent in the skin, ovaries, testis, and gut; conversely, ERβ expression has been detected in the ovaries, prostate, colon, kidneys, cardiovascular system, and central nervous system (Brandenberger et al., 1997). Notably, ERβ has been described by some groups to be the sole ER expressed in specific cell types within the endometrium, such as the endothelium and uterine natural killer (uNK) cells, which are the most abundant subset of immune cells in the endometrium (Taylor and Al-Azzawi, 2000; Critchley et al., 2001; Henderson et al., 2003). Notwithstanding, the number of studies investigating the potential roles of ERβ in the endometrium is remarkably modest.
Moreover, although ER expression varies over time during the menstrual cycle, being more prominent in the nuclei of the epithelial layer of the endometrium during the proliferative phase, mRNAs specific for both ER types have been reported in glandular epithelial, stromal, and myometrial cells of the human uterus during all stages of the menstrual cycle (Xu et al., 2021). Three ERα and four ERβ isoforms, generated by alternative splicing of pre-mRNA from ER1 and ER2 genes, have been described. Although their role has only marginally been elucidated so far (Jia et al., 2015; Yu et al., 2022), several reports indicate that ER isoforms can differentially modulate estrogen signaling and, as a consequence, impact target gene regulation (Ramsey et al., 2004; Elhasnaoui et al., 2021; Costa et al., 2022). Upon binding estrogen, ERα and ERβ dimerize and move to the cell nucleus, exerting their effect by activating or suppressing the transcription of a plethora of different genes, which are regulators of various physiological processes (Mal et al., 2020) including uterine development and fertility, blastocyst implantation and the early stages of embryo development (Park et al., 2012; Vasquez and DeMayo, 2013). Although responsible for several physiological functions in both females and males, studies performed on ERα and ERβ double knockout mice show that life is feasible even without E2 action, regardless of ubiquitous ER expression in almost all anatomical areas (Lubahn et al., 1993; Dupont et al., 2000; Weihua et al., 2000). Besides, E2 is indispensable for reproductive function in females, but only ERα seems to be crucial in maintaining fertility (Lubahn et al., 1993). Thus, the key task of ERβ is thought to counteract undesired ERα-mediated actions of E2.
The periconceptional stage
Every successful pregnancy starts with embryo implantation in the uterine endometrium. The crucial steps required to prepare a receptive endometrium suitable for the implantation and the subsequent growth of a competent blastocyst are tightly regulated by a number of biochemical factors, including a proper hormonal milieu, and by a strict cross-talk between blastocyst and endometrium (Zhang et al., 2013). The exposure of the endometrium to the fluctuation of ovarian sex hormones during the menstrual cycle periodically creates and maintains a receptive environment for further blastocyst development. However, limited data are available on the role of estrogens in fertile women during the periconceptional period, here defined as the critical time window starting one month before conception, and most of our understanding relies on animal models and in vitro cell systems. Previous studies investigated the reference values of serum E2 during natural menstrual cycles (Stricker et al., 2006; Anckaert et al., 2021), but only few focused on its concentration at the LH peak of natural conceiving cycles (Lenton et al., 1982; Stewart et al., 1993). At peak maturation, a single oocyte is estimated to secrete 200–300 pg/ml of E2 (Speroff and Fritz, 2011). Owing to the higher number of retrieved oocytes in IVF/ET cycles (Fig. 1), serum E2 concentration at time of the LH surge in natural conception cycles is significantly lower compared to autologous ART conception. In the following paragraphs, we compiled all available evidence in an attempt to better characterize the potential impact of such difference on pregnancy outcome.
pg/ml of E2 (Speroff and Fritz, 2011). Owing to the higher number of retrieved oocytes in IVF/ET cycles (Fig. 1), serum E2 concentration at time of the LH surge in natural conception cycles is significantly lower compared to autologous ART conception. In the following paragraphs, we compiled all available evidence in an attempt to better characterize the potential impact of such difference on pregnancy outcome.
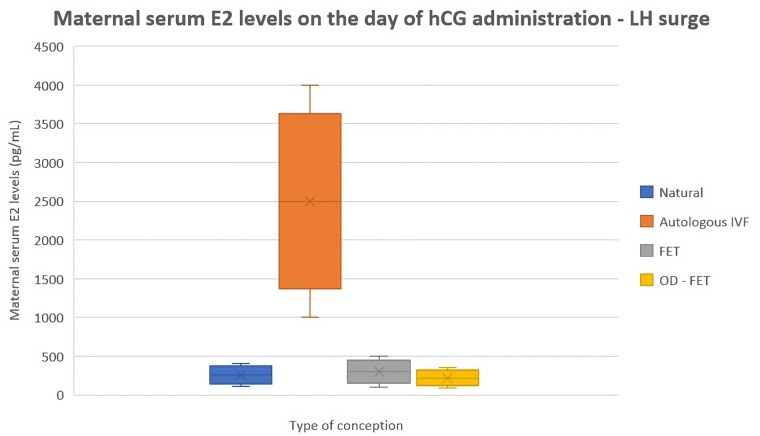
Serum estrogen concentration in women at time of the LH surge in natural conception cycles compared to autologous ART conception and oocyte donation. E2, estradiol; FET, frozen embryo transfer; OD-FET, oocyte-donation FET.
Natural conception
Highly complex and still understudied biological processes occur within the periconceptional period, including follicular development, fertilization, implantation, and early organogenesis, all rigorously orchestrated by hormonal fluctuation, epigenetic changes, and inflammatory signaling (Steegers-Theunissen et al., 2013). Most of the studies are consistent in demonstrating that estrogen levels vary from cycle to cycle in healthy fertile women. In particular, lower serum, urinary, and salivary estrogen concentrations were associated with non-conception cycles compared to conception cycles (Baird et al., 1991; Stewart et al., 1993; Lipson and Ellison, 1996; Baird et al., 1997, 1999; Li et al., 2001; Chen et al., 2003; Venners et al., 2006). Previous literature showed an excellent agreement between serum, urinary and salivary estrogen biomarkers. In particular, urinary metabolites exhibit cyclic patterns similar to serum steroids during the menstrual cycle, but smaller variations between baseline and peak concentrations and about a 1-day delay compared to the serum peak were described (Cekan et al., 1986; Hardiman et al., 1990; Roos et al., 2015). Venners et al. (2006) evaluated urinary concentrations of E1 conjugates (E1C) in a population of 347 healthy and fertile women who were attempting to get pregnant naturally, finding higher estrogen concentrations during cycles resulting in clinical pregnancy compared to non-conception cycles (Venners et al., 2006). Lipson and Ellison (1996) previously evaluated salivary estrogen and progesterone levels among conception and non-conception cycles, finding no differences in progesterone concentrations and higher salivary estrogen in conception cycles, especially in the mid-follicular phase (Lipson and Ellison, 1996). In line with these results, Stewart et al. (1993) and Baird et al. (1997) found higher mid-luteal estrogen concentrations in fertile women with conception compared to non-conception cycles (Stewart et al., 1993; Baird et al., 1997). While the former hypothesizes a possible effect of the preimplantation embryo on ovarian steroid production, the latter suggests that the detected differences in steroid concentrations may reflect a higher quality of cycles resulting in improved chances of conception. Additionally, data on estrogen levels of conception cycles with early pregnancy loss were inconclusive. Baird et al. (1991) described similar pre-implantation estrogen and progesterone levels among 20 cycles with early pregnancy loss and 20 cycles with successful pregnancy in the same study population, whereas Venners et al. (2006) found a trend towards lower urinary E1C levels in 63 early pregnancy loss cycles, especially during the ovulation and the early luteal phase, compared with 266 successful pregnancy cycles (Baird et al., 1991; Venners et al., 2006).
Autologous ART
The available data show controversial results. Five out of the 22 identified studies suggest no correlation between serum E2 levels on the day of luteinizing trigger and pregnancy outcomes. Huang et al. (2014) divided 2868 patients stimulated with long GnRH agonist protocol into three groups according to the dynamics of serum E2 levels 12 h before and after hCG administration (ΔE2
h before and after hCG administration (ΔE2 >
> 10%, −10% ≤ ΔE2
10%, −10% ≤ ΔE2 ≤
≤ 10% and ΔE2 < −10%) (Huang et al., 2014). In line with Styer et al. (2005), no significant difference in live birth rate (LBR) and miscarriage rate (MR) was highlighted and pregnancy outcome seemed not to be influenced by E2 dynamics (Styer et al. 2005; Huang et al. 2014; Sunkara et al. 2015). Similar results were achieved by Zavy et al. (2014) who analyzed serum E2 levels on the day of hCG administration in 478 patients undergoing fresh IVF/ET. No difference was detected among the three selected groups (E2
10% and ΔE2 < −10%) (Huang et al., 2014). In line with Styer et al. (2005), no significant difference in live birth rate (LBR) and miscarriage rate (MR) was highlighted and pregnancy outcome seemed not to be influenced by E2 dynamics (Styer et al. 2005; Huang et al. 2014; Sunkara et al. 2015). Similar results were achieved by Zavy et al. (2014) who analyzed serum E2 levels on the day of hCG administration in 478 patients undergoing fresh IVF/ET. No difference was detected among the three selected groups (E2 <
< 2000
2000 pg/ml; E2 2000–4000
pg/ml; E2 2000–4000 pg/ml; E2
pg/ml; E2 >
> 4000
4000 pg/ml) in LBR and MR, thus suggesting that other factors, including maternal age and embryo quality, seem to be the main determinants of pregnancy outcomes (Zavy et al., 2014).
pg/ml) in LBR and MR, thus suggesting that other factors, including maternal age and embryo quality, seem to be the main determinants of pregnancy outcomes (Zavy et al., 2014).
LBR seemed to be mainly affected by embryo quality also in the retrospective study by Mackens et al. (2020), which focused on monitoring serum E2 levels in endometrial preparation protocols of patients undergoing FET. In line with previous results, no significant difference in pregnancy outcome was detected among the three groups defined by serum E2 concentrations at the start of progesterone supplementation (E2 <
< 144
144 pg/ml; E2 145–438
pg/ml; E2 145–438 pg/ml; E2
pg/ml; E2 >
> 439
439 pg/ml) (Mackens et al., 2020). Also, Morales et al. (2021), analyzing 181 clinical records, assumed that serum E2 measurements on the day of hCG trigger represent a valid predictor of oocyte retrieval, maturation and subsequent fertilization, but no significant association further existed between the observed E2 concentrations and pregnancy outcome. Among the studies showing a possible association between serum estrogen levels and pregnancy outcome, Kondapalli et al. (2012) retrospectively focused on E2 dynamics (ΔE2) before and after hCG administration in 1712 patients undergoing IVF. Participants were divided into three groups (ΔE2
pg/ml) (Mackens et al., 2020). Also, Morales et al. (2021), analyzing 181 clinical records, assumed that serum E2 measurements on the day of hCG trigger represent a valid predictor of oocyte retrieval, maturation and subsequent fertilization, but no significant association further existed between the observed E2 concentrations and pregnancy outcome. Among the studies showing a possible association between serum estrogen levels and pregnancy outcome, Kondapalli et al. (2012) retrospectively focused on E2 dynamics (ΔE2) before and after hCG administration in 1712 patients undergoing IVF. Participants were divided into three groups (ΔE2 >
> 10%, −10% ≤ ΔE2
10%, −10% ≤ ΔE2 ≤
≤ 10%, and ΔE2 < −10%): while LBR was significantly higher in women with ΔE2
10%, and ΔE2 < −10%): while LBR was significantly higher in women with ΔE2 >
> 10%, no difference resulted in MR (Kondapalli et al., 2012). Despite the retrospective design and heterogeneous stimulation protocols, the adjustment for multiple confounding factors and the large sample size make the results of interest. Similar results were achieved by Wang et al. (2017), who retrospectively divided 3393 patients undergoing long GnRH-agonist protocol stimulation into two groups according to serum E2 levels on the day of hCG administration. Significantly increased LBRs were detected in the ‘high E2’ group (E2
10%, no difference resulted in MR (Kondapalli et al., 2012). Despite the retrospective design and heterogeneous stimulation protocols, the adjustment for multiple confounding factors and the large sample size make the results of interest. Similar results were achieved by Wang et al. (2017), who retrospectively divided 3393 patients undergoing long GnRH-agonist protocol stimulation into two groups according to serum E2 levels on the day of hCG administration. Significantly increased LBRs were detected in the ‘high E2’ group (E2 >
> 3757
3757 pg/ml) versus the ‘low E2’ group (E2
pg/ml) versus the ‘low E2’ group (E2 <
< 3757
3757 pg/ml), with no differences in delivery mode, gestational age (GA) at birth, birthweight, risk of preterm birth, and fetal malformations between the two groups (Wang et al., 2017). Nevertheless, the poor statistical analysis with no adjustment for any confounding factor makes interpretation of the results difficult. Furthermore, Li et al. (2019) observed that only the interval of medium-high E2 concentrations (1000–5000
pg/ml), with no differences in delivery mode, gestational age (GA) at birth, birthweight, risk of preterm birth, and fetal malformations between the two groups (Wang et al., 2017). Nevertheless, the poor statistical analysis with no adjustment for any confounding factor makes interpretation of the results difficult. Furthermore, Li et al. (2019) observed that only the interval of medium-high E2 concentrations (1000–5000 pg/ml) on hCG administration day correlates with better IVF and pregnancy outcomes. In fact, as previously noted by Steward et al. (2015), supraphysiological estradiol levels were associated with lower LBRs and higher MRs. E2
pg/ml) on hCG administration day correlates with better IVF and pregnancy outcomes. In fact, as previously noted by Steward et al. (2015), supraphysiological estradiol levels were associated with lower LBRs and higher MRs. E2 >
> 5000
5000 pg/ml was additionally identified as an independent risk factor for low birthweight (LBW) newborns, with a reported odds ratio (OR) of 16.8 (Li et al., 2019). These observations may explain the controversial results previously reported, as an inverted U-shaped association between E2 concentrations and IVF/pregnancy outcome probably involves an optimal serum E2 concentration range, outside of which both extremes are determinants of adverse reproductive outcome. Similar results are reported by Joo et al. (2010), who retrospectively analyzed 494 fresh IVF/ET cycles. The five groups defined according to serum E2 levels on the day of hCG administration (E2
pg/ml was additionally identified as an independent risk factor for low birthweight (LBW) newborns, with a reported odds ratio (OR) of 16.8 (Li et al., 2019). These observations may explain the controversial results previously reported, as an inverted U-shaped association between E2 concentrations and IVF/pregnancy outcome probably involves an optimal serum E2 concentration range, outside of which both extremes are determinants of adverse reproductive outcome. Similar results are reported by Joo et al. (2010), who retrospectively analyzed 494 fresh IVF/ET cycles. The five groups defined according to serum E2 levels on the day of hCG administration (E2 <
< 1000
1000 pg/ml; E2 1000–2000
pg/ml; E2 1000–2000 pg/ml; E2 2000–3000
pg/ml; E2 2000–3000 pg/ml; E2 3000–4000
pg/ml; E2 3000–4000 pg/ml; E2
pg/ml; E2 >
> 4000
4000 pg/ml) highlighted a significant difference in implantation, pregnancy, and LBRs, further suggesting optimal age-dependent ranges of serum E2 (3000–4000
pg/ml) highlighted a significant difference in implantation, pregnancy, and LBRs, further suggesting optimal age-dependent ranges of serum E2 (3000–4000 pg/ml for women <38
pg/ml for women <38 years and 2000–3000
years and 2000–3000 pg/ml for women ≥38
pg/ml for women ≥38 years) (Joo et al., 2010). Wu et al. (2012) added that patients with elevated progesterone and E2 concentrations (E2
years) (Joo et al., 2010). Wu et al. (2012) added that patients with elevated progesterone and E2 concentrations (E2 >
> 19124
19124 pmol/ml, i.e. E2
pmol/ml, i.e. E2 >
> 5209
5209 pg/ml) on the day of hCG administration have significantly lower LBRs and higher ectopic pregnancy rates, assuming that, when combined with premature elevation of progesterone, high E2 concentrations may have a potential detrimental effect on pregnancy outcome (Wu et al., 2012).
pg/ml) on the day of hCG administration have significantly lower LBRs and higher ectopic pregnancy rates, assuming that, when combined with premature elevation of progesterone, high E2 concentrations may have a potential detrimental effect on pregnancy outcome (Wu et al., 2012).
