Abstract
Free full text

Rapid Characterization of the Functional and Pharmacological Consequences of Cantú Syndrome KATP Channel Mutations in Intact Cells
Abstract
Gain-of-function of KATP channels, resulting from mutations in either KCNJ8 (encoding inward rectifier sub-family 6 [Kir6.1]) or ABCC9 (encoding sulphonylurea receptor [SUR2]), cause Cantú syndrome (CS), a channelopathy characterized by excess hair growth, coarse facial appearance, cardiomegaly, and lymphedema. Here, we established a pipeline for rapid analysis of CS mutation consequences in Landing pad HEK 293 cell lines stably expressing wild type (WT) and mutant human Kir6.1 and SUR2B. Thallium-influx and cell membrane potential, reported by fluorescent Tl-sensitive Fluozin-2 and voltage-sensitive bis-(1,3-dibutylbarbituric acid)trimethine oxonol (DiBAC4(3)) dyes, respectively, were used to assess channel activity. In the Tl-influx assay, CS-associated Kir6.1 mutations increased sensitivity to the ATP-sensitive potassium (KATP) channel activator, pinacidil, but there was strikingly little effect of pinacidil for any SUR2B mutations, reflecting unexpected differences in the molecular mechanisms of Kir6.1 versus SUR2B mutations. Compared with the Tl-influx assay, the DiBAC4(3) assay presents more significant signal changes in response to subtle KATP channel activity changes, and all CS mutants (both Kir6.1 and SUR2B), but not WT channels, caused marked hyperpolarization, demonstrating that all mutants were activated under ambient conditions in intact cells. Most SUR2 CS mutations were markedly inhibited by <100 nM glibenclamide, but sensitivity to inhibition by glibenclamide, repaglinide, and PNU37883A was markedly reduced for Kir6.1 CS mutations. Understanding functional consequences of mutations can help with disease diagnosis and treatment. The analysis pipeline we have developed has the potential to rapidly identify mutational consequences, aiding future CS diagnosis, drug discovery, and individualization of treatment.
SIGNIFICANCE STATEMENT
We have developed new fluorescence-based assays of channel activities and drug sensitivities of Cantú syndrome (CS) mutations in human Kir6.1/SUR2B-dependent KATP channels, showing that Kir6.1 mutations increase sensitivity to potassium channel openers, while SUR2B mutations markedly reduce K channel opener (KCO) sensitivity. However, both Kir6.1 and SUR2B CS mutations are both more hyperpolarized than WT cells under basal conditions, confirming pathophysiologically relevant gain-of-function, validating DiBAC4(3) fluorescence to characterize hyperpolarization induced by KATP channel activity under basal, non KCO-activated conditions.
Introduction
ATP-sensitive potassium (KATP) channels are composed of a pore-forming tetramer of four inward rectifier sub-family 6 (Kir6) subunits (Kir6.1 or Kir6.2, encoded by KCNJ8 and KCNJ11, respectively) and four auxiliary sulphonylurea receptor (SUR) subunits (SUR1 or SUR2, encoded by ABCC8 and ABCC9, respectively) (Shyng and Nichols, 1997; Aguilar-Bryan et al., 1998; Li et al., 2017; Martin et al., 2017b; Sung et al., 2021). Two major SUR2 splice variants, SUR2A and SUR2B, differ in the last 42 amino acids (Chutkow et al., 1996; Shindo et al., 1998). ATP inhibition through binding to Kir6 subunits (Tucker et al., 1997) and MgATP or MgADP activation through induction of nucleotide binding domain (NBD) dimerization in the SUR subunits (Nichols et al., 1996; Nichols, 2006; Tinker et al., 2018) dominate channel regulation.
