Abstract
Importance
Smoking causes considerable noncommunicable diseases, perinatal morbidity, and mortality.Objective
To investigate the associations of population-level tobacco-control policies with health outcomes.Data sources
PubMed, EMBASE, Web of Science, Cumulated Index to Nursing and Allied Health Literature, and EconLit were searched from inception to March 2021 (updated on 1 March 2022). References were manually searched.Study selection
Studies reporting on associations of population-level tobacco control policies with health-related outcomes were included. Data were analyzed from May to July 2022.Data extraction and synthesis
Data were extracted by 1 investigator and cross-checked by a second investigator. Analyses were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.Main outcomes and measures
The primary outcomes were respiratory system disease (RSD), cardiovascular disease (CVD), cancer, mortality, hospitalization, and health care utilization. The secondary outcomes were adverse birth outcomes, such as low birth weight and preterm birth. Random-effects meta-analysis was used to estimate pooled odds ratios (ORs) and 95% CIs.Results
Of 4952 records identified, 144 population-level studies were included in the final analysis; 126 studies (87.5%) were of high or moderate quality. The most frequently reported policies were smoke-free legislation (126 studies), followed by tax or price increases (14 studies), multicomponent tobacco control programs (12 studies), and a minimum cigarette purchase age law (1 study). Smoke-free legislation was associated with decreased risk of all CVD events (OR, 0.90; 95% CI, 0.86-0.94), RSD events (OR, 0.83; 95% CI, 0.72-0.96), hospitalization due to CVD or RSD (OR, 0.91; 95% CI, 0.87-0.95), and adverse birth outcomes (OR, 0.94; 95% CI, 0.92-0.96). These associations persisted in all sensitivity and subgroup analyses, except for the country income category, for which a significant reduction was only observed in high-income countries. In meta-analysis, there was no clear association of tax or price increases with adverse health outcomes. However, for the narrative synthesis, all 8 studies reported statistically significant associations between tax increases and decreases in adverse health events.Conclusions and relevance
In this systematic review and meta-analysis, smoke-free legislation was associated with significant reductions in morbidity and mortality related to CVD, RSD, and perinatal outcomes. These findings support the need to accelerate the implementation of smoke-free laws to protect populations against smoking-related harm.Free full text

Evaluation of Population-Level Tobacco Control Interventions and Health Outcomes
Key Points
Question
Are population-level tobacco control interventions associated with lower tobacco-linked adverse health outcomes?
Findings
In this systematic review and meta-analysis including 144 population-level studies, smoke-free legislation was associated with beneficial cardiovascular and respiratory health outcomes as well as birth outcomes. Health outcomes were not clearly associated with tax or price increases or other policies; however, the lack of relevant studies could explain this.
Meaning
These studies support the use of smoke-free legislation to improve population-level cardiovascular, respiratory, and birth outcomes.
Abstract
Importance
Smoking causes considerable noncommunicable diseases, perinatal morbidity, and mortality.
Objective
To investigate the associations of population-level tobacco-control policies with health outcomes.
Data Sources
PubMed, EMBASE, Web of Science, Cumulated Index to Nursing and Allied Health Literature, and EconLit were searched from inception to March 2021 (updated on 1 March 2022). References were manually searched.
Study Selection
Studies reporting on associations of population-level tobacco control policies with health-related outcomes were included. Data were analyzed from May to July 2022.
Data Extraction and Synthesis
Data were extracted by 1 investigator and cross-checked by a second investigator. Analyses were conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline.
Main Outcomes and Measures
The primary outcomes were respiratory system disease (RSD), cardiovascular disease (CVD), cancer, mortality, hospitalization, and health care utilization. The secondary outcomes were adverse birth outcomes, such as low birth weight and preterm birth. Random-effects meta-analysis was used to estimate pooled odds ratios (ORs) and 95% CIs.
Results
Of 4952 records identified, 144 population-level studies were included in the final analysis; 126 studies (87.5%) were of high or moderate quality. The most frequently reported policies were smoke-free legislation (126 studies), followed by tax or price increases (14 studies), multicomponent tobacco control programs (12 studies), and a minimum cigarette purchase age law (1 study). Smoke-free legislation was associated with decreased risk of all CVD events (OR, 0.90; 95% CI, 0.86-0.94), RSD events (OR, 0.83; 95% CI, 0.72-0.96), hospitalization due to CVD or RSD (OR, 0.91; 95% CI, 0.87-0.95), and adverse birth outcomes (OR, 0.94; 95% CI, 0.92-0.96). These associations persisted in all sensitivity and subgroup analyses, except for the country income category, for which a significant reduction was only observed in high-income countries. In meta-analysis, there was no clear association of tax or price increases with adverse health outcomes. However, for the narrative synthesis, all 8 studies reported statistically significant associations between tax increases and decreases in adverse health events.
Conclusions and Relevance
In this systematic review and meta-analysis, smoke-free legislation was associated with significant reductions in morbidity and mortality related to CVD, RSD, and perinatal outcomes. These findings support the need to accelerate the implementation of smoke-free laws to protect populations against smoking-related harm.