In autologous FET cycles, where exogenous estrogen administration is an essential step for endometrial preparation, Fritz et al. (2017) observed a significant inverse association between LBRs and average and peak serum E2 levels, measured from initiation of the artificial FET cycle to the last level prior to progesterone supplementation (LBRs from 54% for peak serum E2 levels below 234 pg/ml to 9% for peak serum E2 levels above 692
pg/ml to 9% for peak serum E2 levels above 692 pg/ml) (Fritz et al., 2017). Again, these results highlight that, even in FET cycles and in line with fresh IVF, serum E2 levels should be monitored and held in a relatively narrow range for optimizing endometrial receptivity without detrimental effects of supraphysiological concentrations. Furthermore, Li et al. (2022), in the setting of a retrospective study on 776 FET cycles and in multi-adjusted models including confounding factors, suggested that elevated E2 levels on progesterone-initiation-day may negatively affect pregnancy outcomes only after cleavage-stage embryo transfers and not when transferring blastocysts (Li et al., 2022). Pregnancy outcomes following frozen-thawed blastocyst transfer appear not to be influenced by estrogen levels, as also shown in the study by Choi et al. (2021). Again, these results suggest a shortened receptivity window, caused by supraphysiological E2 levels, that may be missed by a cleavage-stage ET, with no impact on a late-growing blastocyst. This is also confirmed in the retrospective study by Romanski et al. (2021) on patients undergoing natural FET. In this study, frozen blastocysts were transferred in natural cycles without exogenous hormonal stimulation. In this setting, the length of endogenous estrogenic stimulation may have a positive impact on pregnancy outcome: in fact, the authors reported a significantly lower LBR (46.6%) in patients with E2
pg/ml) (Fritz et al., 2017). Again, these results highlight that, even in FET cycles and in line with fresh IVF, serum E2 levels should be monitored and held in a relatively narrow range for optimizing endometrial receptivity without detrimental effects of supraphysiological concentrations. Furthermore, Li et al. (2022), in the setting of a retrospective study on 776 FET cycles and in multi-adjusted models including confounding factors, suggested that elevated E2 levels on progesterone-initiation-day may negatively affect pregnancy outcomes only after cleavage-stage embryo transfers and not when transferring blastocysts (Li et al., 2022). Pregnancy outcomes following frozen-thawed blastocyst transfer appear not to be influenced by estrogen levels, as also shown in the study by Choi et al. (2021). Again, these results suggest a shortened receptivity window, caused by supraphysiological E2 levels, that may be missed by a cleavage-stage ET, with no impact on a late-growing blastocyst. This is also confirmed in the retrospective study by Romanski et al. (2021) on patients undergoing natural FET. In this study, frozen blastocysts were transferred in natural cycles without exogenous hormonal stimulation. In this setting, the length of endogenous estrogenic stimulation may have a positive impact on pregnancy outcome: in fact, the authors reported a significantly lower LBR (46.6%) in patients with E2 >
> 100
100 pg/ml for <4
pg/ml for <4 days in the follicular phase compared to patients with E2
days in the follicular phase compared to patients with E2 >
> 100
100 pg/ml for more than 4
pg/ml for more than 4 days (52.0%) (Romanski et al., 2021). This result supports the idea that sustained physiological E2 concentrations can result in adequate endometrial receptivity, unlike the detrimental endometrial effects of prolonged supraphysiological E2 concentrations. Regarding the association between follicular estrogenic concentrations, placentation and pregnancy complications, the results in humans seem concordant when pregnancies are achieved in the setting of high E2 levels after controlled ovarian stimulation for IVF. An exception is the Dunne et al. (2017) study, which did not find associations between estrogen concentrations, early markers of placental development (pregnancy-associated plasma protein-A (PAPP-a)) and composite adverse maternal and neonatal birth outcomes associated with placental dysfunction (i.e. fetal growth restriction, pregnancy-induced hypertension). Farhi et al. (2010) retrospectively reported higher rates (10.1%) of placenta-related pregnancy complications (fetal growth restriction, pregnancy-induced hypertension and abnormal implantation of the placenta) in patients with E2 concentrations above the 75th percentile (=9000
days (52.0%) (Romanski et al., 2021). This result supports the idea that sustained physiological E2 concentrations can result in adequate endometrial receptivity, unlike the detrimental endometrial effects of prolonged supraphysiological E2 concentrations. Regarding the association between follicular estrogenic concentrations, placentation and pregnancy complications, the results in humans seem concordant when pregnancies are achieved in the setting of high E2 levels after controlled ovarian stimulation for IVF. An exception is the Dunne et al. (2017) study, which did not find associations between estrogen concentrations, early markers of placental development (pregnancy-associated plasma protein-A (PAPP-a)) and composite adverse maternal and neonatal birth outcomes associated with placental dysfunction (i.e. fetal growth restriction, pregnancy-induced hypertension). Farhi et al. (2010) retrospectively reported higher rates (10.1%) of placenta-related pregnancy complications (fetal growth restriction, pregnancy-induced hypertension and abnormal implantation of the placenta) in patients with E2 concentrations above the 75th percentile (=9000 pmol/l) compared with patients with E2 lower than the 75th percentile (1.4%), identifying high E2 concentration on hCG day as the only independent variable associated with increased risks for pregnancy complications in multi-adjusted regression models (adjusted OR 14.1) (Farhi et al., 2010). In line with these results, Pereira et al. (2015) reported a significantly raised risk of LBW newborns in patients undergoing fresh IVF/ET cycles, with peak E2 levels > 3069
pmol/l) compared with patients with E2 lower than the 75th percentile (1.4%), identifying high E2 concentration on hCG day as the only independent variable associated with increased risks for pregnancy complications in multi-adjusted regression models (adjusted OR 14.1) (Farhi et al., 2010). In line with these results, Pereira et al. (2015) reported a significantly raised risk of LBW newborns in patients undergoing fresh IVF/ET cycles, with peak E2 levels > 3069 pg/ml (Pereira et al., 2015). The same group observed that the risk of LBW increases up to 6.1–7.9 times when serum E2 levels on hCG administration day are above 2500
pg/ml (Pereira et al., 2015). The same group observed that the risk of LBW increases up to 6.1–7.9 times when serum E2 levels on hCG administration day are above 2500 pg/ml, revealing E2 as an independent predictor for LBW rate with adjusted OR of 10.8 (Pereira et al., 2015). LBW rates in IVF-conceived pregnancies are also reported to be associated with the number of retrieved oocytes during controlled ovarian stimulation. Sunkara et al. (2015) observed that high responder patients (cutoff: >20 follicles retrieved) are significantly more prone to develop fetal growth restriction, as a possible indirect effect of extreme supraphysiological hyper-estrogenism (Sunkara et al., 2015). Tarlatzi et al. (2021), as previously noted by Liu et al. (2017) and Kalra et al. (2011), confirmed the association between supraphysiologic E2 levels and LBW, highlighting a significant difference in birthweight by comparing fresh IVF cycles and FET. Although fetal growth restriction burdens both ART procedures, fresh cycles have shown significantly lower birthweight compared to FET, probably as a consequence of higher periconceptional estrogenic concentrations, which are reported to be related to lower β-hCG levels during the first trimester and abnormal placentation (Kalra et al., 2011; Liu et al., 2017; Tarlatzi et al., 2021).
pg/ml, revealing E2 as an independent predictor for LBW rate with adjusted OR of 10.8 (Pereira et al., 2015). LBW rates in IVF-conceived pregnancies are also reported to be associated with the number of retrieved oocytes during controlled ovarian stimulation. Sunkara et al. (2015) observed that high responder patients (cutoff: >20 follicles retrieved) are significantly more prone to develop fetal growth restriction, as a possible indirect effect of extreme supraphysiological hyper-estrogenism (Sunkara et al., 2015). Tarlatzi et al. (2021), as previously noted by Liu et al. (2017) and Kalra et al. (2011), confirmed the association between supraphysiologic E2 levels and LBW, highlighting a significant difference in birthweight by comparing fresh IVF cycles and FET. Although fetal growth restriction burdens both ART procedures, fresh cycles have shown significantly lower birthweight compared to FET, probably as a consequence of higher periconceptional estrogenic concentrations, which are reported to be related to lower β-hCG levels during the first trimester and abnormal placentation (Kalra et al., 2011; Liu et al., 2017; Tarlatzi et al., 2021).
Finally, Imudia et al. (2012) showed that elevated E2 levels (E2 >
> 3450
3450 pg/ml) on the day of hCG administration during ovarian stimulation is associated with greater odds of developing pre-eclampsia and small-for-GA infants in singleton pregnancies resulting from IVF cycles. On the other hand, Chen et al. (2020) revealed an opposite association between serum E2 concentration and the risk of pre-eclampsia in fresh IVF/ET-conceived pregnancies, reporting lower estrogenic levels, together with excessive maternal weight gain, as the main risk factor for pre-eclampsia in a multivariate analysis (Chen et al., 2020). Despite the apparently contradictory results, different E2 concentrations are reported as a risk factor: in fact, Chen et al. defined E2 lower than 1200
pg/ml) on the day of hCG administration during ovarian stimulation is associated with greater odds of developing pre-eclampsia and small-for-GA infants in singleton pregnancies resulting from IVF cycles. On the other hand, Chen et al. (2020) revealed an opposite association between serum E2 concentration and the risk of pre-eclampsia in fresh IVF/ET-conceived pregnancies, reporting lower estrogenic levels, together with excessive maternal weight gain, as the main risk factor for pre-eclampsia in a multivariate analysis (Chen et al., 2020). Despite the apparently contradictory results, different E2 concentrations are reported as a risk factor: in fact, Chen et al. defined E2 lower than 1200 pg/ml as a risk factor for pre-eclampsia, while Imudia et al. reported high supraphysiological levels (>3450
pg/ml as a risk factor for pre-eclampsia, while Imudia et al. reported high supraphysiological levels (>3450 pg/ml) as a detrimental risk factor.
pg/ml) as a detrimental risk factor.
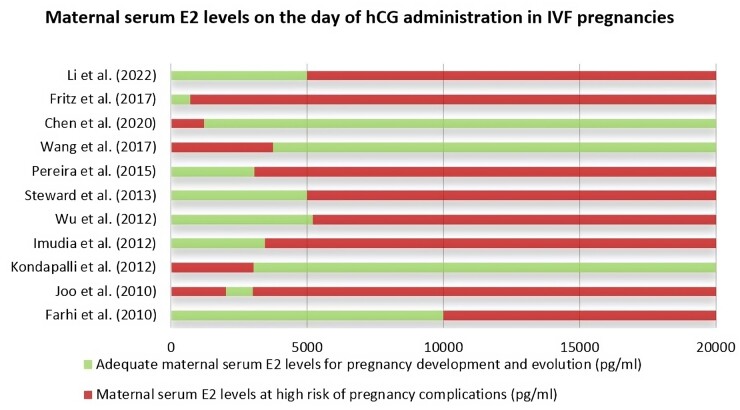
While all the presented studies are surely limited by the retrospective design and comparisons may be affected by differences in study populations, protocols used for ovarian stimulation or endometrial preparation, and day on which steroid hormone levels were measured, a strict serum E2 concentration window in artificial settings with hormonal manipulation appears to be optimal in order to improve reproductive outcomes in terms of both LBR and placental efficiency. Figure 2 shows the E2 levels on the day of hCG administration described in the literature as adequate for pregnancy progression or at risk for pregnancy complications.
Oocyte donation
Advances in ART have allowed women who cannot use their own oocytes to achieve a pregnancy through OD. OD is associated with the highest success rates among ART procedures and nowadays has become a common treatment option, especially to overcome infertility linked to advanced maternal age. This success has led to an increasing interest in the impact of OD on several aspects of obstetrical care, especially pregnancy outcomes (Savasi et al., 2016). However, despite increasing interest in this ART procedure, many issues remain unclear, such as the hormonal environment in both the donor and recipient, and its possible manipulation. In OD, the donor's gametogenesis and ovarian steroidogenesis are dissociated from the recipient’s endometrial development and receptivity, allowing the effects of elevated E2 concentrations on the embryo to be distinguished from those on the endometrium.
Considering endometrial preparation, the most recent meta-analysis concluded that it is not yet possible to recommend one protocol over another in FET cycles (Groenewoud et al., 2013). However, for fresh or FET two methods can be used to attain a receptive endometrium for embryo implantation: through a ‘natural cycle’ (synchronization of the embryo transfer with recipient’s ovulation) or through the administration of exogenous steroids: in the latter case, estrogens are supplied either orally or transdermally from Day 1 of the menstrual cycle in which the embryo transfer will be performed, and estrogen can be administered either in an increasing dose or a constant dose from the beginning of the menstrual cycle. Madero et al. (2016) compared a constant versus an increasing E2 dose protocol in 8236 heterologous ETs. No significant difference resulted in terms of LBR, as well as in MR, between the two protocols. No difference has been detected among various routes of estrogen administration. These data, in contrast with previous findings in autologous ART procedures, suggest that the estrogen dose for endometrial preparation does not impact reproductive outcomes as much as other variables, such as alterations of the BMI, embryo quality and oocyte donor’s age (Madero et al., 2016).
On the donor side, Bianco et al. (2009) compared 58 oocyte donors undergoing controlled ovarian stimulation, distributed into two groups according to E2 levels on the day of hCG administration (E2 <
< 2000
2000 pg/ml and E2
pg/ml and E2 >
> 2000
2000 pg/ml). Significantly higher oocyte retrieval was achieved in the ‘high E2’ group, in line with evidence reported by Peña et al. (2002). However, no difference in terms of LBR and other obstetric outcomes occurred following embryo transfer in the recruited recipients (Bianco et al., 2009). Surprisingly, Palmerola et al. (2018) observed in 366 OD cycles that low and very low E2 levels on the day of hCG trigger (low E2: 50–100
pg/ml). Significantly higher oocyte retrieval was achieved in the ‘high E2’ group, in line with evidence reported by Peña et al. (2002). However, no difference in terms of LBR and other obstetric outcomes occurred following embryo transfer in the recruited recipients (Bianco et al., 2009). Surprisingly, Palmerola et al. (2018) observed in 366 OD cycles that low and very low E2 levels on the day of hCG trigger (low E2: 50–100 pg/ml per retrieved oocyte; very low E2
pg/ml per retrieved oocyte; very low E2 <
< 50
50 pg/ml per retrieved oocyte) are common (20.2%) and not associated with poorer pregnancy rates and outcomes. Unlike autologous IVF cycles, serum E2 levels in the donor seem to have no effect on recipient pregnancy outcomes, indicating that the effects of E2 on reproductive outcomes are mainly related to its effect on endometrial preparation and receptivity. Furthermore, these differences may have other explanations, including reduced ovarian reserve, oocyte quality, and the effects of aging in women undergoing autologous IVF/ET cycles (Palmerola et al., 2018).
pg/ml per retrieved oocyte) are common (20.2%) and not associated with poorer pregnancy rates and outcomes. Unlike autologous IVF cycles, serum E2 levels in the donor seem to have no effect on recipient pregnancy outcomes, indicating that the effects of E2 on reproductive outcomes are mainly related to its effect on endometrial preparation and receptivity. Furthermore, these differences may have other explanations, including reduced ovarian reserve, oocyte quality, and the effects of aging in women undergoing autologous IVF/ET cycles (Palmerola et al., 2018).
Unfortunately, very limited evidence is available on this topic. Further investigation is necessary to better understand the mechanisms and clinical implications of estrogenic concentrations on both sides of the donor–recipient couple. On the donor side, as seen in autologous ovarian hyperstimulation, reference E2 ranges should be established in order to maximize oocyte retrieval and embryo quality. On the recipient side, instead, suitable endometrial preparation protocols should be created, with the goal of optimizing implantation and placentation. Considering that OD pregnancies are per se associated with a higher rate of placental disorders of pregnancy compared to other IVF pregnancies, avoiding further risk factors for abnormal trophoblastic invasion is highly warranted (van der Hoorn et al., 2010; Savasi et al., 2016) (Table 1).
Table 1.
