Abstract
Free full text

Stereoselective amino acid synthesis by synergistic photoredox–pyridoxal radical biocatalysis
Abstract
Advancing general biocatalytic activation modes which are both new-to-nature and new-to-chemistry constitutes a premier challenge. Through the synergistic merger of photoredox catalysis and pyridoxal 5’-phosphate (PLP) biocatalysis, we developed a new form of radical pyridoxal biocatalysis that is not known in either natural PLP enzymology or synthetic carbonyl catalysis, enabling the protecting-group-free preparation of valuable non-canonical amino acids, including those bearing a stereochemical dyad or triad. Using engineered PLP enzymes, the α-stereochemistry of amino acids could be edited, giving rise to either enantiomeric product in a biocatalyst-controlled fashion. Together, synergetic photoredox–pyridoxal radical biocatalysis represents a powerful platform to discover new catalytic reactions and to tame radical intermediates for asymmetric catalysis.
One-Sentence Summary:
Synergistic photoredox and pyridoxal radical biocatalysis, a novel activation mode which is both new-to-nature and new-to-chemistry, was developed to enable the stereoselective synthesis of diverse valuable non-canonical amino acids.
The past decade has witnessed the development of an array of useful biocatalytic processes that are not encountered in biology (1–6). Drawing inspirations from small-molecule catalysis, biocatalysis researchers repurposed natural flavin- and nicotinamide-dependent enzymes (4, 5) as well as metalloenzymes (2, 3, 7, 8) to catalyze unnatural reactions, particularly stereoselective free radical-mediated processes as demonstrated by Hyster, Zhao, Huang and our group (7–15). Despite these venerable breakthroughs, the vast majority of previously developed unnatural biocatalytic reactions are known to synthetic chemistry (7–14). Oftentimes, the same transformations could also be achieved using small-molecule catalysts, albeit with no or lower levels of stereocontrol. To further advance the field of biocatalysis, it is thus essential to develop general and synthetically useful enzymatic activation modes that are both new-to-chemistry and new-to-biology. We envisioned that through the productive merger of two distinct catalytic cycles (16) involving an enzyme and a small-molecule catalyst, we would be able to devise new activation modes which are not previously accessible in conventional enzymology and synthetic chemistry.
In this context, we initiated a research program using visible light photoredox catalysis to unlock the potential of pyridoxal phosphate (PLP) enzymes (17, 18) for novel stereoselective radical reactions, thereby providing access to valuable non-canonical amino acids (ncAAs) (Fig. 1(A)). Due to the ubiquity of ncAAs in bioactive natural products (19), peptide therapeutics (20) and functional unnatural proteins (21), their efficient stereoselective synthesis has been widely recognized as a preeminent objective in synthetic chemistry (22) and synthetic biology (23). Traditional chemical synthesis of ncAAs has relied on the tedious installation and removal of amino- and carboxylate-protecting groups (22). In contrast, in nature, PLP enzymes facilitate a myriad of biochemical processes constructing and degrading free amino acids with outstanding chemical fidelity, thus underscoring their potential as promising biocatalysts for ncAA synthesis without protecting group manipulation. As a family of biosynthetic machineries with unrivaled structural and functional diversity (17), PLP-dependent enzymes catalyze diverse C–C and C–heteroatom bond forming reactions at the α- (24, 25), β- (26–33), and γ- (34, 35) positions of amino acids via a unique carbonyl catalysis mechanism (Fig. 1(A)) (17, 36). Although open-shell intermediates have been proposed and investigated in [4Fe-4S]/SAM- and cobalamin-dependent PLP aminomutases (37) and O2-dependent PLP oxidases (38, 39), almost all biotechnologically useful PLP enzymes operate through classic closed-shell mechanisms for substrate activation.