Distinct subunit combinations are expressed in different tissues, with Kir6.2/SUR1, Kir6.2/SUR2A, and Kir6.1/SUR2B prominent in pancreas, striated muscle, and vascular smooth muscle, respectively (Inagaki et al., 1995; Teramoto et al., 2006; Flagg et al., 2010; Foster and Coetzee, 2016; Tinker et al., 2018). Loss-of-function (LOF) and gain-of-function (GOF) mutations in Kir6.2 or SUR1 cause congenital hyperinsulinism (Huopio et al., 2002) and neonatal diabetes (Koster et al., 2000; Gloyn et al., 2004; Babenko et al., 2006), respectively. LOF consequences of Kir6.1 and SUR2 remain incompletely described, but GOF mutations in either subunit cause Cantú syndrome (CS), a complex condition including excess hair growth, coarse facial appearance, cardiomegaly, and lymphedema (Harakalova et al., 2012; van Bon et al., 2012; Brownstein et al., 2013; Cooper et al., 2014, 2017; McClenaghan et al., 2018; Singh et al., 2022). CS incidence is unknown, but there are now >100 individuals with heterozygous pathogenic variants in the International CS Registry (Grange et al., 2019), and increased awareness of the clinical phenotype is leading to improved recognition, with probably thousands of affected individuals world-wide. Mortality is an issue both in the neonatal period, as a result of cardio-pulmonary issues (Grange et al., 2019), and potentially in old age as a result of developing heart failure (Singh et al., 2022). There is as yet no clear correlation to molecular phenotype (Grange et al., 2019), and there is the potential for misdiagnosis of CS, or missed diagnosis of CS, based on identification of variants of unknown significance (Gao et al., 2023). CS mouse models implicate SUR2B rather SUR2A in the major features of the disease, primarily through decreased contractility of smooth muscles (Huang et al., 2018; Davis et al., 2020; York et al., 2020), which then lead to secondary cardiomegaly (McClenaghan et al., 2020b) and skeletal muscle pathology (Scala et al., 2020, 2021).
The unique composition of KATP channels gives rise to a rich pharmacology (Kharade et al., 2016). Sulfonylureas such as glibenclamide inhibit channel activity by binding to SUR1 and have proven very effective in treating neonatal diabetes (Pearson et al., 2006). Glibenclamide also inhibits SUR2 containing channels but with a lower potency (Meyer et al., 1999), and there is some evidence that glibenclamide may treat CS; it reversed CS-associated cardiovascular abnormalities in SUR2[A478V] mice (McClenaghan et al., 2020b) and restored gastric motility (York et al., 2020). A single published case study suggests that sulfonylureas may be beneficial in treating CS pathologies in humans (Ma et al., 2019), but clinical effectiveness remains to be established and whether drug-desensitizing effects of certain mutations (Cooper et al., 2015; McClenaghan et al., 2018; Houtman et al., 2019) will restrict glibenclamide use to a subset of CS mutations, remains unknown.
Thus, there is need for efficient characterization of the functional consequences of potential CS mutations under normal physiologic conditions, both for accurate clinical diagnosis, and for testing potential therapeutics. Direct measurement of channel activities using patch-clamp is sensitive and accurate and has shown that CS mutations can decrease sensitivity to ATP inhibition or increase sensitivity to Mg-nucleotide activation (Cooper et al., 2014, 2017; McClenaghan et al., 2018; Houtman et al., 2019). For Kir6.1/SUR2B channels, a technical issue is that the channels quickly “rundown” in excised patches, and so for practical purposes, CS mutations have generally been assessed in mouse or rat SUR2A or introduced to the Kir6.2 analog, leading to potential errors in extrapolating to native subunit combinations. Patch-clamp techniques are also very labor- and time-intensive and not readily amenable to high throughput approaches. To overcome these issues, we have here developed alternative fluorescence-based high-throughput assays of stably expressed wild type (WT) or mutant human Kir6.1/SUR2B in HEK cells, highlighting complex CS mutation effects on drug sensitivities and offering an activator-independent approach to assessing inhibitor action.
Materials and Methods
Molecular Biology
The cDNA sequence of human Kir6.1 is identical to NM_004982.4. The synthesized cDNA of human SUR2A (Supplemental Fig. 1) is a gift from Dr. Rod MacKinnon, (Rockefeller University, New York, NY). Human SUR2B was generated by replacing the last 519 nucleotides of hSUR2A with the corresponding sequence of hSUR2B(NM_020297.4). The attB containing plasmid with mCherry and puromycin resistance gene (Matreyek et al., 2017) was used as vector. Restriction endonuclease sites for MluI, BglII, NheI, and EcoRI were used to sequentially insert hKir6.1, an internal ribosome entry sites (IRES) sequence, and hSUR2B into the vector. Site-directed mutations were introduced to the final attB vector using Q5 polymerase and confirmed by Sanger sequencing. Mutations that were difficult to introduce were first generated in hKir6.1 and IRES containing vectors or hSUR2B in pcDNA3.1(–) and then sub-cloned into the final attB plasmid by restriction digestion and ligation.