Studies evaluating the association between estradiol levels during the periconceptional period and pregnancy outcomes in ART-conceived pregnancies.
| Study (year) | Sample size | Age of patients | Stimulation protocol | Type of ART procedure | Type of association between E2 levels during the periconceptional period and pregnancy outcomes |
|---|---|---|---|---|---|
| Styer et al. (2005) | 3653 IVF cycles | 31 ± ± 5 5 years years | GnRH agonist long protocol | Fresh IVF/ET | No association |
| Farhi et al. (2010) |
| <38 years years | GnRH agonist long protocol | Fresh IVF/ET | Higher placenta-related pregnancy complications for E2 >10.000 pg/ml pg/ml |
| Joo et al. (2010) | 455 IVF cycles | 34 ± ± 4.5 4.5 years years | GnRH agonist long or short protocol | Fresh IVF/ET |
|
| Kondapalli et al. (2012) | 1712 IVF cycles | 21–45 years years | GnRH agonist protocol, GnRH antagonist protocol, microdose flare protocol | Fresh IVF/ET |
|
| Imudia et al. (2012) | 1301 fresh IVF/ET cycles | 34 ± ± 4 4 years years | GnRH agonist protocol or GnRH antagonist protocol | Fresh IVF/ET | Higher risk of pre-eclamspia and SGA for E2 >3450 pg/ml pg/ml |
| Wu et al. (2012) | 2921 patients | 31 ± ± 4 4 years years | GnRH agonist long or short protocol | Fresh IVF/ET | Lower LBR and higher ectopic pregnancy rates for E2 >5209 pg/ml pg/ml |
| Steward et al. (2014) | 71 cycles | 33 ± ± 4 4 years years | LPS protocol | Fresh IVF/ET |
|
| Huang et al. (2014) | 2868 patients | 31 ± ± 4 4 years years | GnRH agonist long or short protocol | Fresh IVF/ET | No association |
| Zavy et al. (2014) | 478 patients | <40 years years |
| Fresh IVF/ET | No association |
| Pereira et al. (2015) | 5618 patients | 35 ± ± 4 4 years years | GnRH agonist long protocol | Fresh IVF/ET | Higher risk of LBW and SGA for E2 >3069 pg/ml pg/ml |
| Sunkara et al. (2015) | 402 185 IVF cycles 185 IVF cycles | Not mentioned | Not mentioned | Fresh IVF/ET | Higher risk of FGR in high responder patients |
| Dunne et al. (2017) | 216 patients | Not mentioned | Not mentioned | Fresh IVF/ET | No association |
| Wang et al. (2017) | 3393 patients | <40 years years | GnRH agonist long protocol | Fresh IVF/ET | Higher LBR in ‘High E2 group’ |
| Chen et al. (2020) | 622 patients | 35 ± ± 5 5 years years | GnRH agonist long protocol or GnRH antagonist protocol | Fresh IVF/ET | Higher risk of Preeclampsia for low E2 levels |
| Morales et al. (2021) | 181 patients | 18–56 years years | GnRH antagonist protocol | Fresh IVF/ET | No association |
| Fritz et al. (2017) |
| 30–36 years years | GnRH agonist suppression + transdermal or intramuscular estradiol valerate | FET | Lower LBR for E2 >692 pg/ml pg/ml |
| Mackens et al. (2020) |
| 31–34 ± ± 5 5 years years | Oral estradiol valerate | FET | No association |
| Romanski et al. (2021) |
|
| GnRH antagonists or short GnRH agonist protocols | FET | Lower LBR in patients with an elevated estradiol to surge of <4 days compared to patients with an elevated estradiol to surge of >4 days compared to patients with an elevated estradiol to surge of >4 days days |
| Li et al. (2022) |
| 31 ± ± 4 4 years years | Not mentioned | FET | Worse pregnancy outcomes in cleavage-stage embryos in elevated E2 levels |
| Choi et al. (2021) |
| 18–43 years years | Vaginal estradiol | FET | No association between E2 levels in blastocyst embryo-transfer |
| Kalra et al. (2011) | 38 626 fresh IVF/ET cycles and 18 626 fresh IVF/ET cycles and 18 166 FET cycles 166 FET cycles | 34 ± ± 4 4 years years | Not mentioned | Fresh IVF/ET vs FET | Higher risk of LBW in fresh IVF/ET cycles |
| Liu et al. (2017) | 3191 FET cycles and 3770 fresh IVF/ET cycles | 31 ± ± 4 4 years years |
| Fresh IVF/ET vs FET | Higher risk of LBW in fresh IVF/ET cycles |
| Tarlatzi et al. (2021) | 2885 fresh IVF/ET cycles and 746 FET cycles | Mean age 32 years years |
| Fresh IVF/ET vs FET | Higher risk of LBW in fresh IVF/ET cycles |
| Bianco et al. (2009) | 58 patients (oocyte donors) | 21–34 years years | Oral micronized estradiol | OD | No association |
| Madero et al. (2016) |
| Not mentioned |
| OD | No association |
FET, frozen embryo-transfer; IVF/ET, IVF/embryo-transfer; E2, estradiol; IR, implantation rate; PR, pregnancy rate; LBR, live birth rate; MR, miscarriage rate; LBW, low birthweight; CD, constant dose; ID, increasing dose; OD, oocyte donation.
Molecular mechanisms
Uterine receptivity falls within a discrete temporal window of the menstrual cycle, although the underlying molecular mechanisms are not fully understood (Critchley et al., 2020). Estrogens participate in both phases of the menstrual cycle, although they are prominent in the follicular phase by promoting epithelial cell proliferation and tissue regeneration upon menses. ERα expression in the endometrium increases during the proliferative phase, to decrease after ovulation during the subsequent secretory phase that is dominated by progesterone. An abnormal increase of local ERα expression during the secretory phase has been associated with infertility, PCOS, and endometriosis (Lessey et al., 2006; Young and Lessey, 2010). For instance, in infertile women local ERα overexpression in the mid-secretory stage was accompanied by reduced levels of glycodelin-A (Dorostghoal et al., 2018), one of the major progesterone-regulated proteins secreted into the uterine luminal cavity by secretory/decidualized endometrial glands, and with immunosuppressive properties required for successful implantation (Seppälä et al., 2007). On the other hand, studies in mice models showed a specific role for estrogen signaling in altering protease activity in the oviduct, a physiological regulation required for successful fertilization and preimplantation embryo development, which is impaired in transgenic mice lacking ERα (Winuthayanon et al., 2015). Therefore, a strict spatio-temporal regulation of estrogen signaling is needed during the early stages of blastocyst implantation and survival. Likewise, local ERβ expression seems to be upregulated by estrogens and downregulated by progesterone in mice (Wada-Hiraike et al., 2006). Although widely used, the mouse model exhibits some limitations and discrepancies compared to humans, and observations should be carefully considered (Ajayi and Akhigbe, 2020). In fact, in humans such changes are less prominent compared to ERα changes across the menstrual cycle, and the role of ERβ in the pathophysiological events surrounding reproduction remains poorly investigated (Lecce et al., 2001; Hapangama et al., 2015).
Based on this evidence, a possible explanation proposed during the past two decades relies on the ‘implantation window’ theory (Simón et al., 1996). While local ovarian (i.e. follicular fluid) estrogen concentrations are better defined, local uterine estrogen concentrations are understudied, thus making speculation on local steroid effects incomplete. Nevertheless, studies investigating serum and endometrial specimens were performed in patients with endometriosis and controls, eventually revealing tissue concentrations similar to the serum levels during the menstrual cycle (Huhtinen et al., 2014). At the cellular level, estrogens might prime the functional activation of the endometrium facilitating a receptive/permissive phase, then maintained by paracrine/autocrine signals, rather than a direct regulatory action (Ghosh et al., 1994; Simón et al., 1996; Borini et al., 2001; Niu et al., 2008). Following this theory, estrogens would need to reach the threshold in order to trigger the morphological and biological changes resulting in endometrial receptivity, in both the epithelium and stromal compartments, favoring both embryo attachment and implantation maintenance, respectively (Simón et al., 1996; Borini et al., 2001; Niu et al., 2008). This might explain the apparent discrepancies observed in vivo between estrogen levels and pregnancy/implantation rate, and thickness of endometrium (Sauer et al., 1990; De Ziegler et al., 1991).
Following an estrogenic trigger, a number of effector molecules are secreted either by direct action of estrogens or via paracrine cell signaling, including growth factors, cytokines, and tissue remodeling factors, such as matrix metalloproteases and their inhibitors, along with cell surface and adhesion molecules, which may facilitate embryo attachment and decidua invasion (Simón et al., 1996). Among these, here we mention selected molecules that have long been implicated in the early events of reproduction, also based on their relevance to clinical observations. The glycoprotein leukemia-inhibiting factor (LIF) is produced by the endometrium during the secretory phase of the menstrual cycle under the control of both E2 and progesterone (Aghajanova, 2004). LIF is a pleiotropic cytokine of the IL-6 family, with effects on multiple cells of different lineages. LIF was shown to inhibit embryonic stem cell differentiation in vitro and is essential for embryo implantation in mice. Although, endometrial fluids and tissue from infertile women display reduced levels of LIF, and LIF receptors are present in the human blastocyst, its crucial role remains to be demonstrated in human pregnancy (Lass et al., 2001).
Changes in the composition of the uterine sugar molecules expressed on the surface of epithelial cells, i.e. glycocalyx, have also been observed during the peri-implantation period. Expression of the large transmembrane glycoprotein mucin 1 (MUC-1) achieves peak levels during the secretory phase and is lost at the site of embryo attachment, perhaps triggered directly or indirectly by the blastocyst upon interacting with the endometrium (Brayman et al., 2004). Although a clear role of estrogens in the regulation of MUC-1 has yet to be demonstrated, this process may affect endometrial receptivity by modulating the embryo’s access to adhesion molecules, e.g. cadherins and integrins, which would be otherwise masked by larger molecules such as MUC-1. In line with this hypothesis, women who suffer recurrent spontaneous miscarriages display reduced levels of MUC-1 in endometrial fluids, suggesting a role for defects in the MUC-1 release system in infertility (Hey et al., 1995). On the other hand, extremely low levels of MUC-1, as well as of glycodelin A, in endometrial biopsies have also been associated with recurrent implantation failure (Bastu et al., 2015), thus highlighting the importance of location, as well as timing, in the study of early reproduction.
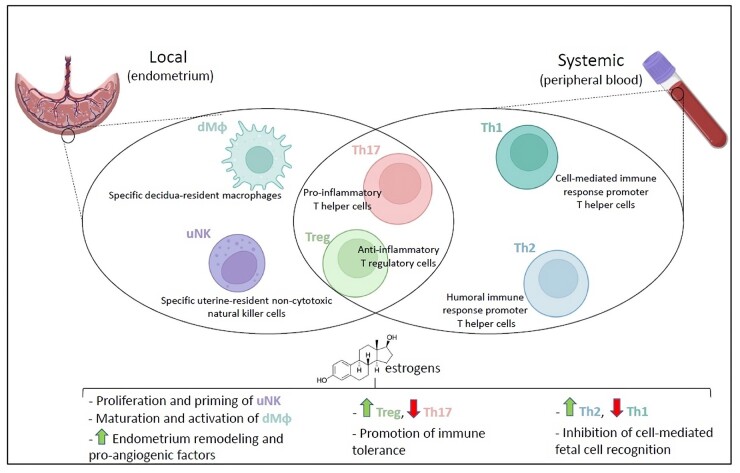
Immune cells react to the hormonal fluctuations of the menstrual cycle, both at the peripheral and locally at the uterine level, although the underlying molecular mechanisms are not fully elucidated (Oertelt-Prigione, 2012). The main immune cell types involved in such fluctuation and, later on, in pregnancy are uNK cells, macrophages and T lymphocytes, whose contribution consist of approximatively 60%, 25%, and 10% of the leukocytes found locally in the decidua, respectively (Trundley and Moffett, 2004). However, the percentages reported herein may greatly vary throughout the menses. Typically, uNK proliferate during the follicular phase, to reach a peak during the late follicular phase and part of the luteal phase (Lee et al., 2010). This is compatible with the observation proposed by Borzychowski et al. (2003) that uNK cells require an estrogen priming to proliferate and reach their functional stage, although they respond to both estrogens and progesterone once activated (Kalkunte et al., 2008). During the follicular phase, uNK cells contribute to the endometrium tissue and vascular remodeling by secreting vascular endothelial growth factor (VEGF) and chemokines in an estrogen-dependent manner, ultimately resulting in endometrial receptivity (Gibson et al., 2015; Wang et al., 2021). Decidual macrophages are endometrium-resident specialized cells that are involved in the menstrual cycle and during pregnancy (Tian et al., 2020). Unlike uNK cells, which robustly proliferate during the follicular phase and are reduced in numbers during the late luteal phase and throughout the gestation (Aghaeepour et al., 2017), decidual macrophages are thought to maintain an overall fairly constant number (Tian et al., 2020). Unexpectedly, during the estrogen-driven follicular phase, the implantation window and at the very beginning of the pregnancy, decidual macrophages build a pro-inflammatory environment by secreting IL-1, following the estrogen peak (Mathur et al., 1979). Such a transitory pro-inflammatory environment was confirmed by observations reported in the mouse model. Indeed, within hours of the insemination, uterine cells synthesize the granulocyte macrophage colony-stimulating factor (GM-CSF) (Robertson et al., 1992), a cytokine that promotes proliferation, maturation and activation of macrophages, which were found mainly skewed towards the pro-inflammatory classical phenotype M1 at this early stage (Jaiswal et al., 2012). Moreover, in an in vitro experiment performed on cultured human endometrial cells, the expression of IL-8, which serves to both promote leukocyte recruitment and activate neutrophils, was increased in an estrogen dose-dependent manner (DeLoia et al., 2002), as confirmed in vivo (Arici et al., 1998). It is controversial how this transitory pro-inflammatory environment could be beneficial. It was proposed that this may support the blastocyst during the ‘break through’ of the endometrial tissue, in order to invade (Mor et al., 2011). On the other hand, it could promote menses in the absence of implantation (DeLoia et al., 2002). Finally, T lymphocytes are subjected to estrogens fluctuations. The T regulatory cell (Treg) subpopulation expands during the follicular phase, reaching a pre-ovulatory mid-cycle peak, to then significantly decrease during the luteal phase (Oertelt-Prigione, 2012). Low estrogen levels were correlated with a lower Treg activity, rather than a lower frequency (Arruvito et al., 2007), suggesting that Treg proliferation is not entirely dependent on estrogens. The extent and the magnitude of a direct effect of estrogens on immune cells has yet to be defined.
The early stages of pregnancy
Although the associations between maternal sex steroid levels and short-term (pregnancy loss, pre-eclampsia) and long-term health outcomes (risk of maternal and offspring steroid-sensitive cancers, autistic spectrum and atopic disorders in children) are becoming progressively evident, the role of estrogens in early naturally conceived pregnancies is still poorly understood (Holl et al., 2009). From promoting endometrial cell proliferation and embryo implantation, to modulating maternal cardiovascular adaptations to pregnancy, the effect of estrogens on trophoblast development and function remains controversial (Chang and Lubo, 2008; Burton et al., 2009; King and Critchley, 2010; Baud and Berkane, 2019).