(A) Synergistic photoredox and pyridoxal radical biocatalysis. (B) Enantio- and diastereoselective biocatalytic synthesis of non-canonical amino acids (ncAAs) with up to three contiguous stereogenic centers. Red, blue and yellow spheres are generic substituents of the molecule. In the present study, yellow sphere = H or Me, red sphere = aryl or alkyl, blue sphere = H or alkyl. ncAA = non-canonical amino acid. (C) Enantiodivergent synthesis of l- and d-amino acids using an orthogonal set of engineered PLP enzymes. (D) Synergistic photoredox and pyridoxal radical biocatalysis: dual catalytic cycle. PLP = pyridoxal 5’-phosphate. K = lysine, S = serine, D = aspartate. [RhB] = rhodamine B. ET/PT = electron transfer/proton transfer, PCET = proton-coupled electron transfer. Enzyme illustration is made from 5VM5 (PDB ID (40)).
We postulated that if natural PLP enzymes can be reprogrammed to catalyze unnatural radical C–C bond formation, they would allow us to access a broad spectrum of valuable ncAAs with exquisite diastereo- and enantiocontrol (Fig. 1(B) and ((C)).C)). In particular, we were intrigued by the possibility of synergistically coupling visible light photoredox catalysis (41–44) and PLP biocatalysis (17) to furnish a new paradigm of pyridoxal radical biocatalysis (Fig. 1(D)). In this catalysis mode, the photocatalytically generated free radical intermediate is captured by an enzymatically generated covalent intermediate derived from pyridoxal, thereby enabling stereoselective C–C bond formation in an intermolecular fashion. In contrast to previously investigated natural (45–47) and unnatural photoenzymatic catalysis (4, 5), the present synergistic photoredox–pyridoxal biocatalysis separates photoinduced radical formation and enzymatic radical interception in two discrete catalytic cycles. Not relying on demanding photochemical properties of the cofactor of the enzyme, this strategy could open up numerous opportunities for the further development of new-to-nature radical biocatalysis. Furthermore, by engaging reactive catalytic intermediates in PLP enzymes that remain out of the reach of mechanistically related small-molecule carbonyl catalysis (36), this synergistic catalysis will facilitate the discovery of fundamental reactivities that are not only new-to-nature but also new-to-chemistry.
Design of synergistic photoredox–pyridoxal radical biocatalysis
Guided by these design principles, we were intrigued by the synergistic merger of photoredox catalysis and PLP biocatalysis to advance a stereoselective radical-mediated synthesis of non-canonical amino acids (Fig. 1). We focused our efforts on PLP enzymes capable of functionalizing the β-position of serine, an abundant amino acid building block, and its derivatives, in part due to the diverse enzymes in this superfamily, including tryptophan synthases (26, 27), tyrosine phenol lyases (28, 29), and O-acetylserine sulfhydrylases (30, 31). Mechanistically, we postulated that visible light irradiation of an appropriate photoredox catalyst (IV) would produce an excited-state photooxidant (IV*) (Fig. 1(D)). Single electron oxidation of the alkyltrifluoroborate substrate I with IV* would produce a carbon-centered radical (VI) and the reduced photocatalyst V. Concurrent to this photoredox catalytic cycle, we posited that the use of a β-functionalization PLP-dependent enzyme (VII), such as a tryptophan synthase, would convert serine and other β-hydroxy-α-amino acids (II) into an external aldimine (VIII). α-Deprotonation of VIII would lead to a quinonoid intermediate (IX), which would then expel the β-hydroxy group and lead to the formation of an electrophilic aminoacrylate intermediate (X). If the photocatalytically generated alkyl radical could enter the active site and engage the biocatalytically formed aminoacrylate X, it would lead to an enzyme-bound azaallyl radical (XI) (48), an elusive species in natural PLP biochemistry (17, 18). In particular, if the PLP biocatalyst is able to impose excellent stereocontrol over this radical addition step, up to two contiguous stereocenters would form with high levels of stereochemical fidelity. Subsequent electron transfer/proton transfer (ET/PT) or proton-coupled electron transfer (PCET) (49, 50) involving the reduced photocatalyst V would furnish a new external aldimine XII, which upon hydrolysis would release the radical C–C bond formation product III and regenerate the pyridoxal coenzyme VII.