Landing Pad Stable Cell Line Generation
Stable cell lines were introduced into the landing pad HEK293 cell line as described previously (Matreyek et al., 2017) with minor adjustments (Fig. 1A). Landing pad HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin, and plated into 6-well plates 1 day before transfection. For cells in one well of the plate, 1.5 μg pCAG-NLS-Bxb1 and 1.5 μg attB recombination plasmid (containing the KATP channel subunits) were cotransfected with 6 μl Fugene 6. Two days after transfection, the medium was replaced by the culture media supplemented with doxycycline (4 μg/ml) and puromycin (10 mM). Death of cells without DNA recombination was observed within 2 days. The cells were then cultured for 2–3 weeks with occasional replacement of the culture medium. The established stable cell lines lost blue fluorescence and uniformly acquired red fluorescence under a fluorescent microscope (Fig. 1B, Supplemental Fig. 2).
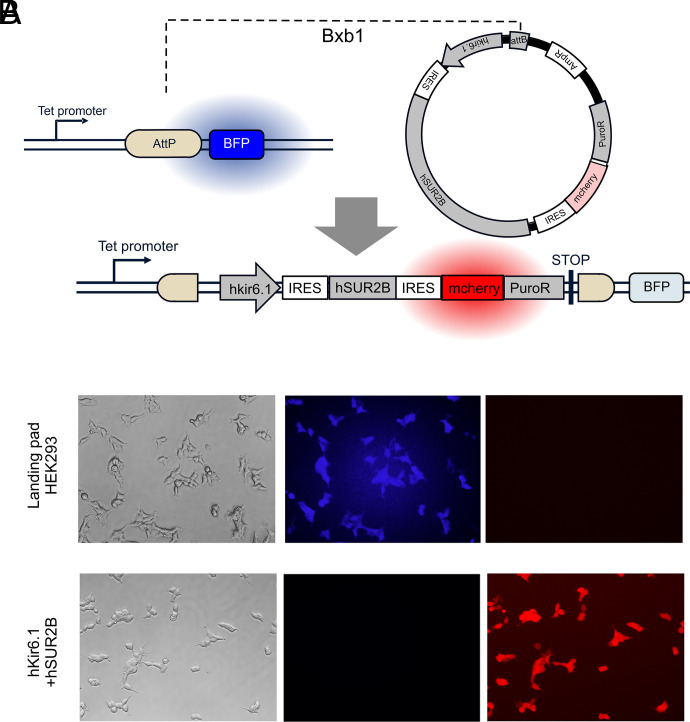
Landing pad system for generation of stable cell lines. (A) Insertion of cDNAs containing Kir6.1 and SUR2B into the genome of Landing pad HEK293 cells. (B) Representative micrographs of untransfected Landing pad cells (top) and cells transfected with hKir6.1/hSUR2B channel constructs (bottom). 4′,6-Diamidino-2-phenylindole (DAPI)–filtered images show blue fluorescence in untransfected cells disappears in transfected cells, and red fluorescent protein filter shows appearance of red fluorescence uniformly in stably modified cells.
Thallium Flux Assay
This procedure is adapted from a previously published paper (Raphemot et al., 2013) with minor adjustment. One day before the assay, Landing pad HEK cells, WT, and mutant hKir6.1+hSUR2B stable cell lines were separately suspended in medium with doxycycline at a density of ~800–1000 k/ml, and 100 μl cell suspensions were plated into each well of a 96-well plate precoated with 0.01% poly-L-lysine. Approximately 2 hours before the assay, 5 μl Fluozin-2 (0.5 μg/μl in DMSO) was mixed with 2.5 μl Pluronic F-127/DMSO solution (20% w/v) and then dissolved in 5 ml Hanks’ balanced salt solution (HBSS) buffer forming the dye solution. Tl+ solution (1 mM) was prepared and added to two columns (300 μl/well) of another 96-well plate as compound source.