Natural conception
Unexplained large variations in E2 levels have been detected among pregnant women. Estrogen concentrations in early natural pregnancy are strongly correlated with GA (r =
= 0.71) (Toriola et al., 2011). Our group built GA-specific reference intervals for maternal E2 concentrations during early singleton natural pregnancies, further providing a mathematic model for E2 assessment through an equation that takes into account progesterone concentrations and GA (logE2
0.71) (Toriola et al., 2011). Our group built GA-specific reference intervals for maternal E2 concentrations during early singleton natural pregnancies, further providing a mathematic model for E2 assessment through an equation that takes into account progesterone concentrations and GA (logE2 =
= 1.96
1.96 +
+ 0.01
0.01 ×
× GA + 0.004
GA + 0.004 ×
× progesteron) (Grossi et al., 2019). In this study, women aged at least 18
progesteron) (Grossi et al., 2019). In this study, women aged at least 18 years, without any known disease or drug consumption, as well as with no previous obstetric adverse outcomes (i.e. pre-eclampsia, intrauterine growth restriction) and carrying a singleton ongoing pregnancy (5+0–13+6 gestational weeks) with no obstetric complications, were enrolled and considered as a ‘physiological’ reference optimizing the external validity of the results. Data examining the associations between feto-maternal characteristics and estrogen concentrations during the first trimester of natural pregnancies showed higher E2 levels in younger nulliparous women carrying a female fetus (Toriola et al., 2011). In particular, E2 concentrations were 6% lower, 16% lower, and 9% higher in case of age higher than 30
years, without any known disease or drug consumption, as well as with no previous obstetric adverse outcomes (i.e. pre-eclampsia, intrauterine growth restriction) and carrying a singleton ongoing pregnancy (5+0–13+6 gestational weeks) with no obstetric complications, were enrolled and considered as a ‘physiological’ reference optimizing the external validity of the results. Data examining the associations between feto-maternal characteristics and estrogen concentrations during the first trimester of natural pregnancies showed higher E2 levels in younger nulliparous women carrying a female fetus (Toriola et al., 2011). In particular, E2 concentrations were 6% lower, 16% lower, and 9% higher in case of age higher than 30 years, multiparity, and a female fetus, respectively, whereas no associations were detected with maternal smoking (Toriola et al., 2011). More recently, a multi-centre American study on 548 singleton pregnancies showed a 1–2% decrease in estrogen levels for every unit increase in maternal BMI, whereas no associations were detected between early estrogen levels and gestational weight gain or dietary fat intake in a prospective Swedish study, thus indicating a likely independence of estrogen levels from maternal nutritional habits (Lof et al., 2009; Barrett et al., 2019). Despite being surprising, the authors hypothesize that the inverse association between BMI and estrogens could be mainly related to the fetoplacental origin of these steroids, that tend to be more diluted in the circulation of heavier mothers compared to normal weight controls. Finally, estrogen concentrations were found to be significantly lowered by alcohol use during pregnancy (Troisi et al., 2008).
years, multiparity, and a female fetus, respectively, whereas no associations were detected with maternal smoking (Toriola et al., 2011). More recently, a multi-centre American study on 548 singleton pregnancies showed a 1–2% decrease in estrogen levels for every unit increase in maternal BMI, whereas no associations were detected between early estrogen levels and gestational weight gain or dietary fat intake in a prospective Swedish study, thus indicating a likely independence of estrogen levels from maternal nutritional habits (Lof et al., 2009; Barrett et al., 2019). Despite being surprising, the authors hypothesize that the inverse association between BMI and estrogens could be mainly related to the fetoplacental origin of these steroids, that tend to be more diluted in the circulation of heavier mothers compared to normal weight controls. Finally, estrogen concentrations were found to be significantly lowered by alcohol use during pregnancy (Troisi et al., 2008).
Estrogens stimulate endometrial cell proliferation, myometrium thickness, uterine vascularization, and contraction force. As discussed above, E2 may represent a key factor in establishing and maintaining pregnancy (Verma et al., 2019; Zhang et al., 2019; Deng et al., 2022). In fact, E2 levels during early natural pregnancy may reflect the quality of the dominant follicle and the proper development and function of the corpus luteum (Salazar and Calzada, 2007; Gao et al., 2008). In line with this hypothesis, serum E2 concentrations were significantly lower in pregnant women undergoing spontaneous abortion than in those with a normal pregnancy (Gao et al., 2008). More recently, E2 concentrations showed a marked deviation in pregnancies evolving to miscarriage compared to normal pregnancies, reflecting a deficiency in the ovarian response (Whittaker et al., 2018). It has been hypothesized that E2 could prevent allogeneic fetal rejection by acting as a potent local immunomodulator, possibly explaining increased rates of miscarriage in case of E2 deficiency.
During pregnancy, the placenta represents the main site of estrogen release through the conversion of both maternal and fetal adrenal androgen precursors. Estrogens may play a role in vascular adaptations and development in both uterus and placenta. Firstly, on the uterine side, adaptations to pregnancy involve the growth of an arterial and venous axis in order to lower uterine vascular resistance and increase capacitance. Additionally, changes in the uterine vascular walls at cellular level (via endovascular cytotrophoblast invasion) and in matrix composition (impacting on vascular distensibility through changes of the extracellular matrix composition caused by metalloproteinases) are essential for modifying local vascular resistance and increasing blood flow to the intervillous space. The regulation of uterine vascular remodeling by estrogens has been suggested by the correlation between steroid plasma levels and uteroplacental blood flow modifications during pregnancy and the follicular phase of the ovarian cycle (Bernstein, 2002). To corroborate this hypothesis, exogenous estrogen administration to non-pregnant ovariectomized ewes induces uterine vasodilation and increases uterine blood flow, which was a reversible effect after the administration of ER inhibitors (Magness et al., 2005; Mandalà, 2020). ERs were detected both in endothelial and vascular smooth muscle of the uterine artery and are thought to modulate vasculature contractility and myogenic tone both directly (via transcriptional factor activity) and indirectly (i.e. via a nitric oxide (NO)-dependent pathway), eventually driving the described local adaptations to pregnancy (Chang and Lubo, 2008). Again, ovariectomized Guinea pigs injected with E2 showed uterine vascular and cellular changes similar to those seen in pregnancy (Makinoda and Moll, 1986). Secondly, estrogens, more specifically E2, may drive angiogenesis and vascular development on the placental side in multiple ways. Estrogens are thought to induce placental angiogenesis and vasodilation mainly by increasing vasoactive mediator (NO and prostacyclin) and angiogenic factor release (VEGF, placental growth factor: PlGF) (Rosenfeld and Rivera, 1978; Cullinan-Bove and Koos, 1993; Shifren et al., 1996; Caulin-Glaser et al., 1997; Hisamoto et al., 2001; Simoncini et al., 2002; Hervé et al., 2006; Johnson et al., 2006). In vitro human and animal studies demonstrated that E2 led to enhanced endothelial cell proliferation and migration, thus resulting in new vessel proliferation (Powazniak et al., 2009; Zhang et al., 2016; Liu et al., 2018). The same models additionally demonstrated that the suppression of estrogen in the first stages of pregnancy through specific aromatase inhibitors (letrozole) led to a marked suppression of maternal estrogen concentrations, increased androgen levels, reduced placental vessel network and pro-angiogenic gene expression, eventually resulting in increased pregnancy loss and reduced birthweight. This suggests an estrogen-dependent placental vasculogenesis and development as early as the first half of pregnancy (Albrecht et al., 2004; Albrecht and Pepe, 2010; Haneda et al., 2021). Moreover, estrogens may guide placental development by involving structural uterine reorganization of the extracellular matrix, as indicated by the correlation between local expression of metalloproteinases and maternal estrogen concentrations, as well as by the induced upregulation of metalloproteinase expression by estrogen in animal models (Schäfer-Somi et al., 2005; Dang et al., 2013).
Since proper vascular remodeling and maternal adaptation to pregnancy represent crucial determinants of appropriate placentation and pregnancy progression, a potential association between estrogen levels and the development of human placental insufficiency diseases has been widely investigated (Fig. 3). Whereas previous studies reported conflicting results on the association between low maternal blood estrogen concentrations and the development of pre-eclampsia, recent research has consistently shown lower serum estrogen levels in women diagnosed with pre-eclampsia compared to controls (Rahman et al., 1975; Notation and Tagatz, 1977; Rosing and Carlström, 1984; Zamudio et al., 1994; Hertig et al., 2010; Jobe et al., 2013). Although lower, estrogen serum concentration did not correlate with disease severity (Salas et al., 2006; Hertig et al., 2010; Bussen and Bussen, 2011; Jobe et al., 2013). Nevertheless, whether the E2 deficiency takes part in the pathogenic process or rather is a consequence of the pre-eclamptic insult is still a matter of debate. The lower estrogen concentrations of pre-eclamptic women can be linked to abnormal activity of the aromatase as a consequence of chronic hypoxia or abnormal hormonal signaling (i.e. increased leptin in obese pre-eclamptic women decreases placental estrogen production) (Coya et al., 2006; Perez-Sepulveda et al., 2015). Promising results have been shown using a therapeutic approach with short-term estrogens in ameliorating placental oxidative stress and clinical features of pre-eclampsia (Djordjević et al., 2010; Babic et al., 2018).
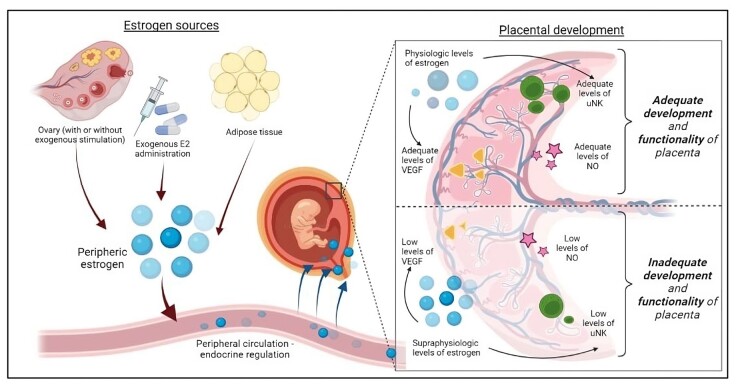
Influence of estrogen levels on appropriate placentation and pregnancy progression. The schematic represents crucial determinants of the development of human placental insufficiency diseases and compares the effects of adequate and supraphysiologic E2 levels on vascular remodeling and angiogenesis at the materno-fetal interface. E2, estradiol; VEGF, vascular endothelial growth factor; NO, nitric oxide; uNK, uterine natural killer.
Autologous ART
Limited data are available on this topic. The hormonal profile in the mid-luteal phase and initial stages of ART-conceived pregnancy has mainly been studied to find an early predictor of IVF/ET outcome as an alternative to β-hCG. Kumbak et al. (2006) and Sonntag et al. (2013) observed that serum E2 levels were significantly higher in conception cycles than in non-conception cycles from the very beginning of the luteal phase (4–8 days after ET). (Kumbak et al., 2006; Sonntag et al., 2013). Similar results emerged from the Vanderlelie et al. (2003) study, which showed that E2 concentrations on Day 6 after ET were significantly lower in those patients with no conception (Vanderlelie et al., 2003). Further analysis of the concentration of E2 on Day 6 found that patients who conceived had only a 7.9% chance of pregnancy success when E2 levels were below 600
days after ET). (Kumbak et al., 2006; Sonntag et al., 2013). Similar results emerged from the Vanderlelie et al. (2003) study, which showed that E2 concentrations on Day 6 after ET were significantly lower in those patients with no conception (Vanderlelie et al., 2003). Further analysis of the concentration of E2 on Day 6 found that patients who conceived had only a 7.9% chance of pregnancy success when E2 levels were below 600 pg/ml (Vanderlelie et al., 2003). In line with these findings, Melnick et al. (2016) highlighted a correlation between serum E2 levels on the 28th cycle day and pregnancy outcomes: LBRs were higher in those groups with medium (E2 51–100
pg/ml (Vanderlelie et al., 2003). In line with these findings, Melnick et al. (2016) highlighted a correlation between serum E2 levels on the 28th cycle day and pregnancy outcomes: LBRs were higher in those groups with medium (E2 51–100 pg/ml) and medium-high (E2
pg/ml) and medium-high (E2 >
> 100
100 pg/ml) E2 concentrations. Instead, biochemical pregnancy rates were higher in the low estrogen group (E2
pg/ml) E2 concentrations. Instead, biochemical pregnancy rates were higher in the low estrogen group (E2 <
< 50
50 pg/ml), suggesting that estrogen support is fundamental in early gestational phases (Melnick et al., 2016).
pg/ml), suggesting that estrogen support is fundamental in early gestational phases (Melnick et al., 2016).
Indeed, during the late first trimester, steroid sex hormones concentrations are significantly higher in in IVF, after pregnancies conceived after controlled ovarian stimulation + ET, compared to natural pregnancies. As already observed in the late 1980s and 1990s, and corroborated by Sun et al. (2019), higher steroid sex hormones concentrations influence the placenta metabolomic profile, biophysical aspects (i.e. the uterine artery Doppler velocimetry) and epigenetic reprogramming. However, so far, no conclusive literature supports a clear-cut scenario. Low levels of E2 are required for normal trophoblastic invasion in human pregnancies to avoid adverse pregnancy outcomes (Yovich et al., 1985; Johnson et al., 1993; Inversetti et al., 2018; Sun et al., 2019).
Comparing fresh IVF/ET cycles and FET, E2 levels at 4 and 8 gestational weeks are significantly higher in the former group, suggesting that the effect of controlled ovarian stimulation is stronger than any other endometrial preparation protocol. This may be related to the important role of the corpus luteum in hormone production during the first weeks of gestation: multiple corpora lutea can be found in patients undergoing oocyte retrieval, while the exogenous hormone administration for endometrial preparation used during IVF protocols produces pregnancy with no corpus luteum (Conrad et al., 2019). However, as previously mentioned, elevated estrogenic concentrations are associated with increased LBW rates in autologous ART pregnancies: better pregnancy outcomes were detected in FET cycles than in fresh ones (Hu et al., 2014). Moreover, as observed by Johnson et al. (1993) and Póvoa et al. (2018), E2 concentration during late first trimester (>8 gestational weeks) is greater in multiple pregnancies than in singleton ones (Johnson et al., 1993; Póvoa et al., 2018). Maternal serum E2 level at 8 weeks of gestation increased with the number of fetuses, showing a direct correlation with the number of placentas, the main responsible for hormone production from the 8th to 10th gestational week.
weeks of gestation increased with the number of fetuses, showing a direct correlation with the number of placentas, the main responsible for hormone production from the 8th to 10th gestational week.
In conclusion, as mentioned above, high estrogenic concentrations seem negatively associated with offspring birthweight. Evidence from animal and human models indicated that certain transient environmental influences could produce persistent changes in epigenetic marks, such as on imprinted genes, which are implicated in the regulation of fetal growth and development. As well as endocrine disruptors that can alter DNA methylation, resulting in transcriptional changes, high maternal E2 levels might induce epigenetic modifications, leading to LBW in a dose-dependent manner (Hu et al., 2022). However, evidence on this topic is still very limited and heterogeneous. Further studies could highlight optimal estrogenic levels to minimize adverse outcomes of pregnancy and a possible use of E2 concentration as an early marker of ART and pregnancy success.
Oocyte donation
A review of the literature has highlighted only a few studies, mainly from our study group. Mandia et al. (2020) compared serum E2 levels in four different groups (55 OD pregnancies, 48 autologous fresh IVF/ET pregnancies, 10 autologous FET pregnancies and 122 naturally conceived pregnancies) during the late first trimester of pregnancy (from 11 +
+ 0 to 13
0 to 13 +
+ 6 gestational weeks). Unexpectedly, significantly lower E2 concentrations were detected in OD pregnancies compared to natural conception or autologous IVF pregnancies. However, no difference in pregnancy outcomes and complications emerged (Mandia et al., 2020).
6 gestational weeks). Unexpectedly, significantly lower E2 concentrations were detected in OD pregnancies compared to natural conception or autologous IVF pregnancies. However, no difference in pregnancy outcomes and complications emerged (Mandia et al., 2020).
Owing to the lack of evidence, this result could be variously explained: first, we could hypothesize that the smaller placental volumes detected in OD placentas could imply a lower estrogen release compared to natural and autologous IVF pregnancies (Rizzo et al., 2016; Mandia et al., 2020); and second, we could speculate that oral estrogen administration might inhibit placental production, in a sort of feedback mechanism that is common in many endocrine systems. Undoubtedly, placental function in OD is reduced compared to naturally conceived and autologous IVF pregnancies: as observed by Savasi et al. (2015), first trimester placental markers, such as free β-hCG and PAPP-A, are significantly altered, suggesting a reduced crosstalk at the fetal–maternal interface (Savasi et al., 2015). Moreover, exogenous steroid supplementation may not replace the total absence of a corpus luteum during the very early stages of an OD pregnancy, as well as playing a part in a negative feedback regulation on placental function. Eventually, altered serum estrogen levels during the late first trimester may induce improper modelling of uterine arteries, resulting in pregnancy complications, such as pre-eclampsia and fetal growth restriction (Jauniaux et al., 1992; Mandia et al., 2020). As for periconceptional stages, further investigation is needed to find an optimal range for estrogenic concentrations during the first trimester. This may improve pregnancy outcomes in OD programs, reducing the risk of feto-maternal complications (Table 2).
Table 2.