In contrast to traditional PLP biochemistry, in pyridoxal radical biocatalysis, the α-stereochemistry of the amino acid product III is determined by the ET/PT or PCET step. As such ET/PT or PCET mechanism is unprecedented in native PLP biochemistry, it offers an exciting opportunity to develop stereodivergent access to both l- and d-amino acids via a protein engineering approach (Fig. 1(C)). Moreover, if successfully implemented, this synergistic photoredox and pyridoxal radical biocatalysis would allow the convergent synthesis of ncAAs with a well-defined stereochemical dyad or triad in a single manipulation (Fig. 1(B)), thereby greatly simplifying the enantio- and diastereoselective assembly of these challenging targets. Notably, the successful execution of this proposal requires the chemically formed free radical species VI to diffuse into the enzyme’s active site before its unproductive annihilation, a challenging process in photoenzymatic catalysis (4, 5).
Development of enantiodivergent synergistic photoredox–pyridoxal biocatalytic C–C coupling
We commenced this study by evaluating the synergistic use of β-functionalization PLP enzymes (26–33) and photoredox catalysts for radical β-carbofunctionalization of their native substrates (see Table S1 for additional results). Benzyltrifluoroborate salt 1a was selected as the model substrate, due to its relatively low redox potential (1.09 V versus saturated calomel electrode (SCE) in MeCN) and the enhanced stability of the resulting benzyl radical (51). Among the PLP enzymes we evaluated, the previously evolved “2B9” variant of the Pyrococcus furiosus tryptophan synthase β-subunit elegantly developed by Arnold and Buller (33, 40, 52) showed encouraging activity when combined with an appropriate photoredox catalyst (Fig. 2(A)). These tryptophan synthase β-subunit variants are uniquely powerful for biocatalysis (27), as they do not require the presence of tryptophan synthase α-subunit for function (33, 40). Herein, this “2B9” variant is named as l-PfPLPβ. Through a survey of transition-metal and organic photoredox catalysts ((Fig. 2(B)), it was found that organic photoredox catalysts (43), particularly rhodamine dyes, facilitated the reaction with the highest efficiency (Fig. 2(A), entry 1). Other organic photocatalysts such as acridinium (entry 2) and transition-metal photocatalysts such as Ru(bpy)3Cl2, Ru(bpz)3Cl2 and [Ir[dF(CF3)ppy]2(dtbbpy)](PF6) (entries 3–5, see Table S2 for additional results) furnished inferior results. Ultimately, using 1.0 mol% l-PfPLPβ biocatalyst and 10 mol% rhodamine B (RhB), under slightly acidic conditions (pH = 6.0), this dual photoredox and PLP radical biocatalysis furnished the C–C coupling product 3a in 73% yield and 93:7 enantiomeric ratio (e.r.) favoring the l-amino acid (Fig. 2(A), entry 1). Omitting the enzyme catalyst l-PfPLPβ (entry 6), the photoredox catalyst RhB (entry 8), or the light source (entry 9) led to no product formation, confirming the dual catalytic nature of this process (see Table S3–S5 for additional results). In the current pyridoxal radical biocatalysis, replacing the enzyme catalyst with 5 mol% free PLP cofactor afforded no product (entry 7), further underscoring the synergy between the PLP cofactor and the protein scaffold in enabling this novel reactivity. Finally, the biocatalyst was able to effectively discriminate l- and d-serine (2a), converting racemic dl-serine ((rac)-2a) into enantioenriched products with the same yield and enantiopurity (entry 10, see Fig. S3 for further details)).

(A) Discovery and development of a synergistic photoredox and pyridoxal radical biocatalytic reaction with l-PfPLPβ. (B) Selected examples of photoredox catalysts evaluated in this study. (C) Enantiodivergent pyridoxal radical biocatalysis with d-PfPLPβ (l-PfPLPβ E104G). e.r. = enantiomeric ratio (l-amino acid/ d-amino acid). Reaction conditions: 1a (1 equiv, 4.0 mM), 2a (3 equiv, 12.0 mM), 1 mol% PLP enzyme (40 μM), 10 mol% RhB (400 μM), hν (440 nm), 200 mM KPi buffer, 50 °C, 12 h. Yields are of an average of three runs. See the Supplementary Material for details. Enantiomeric ratios (e.r.) were determined by Marfey’s analysis (53), see Fig. S1 for details. (D) Active site structure of l-PfPLPβ. Illustration is made from 5VM5 (PDB ID) (40).