The medium in the 96-well plate was discarded and the cells were washed three times (“flick and slam”) with 80 μl HBSS for each well. Fluozin-2 dye solution (45 μl) was added to each well for 1 hour, after which the cells were washed another three times with HBSS. HBSS solution (160 μl) was then added to cover the cells, or only 80 μl if there were additional components needed for the assay. In this case, another 80 μl, with these components, was added immediately (4 minutes) prior to assay.
A Flexstation 3 fluorescent plate reader was used to conduct fluorescence measurements. Fluorescence mode was used for the whole assay with excitation wavelength of 485 nm and emission wavelength of 528 nm. The signal gain level was set at medium, any required additions for this assay were at t = –4 minutes, and then fluorescence was measured every 2 seconds for 2 minutes. At the 20 second timepoint, Tl+ solution was added (40 μl/well) and at a height equal to 150 μl liquid. Signals from replicate wells were averaged and plotted versus time for each experiment. Data were saved as Excel files for later calculation.
The recipe for HBSS is: NaCl 137 mM, KCl 5.4 mM, CaCl2 1.3 mM, MgSO4 0.4 mM, MgCl2 0.5 mM, Na2HPO4 0.4 mM, KH2PO4 0.4 mM, Glucose 5.6 mM, NaHCO3 4.2 mM, HEPES 20 mM. The recipe for 4× Tl+ solution is: CaSO4 1.8 mM, MgSO4 1 mM, glucose 5 mM, HEPES 10 mM, TlSO4 4 mM. 4× Tl+ was diluted with HBSS to get the final 1 mM thallium solution.
Thallium Influx Curve Fitting
For analysis (Fig. 2), signal intensity of wells without cells and dye was subtracted, and the data were then normalized (relative fluorescence units [RFU]) to the dye fluorescence prior to Tl+ addition. RFU versus time traces were fit with single exponential eq. 1 using Origin 8:
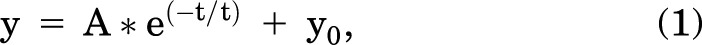
where y = RFU, A = maximum amplitude, t = time, τ = time constant, and y0 = offset.
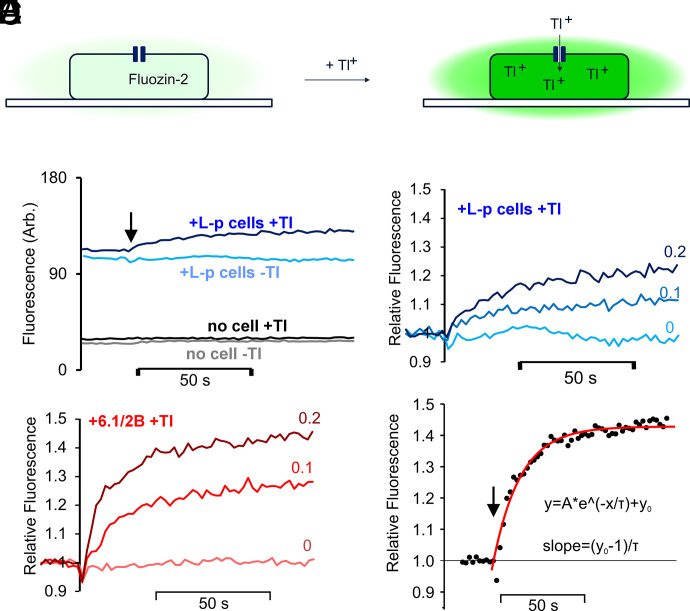
Principle of cell-based, fluorescence high-throughput screening assay. (A) Potassium channel-facilitated thallium influx is measured from the increase of Fluozin-2 fluorescence on binding to thallium. (B) Representative raw data traces of Landing pad only (L-p) cells (or blank wells, no cell) in the presence or absence of 0.2 mM Tl+ added at 20s. In (B)–(E), experiments were all carried out in the presence of 30 μM pinacidil. (C) Fluorescence changes with increasing [Tl+] (0, 0.1 or 0.2 mM as indicated) in Landing pad only cells, normalized to initial fluorescence after subtraction of the fixed fluorescence of empty wells. (D) Fluorescence changes with increasing [Tl+] (0, 0.1 or 0.2 mM as indicated) in cells transfected with WT hKir6.1 and hSUR2B, normalized to initial fluorescence after subtraction of the fixed fluorescence of empty wells. (E) Time-dependent fluorescence change (WT transfected cells with 0.2 mM Tl+, normalized as in D), was fit with a single exponential function, used to calculate initial rate of uptake.