Studies evaluating E2 levels and its implications during the first trimester in ART-conceived pregnancies.
| Study (year) | Sample size | Age of patients | Way of conception | Results |
|---|---|---|---|---|
| Vanderlelie et al. (2003) | 310 patients | 33 ± ± 3 3 years years | IVF/ET | Higher E2 levels in conceiving cycles than non-conceiving cycles |
| Kumbak et al. (2006) | 2035 IVF cycles | 31 ± ± 5 5 years years | IVF/ET | Higher E2 levels in conceiving cycles than non-conceiving cycles |
| Sonntag et al. (2013) | 71 IVF cycles | Not mentioned | IVF/ET | Higher E2 levels in conceiving cycles than non-conceiving cycles |
| Melnick et al. (2016) | 5471 IVF cycles | 37 ± ± 4.5 4.5 years years | IVF/ET | Higher LBR for E2 50–100 pg/ml on 28th cycle day (14th day after ET) pg/ml on 28th cycle day (14th day after ET) |
| Póvoa et al. (2018) |
| 23–40 years years | IVF/ET | Higher E2 levels in multiple pregnancies than in singleton ones |
| Hu et al. (2014) |
| IVF/FET vs FET vs SC | Higher E2 levels in early pregnancies after COH than EP protocols. Higher risk of LBW in fresh IVF/ET conceived pregnancies | |
| Conrad et al. (2019) | 65 patients | 23–46 years years | IVF/ET vs FET | Higher E2 levels in early pregnancies after COH than EP protocols |
| Sun et al. (2019) |
| 40 ± ± 3 3 years years | IVF/ET vs SC | Higher E2 levels in ART-conceived pregnancies |
| Mandia et al. (2020) |
| 27–48 years years | OD vs IVF vs SC | Lower E2 levels in OD than in SC or autologous IVF |
FET, frozen embryo-transfer; IVF/ET, in vitro fertilization/embryo-transfer; SC, spontaneous conception; COH, controlled ovarian hyperstimulation; EP, estro-progestinic; E2, estradiol; LBR, live birth rate; LBW, low birth weight; OD, oocyte donation.
Molecular mechanisms
Upon interacting with the endometrium, cytotrophoblast and syncytiotrophoblast cells are generated that facilitate penetration of the blastocyst into the decidua and allow materno-fetal exchanges, as well as sex hormone production (Albrecht and Pepe, 2010). These cells will eventually engage uterine stromal, endothelial, and immune cells to promote tissue remodeling. Among the most important and studied morphological changes occurring during early pregnancy is the development of a uterine vascular network aimed at facilitating blood flow to the feto-placental unit. The role of estrogens in enhancing uteroplacental flow is well established and may not only reflect changes in vascular reactivity, E2 being a potent vasodilator via the induction of endothelial NO synthase (eNOS) and subsequent NO release, but also enhanced angiogenesis (Berkane et al., 2017). In fact, although hypoxia is a potent stimulus of angiogenesis, multiple studies point at a crosstalk between estrogens and angiogenic factors as a determinant of placental vascularization. ERβ is thought to have a role in the control of the angiogenic and vascular changes that happen during embryo implantation and early placentation as well as for the maintenance of pregnancy (Su et al., 2012). The VEGF gene contains estrogen responsive elements and is upregulated by estrogens that in turn upregulate VEGF receptor 1 (VEGFR-1) and additional growth factors, such as PlGF, in endothelial cells (Mueller et al., 2000; Zhang et al., 2010). Interference with this process, for instance caused by increased levels of the soluble fms-like tyrosine kinase 1 (sFLT1), an antagonist of VEGF and PlGF, has long been associated with vasoconstriction and higher risk of developing pre-eclampsia (Zeisler et al., 2016). Recent reports stemming from the seminal model of estrogen regulation of early pregnancy in baboons not only confirmed the previous observation that supraphysiological E2 levels inhibit VEGF expression in the trophoblast thus impairing uterine artery remodeling, which could be restored by exogenous VEGF, but also identified sFLT-1 increase as the mechanism linking E2 overdose and reduced availability of VEGF (Babischkin et al., 2019; Aberdeen et al., 2022). In spite of disease heterogeneity and the complex pathogenesis, vascular remodeling and angiogenesis at the materno-fetal interface provide an example of how opposed settings, i.e. low versus high estrogens, can lead to the same outcome via an apparently common mechanism (Fig. 3). However, additional mechanisms may explain the link between the observed low circulating E2 levels and impaired angiogenesis and placenta development in pre-eclampsia. In fact, estrogens can alter blood pressure by regulating the expression of the enzyme HSD11B2 (hydroxysteroid 11-beta dehydrogenase 2) that converts cortisol, a vasoconstrictor, into cortisone (Baggia et al., 1990). In pre-eclampsia, elevated fetal cortisol caused by low HSD11B2 expression in the placenta not only affects blood pressure but also prevents an increase of estrogen production by limiting the synthesis of the precursor DHEA in the fetal adrenal glands, which has a central role in supporting the local production of estrogens in the primate placenta (see above). Additionally, E2 administered in vitro to human first-trimester villous explant cultures promoted trophoblast differentiation into an invasive phenotype in a hypoxia-inducible factor-1α dependent manner (Cho et al., 2018). The authors speculate that inappropriately elevated E2 levels could lead to the ‘shallow’ invasion of trophoblast into the spiral arteries and the uterine wall, contributing to complications associated with abnormal trophoblast invasion, as previously observed in pre-eclampsia (Cho et al., 2018). On the other hand, an elevated E2 level was able to induce apoptosis in the first-trimester human cytotrophoblast cell line HTR-8, highlighting a potential role in infertility and placental insufficiency (Patel et al., 2015).
Although the local immune-regulatory activity of sex hormones in the endometrium has been mainly investigated in the context of inflammatory and infectious diseases (Wira et al., 2015), with E2 being regarded as protective in contrast to progesterone, the role of immune cells in endometrial tissue remodeling during early pregnancy represents a good example of the complexity of estrogen effects. Estrogens are sensed by a number of immune cells, such as NK cells, myeloid cells, glial cells, T and B lymphocytes (Medina et al., 2001). Through multiple receptors differentially expressed according to the cell type and differentiation stage, estrogens cover a wide range of immune processes, by regulating cell proliferation, activation, differentiation, and the response to other immunomodulatory hormones and cytokines, such as the GnRH (Igarashi et al., 2001; Tanriverdi et al., 2003; Lang, 2004; Shao et al., 2013). The uNK cells are a peculiar subset of non-cyto-toxic NK cells that accounts for the majority of immune cells present in the endometrium, typically of large primates including humans. They proliferate and differentiate according to the menstrual cycle or pregnancy stage in response to both estrogens and progesterone, among other factors (Kalkunte et al. 2008). In particular, the estrogens synthesized by decidualized stromal cells promote the secretion of angiogenic and vascular-remodeling factors by uNK cells, such as the chemokine (C–C motif) ligand 2 (CCL2) and VEGF (Gibson et al., 2015; Wang et al., 2021). It has been proposed that uNK cells in vivo require estrogen priming to reach their mature and functional stage (Borzychowski et al., 2003), but whether estrogens have a direct receptor-mediated activity on uNK cells or act via paracrine signaling remains controversial (Borzychowski et al., 2003; Gaynor and Colucci, 2017). Likewise, the association between disorders of pregnancy entailing impaired tissue remodeling, such as pre-eclamspia, and alterations in uNK cell number and function (Moffett and Colucci, 2015), as well as their predictive value of pregnancy outcome in ART need further investigation (Tuckerman et al., 2007; Chen, 2019; Donoghue et al., 2019).
In addition to tissue remodeling, the main task of endometrial/decidual immune cells is to shield the semi-allogenic embryo from maternal immunity and at the same time provide protection against potential invading pathogens (Robinson and Klein, 2012; Schumacher et al., 2014). The fulfilment of these important tasks is likely achieved by estrogens, although our understanding of their immune-regulatory action relies on systemic correlates of inflammation, rather than localized at the maternal–fetal interface in human pregnancy (Straub, 2007; Vallvé-Juanico et al., 2019). If the general consensus is that an overall shift from inflammatory responses is needed for a successful pregnancy, the traditional paradigm of a switch occurring among peripheral CD4 T helper (Th) cells from pro-inflammatory Th1 to Th2 cells, supporting cell-mediated versus humoral immunity, has been revised with the inclusion of other relevant immune subsets over the years (Marzi et al., 1996). For instance, the expansion of another pro-inflammatory subset, namely Th17 cells, as well as an altered balance between these and tolerogenic Treg lymphocytes, was previously associated with implantation failure, recurrent pregnancy loss, preterm birth, intrauterine fetal growth restriction, and pre-eclampsia (Harrington et al., 2005; Santner-Nanan et al., 2009; Lee et al., 2011, 2012). Estrogens regulate both Th17 and Treg phenotype, activation, and localization (Laurence and O'Shea, 2007; Polanczyk et al., 2007; Tai et al., 2008; Javadian et al., 2014; Andersson et al., 2015; Fuseini et al., 2019). Treg cells are a specialized subset of T cells that provide an immune-tolerant environment for implantation and fetal development by inhibiting alloreactive immune responses of the maternal immune system against the fetus, also through secretion of cytokines such as IL-10 and transforming growth factor-β (Arruvito et al., 2007; Tai et al., 2008). These cytokines contribute to maintain a tolerogenic environment and their production is further supported by other cell types including antigen presenting cells, such as macrophages and dendritic cells under estrogen regulation, as shown, for example, in ex vivo-treated human chorionic villi and decidua (Wang et al., 2020a) (Fig. 4).
Discussion
Most evidence available to date endorses a relation between periconceptional estrogens levels and pregnancy outcomes, both in terms of LBR and pregnancy complications. In IVF/ET protocols, either extremely low or extremely elevated E2 blood titers appear inadequate to support a pregnancy. In the case of low E2, the lack of appropriate hormonal support may be responsible for reduced pregnancy and implantation rates. For elevated E2, gonadotrophin-stimulated multi-follicular development and production of supraphysiological levels of sex steroid hormones immediately before embryo implantation may represent an independent risk factor of developing LBW and other disorders of abnormal placentation, such as pre-eclampsia. We speculate that a reduced extravillous trophoblast invasion of the decidua owing to a premature and excessive exposure of the endometrium to E2, as modelled in early baboon pregnancy, might be one of the causes underlying such observations.
A few considerations should be noted. First, this narrative review has limitations owing to the non-systematic nature and the available literature: despite the big sample size of most studies, they all have a retrospective design. In addition, different stimulation protocols, techniques, and procedures were compared between study populations that have different demographic characteristics, thus representing a confounding factor. Second, none of the research groups has highlighted a fixed cutoff to define ‘excessive supraphysiological E2 levels’, probably owing to both the heterogeneity of patients and the different analytical methods used to measure E2 concentrations. Therefore, we suggest that an E2 concentration above 2500–3000 pg/ml on the day of hCG administration in fresh IVF/ET cycles might be referred as detrimental, i.e. resulting in poor pregnancy outcomes, although no reference value could be identified for FET procedures. We know that high serum E2 levels on the day of hCG administration and in the first weeks of pregnancy during controlled ovarian stimulation are associated with an increased risk for small for GA and pre-eclampsia, which can be overcome by freezing all of the embryos and transferring them after thawing in an unstimulated cycle (Imudia et al., 2012, 2014). It is difficult to set reference values, either upper or lower, because of the great heterogeneity of patients and confounding variables. We could suggest that the upper E2 limit at transfer should not be higher than 2500/3000
pg/ml on the day of hCG administration in fresh IVF/ET cycles might be referred as detrimental, i.e. resulting in poor pregnancy outcomes, although no reference value could be identified for FET procedures. We know that high serum E2 levels on the day of hCG administration and in the first weeks of pregnancy during controlled ovarian stimulation are associated with an increased risk for small for GA and pre-eclampsia, which can be overcome by freezing all of the embryos and transferring them after thawing in an unstimulated cycle (Imudia et al., 2012, 2014). It is difficult to set reference values, either upper or lower, because of the great heterogeneity of patients and confounding variables. We could suggest that the upper E2 limit at transfer should not be higher than 2500/3000 pmol/l, while the lower limit not be lower than 200/300
pmol/l, while the lower limit not be lower than 200/300 pmol/l, which is the physiological value during embryo implantation.
pmol/l, which is the physiological value during embryo implantation.
Moreover, in all studies the estrogen concentrations refer to peripheral blood, while the local concentrations in the female reproductive tract may differ.
How estrogens co-operate with other factors to maintain a fine balance between local tolerance towards the developing fetus and immune responses to invading pathogens and/or commensals remains elusive. In recent years, the interaction between the host and the local microbiota in the female reproductive tract, as well as in the intestine, has gained more attention also with respect to its potential effects on host metabolism, including estrogen processing, i.e. estrobolome (Salliss et al., 2021). A careful spatial-temporal mapping of the changes in the levels of estrogens and correlates of immunity, locally in the endometrium as well as systemically, may provide a mechanistic insight into the role of estrogen in the physiopathological changes occurring during pregnancy. Although obvious ethical issues restrict sample access and the opportunity to conduct longitudinal studies in women seeking pregnancy, by leveraging transcriptomic analysis of uterine biopsies collected across the menstrual cycle, multiple studies have attempted to unbiasedly identify the molecular determinants of endometrial receptivity (Díaz-Gimeno et al., 2011; Gómez et al., 2015). This approach has been further refined by single-cell and spatial transcriptomics that allows a better resolution in identifying the cell types, as well as their interaction, behind the main changes observed in bulk endometrial biopsies (Wang et al., 2020b; Garcia-Alonso et al., 2021). However, studies in humans do not allow us to accurately discriminate the activity of individual factors of interest to establish a direct unequivocal relationship between observations. To this end, innovative technical solutions in the culture of primary endometrial epithelial cells, which have been historically challenging to maintain for long periods, i.e. organoids, have been employed to study the hormonal regulation of epithelial cells (Turco et al., 2017; Nikolakopoulou and Turco, 2021). By combining advanced genomic analysis of the endometrium and organoid studies, recent work has led to the identification of potential binding sites for ERα across the genome (Hewitt et al., 2022), and to the partial characterization of molecular determinants of the proliferation and differentiation responses in epithelial cells to E2 and progesterone stimulation (Garcia-Alonso et al., 2021). Although organoids provide significant advantages over traditional cell lines and in vitro 2D cell systems, they are still far from recapitulating the full complexity of the endometrium, and therefore their potential translational value relies on the inclusion of stromal as well as immune components, in addition to the above-mentioned microbial partners, in a relevant spatial and functional interface (Murphy et al., 2022). Nevertheless, organoids, along with any other cell line that can be established from a patients’ endometrium, capture their genetic diversity, which is an indispensable factor for developing personalized interventions (Boretto et al., 2019).
Overall, the scenario depicted by our review for the role of estrogens and their optimal concentration in the early events surrounding reproduction remains incomplete, with some elements of controversy. The urgent necessity to investigate and define reference estrogens levels remains, in order to further ameliorate women’s health as well as ART, particularly when considering a steadily increasing demand. Hence, further prospective and experimental studies are highly warranted, also with the goal of identifying the determinants of estrogen response and biomarkers for personalized estrogen-administration regimes in ART.
Contributor Information
F Parisi, Department of Woman, Mother and Neonate, ‘V. Buzzi’ Children Hospital, ASST Fatebenefratelli Sacco, Milan, via L. Castelvetro 32, , Milan, Italy.
C Fenizia, Department of Pathophysiology and Transplantation, University of Milan, Milan, via F. Sforza 35, , Milan 20122, Italy. Department of Biomedical and Clinical Sciences, “L.Sacco” Hospital, University of Milan, Milan, via G.B. Grassi 74, Milan 20157, Italy.
A Introini, Division of Infectious Diseases, Department of Medicine Solna, Karolinska Institutet, Karolinska University Hospital, Stockholm, Nobels väg 5, , Stockholm, Sweden.
A Zavatta, Department of Woman, Mother and Neonate, ‘V. Buzzi’ Children Hospital, ASST Fatebenefratelli Sacco, Milan, via L. Castelvetro 32, , Milan, Italy.
C Scaccabarozzi, Department of Biomedical and Clinical Sciences, “L.Sacco” Hospital, University of Milan, Milan, via G.B. Grassi 74, Milan 20157, Italy.