Introduction
The global health burden associated with tobacco use is high. Despite the implementation of numerous tobacco control policies to change smoking behaviors over the past decades,1 tobacco use remains the second leading risk factor of mortality, with 8.7 million attributable deaths worldwide in 2019.2 Exposure to tobacco smoke, including secondhand smoke (SHS), has been linked to adverse health outcomes in children and adults, particularly chronic noncommunicable diseases, such as cardiac, cerebrovascular, and respiratory diseases and cancers.3,4,5,6,7,8,9 Over 2 decades, to reduce smoking-related morbidity and mortality, various evidence-based tobacco control policies have been proposed, such as the Framework Convention on Tobacco Control by the World Health Organization.10 Framework Convention on Tobacco Control policies, including taxations, regulations, and nicotine replacement therapies, have been found to be associated with lowering the incidence of adverse health outcomes in multiple settings.11,12
To our knowledge, 12 systematic reviews and meta-analyses have synthesized evidence of the associations of population-level tobacco control interventions with health outcomes (eTable 1 in Supplement 1).13,14,15,16,17,18,19,20,21,22,23,24,25 The evidence suggests that regulatory policies, particularly those prohibiting smoking, such as local or public smoking bans, were associated with reduced risks of cardiovascular, cerebrovascular, and respiratory diseases16,24 and improved perinatal outcomes.13,15 However, these syntheses focused on the associations of single or select interventions separately with specific outcomes or populations (children13,15,25 or adults14,16,17,18,19,20,21,22,23,24). In a 2012 meta-analysis, Tan and Glantz24 assessed the pooled risk estimation for the association between smoke-free legislation and hospitalization due to cardiovascular disease (CVD) or respiratory system diseases (RSD) but did not focus on the incidence or prevalence of these conditions. Moreover, associations with other types of population-level policies, such as advertisement campaigns, health warnings on product packages, and taxation of tobacco products, have not been fully explored. While tobacco taxation has been found to be associated with reductions in neonatal and infant mortality,26 there is no systematic review and meta-analysis of its associations with other health outcomes, to our knowledge. Despite these gaps, many countries have continued implementing the tobacco control policies in their endeavors to improve population health and reduce health care costs.16 Systematic reviews and meta-analyses are crucial to quantify the extent to which each tobacco control policy is associated with improving health outcomes and would inform the design and roll-out of more cost-effective policies.
This study provides a comprehensive synthesis of the associations between population-level tobacco control policies and a range of health-related outcomes. Through systematic review and meta-analysis, we aimed to summarize the associations of implementation of all available population-level tobacco control policies with health-related outcomes and estimate a pooled effect size for each relevant combination of policies with outcomes of interest.
Methods
Search Strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. The protocol was registered with PROSPERO (registration No. CRD42022340141). A global search was conducted for studies that report on associations of population-level tobacco control policies with health-related outcomes. We searched for peer-reviewed journal articles and gray literature published from inception to March 2021 (updated on March 1, 2022) using 5 electronic databases: PubMed, Embase, Cumulated Index to Nursing and Allied Health Literature, Web of Science, and EconLit. Complementary searches included reference lists of primary studies and systematic reviews, Google Scholar, and leading organizational websites. Letters, case series, systematic or scoping reviews, commentaries, and editorials without original research findings were excluded. Details of the search strategy and results are presented in eTables 2 through 6 in Supplement 1.
Eligibility Criteria
We summarize eligibility criteria using the PICOS (population, intervention, comparator, outcome, study type) framework. No restriction was applied on population type, condition, or age. Included interventions were population-level policies aimed at reducing tobacco use, ie, interventions or programs implemented outside controlled settings. All policies implemented by governments and nongovernmental organizations were included, including smoking bans, taxation, and minimum cigarette purchase age. Outcomes of interest were respiratory symptoms and related diseases (asthma, chronic obstructive pulmonary disease, pneumonia, bronchitis, spontaneous pneumothorax, and lung cancer), cardiovascular symptoms and related diseases (acute myocardial infarction, ischemic heart disease, acute coronary syndrome, sudden circulatory arrest, angina, congestive heart failure, hypertension), cerebrovascular diseases (stroke, transient ischemic attack), sudden cardiac death, cancers, as well as overall mortality and mortality associated with these conditions. Also, hospitalization, health care utilization, and attendance at health check-ups due to CVD, RSD, or related symptoms were assessed. Finally, we considered adverse perinatal outcomes, such as low birth weight, preterm birth, small for gestational age, still birth, infant mortality, and neonatal mortality. No restriction was applied to the type of comparator.
Study Design
Included studies were observational studies of any design, such as cross-sectional, case-control, cohort studies, and quasi-experimental studies (eg, interrupted time series, before and after, and controlled before and after). Studies that used a controlled environment to estimate intervention effects (eg, randomized clinical trials) and simulation or modeling studies were excluded.
Study Selection
Two assessors independently screened the titles and abstracts (first stage) and critically reviewed the full texts of the selected studies (second stage) to assess eligibility. Disagreements at either stage were resolved through discussion with S.A. and R.N. Study authors were contacted if relevant information on eligibility appeared to be missing at the full-text screening stage.
Data Extraction
A preconceived and standardized data extraction form was used to collect information on the first author’s last name, study country, publication year, survey year, study design, sample size, age range, type of intervention or policy, outcome variable, and quantitative estimates of the associations between policy interventions and the outcomes of interest (eg, percentage, prevalence, odds ratio [OR], risk ratio, hazard ratio [HR]) (eAppendix 1 in Supplement 1). One investigator independently extracted data from the selected primary studies. The extracted data were then cross-checked by a second investigator (S.A. or R.S.N.). Disagreements were resolved consensually.