The derivative function:
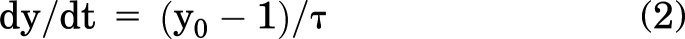
was calculated at t = 0 as the initial rate of uptake after obtaining value y0 and τ.
Pinacidil and glibenclamide dose-response relationships were fitted with modified Hill equations:
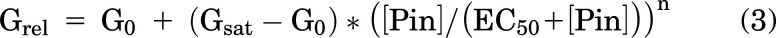
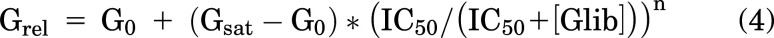
where Grel = relative conductance, G0 = conductance in zero drug, Gsat = conductance in saturating [drug], [Pin] and [Glib] are pinacidil or glibenclamide concentration, EC50 and IC50 are the half-maximally effective drug concentrations, and n = Hill coefficient using Origin 8. Because pinacidil activation curves resulted in both shifts of apparent EC50 to lower values and higher Gsat, we calculated an activity index:
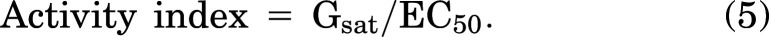
Cell Imaging
Landing pad HEK293 cells, routinely cultured in 35-mm dishes with doxycycline, were imaged using an inverted EVOS M5000 microscope (Thermo Fisher Scientific, Carlsbad, CA) with a 20× objective lens. Images in Fig. 1 were made with transmitted light and 4′,6-diamidino-2-phenylindole (DAPI) or red fluorescent protein filter sets.
Membrane Potential Assessments by Voltage-Sensitive Fluorescent Dye
Transparent cell-culture 96-well plates were coated with poly-L-lysine (0.1 mg/ml) 1 day before stable cells were plated at an appropriate density to ensure an even distribution without layering.
Cells were cultured with 100 μl Dulbecco’s modified Eagle’s medium containing 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 4 μg/ml doxycycline. After 1 day in culture, the medium was discarded and the cells were washed twice with low [K] (1 mM) buffer (80 μl/well). Cells were then incubated with 3 μM DiBAC4(3) in low K buffer (or mixtures of low K and high K buffer), with or without additional channel modulators, for at least 30 minutes. Separate images of green and red fluorescence were generated with EVOS M5000 imaging system (10× lens). In the built-in software, the intensity was 0.005 for green fluorescence and 0.379 for red fluorescence.
The recipe for low K buffer is: NaCl 139 mM, KCl 1 mM, CaCl2 2 mM, MgCl2 1 mM, HEPES 10 mM, glucose 10 mM. The pH was adjusted to 7.4 with NaOH.
The recipe for high K buffer is: KCl 140 mM, CaCl2 2 mM, MgCl2 1 mM, HEPES 10 mM, glucose 10 mM. The pH was adjusted to 7.4 with potassium hydroxide (KOH).
Exported tiff images were analyzed by CellProfiler with custom designed programs for Landing pad cells and generated stable cell lines. Briefly, cells were detected using red fluorescence (or green for depolarized nontransfected Landing pad cells) and defined as objects by the software, then regions of noncellular background fluorescence were identified and subtracted. Multiple measurements of each cell were exported as a csv format file and the mean intensities of all cells in each image were extracted with a custom script written in Python. These mean intensities were used to describe the membrane potential for each specific cell line with a certain treatment in one image. The average intensity value of duplicate images was calculated as one data point for the statistical analysis and data presentation.
Estimation of Potassium Channel Conductance with DiBAC4(3) Assay Results
To estimate conductance from DiBAC4(3) measurements, we used a simplified Goldman-Hodgkin-Katz equation that assumed that permeability is conductance, and that all non-K conductances can be lumped into a single “sodium” conductance:
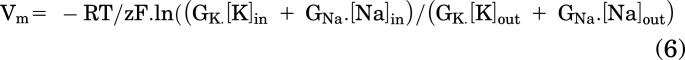
where Vm = membrane potential, R = gas constant, T = temperature (°K), z = 1, F = Faraday’s constant, GK = K conductance, GNa = sodium conductance, and [K]in, [K]out, [Na]in, [Na]out are the ion concentrations on either side of the membrane.