M Biasin, Department of Biomedical and Clinical Sciences, “L.Sacco” Hospital, University of Milan, Milan, via G.B. Grassi 74, Milan 20157, Italy.
V Savasi, Department of Biomedical and Clinical Sciences, “L.Sacco” Hospital, University of Milan, Milan, via G.B. Grassi 74, Milan 20157, Italy.
Authors’ roles
S.V. conceived the original idea and supervised the project. P.F. and F.C. wrote the manuscript with support from I.A., Z.A., S.C., and M.B. All authors discussed the results and contributed to the final manuscript. P.F and F.C. contributed to the final version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and publication of this manuscript.
References
- Aberdeen GW, Babischkin JS, Lindner JR, Pepe GJ, Albrecht ED. Placental sFlt-1 gene delivery in early primate pregnancy suppresses uterine spiral artery remodeling. Endocrinology 2022;163:bqac012. [Europe PMC free article] [Abstract] [Google Scholar]
- Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R et al. An immune clock of human pregnancy. Sci Immunol 2017;2:eaan2946. [Europe PMC free article] [Abstract] [Google Scholar]
- Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Ann N Y Acad Sci 2004;1034:176–183. [Abstract] [Google Scholar]
- Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 2020;6:5. [Europe PMC free article] [Abstract] [Google Scholar]
- Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol 2010;54:397–408. [Europe PMC free article] [Abstract] [Google Scholar]
- Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab 2004;89:5803–5809. [Abstract] [Google Scholar]
- Anckaert E, Jank A, Petzold J, Rohsmann F, Paris R, Renggli M, Schönfeld K, Schiettecatte J, Kriner M. Extensive monitoring of the natural menstrual cycle using the serum biomarkers estradiol, luteinizing hormone and progesterone. Pract Lab Med 2021;25:e00211. [Europe PMC free article] [Abstract] [Google Scholar]
- Andersson A, Stubelius A, Karlsson MN, Engdahl C, Erlandsson M, Grahnemo L, Lagerquist MK, Islander U. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res Ther 2015;17:32. [Europe PMC free article] [Abstract] [Google Scholar]
- Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab 1998;83:1783–1787. [Abstract] [Google Scholar]
- Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 2007;178:2572–2578. [Abstract] [Google Scholar]
- Babic GM, Markovic SD, Varjacic M, Djordjevic NZ, Nikolic T, Stojic I, Jakovljevic V. Estradiol decreases blood pressure in association with redox regulation in preeclampsia. Clin Exp Hypertens 2018;40:281–286. [Abstract] [Google Scholar]
- Babischkin JS, Aberdeen GW, Lindner JR, Bonagura TW, Pepe GJ, Albrecht ED. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology 2019;160:1492–1505. [Europe PMC free article] [Abstract] [Google Scholar]
- Baggia S, Albrecht ED, Pepe GJ. Regulation of 11 beta-hydroxysteroid dehydrogenase activity in the baboon placenta by estrogen. Endocrinology 1990;126:2742–2748. [Abstract] [Google Scholar]
- Baird D, Weinberg C, Zhou H, Kamel F, McConnaughey D, Kesner J, Wilcox A. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril 1999;71:40–49. [Abstract] [Google Scholar]
- Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI, Collins DC. Hormonal profiles of natural conception cycles ending in early, unrecognized pregnancy loss. J Clin Endocrinol Metab 1991;72:793–800. [Abstract] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR, Kamel F, McConnaughey DR, Musey PI, Collins DC. Preimplantation hormonal differences between the conception and non-conception menstrual cycles of 32 normal women. Hum Reprod 1997;12:2607–2613. [Abstract] [Google Scholar]
- Barbieri E, Kashyap S, Chung PH. Chapter 35 - Infertility and in vitro fertilization. In: Legato MJ (ed). Principles of Gender-Specific Medicine, 2nd edn. Amsterdam: Academic Press, 2010, 381–399. [Google Scholar]
- Barrett ES, Mbowe O, Thurston SW, Butts S, Wang C, Nguyen R, Bush N, Redmon JB, Sheshu S, Swan SH et al. Predictors of steroid hormone concentrations in early pregnancy: results from a multi-center cohort. Matern Child Health J 2019;23:397–407. [Europe PMC free article] [Abstract] [Google Scholar]
- Bastu E, Mutlu MF, Yasa C, Dural O, Nehir Aytan A, Celik C, Buyru F, Yeh J. Role of Mucin 1 and glycodelin A in recurrent implantation failure. Fertil Steril 2015;103:1059–1064.e2. [Abstract] [Google Scholar]
- Baud O, Berkane N. Hormonal changes associated with intra-uterine growth restriction: impact on the developing brain and future neurodevelopment. Front Endocrinol (Lausanne) 2019;10:179. [Europe PMC free article] [Abstract] [Google Scholar]
- Berkane N, Liere P, Oudinet J-P, Hertig A, Lefèvre G, Pluchino N, Schumacher M, Chabbert-Buffet N. From pregnancy to preeclampsia: a key role for estrogens. Endocr Rev 2017;38:123–144. [Abstract] [Google Scholar]
- Bernstein IM, Ziegler WF, Leavitt T, Badger GJ. Uterine artery hemodynamic adaptations through the menstrual cycle into early pregnancy. Obstet Gynecol 2002;99:620–624. [Abstract] [Google Scholar]
- Bianco K, Mahutte NG, Arici A, Sakkas D, Taylor HS. Effect of estradiol on oocyte development. Int J Gynaecol Obstet 2009;104:230–232. [Europe PMC free article] [Abstract] [Google Scholar]
- Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 2019;21:1041–1051. [Abstract] [Google Scholar]
- Borini A, Dal Prato L, Bianchi L, Violini F, Cattoli M, Flamigni C. Effect of duration of estradiol replacement on the outcome of oocyte donation. J Assist Reprod Genet 2001;18:185–190. [Europe PMC free article] [Abstract] [Google Scholar]
- Borzychowski AM, Chantakru S, Minhas K, Paffaro VA, Yamada AT, He H, Korach KS, Croy BA. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta 2003;24:403–411. [Abstract] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab 1997;82:3509–3512. [Abstract] [Google Scholar]
- Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol 2004;2:4. [Europe PMC free article] [Abstract] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30:473–482. [Europe PMC free article] [Abstract] [Google Scholar]
- Bussen S, Bussen D. Influence of the vascular endothelial growth factor on the development of severe pre-eclampsia or HELLP syndrome. Arch Gynecol Obstet 2011;284:551–557. [Abstract] [Google Scholar]
- Caulin-Glaser T, García-Cardeña G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res 1997;81:885–892. [Abstract] [Google Scholar]
- Cekan SZ, Beksac MS, Wang E, Shi S, Masironi B, Landgren BM, Diczfalusy E. The prediction and/or detection of ovulation by means of urinary steroid assays. Contraception 1986;33:327–345. [Abstract] [Google Scholar]
- Chang K, Lubo Z. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci 2008;15:336–348. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen X. Prognostic value of uterine natural killer (uNK) cells density in peri-implantation endometrium from women with recurrent implantation failure. Fertility and Sterility 2019;112:e167. [Google Scholar]
- Chen J, Qiu Q, Lohstroh PN, Overstreet JW, Lasley BL. Hormonal characteristics in the early luteal phase of conceptive and nonconceptive menstrual cycles. J Soc Gynecol Invest 2003;10:27–31. [Abstract] [Google Scholar]
- Chen Y-C, Lai Y-J, Su Y-T, Tsai N-C, Lan K-C. Higher gestational weight gain and lower serum estradiol levels are associated with increased risk of preeclampsia after in vitro fertilization. Pregnancy Hypertens 2020;22:126–131. [Abstract] [Google Scholar]
- Cho GJ, Lee LH, Lee B, Lee J, Ahn K-H, Hong S-C, Kim H-J, Oh M-J. Effects of estradiol on HIF-1α expression and trophoblast differentiation in first trimester villous explant cultures. Obstet Gynecol Sci 2018;61:71–78. [Europe PMC free article] [Abstract] [Google Scholar]
- Choi L, Bowers C, Liu A, Pier B, Levy G. Elevated serum estradiol levels do not inhibit implantation during frozen embryo transfer cycles. Reprod Sci 2021;28:2855–2860. [Abstract] [Google Scholar]
- Conrad KP, Petersen JW, Chi Y-Y, Zhai X, Li M, Chiu K-H, Liu J, Lingis MD, Williams RS, Rhoton-Vlasak A et al. Maternal cardiovascular dysregulation during early pregnancy after in vitro fertilization cycles in the absence of a Corpus Luteum. Hypertension 2019;74:705–715. [Europe PMC free article] [Abstract] [Google Scholar]
- Costa AJ, Oliveira RB, Wachilewski P, Nishino MS, Bassani TB, Stilhano RS, Cerutti JM, Nozima B, Porto CS, Pereira D et al. Membrane estrogen receptor ERα activation improves tau clearance via autophagy induction in a tauopathy cell model. Brain Res 2022;1795:148079. [Abstract] [Google Scholar]
- Coya R, Martul P, Algorta J, Aniel-Quiroga MA, Busturia MA, Señarís R. Effect of leptin on the regulation of placental hormone secretion in cultured human placental cells. Gynecol Endocrinol 2006;22:620–626. [Abstract] [Google Scholar]
- Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation–a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. [Abstract] [Google Scholar]
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev 2020;100:1149–1179. [Abstract] [Google Scholar]
- Cui J, , ShenY, , Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends in Molecular Medicine 2013;19:197–209. [Europe PMC free article] [Abstract] [Google Scholar]
- Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 1993;133:829–837. [Abstract] [Google Scholar]
- Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol 2013;86:734–747. [Europe PMC free article] [Abstract] [Google Scholar]
- De Ziegler D, , BessisR, , Frydman R. Vascular resistance of uterine arteries: physiological effects of estradiol and progesterone. Fertility and Sterility 1991;55:775–779. [Abstract] [Google Scholar]
- DeLoia JA, Stewart-Akers AM, Brekosky J, Kubik CJ. Effects of exogenous estrogen on uterine leukocyte recruitment. Fertil Steril 2002;77:548–554. [Abstract] [Google Scholar]
- Deng W, Sun R, Du J, Wu X, Ma L, Wang M, Lv Q. Prediction of miscarriage in first trimester by serum estradiol, progesterone and β-human chorionic gonadotropin within 9 weeks of gestation. BMC Pregnancy Childbirth 2022;22:112. [Europe PMC free article] [Abstract] [Google Scholar]
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, Simón C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 2011;95:50–60, 60.e1. [Abstract] [Google Scholar]
- Djordjević NZ, Babić GM, Marković SD, Ognjanović BI, Stajn AS, Saicić ZS. The antioxidative effect of estradiol therapy on erythrocytes in women with preeclampsia. Reprod Toxicol 2010;29:231–236. [Abstract] [Google Scholar]
- Donoghue JF, , PaivaP, , TehWT, , CannLM, , NowellC, , ReesH, , BittingerS, , ObersV, , BulmerJN, , Stern C et al. Endometrial uNK cell counts do not predict successful implantation in an IVF population. Hum Reprod 2019;34:2456–2466. [Abstract] [Google Scholar]
- Dorostghoal M, Ghaffari H-O-A, Marmazi F, Keikhah N. Overexpression of endometrial estrogen receptor-alpha in the window of implantation in women with unexplained infertility. Int J Fertil Steril 2018;12:37–42. [Europe PMC free article] [Abstract] [Google Scholar]
- Dunne C, Cho K, Shan A, Hutcheon J, Durland US, Seethram K, Havelock JC. Peak serum estradiol level during controlled ovarian stimulation is not associated with lower levels of pregnancy-associated plasma protein-A or small for gestational age infants: a cohort study. J Obstet Gynaecol Can 2017;39:870–879. [Abstract] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 2000;127:4277–4291. [Abstract] [Google Scholar]
- Elhasnaoui J, Ferrero G, Miano V, Cutrupi S, de Bortoli M. The estrogen receptor α signaling pathway controls alternative splicing in the absence of ligands in breast cancer cells. Cancers (Basel) 2021;13:6261. [Europe PMC free article] [Abstract] [Google Scholar]
- Farhi J, Ben-Haroush A, Haroush AB, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online 2010;21:331–337. [Abstract] [Google Scholar]
- Fritz R, Jindal S, Feil H, Buyuk E. Elevated serum estradiol levels in artificial autologous frozen embryo transfer cycles negatively impact ongoing pregnancy and live birth rates. J Assist Reprod Genet 2017;34:1633–1638. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuseini H, Cephus J-Y, Wu P, Davis JB, Contreras DC, Gandhi VD, Rathmell JC, Newcomb DC. ERα signaling increased IL-17A production in Th17 cells by upregulating IL-23R expression, mitochondrial respiration, and proliferation. Front Immunol 2019;10:2740. [Europe PMC free article] [Abstract] [Google Scholar]
- Gao Q, Wang M, Tang R. Serum estradiol and threatened abortion in pregnancy of 4 to 8 weeks. Journal of Shandong University (Health Science) 2008;46:884–886. [Google Scholar]
- Garcia-Alonso L, Handfield L-F, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, Woodhams B, Arutyunyan A, Polanski K, Hoo R et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet 2021;53:1698–1711. [Europe PMC free article] [Abstract] [Google Scholar]
- Gaynor LM, , Colucci F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front Immunol 2017;8:10.3389/fimmu.2017.00467 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ghosh D, De P, Sengupta J. Luteal phase ovarian oestrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Hum Reprod 1994;9:629–637. [Abstract] [Google Scholar]
- Gibson DA, Greaves E, Critchley HOD, Saunders PTK. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum Reprod 2015;30:1290–1301. [Europe PMC free article] [Abstract] [Google Scholar]
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2020;10:CD006359. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldberg JM, Falcone T, Attaran M. In vitro fertilization update. Cleve Clin J Med 2007;74:329–338. [Abstract] [Google Scholar]
- Gómez BI, Gifford CA, Hallford DM, Hernandez Gifford JA. Protein kinase B is required for follicle-stimulating hormone mediated beta-catenin accumulation and estradiol production in granulosa cells of cattle. Anim Reprod Sci 2015;163:97–104. [Abstract] [Google Scholar]
- Groenewoud ER, Cantineau AEP, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2013;19:458–470. [Abstract] [Google Scholar]
- Grossi E, Parisi F, Duca P, Savasi VM. Maternal estradiol and progesterone concentrations among singleton spontaneous pregnancies during the first trimester. J Endocrinol Invest 2019;42:633–638. [Abstract] [Google Scholar]
- Haneda S, Dini P, Esteller-Vico A, Scoggin KE, Squires EL, Troedsson MH, Daels P, Nambo Y, Ball BA. Estrogens regulate placental angiogenesis in horses. Int J Mol Sci 2021;22:12116. [Europe PMC free article] [Abstract] [Google Scholar]
- Hapangama DK, , KamalAM, , Bulmer JN. Estrogen receptor β: the guardian of the endometrium. Human Reproduction Update 2015;21:174–193. [Abstract] [Google Scholar]
- Hardiman P, Thomas M, Osgood V, Vlassopoulou V, Ginsburg J. Are estrogen assays essential for monitoring gonadotropin stimulant therapy? Gynecol Endocrinol 1990;4:261–269. [Abstract] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–1132. [Abstract] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007;87:905–931. [Abstract] [Google Scholar]
- Henderson TA, Saunders PTK, Moffett-King A, Groome NP, Critchley HOD. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab 2003;88:440–449. [Abstract] [Google Scholar]
- Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstet Gynecol 2010;203:477.e1-9–477.e9. [Abstract] [Google Scholar]
- Hervé MAJ, Meduri G, Petit FG, Domet TS, Lazennec G, Mourah S, Perrot-Applanat M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol 2006;188:91–99. [Abstract] [Google Scholar]
- Hewitt SC, Wu S-P, Wang T, Ray M, Brolinson M, Young SL, Spencer TE, DeCherney A, DeMayo FJ. The estrogen receptor α cistrome in human endometrium and epithelial organoids. Endocrinology 2022;163:bqac116. [Europe PMC free article] [Abstract] [Google Scholar]