Identification of enantiodivergent PLP enzymes for non-canonical amino acid synthesis
Canonical two-electron PLP biocatalysis usually operates under neutral to basic conditions (pH = 7–10) (33, 52), and the reaction enantioselectivity typically does not change significantly as a function of pH. Surprisingly, with l-PfPLPβ, the enantioselectivity of PLP radical biocatalysis was found to be highly pH dependent. Increasing the pH of the aqueous buffer from 6.0 to 8.0 resulted in drastically reduced enantioselectivities (Fig. 2(A), entry 1 and entries 11–13, see Table S6 for additional results). Performing this reaction at pH 8.0 (entry 13) resulted in the formation of almost racemic 3a (49:51 e.r.). The unusual pH sensitivity of the present system indicated a potential pH-dependent switch of enantiodetermining mechanism.
In light of this finding, we questioned if the α-stereochemistry of the amino acid product could be reversed through protein engineering. Toward this end, we tested a small library of PLP enzyme variants (Fig. 2(C), see Table S1 for additional results). To our satisfaction, the use of a single mutant of l-PfPLPβ, namely l-PfPLPβ E104G, allowed the complete reversal of enantioselectivity (Fig. 2(C), entry 1). We thus named l-PfPLPβ E104G as d-PfPLPβ. Unlike other unnatural biocatalytic processes using heme and flavoenzymes where enantiopreference reversal has become common (7, 14), in PLP biochemistry, the reversal of α-stereochemistry through protein engineering is notoriously difficult, as the α-configuration of amino acids in traditional PLP enzymology is tightly regulated by the enantiodetermining protonation with a conserved lysine residue (17, 26). As an active site residue relatively far from the PLP cofactor, E104 facilitates the deprotonation of the indole nucleophile in native tryptophan synthase biochemistry (Fig. 2(D)) (52). Our studies showed that in the native tryptophan synthase activity, both l-PfPLPβ and d-PfPLPβ favored the formation of the same natural l-tryptophan (98:2 e.r. and 96:4 e.r., respectively, see Fig. S5 for details), suggesting a new enantiodetermining mechanism in the current pyridoxal radical biocatalysis. Furthermore, biocatalysis using d-PfPLPβ was found to be largely insensitive to the pH of the media. Contrary to the substantial enantioselectivity decrease at higher pH as observed with l-PfPLPβ, using d-PfPLPβ, increasing the pH of the reaction buffer afforded slightly increased enantioselectivity (Fig. 2(C), entries 1–4, see Table S6 for additional results). Under optimized conditions (pH = 7), d-PfPLPβ furnished the enantiomeric product d-homophenylalanine d-3a in 79% yield and 6:94 e.r.. Similar to l-PfPLPβ , d-PfPLPβ was able to convert dl-serine ((rac)-2a) into d-3a with identical yield and enantioselectivity (entry 5). Further studies revealed that while l-PfPLPβ accepted d-serine as a substrate with a relatively low activity toward l-3a formation, d-PfPLPβ exhibited almost no activity toward d-serine (see Fig. S3 for further details).