Whole-Cell Patch-Clamp Experiments
Micropipettes were pulled from soda-lime glass microhematocrit tubes (Kimble-Chase 2502) using a P-97 puller (Sutter Instruments) and pipette tips with resistance of 2–4 MΩ were used for recordings. Whole-cell currents were recorded at a Vm –60 mV, using an Axopatch 200B amplifier, Digidata 1322A (Molecular Devices) and Axon pCLAMP software (Molecular Devices, LLC). High-Na+ bath solution was (mM): NaCl 136, KCl 4, CaCl2 2, MgCl2 1, HEPES 10, and glucose 10, pH 7.4 adjusted with NaOH. High-K+ bath solution was (mM): KCl 140, CaCl2 2, MgCl2 1, HEPES 10, and glucose 10, pH 7.4 adjusted with KOH. The pipette solution was: potassium aspartate 110, KCl 30, NaCl 10, MgCl2 1, HEPES 10, CaCl2 0.5, K2HPO4 4, ATP 0.1, and EGTA 5, pH 7.2 adjusted with KOH.
Noise Analysis
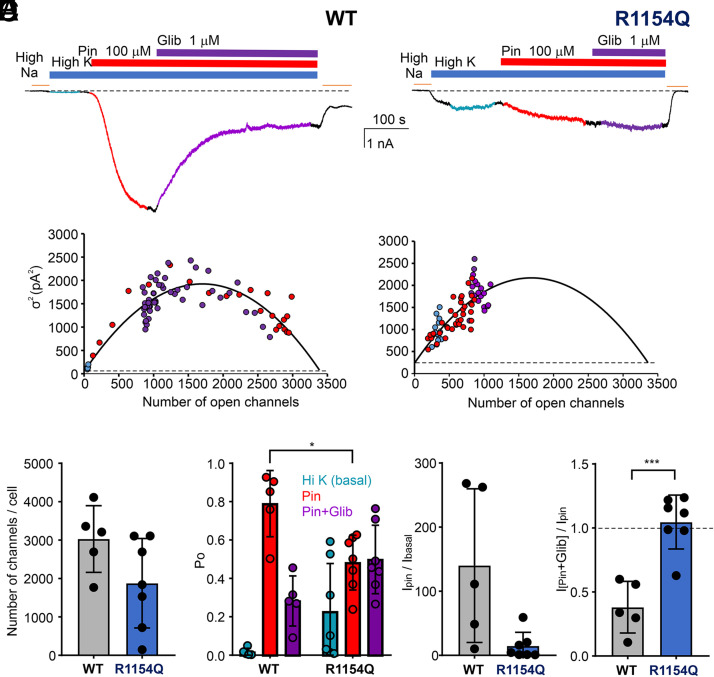
Recorded traces were offline sampled at 100Hz and divided into small fragments, each of 6.4 second Mean current and variance (Var2) were estimated in each fragment. In high Na+, theoretical KATP-specific current ≈0 pA, and mean currents and variance in high Na were subtracted from each high K fragment. Var2 was plotted versus number of open channels (n = mean current/single channel current 2.1 pA) in each fragment and fitted with the equation:
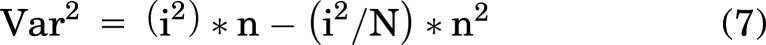
where i (single channel current) = 35 pS * (–60 mV) = –2.1 pA and n (number of open channel) = average current value/(–2.1). N denotes the total number of channels on the cell membrane.
Statistical Analysis
All statistical analyses were performed using Prism. Significance values were calculated using one-way ANOVA, with subsequent post hoc tests as indicated in the figure legends for pairwise comparison. All values are expressed as mean ± S.D.