Estimates for each disease or health measure in studies that reported multiple outcomes (eg, CVDs, cancers, and RSDs) were identified and separately listed. Regression coefficients were reported using logistic regression, and ORs were calculated using exponentiation and P values. Where regression coefficients were reported using linear regression, we calculated the pooled regression coefficient using each coefficient value and the SE or P value. A study that provided multiple ORs, relative risks (RRs), or HRs due to the use of categorical exposure data, such as by sex, geographic area, or age group, were combined into a single overall estimate by running a meta-analysis. Whenever a study provided multiple effect size estimates from statistical models adjusted for different covariates, we selected the estimate that had been adjusted for the greatest number of variables. Finally, when a study provided a percentage change or mean difference with or without P values, we listed those values and summarized the evidence.
Study Quality Assessment
The Newcastle-Ottawa Scale was used to assess the quality of observational studies.27 The quality of controlled before and after, interrupted time series, and other quasi-experimental studies were coded using the Cochrane Effective Practice and Organisation of Care Tools28 (eAppendix 2 in Supplement 2).
Statistical Analysis
A meta-analysis was performed for studies that presented complete data with statistical testing. A narrative synthesis was performed for studies without statistical testing or with high heterogeneity across measures. For meta-analysis, to summarize effect sizes, we performed fixed- or random-effects meta-analyses depending on the degree of heterogeneity. For dichotomous outcomes, ORs, RRs, or HRs with 95% CIs were used to calculate pooled estimates. Regression coefficients with SDs or 95% CIs were used for continuous outcome variables. In line with a previous study,29 we treated ORs as equal to RRs (the most commonly reported type of estimate) each time the incidence of the outcome of interest was low (<10%) in the study population. Funnel plots and Egger test30 were used to assess publication bias. We performed trim and fill procedures to further evaluate possible effects of publication bias.31 We conducted subgroup analyses according to study designs, country-income categories, study quality, and comprehensiveness of smoke-free legislations. Sensitivity analyses were performed by excluding highly influential studies with large sample sizes. Data analyses were performed using Stata software version 16.1 MP (StataCorp) and R software version 3.6.4 (R Project for Statistical Computing). P values were 2-sided, and statistical significance was set at P <
< .05. Data were analyzed from May to July 2022.
.05. Data were analyzed from May to July 2022.
Results
Study Selection
A total of 4952 studies were identified from the initial search, of which 192 studies were added after a manual search and the gray literature. After removal of duplicates, 4408 unique citations were screened for title and abstract, and 515 full-text articles were screened for eligibility. Of these, 144 population-level studies32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176 met the eligibility criteria and were included in analysis (eFigure 1 in Supplement 1).
Study Characteristics
eTable 7 in Supplement 1 shows characteristics of the included studies. Of 144 studies, 26 studies39,46,51,52,55,71,75,97,99,104,106,107,108,110,126,131,135,138,141,142,146,153,154,156,158,173 were cohort or longitudinal, 66 studies32,34,36,37,38,43,44,45,48,49,50,53,54,56,58,59,61,65,66,67,69,73,74,78,79,80,81,82,83,84,85,87,88,89,91,92,93,94,98,111,112,113,114,115,117,119,120,121,122,124,127,137,140,143,144,147,148,150,151,152,164,165,170,171,172,173,174 were interrupted time series, 25 studies40,42,62,63,72,76,77,90,96,102,116,123,125,130,133,134,145,149,155,157,159,162,167,168,175 were controlled before and after, and 27 studies33,35,41,47,52,57,60,64,69,70,86,95,100,101,105,109,118,129,132,136,139,160,161,163,166,176 were cross-sectional. The plurality of the studies (72 studies33,40,41,42,46,47,49,51,53,54,56,57,59,60,61,62,63,65,66,67,69,72,74,75,76,77,79,80,90,91,93,95,98,99,105,108,109,110,114,115,117,119,120,121,122,123,124,125,126,128,130,132,133,136,137,143,144,145,146,149,151,155,157,158,159,160,161,162,168,172,173,175 [50.0%]) were conducted in the United States, 45 studies32,35,37,38,39,48,50,52,55,58,64,68,70,71,82,83,84,85,86,92,94,96,100,101,102,103,104,106,107,111,112,113,116,118,129,142,150,152,154,156,163,166,167,170,171 (31.2%) were conducted in Europe, 14 studies34,43,44,45,73,78,97,138,139,140,141,148,153,164 (9.7%) were conducted in the United Kingdom, and 13 studies36,81,87,88,89,127,131,134,135,147,165,174,176 (9.0%) were conducted elsewhere (mostly in Asia and Latin America). Most studies evaluated smoke-free legislation (126 studies32,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,58,59,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,96,97,99,100,101,102,103,104,105,106,107,108,110,111,112,113,114,115,116,117,118,119,120,121,122,123,125,128,129,130,131,133,134,135,136,138,139,140,141,142,144,145,146,147,148,149,150,153,154,155,156,157,158,160,161,162,163,164,165,166,167,168,170,171,172,173,176 [87.5%]), 14 studies6,60,61,63,66,81,109,121,122,137,144,145,151,159 (9.7%) evaluated tax or price increases, 12 studies53,57,76,95,98,124,126,127,132,143,152,174 (8.3%) assessed multicomponent tobacco control programs, and 1 study175 (0.7%) focused only on minimum cigarette purchase age. The included studies were published between 1998 and 2021. In terms of quality, 86 studies33,39,40,41,43,44,47,49,51,55,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,77,80,81,82,83,85,86,89,90,91,96,99,100,103,105,109,111,112,115,117,118,119,120,121,122,127,128,129,130,132,134,136,137,139,140,141,143,145,146,147,150,151,152,153,155,156,159,160,161,162,163,164,166,167,171,174,176 were rated high, 40 studies32,34,35,36,37,38,45,46,48,50,76,78,79,87,88,92,93,94,95,97,98,102,104,108,110,114,123,124,125,131,133,135,138,142,144,148,149,157,158,173 were rated moderate, and 18 studies42,52,53,54,56,84,101,106,107,113,116,126,154,165,168,170,172,175 were rated poor. Of 144 studies, 60 studies32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 were included in the meta-analysis, 84 studies33,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176 were included in the narrative summary, and 1 study33 was included in both the meta-analysis and narrative summary.