Substrate scope of synergistic photobiocatalytic ncAA synthesis
With a set of enantiodivergent protocols in hand, we next examined the substrate scope of this dual catalytic process (Fig. 3). Both the l- and the d-amino acid forming enzymes l-PfPLPβ (Fig. 3(A)) and d-PfPLPβ (Fig. 3(B)) promoted the transformations of a diverse array of trifluoroborate salts. Benzyltrifluoroborate substrates bearing a para- (3b), a meta- (3c), and an ortho- (3d) methyl substituent on the aromatic ring were compatible, furnishing homophenylalanine derivatives in excellent yields. With l-PfPLPβ, para- (l-3b) substituted substrates afforded higher enantioselectivities than meta- (l-3c) and ortho- (l-3d) substituted ones. When d-PfPLPβ was applied, para-, meta-, and ortho-substituted benzylic substrates furnished uniformly excellent levels of enantiocontrol (d-3a–d-3l). Synthetically useful halogen substituents, such as a fluorine (3f) and a chlorine (3g), as well as sensitive functional groups such as a methyl ester (3h) and a cyano group (3i), were tolerated under the current conditions. Additionally, both electron-deficient (3f, 3g, 3h, and 3i) and electron-rich (3j) benzyltrifluoroborates underwent smooth transformations with excellent enantioselectivities. Bulkier bicyclic substrates possessing a benzodioxole (3k) and a naphthyl (3l) core were also accepted by the enzyme. Furthermore, this dual catalytic process is amenable to the transformation of non-benzylic radical precursors, albeit in relatively low yields (3m–3o). Heteroatom-stabilized (3m), unstabilized primary (3n), and unstabilized secondary (3n) alkyltrifluoroborates all represented viable substrates, furnishing the corresponding non-canonical amino acid products in an enantioselective fashion. These encouraging initial activities constitute an excellent starting point for further optimization via protein and photocatalyst engineering. Unexpectedly, using d-PfPLPβ, the major enantiomer of 3m was found to be l-3m.
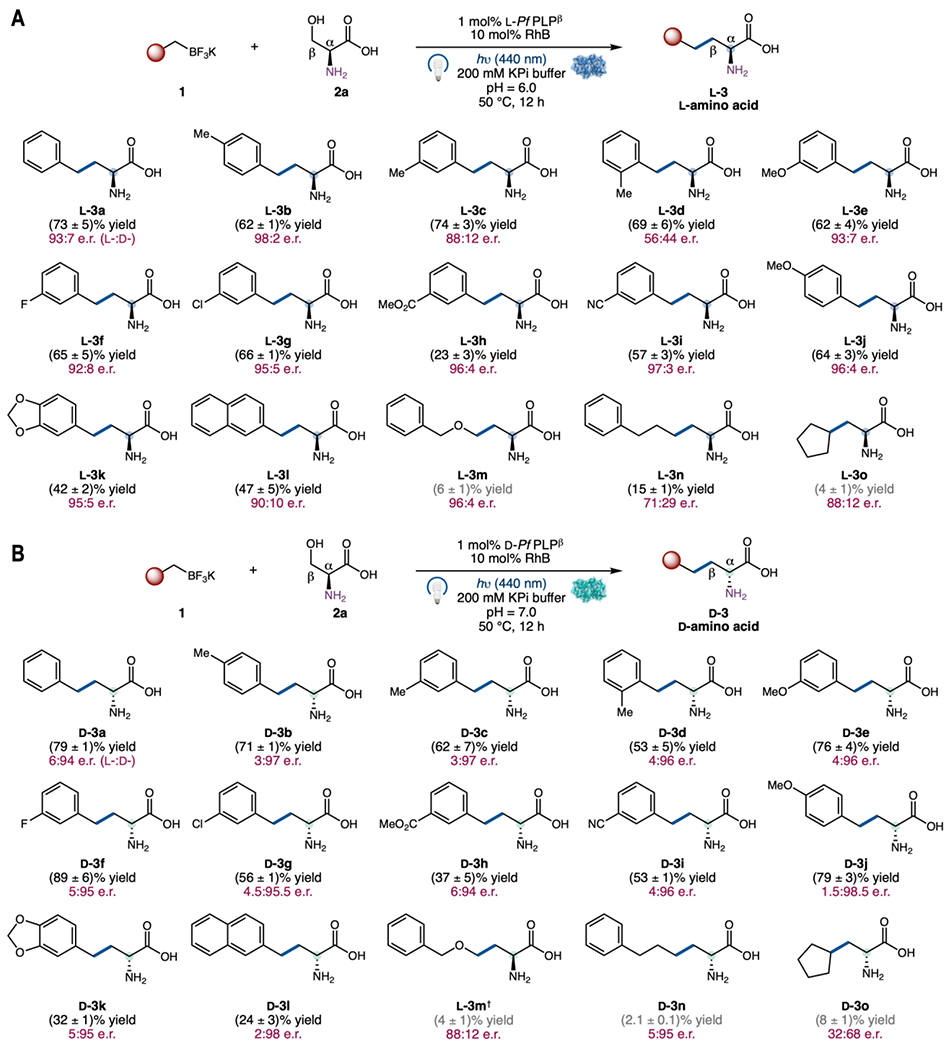
1 (1 equiv, 4.0 mM), 2a (3 equiv, 12.0 mM), 1 mol% l-PfPLPβ or d-PfPLPβ (40 μM), 10 mol% RhB (400 μM), hν (440 nm), 200 mM KPi buffer, 50 °C, 12 h. Yields are of an average of three runs. Enantiomeric ratios (e.r.) were determined by Marfey’s analysis (53), see Fig. S1 for details. The variation of e.r. values is ≤1%. †l-amino acid was found to be the major product.