Results
Establishment of a Thallium Influx Assay with a Stable Cell Line Expressing hKir6.1/hSUR2B
HEK cells stably expressing WT hKir6.1/hSUR2B were generated by incorporating a recombinant DNA cassette encoding hKir6.1, IRES, hSUR2B, IRES, and mcherry-P2A-puromycin resistant gene into the genome of Landing pad cells (Fig. 1A) (Matreyek et al., 2017). As shown by example micrographs in Fig. 1B, incorporation of WT or mutant channels into the landing pad uniformly reduced blue fluorescent protein fluorescence and increased mCherry fluorescence, indicating high level expression in all cells. A Tl+ flux assay (Raphemot et al., 2013) was developed (Fig. 2A), as described in the Materials and Methods. Fig. 2B shows example raw data for fluorescence changes under different conditions. Fluorescence was very low, and Tl+-insensitive, when no cells were plated in the wells. For wells plated with Landing pad only cells (Fig. 2B), or cells with hKir6.1/hSUR2B expression, there was a marked increase in background, nonspecific, fluorescence. Raw data were background-subtracted and normalized to the fluorescence signal before Tl+ was added and plotted as relative fluorescence versus time (Fig. 2, C–E). There was an approximately monoexponential Tl+-sensitive increase in cell fluorescence in Landing pad only cells (Fig. 2C), reflecting a low background conductance, and there was a bigger increase in uptake when the KATP channel subunits were expressed (Fig. 2D). Initial rates of Tl+ uptake were used to assess K+ conductance (Fig. 2E), as described in the Materials and Methods.
Generation of Stable hKir6.1/hSUR2B Cell Lines with Candidate CS Mutations in hKir6.1 or hSUR2B
Multiple hKir6.1 and hSUR2B mutations were introduced into the attB plasmids and used to generate stable cell lines expressing mutant channel complexes (Fig. 3A). These included previously characterized [C176S] and [V65M] (Brownstein et al., 2013; Cooper et al., 2014, 2017) CS mutations, as well as CS-associated, but uncharacterized [E189K] (Chihara et al., 2020), [A46V], and [D338E]mutations in hKir6.1(the latter two mutations were identified in patients clinically diagnosed with CS using Clinical Laboratory Improvement Amendments-approved sequencing). Mutations in hSUR2B include previously characterized [A478V] (Cooper et al., 2015), [R1154Q], [R1154W] (van Bon et al., 2012; McClenaghan et al., 2018), and indel1055 (Gao et al., 2023) CS mutations. After puromycin treatment, all generated cell lines lost blue fluorescence and showed mCherry red fluorescence in almost every single cell (Fig. 1, Supplemental Fig. 2).
WT and Cantú mutant Kir6.1/SUR2B channel properties characterized by Tl influx. (A) Location of introduced mutations in hKir6.1/hSUR2B complex (PDB: 7MJO) color-coded as in subsequent figures. (B) Representative Tl influx traces of untransfected, WT, Kir6.1[A46V], and SUR2B[R1154Q] cells under basal condition, MI (oligomycin, 2.5 mM and 2-deoxy-D-glucose, 1 mM), forskolin (10 μM), and pinacidil (30 μM). (C) Summary of initial rate from each of the four treatment conditions in (A) (n = 3–6). Data from individual experiments were plotted together with mean ± S.D. Statistical significance compared with the WT group is denoted by ***P < 0.001, **P < 0.01, and *P < 0.01. “No significance” is not marked in the figure and is defined as P ≥ 0.05 according to one-way ANOVA test with Dunnett’s multiple comparisons.
Differential Responses of Kir6.1 and SUR2B Mutants to KCOs, Metabolism Inhibition, and Phosphorylation
Kir6.1/SUR2B is known to be activated by multiple mechanisms (Isomoto et al., 1996), including inhibition of cell metabolism [Metabolic inhibition (MI)] (Li et al., 2016) and by protein kinase A and protein kinase G (PKA/PKG)-dependent pathways (Haynes and Cook, 2006; Yang et al., 2008). We first examined the effect of these activating conditions on WT and mutant hKir6.1/hSUR2B channels (Fig. 3A) using the Tl-flux assay. Cell lines were treated with the potassium channel opener (KCO) pinacidil (100 μM), MI (induced with 2.5 mg/ml oligomycin and 1 mM 2-deoxy-D-glucose), or 10 μM forskolin. In untransfected cells, the Tl-influx rate was not increased by any of these conditions, and 10 μM forskolin actually decreased the influx signal (Fig. 3B). Although basal Tl-influx rate was not markedly different between any transfected cell lines (Fig. 3C), WT and Kir6.1 mutant channels all showed much higher Tl-influx rates in the presence of pinacidil, reflecting increased channel activation (Fig. 3C). Although there was no obvious increase for WT, Tl-influx rates increased for hKir6.1[V65M], [C176S], [E189K], and [D338E] mutations with MI or forskolin (Fig. 3C).