Meta-Analysis
Only 60 studies32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 reported quantitative information (ie, OR, RR, HR, or coefficient) suitable for calculating pooled estimates. Effect sizes reported in the other 84 studies33,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176 were summarized narratively. A standard meta-analysis (ie, with >2 studies) was possible for studies assessing smoke-free legislation. Studies on other types of policies were too heterogeneous in terms of outcomes measured or types of data used. Findings are presented in the form of forest plots in eFigures 2 through 6 in Supplement 1 to provide an overview.
Of 60 studies32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 included in the meta-analysis, 15 studies32,39,48,50,58,65,72,75,79,84,85,86,87,88,92 assessed the association of smoke-free legislation with incidence or prevalence of CVD or CVD mortality (Figure 1; eFigure 2 in Supplement 1). We found that smoke-free legislation was associated with significant reductions in the incidence or prevalence of CVD (OR, 0.91; 95% CI, 0.87-0.94), CVD mortality (OR, 0.90; 95% CI, 0.83-0.97), and occurrence of any type of CVD event (OR, 0.90; 95% CI, 0.86-0.94) (Figure 1).
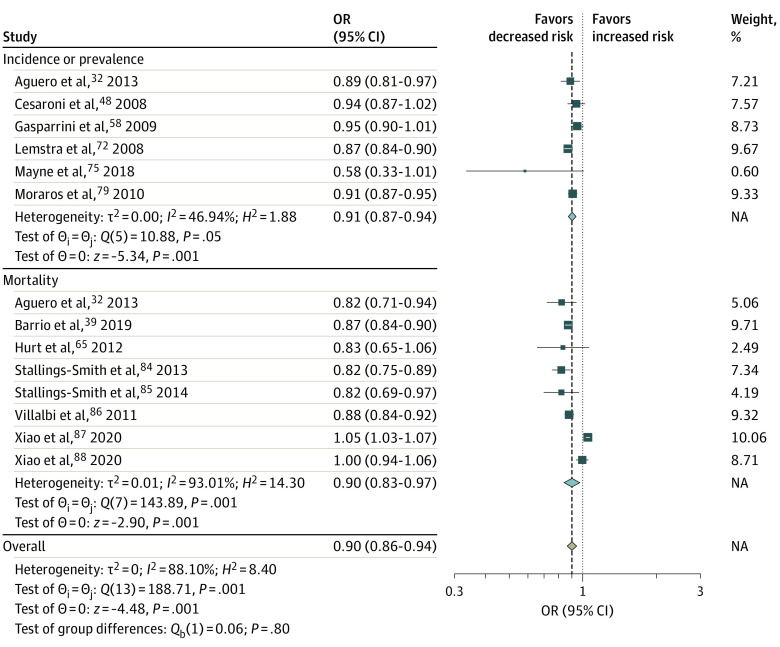
Cardiovascular events included the incidence, prevalence, and mortality due to acute myocardial infarction, heart attack, sudden cardiac death, coronary heart disease, and cerebrovascular disease. Squares indicate estimates; size of squares, study weights; whiskers, 95% CIs; diamond, mean estimate; NA, not applicable; OR, odds ratio.
Twelve studies35,39,45,49,51,55,61,71,79,84,85,105 assessed the association of smoke-free legislation with the prevalence or incidence of RSD, RSD symptoms, or RSD mortality (Figure 2; eFigure 2 in Supplement 1). Smoke-free legislation was not found to be significantly associated with prevalence of RSD or RSD symptoms (OR, 0.83; 95% CI, 0.67-1.03) (Figure 2) but was significantly associated with reductions in RSD mortality (OR, 0.91; 95% CI, 0.85-0.96) and occurrence of any RSD, RSD symptoms, or RSD mortality (OR, 0.83; 95% CI, 0.72-0.96) (Figure 2). The association of smoke-free legislation with hospitalizations induced by CVD was investigated in 24 studies,36,37,38,42,47,52,53,59,62,63,64,65,66,67,70,73,74,81,83,87,89,90,91,99 and the association of smoke-free legislation with hospitalizations induced by RSD or RSD symptoms was investigated in 9 studies44,52,53,55,62,63,64,69,70,78 (Figure 3; eFigure 2 in Supplement 1). In both analyses, the smoke-free legislation was found to be associated with significant reductions in hospitalizations (Figure 3).