Importantly, without further enzyme engineering, the current dual catalytic protocol could be readily extended to the formation of challenging contiguous stereocenters with excellent levels of diastereo- and enantioselectivity (Fig. 4). It was found that l-PfPLPβ readily accepted threonine (2b) (52), an inexpensive and easily available building block, in this dual catalytic process, delivering extended isoleucine analogs (4a–4e) with vicinal α and β stereocenters in excellent yields and stereoselectivities (Fig. 4(A)). Furthermore, this photoredox and biocatalytic system permitted the enantioconvergent transformation of racemic secondary alkyl radical precursors ((rac)-1p), leading to non-canonical amino acids bearing three adjacent stereogenic centers (5a) with outstanding diastereo- and enantiocontrol (Fig. 4(B)). During the course of this stereotriad (5a) formation, recovered substrate 1p showed 50:50 e.r. at varying conversions of 1p, demonstrating that kinetic resolution of (rac)-1p was not involved and confirming the enantioconvergent nature of this process (see Fig. S7 for further details). Finally, l-PfPLPβ was able to convert racemic dl-threonine ((rac)-2b) into enantioenriched products with almost identical yields and enantiopurities (see Fig. S4 for further details). It was found that l-PfPLPβ accepted allo-threonine with low levels of activity and stereoselectivity (see Fig. S4 for further details). d-PfPLPβ displayed very low activities on threonine.

(A) Diastereo- and enantioselective biocatalytic synthesis of non-canonical amino acids with two contiguous stereocenters. (B) Diastereo- and enantioselective biocatalytic synthesis of non-canonical amino acids with three contiguous stereocenters. 1 (1 equiv, 4.0 mM), 2 (5 equiv, 20.0 mM), 1 mol% l-PfPLPβ (40 μM), 10 mol% RhB (400 μM), hν (440 nm), 200 mM KPi buffer, 50 °C, 12 h. Yields are based on an average of triplicates. Standard deviations of yields are provided. Diastereo- and enantiomeric ratios (e.r.) were determined by Marfey’s analysis (53), see Fig. S2 for details. The variation of e.r. values is ≤1%. (C) Transformation and determination of relative and absolute stereochemistries of non-canonical amino acid products. a. NaOH (2 equiv), 1:1 THF/H2O, 0 °C to room temperature, 3 h. For X-ray crystal structures, thermal ellipsoids are set at 50% probability; hydrogen atoms are omitted for clarity. See the Supplementary Material for details.
We note that previously devised synthetic routes toward stereochemical dyads similar to 4 required 7–8 steps starting from commercially available materials (54–56). In addition, these methods relied on the use of a chiral auxiliary (54) or proceeded with low diastereocontrol (55, 56). Moreover, no methods exist for the stereoselective synthesis of stereochemical triad 5. Thus, our synergistic catalytic methods represent a synthetically valuable advance, granting access to non-canonical amino acids bearing multiple adjacent stereocenters in a single operation with excellent diastereo- and enantioselectivities. Finally, the relative and absolute stereochemistries of C–C coupling products 3a, 4a and 5a were ascertained by X-ray single crystal diffraction analysis of their respective N-acylated products 6a, 6b and 6c (Fig. 4(C)).