- Hey NA, Li TC, Devine PL, Graham RA, Saravelos H, Aplin JD. MUC1 in secretory phase endometrium: expression in precisely dated biopsies and flushings from normal and recurrent miscarriage patients. Hum Reprod 1995;10:2655–2662. [Abstract] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N et al. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 2001;276:3459–3467. [Abstract] [Google Scholar]
- Holl K, Lundin E, Surcel H-M, Grankvist K, Koskela P, Dillner J, Hallmans G, Wadell G, Olafsdottir GH, Ogmundsdottir HM et al. Endogenous steroid hormone levels in early pregnancy and risk of testicular cancer in the offspring: a nested case-referent study. Int J Cancer 2009;124:2923–2928. [Abstract] [Google Scholar]
- Hu X-L, Feng C, Lin X-H, Zhong Z-X, Zhu Y-M, Lv P-P, Lv M, Meng Y, Zhang D, Lu X-E et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab 2014;99:2217–2224. [Abstract] [Google Scholar]
- Hu X-L, Shi S, Hou N-N, Meng Y, Li M, Liu A-X, Lu Y-C, Li J-Y, Sheng J-Z, Zhu Y-M et al. High maternal serum estradiol in first trimester of multiple pregnancy contributes to small for gestational age via DNMT1-mediated CDKN1C upregulation. Reprod Sci 2022;29:1368–1378. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang R, Fang C, Wang N, Li L, Yi Y, Liang X. Serum estradiol level change after human chorionic gonadotropin administration had no correlation with live birth rate in IVF cycles. Eur J Obstet Gynecol Reprod Biol 2014;178:177–182. [Abstract] [Google Scholar]
- Huhtinen K, Saloniemi-Heinonen T, Keski-Rahkonen P, Desai R, Laajala D, Ståhle M, Häkkinen MR, Awosanya M, Suvitie P, Kujari H et al. Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J Clin Endocrinol Metab 2014;99:E2188–97. [Abstract] [Google Scholar]
- Igarashi H, , KouroT, , YokotaT, , CompPC, , Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci USA 2001;98:15131–15136. [Europe PMC free article] [Abstract] [Google Scholar]
- Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 2012;97:1374–1379. [Abstract] [Google Scholar]
- Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet 2014;31:65–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Inversetti A, Mandia L, Candiani M, Cetin I, Larcher A, Savasi V, Papaleo E, Cavoretto P. Uterine artery Doppler pulsatility index at 11-38 weeks in ICSI pregnancies with egg donation. J Perinat Med 2018;46:21–27. [Abstract] [Google Scholar]
- Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A, Beaman KD. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012;143:713–725. [Abstract] [Google Scholar]
- Jauniaux E, Jurkovic D, Delogne-Desnoek J, Meuris S. Influence of human chorionic gonadotrophin, oestradiol and progesterone on uteroplacental and corpus luteum blood flow in normal early pregnancy. Hum Reprod 1992;7:1467–1473. [Abstract] [Google Scholar]
- Javadian A, Salehi E, Bidad K, Sahraian MA, Izad M. Effect of estrogen on Th1, Th2 and Th17 cytokines production by proteolipid protein and PHA activated peripheral blood mononuclear cells isolated from multiple sclerosis patients. Arch Med Res 2014;45:177–182. [Abstract] [Google Scholar]
- Jia M, Dahlman-Wright K, Gustafsson J-Å. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 2015;29:557–568. [Abstract] [Google Scholar]
- Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013;61:480–487. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Effects of estradiol-17beta on expression of mRNA for seven angiogenic factors and their receptors in the endometrium of ovariectomized (OVX) ewes. Endocrine 2006;30:333–342. [Abstract] [Google Scholar]
- Johnson MR, Riddle AF, Grudzinskas JG, Sharma V, Campbell S, Collins WP, Lightman SL, Mason B, Nicolaides KH. Endocrinology of in-vitro fertilization pregnancies during the first trimester. Hum Reprod 1993;8:316–322. [Abstract] [Google Scholar]
- Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril 2010;93:442–446. [Abstract] [Google Scholar]
- Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol 2008;59:425–432. [Europe PMC free article] [Abstract] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 2011;118:863–871. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaser DJ, Ginsburg ES, Carrell DT, Racowsky C. Chapter 31 - Assisted reproduction. In: Strauss JF, Barbieri RL (eds). YEN & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia, PA: Elsevier, 2019, 779–822.e16. [Google Scholar]
- King AE, Critchley HOD. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J Steroid Biochem Mol Biol 2010;120:116–126. [Abstract] [Google Scholar]
- Kondapalli LA, Molinaro TA, Sammel MD, Dokras A. A decrease in serum estradiol levels after human chorionic gonadotrophin administration predicts significantly lower clinical pregnancy and live birth rates in in vitro fertilization cycles. Hum Reprod 2012;27:2690–2697. [Europe PMC free article] [Abstract] [Google Scholar]
- Kumbak B, Oral E, Karlikaya G, Lacin S, Kahraman S. Serum oestradiol and β-HCG measurements after day 3 or 5 embryo transfers in interpreting pregnancy outcome. Reprod Biomed Online 2006;13:459–464. [Abstract] [Google Scholar]
- Lang TJ. Estrogen as an immunomodulator. Clinical Immunology 2004;113:224–230. [Abstract] [Google Scholar]
- Lass A, , WeiserW, , MunafoA, , Loumaye E. Leukemia inhibitory factor in human reproduction. Fertility and Sterility 2001;76:1091–1096. [Abstract] [Google Scholar]
- Laurence A, O'Shea JJ. T(H)-17 differentiation: of mice and men. Nat Immunol 2007;8:903–905. [Abstract] [Google Scholar]
- Lecce G, , MeduriG, , AncelinM, , BergeronC, , Perrot-Applanat M. Presence of Estrogen Receptor β in the Human Endometrium through the Cycle: Expression in Glandular, Stromal, and Vascular Cells 1. The Journal of Clinical Endocrinology & Metabolism 2001;86:1379–1386. [Abstract] [Google Scholar]
- Lee S, Kim J, Jang B, Hur S, Jung U, Kil K, Na B, Lee M, Choi Y, Fukui A et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol 2010;185:756–762. [Abstract] [Google Scholar]
- Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A, Kwak-Kim J. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod 2011;26:2964–2971. [Abstract] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol 2012;67:311–318. [Abstract] [Google Scholar]
- Lenton EA, Sulaiman R, Sobowale O, Cooke ID. The human menstrual cycle: plasma concentrations of prolactin, LH, FSH, oestradiol and progesterone in conceiving and non-conceiving women. J Reprod Fertil 1982;65:131–139. [Abstract] [Google Scholar]
- Lessey BA, Palomino WA, Apparao KBC, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol 2006;4(Suppl 1):S9. [Europe PMC free article] [Abstract] [Google Scholar]
- Li H, Nakajima ST, Chen J, Todd HE, Overstreet JW, Lasley BL. Differences in hormonal characteristics of conceptive versus nonconceptive menstrual cycles. Fertil Steril 2001;75:549–553. [Abstract] [Google Scholar]
- Li Q, Ruan L, Zhu L, Yang Z, Zhu M, Luo Y. Elevated estradiol levels in frozen embryo transfer have different effects on pregnancy outcomes depending on the stage of transferred embryos. Sci Rep 2022;12:5592. [Europe PMC free article] [Abstract] [Google Scholar]
- Li X, Zeng C, Shang J, Wang S, Gao X-L, Xue Q. Association between serum estradiol level on the human chorionic gonadotrophin administration day and clinical outcome. Chin Med J (Engl) 2019;132:1194–1201. [Europe PMC free article] [Abstract] [Google Scholar]
- Lipson SF, Ellison PT. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum Reprod 1996;11:2090–2096. [Abstract] [Google Scholar]
- Liu F, Wu K, Wu W, Chen Y, Wu H, Wang H, Zhang W. miR-203 contributes to pre-eclampsia via inhibition of VEGFA expression. Mol Med Rep 2018;17:5627–5634. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu S, Kuang Y, Wu Y, Feng Y, Lyu Q, Wang L, Sun Y, Sun X. High oestradiol concentration after ovarian stimulation is associated with lower maternal serum beta-HCG concentration and neonatal birth weight. Reprod Biomed Online 2017;35:189–196. [Abstract] [Google Scholar]
- Lof M, Hilakivi-Clarke L, Sandin SS, Assis S, de YW, Weiderpass E. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health 2009;9:10. [Europe PMC free article] [Abstract] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 1993;90:11162–11166. [Europe PMC free article] [Abstract] [Google Scholar]
- Mackens S, Santos-Ribeiro S, Orinx E, N de M, Racca A, Roelens C, Popovic-Todorovic B, M de V, Tournaye H, Blockeel C. Impact of serum estradiol levels prior to progesterone administration in artificially prepared frozen embryo transfer cycles. Front Endocrinol (Lausanne) 2020;11:255. [Europe PMC free article] [Abstract] [Google Scholar]
- Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, van Landuyt L, Tournaye H, Blockeel C. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod 2017;32:2234–2242. [Abstract] [Google Scholar]
- Madero S, Rodriguez A, Vassena R, Vernaeve V. Endometrial preparation: effect of estrogen dose and administration route on reproductive outcomes in oocyte donation cycles with fresh embryo transfer. Hum Reprod 2016;31:1755–1764. [Abstract] [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen D-B. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol 2005;565:71–83. [Abstract] [Google Scholar]
- Makinoda S, Moll W. Deoxyribonucleic acid synthesis in mesometrial arteries of Guinea pigs during oestrous cycle, pregnancy and treatment with oestradiol benzoate. Placenta 1986;7:189–198. [Abstract] [Google Scholar]
- Mal R, Magner A, David J, Datta J, Vallabhaneni M, Kassem M, Manouchehri J, Willingham N, Stover D, Vandeusen J et al. Estrogen receptor beta (ERβ): a ligand activated tumor suppressor. Front Oncol 2020;10:587386. [Europe PMC free article] [Abstract] [Google Scholar]
- Mandalà M. Influence of estrogens on uterine vascular adaptation in normal and preeclamptic pregnancies. Int J Mol Sci 2020;21(7):2592. [Europe PMC free article] [Abstract] [Google Scholar]
- Mandia L, Cavoretto P, Duca P, Candiani M, Cetin I, Savasi V. Evaluation of uterine artery Doppler and estrogen milieu in oocyte donation pregnancies—a pilot study. Diagnostics 2020;10:254. [Europe PMC free article] [Abstract] [Google Scholar]
- Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol 1996;106:127–133. [Abstract] [Google Scholar]
- Mathur S, Mathur RS, Goust JM, Williamson HO, Fudenberg HH. Cyclic variations in white cell subpopulations in the human menstrual cycle: correlations with progesterone and estradiol. Clin Immunol Immunopathol 1979;13:246–253. [Abstract] [Google Scholar]
- Medina KL, , GarrettKP, , ThompsonLF, , RossiMID, , PayneKJ, , Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol 2001;2:718–724. [Abstract] [Google Scholar]
- Melnick AP, Pereira N, Murphy EM, Rosenwaks Z, Spandorfer SD. How low is too low? Cycle day 28 estradiol levels and pregnancy outcomes. Fertil Steril 2016;105:905–909.e1. [Abstract] [Google Scholar]
- Melnick AP, Rosenwaks Z. Oocyte donation: insights gleaned and future challenges. Fertil Steril 2018;110:988–993. [Abstract] [Google Scholar]
- Moffett A, , Colucci F. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev 2015;267:283–297. [Abstract] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–87. [Europe PMC free article] [Abstract] [Google Scholar]
- Morales HSG, , GuiotML, , LópezGGP, , CórtesDV, , MaldonadoBF, , HernándezHS, , TorresGCR, , CamachoFMR, , Montoya GA. Serum estradiol level on the day of trigger as a predictor of number of metaphase II oocytes from IVF antagonist cycles and subsequent impact on pregnancy rates. JBRA 2021;10.5935/1518-0557.20210007. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA 2000;97:10972–10977. [Europe PMC free article] [Abstract] [Google Scholar]
- Murphy AR, Campo H, Kim JJ. Strategies for modelling endometrial diseases. Nat Rev Endocrinol 2022;18:727–743. [Europe PMC free article] [Abstract] [Google Scholar]
- Nikolakopoulou K, Turco MY. Investigation of infertility using endometrial organoids. Reproduction 2021;161:R113–R127. [Europe PMC free article] [Abstract] [Google Scholar]
- Niu Z, Feng Y, Sun Y, Zhang A, Zhang H. Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary? J Exp Clin Assist Reprod 2008;5:4. [Europe PMC free article] [Abstract] [Google Scholar]
- Notation AD, Tagatz GE. Unconjugated estriol and 15α-hydroxyestriol in complicated pregnancies. Am J Obstet Gynecol 1977;128:747–756. [Abstract] [Google Scholar]
- Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev 2012;11:A486–92. [Abstract] [Google Scholar]
- Orzołek I, Sobieraj J, Domagała-Kulawik J. Estrogens, cancer and immunity. Cancers (Basel) 2022;14:2265. [Europe PMC free article] [Abstract] [Google Scholar]
- Palmerola KL, Rudick BJ, Lobo RA. Low estradiol responses in oocyte donors undergoing gonadotropin stimulation do not influence clinical outcomes. J Assist Reprod Genet 2018;35:1675–1682. [Europe PMC free article] [Abstract] [Google Scholar]
- Park M-R, Hwang K-C, Bui H-T, Cho S-G, Park C, Song H, Oh J-W, Kim J-H. Altered gene expression profiles in mouse tetraploid blastocysts. J Reprod Dev 2012;58:344–352. [Abstract] [Google Scholar]
- Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biol Reprod 2015;93:74. [Europe PMC free article] [Abstract] [Google Scholar]
- Peña JE, Chang PL, Chan L-K, Zeitoun K, Thornton MH, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod 2002;17:83–87. [Abstract] [Google Scholar]
- Pepe G, , LocatiM, , Della TorreS, , MornataF, , CignarellaA, , MaggiA, , Vegeto E. The estrogen–macrophage interplay in the homeostasis of the female reproductive tract. Human Reproduction Update 2018;24:652–672. [Abstract] [Google Scholar]
- Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet 2015;32:527–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, España-Perrot PP, Venegas-Araneda P, Guzmán-Rojas AM, González MI, Palominos-Rivera M, Irarrazabal CE, Figueroa-Diesel H et al. Placental aromatase is deficient in placental ischemia and preeclampsia. PLoS One 2015;10:e0139682. [Europe PMC free article] [Abstract] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1). Int Immunol 2007;19:337–343. [Abstract] [Google Scholar]
- Póvoa A, Xavier P, Matias A, Blickstein I. First trimester β-hCG and estradiol levels in singleton and twin pregnancies after assisted reproduction. J Perinat Med 2018;46:853–856. [Abstract] [Google Scholar]
- Powazniak Y, Kempfer AC, Dominguez M de LaPaz, Farias C, Keller L, Calderazzo JC, Lazzari MA. Effect of estradiol, progesterone and testosterone on apoptosis- and proliferation-induced MAPK signaling in human umbilical vein endothelial cells. Mol Med Rep 2009;2:441–447. [Abstract] [Google Scholar]
- Rahman SA, Hingorani V, Laumas KR. Biosynthesis of oestrogens and their inter-conversion in human placentae from normal and toxaemic pregnancies. Clin Endocrinol (Oxf) 1975;4:333–341. [Abstract] [Google Scholar]
- Ramsey TL, Risinger KE, Jernigan SC, Mattingly KA, Klinge CM. Estrogen receptor beta isoforms exhibit differences in ligand-activated transcriptional activity in an estrogen response element sequence-dependent manner. Endocrinology 2004;145:149–160. [Abstract] [Google Scholar]
- Rizzo G, Aiello E, Pietrolucci ME, Arduini D. Placental volume and uterine artery Doppler evaluation at 11 + 0 to 13 + 6 weeks' gestation in pregnancies conceived with in-vitro fertilization: comparison between autologous and donor oocyte recipients. Ultrasound Obstet Gynecol 2016;47:726–731. [Abstract] [Google Scholar]
- Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod 1992;46:1069–1079. [Abstract] [Google Scholar]
- Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012;62:263–271. [Europe PMC free article] [Abstract] [Google Scholar]
- Romanski PA, Bortoletto P, Liu Y-L, Chung PH, Rosenwaks Z. Length of estradiol exposure 100 pg/ml in the follicular phase affects pregnancy outcomes in natural frozen embryo transfer cycles. Hum Reprod 2021;36:1932–1940. [Abstract] [Google Scholar]