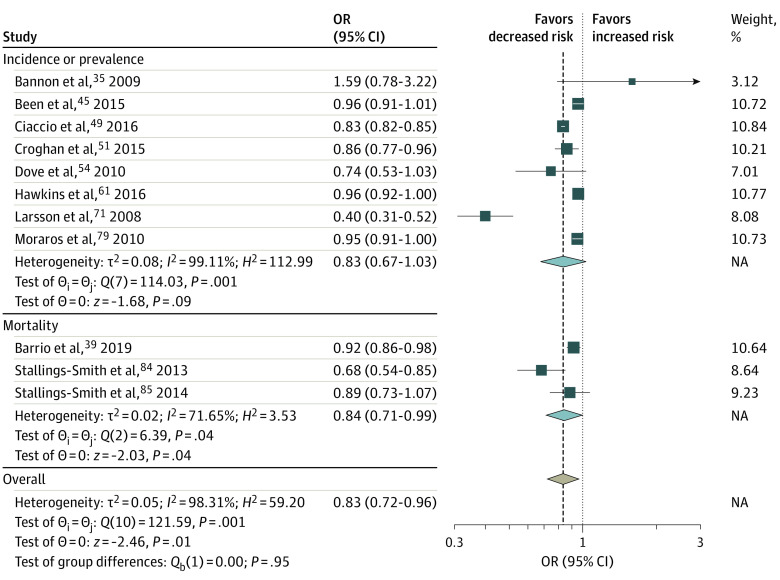
Respiratory disease or respiratory symptoms included the prevalence and mortality of lung cancer, respiratory symptoms, chronic obstructive pulmonary disease, asthma, and bronchitis. Squares indicate estimates; size of squares, study weights; whiskers, 95% CIs; diamond, mean estimate; NA, not applicable; OR, odds ratio.
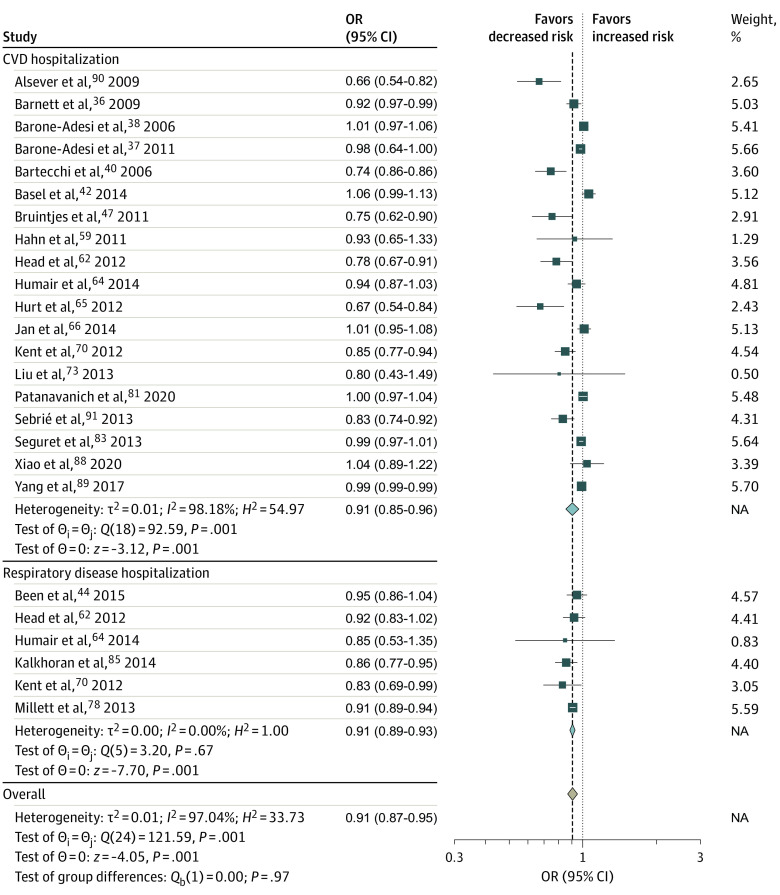
Cardiovascular disease hospital admission rates included admissions due to cardiovascular disease, ischemic heart disease, angina, acute coronary syndrome, coronary heart disease, acute myocardial infarction, heart attack, cerebrovascular disease, and stroke. Hospitalization due to respiratory disease or respiratory symptoms included admission due to lung cancer, respiratory symptoms, chronic obstructive pulmonary disease, asthma, and bronchitis. Squares indicate estimates; size of squares, study weights; whiskers, 95% CIs; diamond, mean estimate; NA, not applicable; OR, odds ratio.
Eight studies33,34,41,43,68,77,80,82 estimated the associations between smoke-free legislation and adverse birth outcomes, including infant mortality, neonatal mortality, stillbirth, preterm birth, very preterm birth, small for gestational age, low birth weight, very low birth weight, and sudden infant death syndrome (Figure 4; eFigure 3 in Supplement 1). Although the pooled estimates for low birth weight, preterm birth, and small for gestational age were not statistically significant, significant associations were found for stillbirth (OR, 0.93; 95% CI, 0.890.97) and overall birth (OR, 0.94; 95% CI, 0.92-0.96) outcomes (Figure 4).
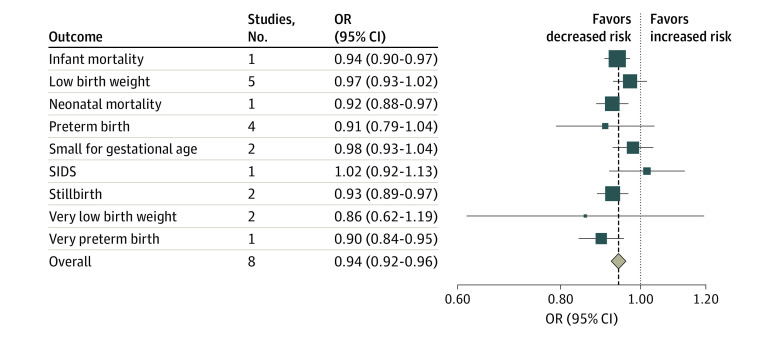
Squares indicate estimates; size of squares, study weights; whiskers, 95% CIs; diamond, mean estimate; OR, odds ratio; SIDS, sudden infant death syndrome.