Mechanistic and computational studies
To probe the open-shell nature of this dual catalytic process, we performed radical trapping experiments using 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO). Under the standard conditions, in the presence of TEMPO, radical trapping product formed in 14% yield (See Fig. S8 and S9 for details). Furthermore, in the model reaction (1a + 2a → L-3a), side products derived from benzyl radical including dibenzyl (ca. 1%) and PhCHO (ca. 5%) were observed by GC-MS analysis. (See Figs. S6, S10 and S11 for details). Together, these results are consistent with the formation of benzyl radical under the reaction conditions. Furthermore, UV-Vis spectroscopic analysis suggested that unlike previously reported unnatural photoenzymatic catalysis with flavin-dependent ene reductases (10, 12), charge transfer (CT) complexes between the substrate and the enzymatic intermediate are likely not involved in the present pyridoxal radical biocatalysis (See Fig. S12 for details).
To further elucidate the reaction mechanism and the origin of regioselectivity for β-functionalization, we performed density functional theory (DFT) calculations using a theozyme model (57) prepared from 5VM5 (40) consisting of catalytically relevant amino acid residues K82, D300, and other residues within 3.0 Å of the PLP cofactor (Fig. 5, see the Supplementary Material for computational details). Consistent with previous computational studies on related PLP-dependent enzymes (58, 59), the conversion between the internal aldimine and external aldimine 7 requires a low activation barrier (Fig. S16). From the external aldimine 7, α-deprotonation by a lysine residue K82 (TS-1) takes place, forming a quinonoid intermediate 8. The β-hydroxy elimination of the quinonoid 8 is facilitated by the acidic aspartate residue D300 (60), leading to the key aminoacrylate species 10 with an activation barrier of 17.0 kcal/mol relative to 8. The addition of the benzyl radical 9 generated from the photoredox catalytic cycle to the β-carbon of aminoacrylate 10 via TS-3 features a low activation barrier, giving rise to the azaallyl radical intermediate 11. Mulliken spin density calculations showed that in this novel azaallyl radical intermediate, the unpaired electron is largely located at the Cα atom of the amino acid (Fig. S17 for details). This azaallyl radical 11 then undergoes electron transfer and proton transfer (ET/PT), generating a new external aldimine 13. ET between azaallyl radical 11 and the reduced photocatalyst [RhB]•−, presumably via a long-range ET, is found to be kinetically and thermodynamically feasible based on Marcus theory calculations (Fig. S21–S23). The succeeding PT step (TS-4) has a relatively low barrier of 16.0 kcal/mol.

(A) Computed energy profile using a theozyme model. Enthalpy values are relative to external aldimine species 7. Except those in 7, active site residues are omitted for clarity. (B) Comparison of activation barriers from the theozyme and the free PLP cofactor models. Enthalpy values are relative to aminoacrylate 10. (C) Optimized structures of radical addition transition states from the theozyme model. Bond distances are in Å.
We next performed transition state calculations to reveal the critical role of the protein scaffold in controlling the regioselectivity during the benzyl radical (9) addition to aminoacrylate 10 (Fig. 5(B) and ((C)).C)). Our calculations showed that in the absence of any active site residues, the radical addition to a free PLP cofactor based model system is barely selective: although the addition to the C3 atom of the pyridine ring (via TS3’’) is kinetically disfavored due to the disruption of aromaticity, the radical additions to the β-position (C1, via TS3) and the pyridoxal aldehyde carbon (C2, via TS3’) of the aminoacrylate intermediate (10) have nearly identical activation barriers (Fig. 5(B), ΔΔH‡ = −0.2 kcal/mol, see Fig. S18 for details). The lack of site selectivity with the free PLP model is ascribed to a combination of electronic effects favoring the more electron-deficient C2 possessing a larger LUMO coefficient and steric effects favoring the more exposed β-position (C1). Strikingly, with the theozyme model, radical addition to the β-position (C1) has a markedly lower barrier than radical addition to C2 (ΔΔH‡ = 3.2 kcal/mol). This result indicates that the enzyme environment plays an essential role in imposing the challenging requisite site selectivity over the radical addition step. Within the enzyme active site, the radical addition to the pyridoxal carbon C2 is disfavored due to the presence of glycine 298 and lysine 98 that block both faces of C2 from the benzyl radical attack (Fig. 5(C)). Thus, these DFT calculations underscored the importance of the enzyme scaffold to ensure excellent site selectivities during pyridoxal radical catalysis, revealing the underappreciated potential of enzymes in the discovery of previously elusive reactivity patterns.