- Roos J, Johnson S, Weddell S, Godehardt E, Schiffner J, Freundl G, Gnoth C. Monitoring the menstrual cycle: comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept Reprod Health Care 2015;20:438–450. [Abstract] [Google Scholar]
- Rosenfeld CR, Rivera R. Circulatory responses to systemic infusions of estrone and estradiol-17α in nonpregnant, oophorectomized ewes. Am J Obstet Gynecol 1978;132:442–448. [Abstract] [Google Scholar]
- Rosing U, Carlström K. Serum levels of unconjugated and total oestrogens and dehydroepiandrosterone, progesterone and urinary oestriol excretion in pre-eclampsia. Gynecol Obstet Invest 1984;18:199–205. [Abstract] [Google Scholar]
- Salas SP, Marshall G, Gutiérrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006;47:203–208. [Abstract] [Google Scholar]
- Salazar EL, Calzada L. The role of progesterone in endometrial estradiol- and progesterone-receptor synthesis in women with menstrual disorders and habitual abortion. Gynecol Endocrinol 2007;23:222–225. [Abstract] [Google Scholar]
- Sallam H, Rizk B. Infertility: an overview. In: Clinical Infertility and in Vitro Fertilization, 1st edn. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd, 2012, p. 45. [Google Scholar]
- Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update 2021;28:92–131. [Abstract] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 2009;183:7023–7030. [Abstract] [Google Scholar]
- Sauer MV, Paulson RJ, Lobo RA. A preliminary report on oocyte donation extending reproductive potential to women over 40. N Engl J Med 1990;323:1157–1160. [Abstract] [Google Scholar]
- Savasi VM, Mandia L, Laoreti A, Cetin I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum Reprod Update 2016;22:620–633. [Abstract] [Google Scholar]
- Savasi VM, Mandia L, Laoreti A, Ghisoni L, Duca P, Cetin I. First trimester placental markers in oocyte donation pregnancies. Placenta 2015;36:921–925. [Abstract] [Google Scholar]
- Schäfer-Somi S, Ali Aksoy O, Patzl M, Findik M, Erünal-Maral N, Beceriklisoy HB, Polat B, Aslan S. The activity of matrix metalloproteinase-2 and -9 in serum of pregnant and non-pregnant bitches. Reprod Domest Anim 2005;40:46–50. [Abstract] [Google Scholar]
- Schumacher A, Costa S-D, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol 2014;5:196. [Europe PMC free article] [Abstract] [Google Scholar]
- Seppälä M, Koistinen H, Koistinen R, Chiu PCN, Yeung WSB. Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations. Hum Reprod Update 2007;13:275–287. [Abstract] [Google Scholar]
- Shao J, , LiM-Q, , MengY-H, , ChangK-K, , WangY, , ZhangL, , Li D-J. Estrogen promotes the growth of decidual stromal cells in human early pregnancy. MHR: Basic Science of Reproductive Medicine 2013;19:655–664. [Abstract] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 1996;81:3112–3118. [Abstract] [Google Scholar]
- Simón C, Gimeno MJ, Mercader A, Francés A, Garcia Velasco J, Remohí J, Polan ML, Pellicer A. Cytokines-adhesion molecules-invasive proteinases. The missing paracrine/autocrine link in embryonic implantation? Mol Hum Reprod 1996;2:405–424. [Abstract] [Google Scholar]
- Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation 2002;105:1368–1373. [Abstract] [Google Scholar]
- Sonntag B, Loebbecke KC, Nofer J-R, Kiesel L, Greb RR. Serum estradiol and progesterone in the mid-luteal phase predict clinical pregnancy outcome in IVF/ICSI cycles. Gynecol Endocrinol 2013;29:700–703. [Abstract] [Google Scholar]
- Speroff L, Fritz MA. Speroff's Clinical Gynecologic Endocrinology and Infertility|Clinical Gynecologic Endocrinology and Infertility, 8th edn. Philadelphia, PA: Wolters Kluwer Health, 2011. [Google Scholar]
- Steegers-Theunissen RPM, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–655. [Abstract] [Google Scholar]
- Steward RG, , LanL, , ShahAA, , YehJS, , PriceTM, , GoldfarbJM, , Muasher SJ. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertility and Sterility 2014;101:967–973. [Abstract] [Google Scholar]
- Steward RG, Zhang CE, Shah AA, Yeh JS, Chen C, Li Y-J, Price TM, Muasher SJ. High peak estradiol predicts higher miscarriage and lower live birth rates in high responders triggered with a GnRH agonist in IVF/ICSI cycles. J Reprod Med 2015;60:463–470. [Abstract] [Google Scholar]
- Stewart DR, Overstreet JW, Nakajima ST, Lasley BL. Enhanced ovarian steroid secretion before implantation in early human pregnancy. J Clin Endocrinol Metab 1993;76:1470–1476. [Abstract] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [Abstract] [Google Scholar]
- Stricker R, Eberhart R, Chevailler M-C, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med 2006;44:883–887. [Abstract] [Google Scholar]
- Styer AK, Jackson KV, Hornstein MD, Racowsky C, Ginsburg ES, Gargiulo AR. Pregnancy outcomes in in vitro fertilization cycles with serum estradiol drop prior to human chorionic gonadotropin. Int J Gynaecol Obstet 2005;89:133–137. [Abstract] [Google Scholar]
- Su EJ, Xin H, Monsivais D. The emerging role of estrogen receptor-β in human reproduction. Semin Reprod Med 2012;30:62–70. [Abstract] [Google Scholar]
- Sun T, Lee B, Kinchen J, Wang ET, Gonzalez TL, Chan JL, Rotter JI, Chen Y-DI, Taylor K, Goodarzi MO et al. Differences in first-trimester maternal metabolomic profiles in pregnancies conceived from fertility treatments. J Clin Endocrinol Metab 2019;104:1005–1019. [Europe PMC free article] [Abstract] [Google Scholar]
- Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod 2015;30:1473–1480. [Abstract] [Google Scholar]
- Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol 2008;214:456–464. [Abstract] [Google Scholar]
- Tanriverdi F, , SilveiraLF, , MacCollGS, , Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. Journal of Endocrinology 2003;176:293–304. [Abstract] [Google Scholar]
- Tarlatzi T, Venetis C, Sassi A, Devreker F, Englert Y, Delbaere A. Higher estradiol levels are associated with lower neonatal birthweight after fresh and frozen embryo transfers. A cohort study of 3631 singleton IVF pregnancies. Gynecol Endocrinol 2021;37:618–623. [Abstract] [Google Scholar]
- Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol 2000;24:145–155. [Abstract] [Google Scholar]
- Tian X, Eikmans M, van der Hoorn M-L. The role of macrophages in oocyte donation pregnancy: a systematic review. Int J Mol Sci 2020;21(3):939. [Europe PMC free article] [Abstract] [Google Scholar]
- Toriola AT, Vääräsmäki M, Lehtinen M, Zeleniuch-Jacquotte A, Lundin E, Rodgers K-G, Lakso H-A, Chen T, Schock H, Hallmans G et al. Determinants of maternal sex steroids during the first half of pregnancy. Obstet Gynecol 2011;118:1029–1036. [Europe PMC free article] [Abstract] [Google Scholar]
- Troisi R, Hoover RN, Thadhani R, Hsieh C-C, Sluss P, Ballard-Barbash R, Potischman N. Maternal, prenatal and perinatal characteristics and first trimester maternal serum hormone concentrations. Br J Cancer 2008;99:1161–1164. [Europe PMC free article] [Abstract] [Google Scholar]
- Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004;63:1–12. [Abstract] [Google Scholar]
- Tuckerman E, , LairdSM, , PrakashA, , Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Human Reproduction 2007;22:2208–2213. [Abstract] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017;19:568–577. [Europe PMC free article] [Abstract] [Google Scholar]
- Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update 2019;25:564–591. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Hoorn MLP, Lashley EELO, Bianchi DW, Claas FHJ, Schonkeren CMC, Scherjon SA. Clinical and immunologic aspects of egg donation pregnancies: a systematic review. Hum Reprod Update 2010;16:704–712. [Abstract] [Google Scholar]
- Vanderlelie J, Bell K, Perkins AV. The serum concentration of estradiol after embryo transfer and the decline from preovulatory levels may influence the success of IVF treatment. Horm Res 2003;59:95–99. [Abstract] [Google Scholar]
- Vasquez YM, DeMayo FJ. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol 2013;24:724–735. [Europe PMC free article] [Abstract] [Google Scholar]
- Venners SA, Liu X, Perry MJ, Korrick SA, Li Z, Yang F, Yang J, Lasley BL, Xu X, Wang X. Urinary estrogen and progesterone metabolite concentrations in menstrual cycles of fertile women with non-conception, early pregnancy loss or clinical pregnancy. Hum Reprod 2006;21:2272–2280. [Abstract] [Google Scholar]
- Verma P, Verma R, Nair RR, Budhwar S, Khanna A, Agrawal NR, Sinha R, Birendra R, Rajender S, Singh K. Altered crosstalk of estradiol and progesterone with Myeloid-derived suppressor cells and Th1/Th2 cytokines in early miscarriage is associated with early breakdown of maternal-fetal tolerance. Am J Reprod Immunol 2019;81:e13081. [Abstract] [Google Scholar]
- Wada-Hiraike O, , HiraikeH, , OkinagaH, , ImamovO, , BarrosRPA, , MoraniA, , OmotoY, , WarnerM, , Gustafsson J-Å.. Role of estrogen receptor β in uterine stroma and epithelium: Insights from estrogen receptor β −/− mice. Proc Natl Acad Sci USA 2006;103:18350–18355. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang J, Chen Z, Xiao Z, Weng Y, Yang M, Yang L, Tu Y, Zhou H, Wu L, Shun F et al. Estrogen induces IDO expression via TGF-β in chorionic villi and decidua during early stages of pregnancy. Int J Mol Med 2020a;46:1186–1196. [Abstract] [Google Scholar]
- Wang F, Qualls AE, Marques-Fernandez L, Colucci F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell Mol Immunol 2021;18:2101–2113. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang M, Hao C, Bao H, Huang X, Liu Z, Zhang W, Li F. Effect of elevated estradiol levels on the hCG administration day on IVF pregnancy and birth outcomes in the long GnRH-agonist protocol: analysis of 3393 cycles. Arch Gynecol Obstet 2017;295:407–414. [Abstract] [Google Scholar]
- Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol 2020b;11:2025. [Europe PMC free article] [Abstract] [Google Scholar]
- Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA 2000;97:5936–5941. [Europe PMC free article] [Abstract] [Google Scholar]
- Whittaker PG, Schreiber CA, Sammel MD. Gestational hormone trajectories and early pregnancy failure: a reassessment. Reprod Biol Endocrinol 2018;16:95. [Europe PMC free article] [Abstract] [Google Scholar]
- Winuthayanon W, , BernhardtML, , Padilla-BanksE, , MyersPH, , EdinML, , LihFB, , HewittSC, , KorachKS, , Williams CJ. Oviductal estrogen receptor α signaling prevents protease-mediated embryo death. eLife 2015;4. 10.7554/eLife.10453. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 2015;15:217–230. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y, Chen X, Liu P, Qiao J. Effect of HCG-day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reprod Biomed Online 2012;24:511–520. [Abstract] [Google Scholar]
- Wyns C, Geyter C, de C-JC, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Goossens V. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open 2022;2022:hoac022. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu X-L, Deng S-L, Lian Z-X, Yu K. Estrogen receptors in polycystic ovary syndrome. Cells 2021;10:459. [Europe PMC free article] [Abstract] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med 2010;28:5–16. [Abstract] [Google Scholar]
- Yovich JL, Stanger JD, Yovich JM, Tuvik AI, Turner SR. Hormonal profiles in the follicular phase, luteal phase and first trimester of pregnancies arising from in-vitro fertilization. Br J Obstet Gynaecol 1985;92:374–384. [Abstract] [Google Scholar]
- Yu K, Huang Z-Y, Xu X-L, Li J, Fu X-W, Deng S-L. Estrogen receptor function: impact on the human endometrium. Front Endocrinol (Lausanne) 2022;13:827724. [Europe PMC free article] [Abstract] [Google Scholar]
- Zamudio S, Leslie KK, White M, Hagerman DD, Moore LG. Low serum estradiol and high serum progesterone concentrations characterize hypertensive pregnancies at high altitude. J Soc Gynecol Investig 1994;1:197–205. [Abstract] [Google Scholar]
- Zavy MT, Craig LB, Wild RA, Kahn SN, O'Leary D, Hansen KR. In high responding patients undergoing an initial IVF cycle, elevated estradiol on the day of hCG has no effect on live birth rate. Reprod Biol Endocrinol 2014;12:119. [Europe PMC free article] [Abstract] [Google Scholar]
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374:13–22. [Abstract] [Google Scholar]
- Zhang M, Muralimanoharan S, Wortman AC, Mendelson CR. Primate-specific miR-515 family members inhibit key genes in human trophoblast differentiation and are upregulated in preeclampsia. Proc Natl Acad Sci USA 2016;113:E7069–E7076. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 2013;34:939–980. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang W, Tian Y, Xie D, Miao Y, Liu J, Wang X. The impact of peak estradiol during controlled ovarian stimulation on the cumulative live birth rate of IVF/ICSI in non-PCOS patients. J Assist Reprod Genet 2019;36:2333–2344. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE. VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ 2010;17:499–512. [Europe PMC free article] [Abstract] [Google Scholar]
- D de Z, Cornel C, Bergeron C, Hazout A, Bouchard P, Frydman R. Controlled preparation of the endometrium with exogenous estradiol and progesterone in women having functioning ovaries. Fertil Steril 1991;56:851–855. [Abstract] [Google Scholar]
Articles from Human Reproduction Update are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/humupd/dmad016
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/humupd/advance-article-pdf/doi/10.1093/humupd/dmad016/50693700/dmad016.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/152040458
Article citations
Effect of estradiol supplementation on luteal support following a significant reduction in serum estradiol levels after hCG triggering: a prospective randomized controlled trial.
Reprod Biol Endocrinol, 22(1):117, 12 Sep 2024
Cited by: 0 articles | PMID: 39267070 | PMCID: PMC11391712
Supraphysiological Dose of Testosterone Impairs the Expression and Distribution of Sex Steroid Receptors during Endometrial Receptivity Development in Female Sprague-Dawley Rats.
Int J Mol Sci, 25(18):10202, 23 Sep 2024
Cited by: 0 articles | PMID: 39337689 | PMCID: PMC11432676
Correlation of Maternal Vitamin D Status in Early Pregnancy and Vitamin D Supplementation during Pregnancy with Atopic Dermatitis in Infants: A Prospective Birth Cohort Study.
Nutrients, 16(13):2168, 08 Jul 2024
Cited by: 0 articles | PMID: 38999915
Follow-Up Study of 17-β Estradiol, Prolactin and Progesterone with the Kinetics and Prevalence of T. gondii Infection in Pregnant Women.
Curr Issues Mol Biol, 46(6):5701-5711, 07 Jun 2024
Cited by: 0 articles | PMID: 38921012 | PMCID: PMC11202031
Associations between mycoestrogen exposure and sex steroid hormone concentrations in maternal serum and cord blood in the UPSIDE pregnancy cohort.
Int J Hyg Environ Health, 260:114405, 14 Jun 2024
Cited by: 2 articles | PMID: 38878407 | PMCID: PMC11441442
Go to all (12) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV-positive pregnancies.
Hum Reprod, 35(8):1781-1796, 01 Aug 2020
Cited by: 7 articles | PMID: 32712670 | PMCID: PMC7398624
The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: a systematic review.
Hum Reprod Update, 25(1):72-94, 01 Jan 2019
Cited by: 24 articles | PMID: 30407510
Review
Endometrial receptivity in women of advanced age: an underrated factor in infertility.
Hum Reprod Update, 29(6):773-793, 01 Nov 2023
Cited by: 17 articles | PMID: 37468438 | PMCID: PMC10628506
Review Free full text in Europe PMC
Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2.
Hum Reprod, 30(6):1290-1301, 27 Mar 2015
Cited by: 56 articles | PMID: 25820695 | PMCID: PMC4498222