Limited research has been conducted on the associations of tobacco tax policies with health-related outcomes. The available data suffer from heterogeneity in terms of measured outcomes (eg, hospitalization, incidence, or mortality for various health conditions) or data types used (eg, some studies presented data as ORs while others as regression coefficient), making a standardized meta-analysis unfeasible. For instance, 4 studies53,63,66,81 examined CVD-related hospitalizations, 2 studies60,61 focused on the prevalence or incidence of RSD and RSD symptoms, 2 studies53,63 explored RSD- and RSD symptom–related hospitalizations, and 1 study46 investigated RSD-related mortality (eFigure 4 and eFigure 5 in Supplement 1). No significant associations were found for any of these outcomes. Additionally, 2 studies46,53 focused on cancer incidence or mortality, and another study46 investigated total mortality (eFigure 4 and eFigure 5 in Supplement 1). While tax policies demonstrated a significant association with reduced cancer mortality and total mortality, no significant association was observed for cancer incidence.
Finally, 3 studies53,57,76 assessed the association of multicomponent tobacco control programs with CVD mortality, CVD-related hospitalizations, RSD- or RSD symptom–related hospitalizations, and cancer incidence (eFigure 6 in Supplement 1). Mixed results have been reported regarding reductions in CVD-related mortality risks.57,76 One study76 found multicomponent tobacco control programs to be significantly associated with decreased CVD mortality, while another study reported a significant increase in CVD mortality.57 The other study53 found that multicomponent tobacco control programs were significantly associated with reduced CVD-related hospitalizations but not cancer incidence or respiratory disease-related hospitalizations.
We found evidence of large heterogeneity in the association between smoke-free legislation and CVD events (I2 =
= 88.1%; P
88.1%; P <
< .001) (Figure 1), RSD and RSD symptom events (I2
.001) (Figure 1), RSD and RSD symptom events (I2 =
= 98.3%; P
98.3%; P <
< .001) (Figure 2), hospitalization due to CVD and RSD and RSD symptom (I2
.001) (Figure 2), hospitalization due to CVD and RSD and RSD symptom (I2 =
= 97.0%; P
97.0%; P <
< .001) (Figure 3), and adverse birth outcomes (I2
.001) (Figure 3), and adverse birth outcomes (I2 =
= 76% to 97%; P
76% to 97%; P <
< .01). However, results of the main meta-analysis were consistent in the sensitivity (eAppendix 3, eFigures 7-9, and eTable 8 in Supplement 1) and stratified (eAppendix 4, eTable 9, and eTable 10 in Supplement 1) analyses, except for country income category, for which a significant reduction was observed in high-income countries.
.01). However, results of the main meta-analysis were consistent in the sensitivity (eAppendix 3, eFigures 7-9, and eTable 8 in Supplement 1) and stratified (eAppendix 4, eTable 9, and eTable 10 in Supplement 1) analyses, except for country income category, for which a significant reduction was observed in high-income countries.
Narrative Summary
The Table presents a narrative summary of the associations of population-level tobacco control policies with health-related outcomes. More details are provided in eTables 11 through 14 in Supplement 1. The narrative summary included 84 studies.33,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176 After the implementation of smoke-free legislation, risk reductions were reported in 13 of 17 studies3,17,27,30,40,55,67,76,90,111,119,120,126,130,134,135,139 (76.4%) for CVD events, 9 of 17 studies94,104,111,112,113,123,130,136,146,147,149,154,165,170,171 (52.9%) for CVD-related hospitalizations, 14 of 18 studies54,94,104,111,112,113,123,130,136,146,147,149,153,154,165,170,171 (77.8%) for RSD and RSD symptoms, 8 of 16 studies90,96,106,107,108,110,116,131,135,138,142,147,148,149,158,170,171,173 (50.0%) for RSD-related hospitalizations, and 10 of 14 studies101,111,112,113,115,123,124,139,147,149,165,168 (71.4%) for risk of adverse birth outcomes. One study161 reported an improvement in self-reported health. After the implementation of tax or price increases, all 8 studies45,62,64,65,100,101,114,125 found significant reductions in CVD- and RSD-related hospitalizations, RSD and RSD symptoms, sudden infant death syndrome, and adverse birth outcomes. Regarding multicomponent tobacco control policies, both studies14,141 assessing risk of CVD-related hospitalizations and RSD and RSD symptoms reported reductions, 2 studies124,127 found reductions in mortality (cancer or smoking-attributable), and 1 study152 reported a reduction in small for gestational age but not for other birth outcomes, such as perinatal mortality rate, preterm birth, very preterm birth, still birth, neonatal mortality, low birth weight, and very low birth weight. The narrative summary of the associations of free or discounted nicotine replacement therapy, minimum cigarette purchase age law, and tobacco retailer density are presented in eTable 14 in Supplement 1.