In summary, we developed a novel synergistic photobiocatalysis strategy to unlock the potential of PLP enzymes for asymmetric intermolecular radical transformations. By interfacing transient radicals generated from photoredox catalysis with reactive covalent intermediates formed in PLP enzymes, this dual catalysis furnishes a fertile ground for the discovery and optimization of unprecedented stereoselective intermolecular free radical reactions that are both new-to-biology and new-to-chemistry, thereby advancing the field of biocatalysis to the next level of sophistication.
Acknowledgments:
We thank Prof. Yang Hai (UCSB), Prof. Liming Zhang (UCSB), Prof. Yiming Wang (University of Pittsburgh) and Prof. Binju Wang (Xiamen University) for helpful discussions.
Funding:
This work is supported by the start-up funds from the University of California Santa Barbara (Y.Y.), the Herman Frasch Foundation Grant (947-HF22 to Y.Y.), and the National Institutes of Health (R35GM128779 to P.L.). We acknowledge the BioPACIFIC MIP (NSF Materials Innovation Platform, DMR-1933487) at UCSB for access to instrumentation. Calculations were performed at the Center for Research Computing at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment.
Footnotes
Competing interests: A provisional patent application (US provisional patent number 63/437,491) has been filed through the University of California Santa Barbara based on the results presented herein.
Data and materials availability:
All data are available in the main text or the supplementary materials. Solid-state structures of 6a, 6b and 6c are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC 2220221, 2220222 and 2220224, respectively. Plasmids encoding engineered PLP enzymes are available from Y.Y. under a material transfer agreement (MTA) with the University of California Santa Barbara.
References and Notes
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/152049638
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1126/science.adg2420
Article citations
Biocatalytic asymmetric aldol addition into unactivated ketones.
Nat Chem, 27 Sep 2024
Cited by: 0 articles | PMID: 39333392
New advances in protein engineering for industrial applications: Key takeaways.
Open Life Sci, 19(1):20220856, 17 Jun 2024
Cited by: 0 articles | PMID: 38911927 | PMCID: PMC11193397
Review Free full text in Europe PMC
A metalloenzyme platform for catalytic asymmetric radical dearomatization.
Nat Chem, 28 Aug 2024
Cited by: 1 article | PMID: 39198700
Machine learning-guided co-optimization of fitness and diversity facilitates combinatorial library design in enzyme engineering.
Nat Commun, 15(1):6392, 29 Jul 2024
Cited by: 2 articles | PMID: 39080249 | PMCID: PMC11289365
Threonine Aldolase-Catalyzed Enantioselective α-Alkylation of Amino Acids through Unconventional Photoinduced Radical Initiation.
J Am Chem Soc, 146(32):22476-22484, 04 Jul 2024
Cited by: 0 articles | PMID: 38961805
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Threonine Aldolase-Catalyzed Enantioselective α-Alkylation of Amino Acids through Unconventional Photoinduced Radical Initiation.
J Am Chem Soc, 146(32):22476-22484, 04 Jul 2024
Cited by: 0 articles | PMID: 38961805
Stereoselective amino acid synthesis by photobiocatalytic oxidative coupling.
Nature, 629(8010):98-104, 01 May 2024
Cited by: 3 articles | PMID: 38693411
Enzymatic Synthesis of Unprotected α,β-Diamino Acids via Direct Asymmetric Mannich Reactions.
J Am Chem Soc, 146(29):20263-20269, 13 Jul 2024
Cited by: 0 articles | PMID: 39001849
Oxygen reactivity with pyridoxal 5'-phosphate enzymes: biochemical implications and functional relevance.
Amino Acids, 52(8):1089-1105, 25 Aug 2020
Cited by: 8 articles | PMID: 32844248 | PMCID: PMC7497351
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: R35 GM128779
Grant ID: R35 GM147387



