Abstract
Free full text

Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction.
Abstract
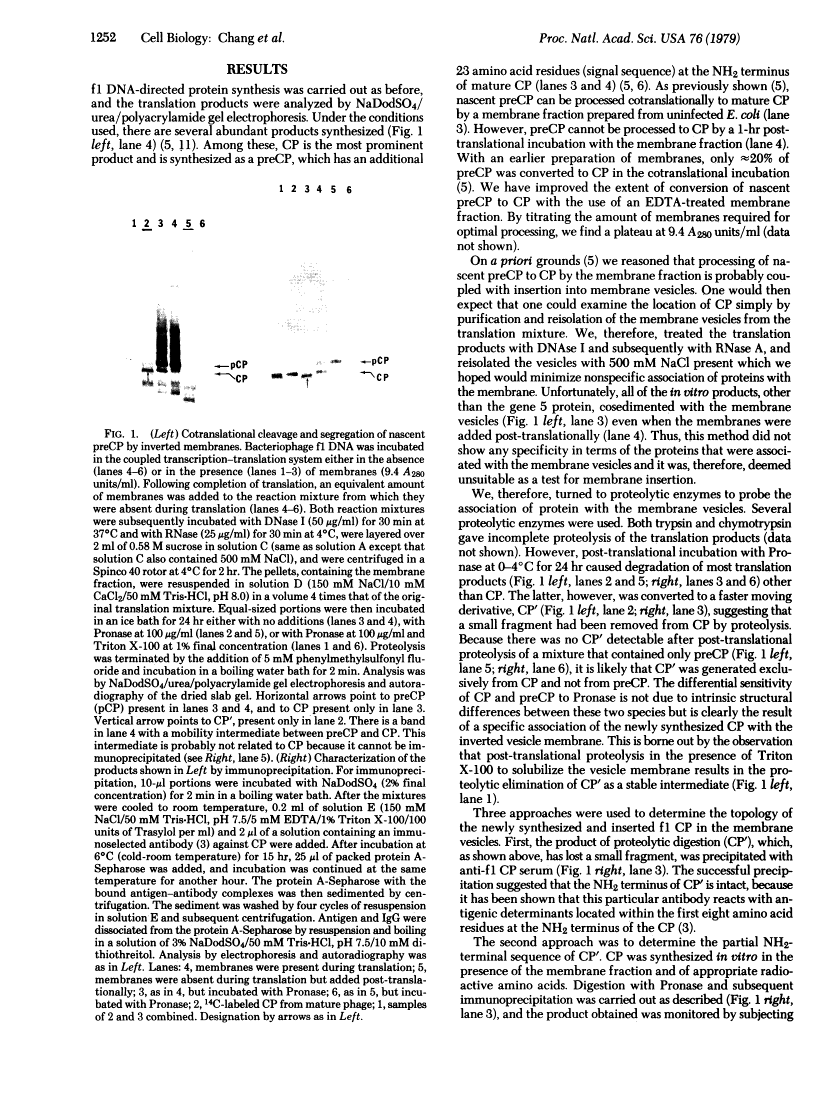
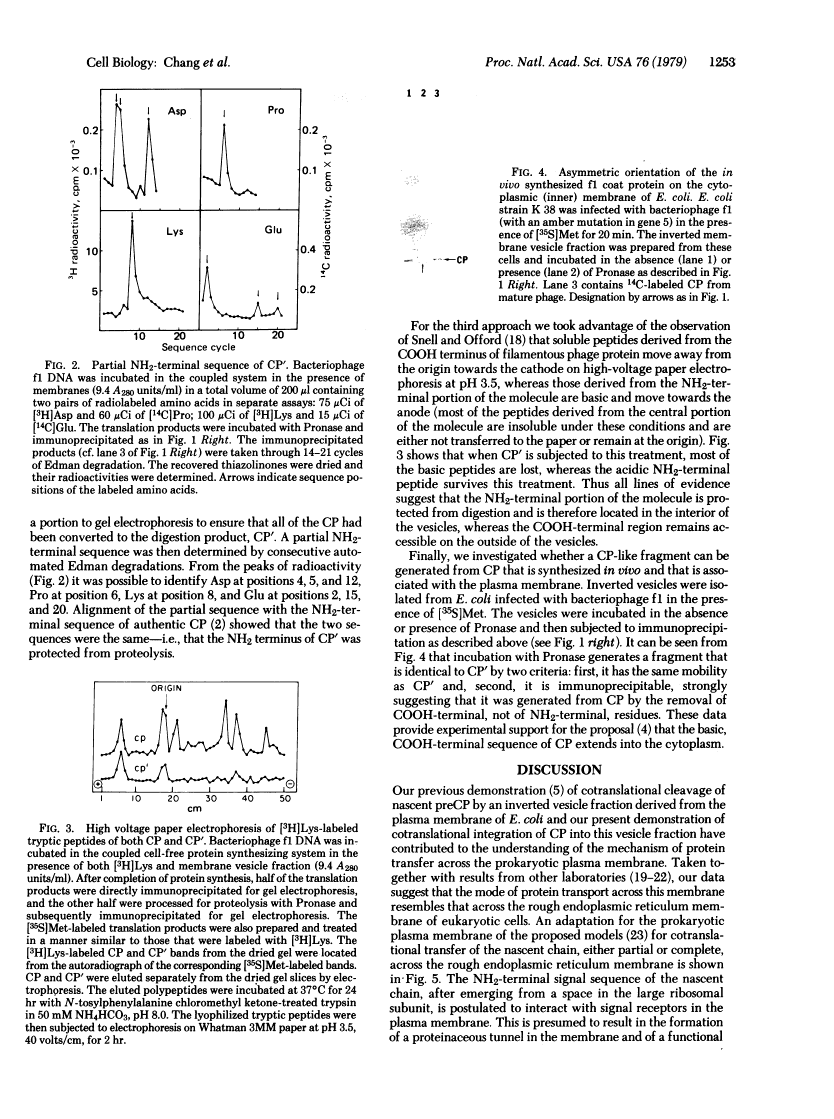
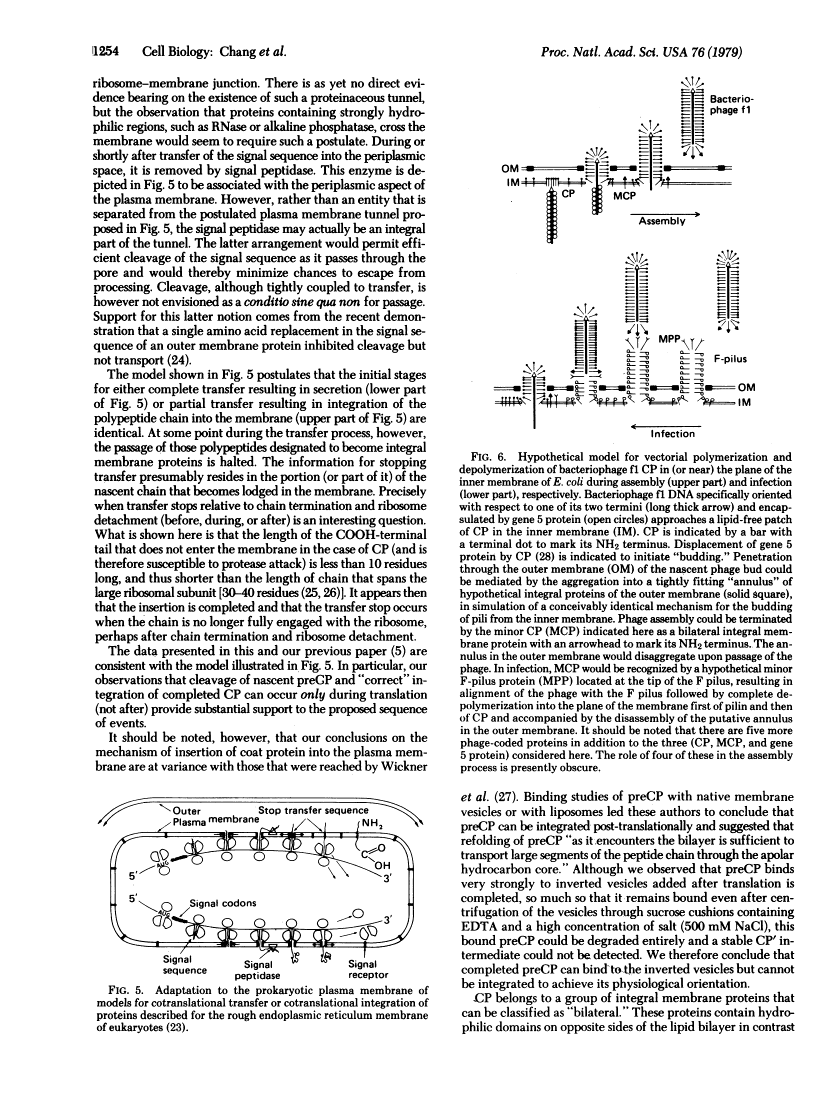
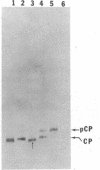
The coat protein (CP) of bacteriophage f1 is integrated into an Escherichia coli plasma membrane fraction consisting of inverted vesicles when it is synthesized in a cell-free, coupled transcription--translation system supplemented with the inverted vesicles. By using proteolytic enzymes as probes, we found by subsequent peptide mapping and determination of the sequence of the proteolytic products that CP was inserted into the inverted vesicles in an orientation indistinguishable from that in inverted vesicles prepared from infected E. coli: only a COOH-terminal portion of approximately 10 residues was accessible to proteolysis, whereas the remainder of CP (CP') was entirely protected. Protection of CP' was dependent on the integrity of the vesicle membrane, because it was abolished when proteolysis was done in the presence of nonionic detergents. Insertion was observed when the inverted vesicles were present during translation in the cell-free system, not when they were added after translation. Thus, the asymmetric insertion of this type of integral membrane protein is strictly coupled to translation. These findings are discussed with respect to prokaryotic membrane biogenesis and are related to bacteriophage f1 assembly and infection.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Smilowitz H, Carson J, Robbins PW. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. [Abstract] [Google Scholar]
- Bailey GS, Gillett D, Hill DF, Petersen GB. Automated sequencing of insoluble peptides using detergent. Bacteriophage fl coat protein. J Biol Chem. 1977 Apr 10;252(7):2218–2225. [Abstract] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. [Europe PMC free article] [Abstract] [Google Scholar]
- Marvin DA, Wachtel EJ. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. [Abstract] [Google Scholar]
- Chang CN, Blobel G, Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. [Europe PMC free article] [Abstract] [Google Scholar]
- Sugimoto K, Sugisaki H, Okamoto T, Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. [Abstract] [Google Scholar]
- Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. [Europe PMC free article] [Abstract] [Google Scholar]
- Blobel G, Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. [Europe PMC free article] [Abstract] [Google Scholar]
- Model P, Zinder ND. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. [Abstract] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. [Abstract] [Google Scholar]
- Tsuchiya T, Rosen BP. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [Abstract] [Google Scholar]
- Cammack KA, Wade HE. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. [Europe PMC free article] [Abstract] [Google Scholar]
- ZINDER ND, VALENTINE RC, ROGER M, STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. [Abstract] [Google Scholar]
- GAREN A, GAREN S. Genetic evidence on the nature of the repressor for alkaline phosphatase in E. coli. J Mol Biol. 1963 May;6:433–438. [Abstract] [Google Scholar]
- Snell DT, Offord RE. The amino acid sequence of the B-protein of bacteriophage ZJ-2. Biochem J. 1972 Mar;127(1):167–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Emr SD, Schwartz M, Silhavy TJ. Mutations altering the cellular localization of the phage lambda receptor, an Escherichia coli outer membrane protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5802–5806. [Europe PMC free article] [Abstract] [Google Scholar]
- Silhavy TJ, Casadaban MJ, Shuman HA, Beckwith JR. Conversion of beta-galactosidase to a membrane-bound state by gene fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3423–3427. [Europe PMC free article] [Abstract] [Google Scholar]
- Silhavy TJ, Shuman HA, Beckwith J, Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5411–5415. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith WP, Tai PC, Thompson RC, Davis BD. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. [Europe PMC free article] [Abstract] [Google Scholar]
- Lin JJ, Kanazawa H, Ozols J, Wu HC. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. [Europe PMC free article] [Abstract] [Google Scholar]
- Malkin LI, Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. [Abstract] [Google Scholar]
- Blobel G, Sabatini DD. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. [Europe PMC free article] [Abstract] [Google Scholar]