Table.
| Outcome | Tobacco policy studies, No. (N = = 80) 80) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multicomponent tobacco lawa | Tax or price increase | Smoking-free legislation | |||||||
| Positive | Negative | Null | Positive | Negative | Null | Positive | Negative | Null | |
| Cardiovascular eventb | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 4 |
| Hospital admission rates due to cardiovascular diseasesc | 1 | 0 | 0 | 1 | 0 | 0 | 9 | 0 | 8 |
| Lung cancer, SIDS, respiratory symptoms and diseasesd | 2 | 0 | 0 | 3 | 0 | 0 | 14 | 0 | 4 |
| Hospital admission and discharge rates due to lung diseasese | 0 | 0 | 0 | 1 | 0 | 0 | 8 | 2 | 6 |
| Birth outcomesf | 1 | 0 | 1 | 3 | 0 | 0 | 10 | 1 | 3 |
| Cancerg | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Health statush | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Abbreviation: SIDS, sudden infant death syndrome.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to evaluate the associations of all types of population-level tobacco control policies with health-related outcomes. We found evidence that smoke-free legislation was significantly associated with reductions in the risk of CVD events, RSD events or symptoms, CVD- and RSD-related hospitalizations, and adverse perinatal outcomes. However, the associations of other types of tobacco control policies remain unclear, primarily due to a limited number of primary studies available.
Our analysis showed that smoke-free legislation was associated with approximately 9% to 10% reduced odds of overall CVD events, including CVD incidence, prevalence, and mortality due to acute myocardial infarction, coronary heart disease, cerebrovascular disease (stroke, transient ischemic attack), and sudden cardiac death. In previous meta-analyses investigating the associations with more specific outcomes, smoke-free legislation was found to be associated with reductions in AMI mortality between 8%17 and 13%.20 Our findings confirm the positive associations of smoke-free legislation with acute myocardial infarction mortality and generally positive outcomes in overall CVD event risk reduction.
Smoke-free legislation was also found to be associated with a 9% reduction in CVD-related hospitalizations. Previous meta-analyses also reported positive associations between the policy and hospitalizations; however, they focused on specific CVD events: a reduction by 15% for coronary events, 16% for cerebrovascular accidents, and 39% for other heart diseases.24 Regarding respiratory diseases or symptoms, we found that smoke-free legislation was associated with a 16% to 17% reduction in mortality and a 9% reduction in related hospitalizations, corroborating the findings of a 24% reduction in respiratory disease24 and a 19% reduction in respiratory symptoms from previous meta-analyses.23 Our results also support the use of smoking bans to improve birth outcomes. We found that smoke-free legislation was associated with a 4% to 9% reduction in the odds of adverse perinatal outcomes. Previous studies have reported mixed findings. One study by Been et al13 found evidence of reductions in the risk of preterm birth by10% but not low birth weight.13 Another study by Faber et al15 reported significant improvements for a range of birth outcomes (eg, 2% reduction in small for gestational age and 10% reduction in the risk of very preterm birth), but not for other birth outcomes. Although tobacco tax policies are significantly associated with reductions in smoking prevalence,1,11,12,15 we did not find any significant associations of tax policies with improvements in the health outcomes of interest. At the primary study level, we observed a large heterogeneity in the size and direction of policy outcomes across studies.60,61 Recent systematic reviews have found that marketing restrictions and warning labels were associated with decreased tobacco consumption,1,177,178 which may in turn be associated with positive health benefits. A 2019 study by Jiang et al127 found that a cigarette advertisement ban was associated with reduced tobacco consumption and overall cancer mortality by −1.43 and −1.24 per 100 000 population, respectively.
000 population, respectively.
Enforcement of smoke-free legislation may be associated with a reduction of the risk of adverse health outcomes. Rapid declines in CVD conditions may be associated with decreases in exposure to SHS after the implementation of smoke-free laws.18 Even low doses of exposure to toxins in tobacco smoke have been found to increase the risk of CVD conditions through various channels, such as activation of blood platelets, increased arterial stiffness, and others.98,179,180 Current evidence for establishing a causal link between tobacco smoke exposure and RSD is only suggestive.84 For instance, SHS exposure has been associated with exacerbations among individuals with chronic obstructive pulmonary disease.181,182 Moreover, a dose-response association has been demonstrated in relation to the comprehensiveness of smoke-free legislation and the incidence of respiratory disease.24 For birth outcomes, active maternal smoking and SHS exposure during pregnancy are known risk factors associated with preterm birth, low birth weight, small for gestational age, stillbirth, and others.1 Maternal smoking and exposure to SHS during pregnancy can pose severe developmental health risks to the fetus due to toxic constituents of tobacco smoke that readily penetrate the placenta.183
Limitations
This study has some limitations. First, we found significant between-study heterogeneity. To minimize its influence, we used a random-effects meta-analysis and stratified by individual health outcomes. In our stratified analyses, we found summary ORs consistently less than 1 across several study-level characteristics, except for the country income category. Second, smoke-free legislation policies are implemented to varying degrees (eg, workplaces only, workplaces and restaurants only, or workplaces, restaurants, and bars). Thus, the potential for differential- or dose-response associations is dependent on the comprehensiveness of interventions.13 Thus, data were analyzed separately according to whether the bans were comprehensive or partial, but we did not find any significant differences. Third, funnel plot analysis showed some asymmetry for all outcomes, except for mortality due to RSD, suggesting the possibility of publication bias and missing gray literature. To address this issue, we used a trim-and-fill method that can capture all unpublished and gray literature. The results did not vary, suggesting that the association was unaffected by unpublished studies. Fourth, as studies included in the meta-analyses were mainly from high-income countries, the findings might not be generalizable to low- and middle-income countries. We performed a stratified analysis according to income category and found a consistent pooled OR greater than 1 in low- and middle-income countries for all outcomes related to CVD. Fifth, emerging trends in tobacco use, such as the increase in the use of alternative tobacco products (eg, electronic cigarettes) and changing levels of air pollution, may impact the effectiveness of tobacco policies on health outcomes. Additionally, an increasing prevalence of cannabis use, which is often associated with tobacco consumption,184 is not fully reflected in this study.