- Wickner W, Mandel G, Zwizinski C, Bates M, Killick T. Synthesis of phage M13 coat protein and its assembly into membranes in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1754–1758. [Europe PMC free article] [Abstract] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection: fate of the parental major coat protein. J Virol. 1974 Jan;13(1):94–99. [Europe PMC free article] [Abstract] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. [Europe PMC free article] [Abstract] [Google Scholar]
- Marco R, Jazwinski SM, Kornberg A. Binding, eclipse, and penetration of the filamentous bacteriophage M13 in intact and disrupted cells. Virology. 1974 Nov;62(1):209–223. [Abstract] [Google Scholar]
- Novotny CP, Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.76.3.1251
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/pnas/76/3/1251.full.pdf
Citations & impact
Impact metrics
Article citations
Unprecedented high-resolution view of bacterial operon architecture revealed by RNA sequencing.
mBio, 5(4):e01442-14, 08 Jul 2014
Cited by: 110 articles | PMID: 25006232 | PMCID: PMC4161252
Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane--distinct translocases and mechanisms.
Biochim Biophys Acta, 1778(9):1735-1756, 09 Aug 2007
Cited by: 271 articles | PMID: 17935691
Review
The phage phi29 membrane protein p16.7, involved in DNA replication, is required for efficient ejection of the viral genome.
J Bacteriol, 189(15):5542-5549, 25 May 2007
Cited by: 8 articles | PMID: 17526715 | PMCID: PMC1951806
Functional display of proteins, mutant proteins, fragments of proteins and peptides on the surface of filamentous (bacterio) phages: A review.
Cytotechnology, 18(1-2):107-112, 01 Jan 1995
Cited by: 2 articles | PMID: 22358642
Go to all (52) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein.
Proc Natl Acad Sci U S A, 75(1):361-365, 01 Jan 1978
Cited by: 112 articles | PMID: 343108 | PMCID: PMC411248
The orientation of the major coat protein of bacteriophage f1 in the cytoplasmic membrane of Escherichia coli.
J Biol Chem, 256(19):9951-9958, 01 Oct 1981
Cited by: 22 articles | PMID: 7024275
Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13.
Proc Natl Acad Sci U S A, 76(3):1199-1203, 01 Mar 1979
Cited by: 36 articles | PMID: 375229 | PMCID: PMC383217
Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli.
Proc Natl Acad Sci U S A, 72(12):4749-4753, 01 Dec 1975
Cited by: 33 articles | PMID: 54916 | PMCID: PMC388808