Conclusions
In this systematic review and meta-analysis, we found that implementation of smoke-free legislation was followed by a significant decrease in multiple adverse health outcomes. The findings support the need to accelerate the uptake of laws restricting smoking in public spaces in efforts to protect people from related cardiovascular, respiratory, and birth health hazards.
Notes
Supplement 1.
eTable 1. Synthesis Past Evidence
eTable 2. PubMed Search Results
eTable 3. EMBASE Search Results
eTable 4. Web of Science Search Results
eTable 5. CINAHL Search Results
eTable 6. EconLit Search Results
eAppendix 1. Data Extraction Form
eAppendix 2. Study Quality Assessment
eFigure 1. Study Selection Flowchart
eTable 7. Study Characteristics
eFigure 2. Forest Plot of Meta-analysis of the Studies Examining the Associations of Smoke-free Legislation With Cardiovascular Disease (CVD), CVD Hospitalization, Respiratory Disease Hospitalization, CVD Mortality, Cancer, Respiratory Symptoms, Birth Weight, and Gestational Age Based on Coefficient Results
eFigure 3. Meta-analysis of the Association of Smoke-free Legislation With Perinatal Mortality and Adverse Birth Outcomes
eFigure 4. Forest Plot of Meta-analysis of Studies Examining the Association of Tax or Price Increase With Hospitalization Due to Cardiovascular Disease or Respiratory Symptoms
eFigure 5. Forest Plot of Meta-analysis of the Studies Examining the Association of Tax or Price Increase With Total Mortality, Cardiovascular Disease Mortality, Cancer Mortality, Respiratory Disease Mortality, Cardiovascular Disease Hospitalization, and Respiratory Disease Hospitalization Based on Coefficient Results
eFigure 6. Forest Plot of Meta-analysis of Studies Examining the Synergistic Associations of Tobacco Control Program (Combination of Several Tobacco Control Laws) With Cardiovascular Disease Hospitalization, Cardiovascular Disease Mortality, and Cancer Based on Coefficient Results
eAppendix 3. Sensitivity Analyses
eFigure 7. Publication Bias Assessment for Cardiovascular Diseases
eFigure 8. Publication Bias Assessment for Smoke-free Legislation and Respiratory Disease and Symptoms
eFigure 9. Publication Bias Assessment for Smoke-free Legislation and Perinatal Mortality and Adverse Birth Outcomes
eTable 8. Summary, Publication Bias, and Trim-and-Fill Estimates
eAppendix 4. Stratified Analysis
eTable 9. Stratified Analysis According to Study Design, Country Income Category and Quality Score
eTable 10. Stratified Analysis According to Comprehensiveness of Smoke-free Legislation
eTable 11. Narrative Summary of Adverse Health Outcomes Following the Implementation of the Smoking Ban
eTable 12. Narrative Summary of Adverse Health Outcomes Following the Implementation of Tobacco Law
eTable 13. Narrative Summary of Adverse Health Outcomes Following the Implementation of an Increase in Cigarette Prices or Cigarette Taxes
eTable 14. Narrative Summary of Adverse Health Outcomes Following the Implementation of Miscellaneous Policies
eReferences.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamanetworkopen.2023.22341
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2807052/akter_2023_oi_230662_1688055895.14775.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/151108779
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jamanetworkopen.2023.22341
Article citations
A systematic review and network meta-analysis of population-level interventions to tackle smoking behaviour.
Nat Hum Behav, 07 Oct 2024
Cited by: 0 articles | PMID: 39375543
Regional insights on tobacco-related tweets: unveiling user opinions and usage patterns.
Front Public Health, 12:1342460, 14 Jun 2024
Cited by: 0 articles | PMID: 38947344
Does Exposure to Ambient Air Pollution Affect Gestational Age and Newborn Weight?-A Systematic Review.
Healthcare (Basel), 12(12):1176, 11 Jun 2024
Cited by: 0 articles | PMID: 38921290 | PMCID: PMC11203000
Review Free full text in Europe PMC
Impact of Total Indoor Smoking Ban on Cardiovascular Disease Hospitalizations and Mortality: The Case of Chile.
Nicotine Tob Res, 26(9):1166-1174, 01 Aug 2024
Cited by: 0 articles | PMID: 38457437 | PMCID: PMC11339173
The relationship between the price and demand of alcohol, tobacco, unhealthy food, sugar-sweetened beverages, and gambling: an umbrella review of systematic reviews.
BMC Public Health, 24(1):1286, 10 May 2024
Cited by: 2 articles | PMID: 38730332 | PMCID: PMC11088175
Review Free full text in Europe PMC
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Cochrane Database Syst Rev, 2(2022), 01 Feb 2022
Cited by: 12 articles | PMID: 36321557 | PMCID: PMC8805585
Review Free full text in Europe PMC
Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis.
Lancet Public Health, 2(9):e420-e437, 05 Sep 2017
Cited by: 82 articles | PMID: 28944313 | PMCID: PMC5592249
Interventions for Tobacco Cessation in Adults, Including Pregnant Women: An Evidence Update for the U.S. Preventive Services Task Force
Agency for Healthcare Research and Quality (US), Rockville (MD), 02 Feb 2021
Cited by: 0 articles | PMID: 33523610
ReviewBooks & documents Free full text in Europe PMC
Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force
Agency for Healthcare Research and Quality (US), Rockville (MD), 25 Jan 2018
Cited by: 1 article | PMID: 29364620
ReviewBooks & documents Free full text in Europe PMC

 1
,
4
1
,
4