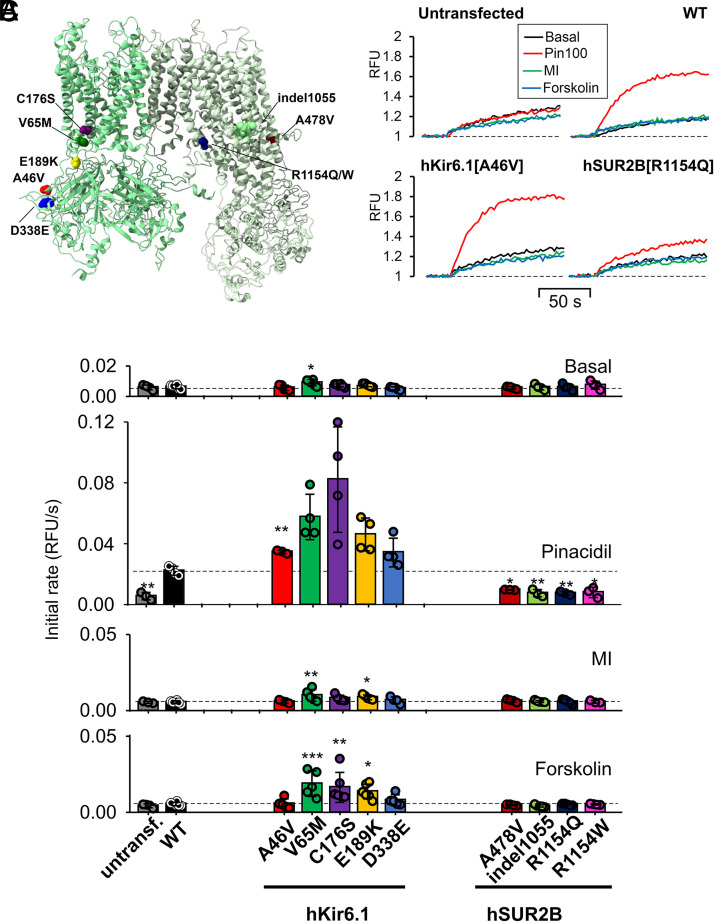
Very surprisingly, all cell lines with hSUR2B mutations were essentially insensitive to pinacidil activation, as well to MI and 10 μM forskolin (Fig. 3C), even though A478V, indel1055, R1154Q, and R1154W have all been shown to be GOF mutations, with enhanced sensitivity to activation by Mg-nucleotides and by pinacidil, in previous studies with Kir6.2/SUR2A mutant channels (Harakalova et al., 2012; Cooper et al., 2015; McClenaghan et al., 2018; Gao et al., 2023). To examine the generality of this markedly different response of hKir6.1 and hSUR2B mutants to the KCO pinacidil, we further tested the response of hKir6.1[V65M] and hSUR2B[R1154Q] mutations to other KCOs (diazoxide, cromakalim, and nicorandil) at presumably maximal concentrations. WT channels were only activated by pinacidil and cromakalim (Fig. 4, A and B), whereas hKir6.1[V65M] showed enhanced activation by these drugs and measurable activation by nicorandil and diazoxide (Fig. 4, A and B). Note, however, that hSUR2B[R1154Q] was not activated by any of the KCOs (Fig. 4, A and B).
Response of WT and Cantú mutant Kir6.1/SUR2B channels to different KCOs. (A) Representative thallium influx traces of WT, Kir6.1[V65M], and SUR2B[R1154Q] cells treated with pinacidil at indicated concentrations (μM). (B) Summary of the mean initial rate values of data as in (A), for treatment with pinacidil, diazoxide, cromakalim, and nicorandil treatments (n = 3–4, concentrations indicated in μM). Data from individual experiments are plotted together with mean ± S.D. Statistical significance compared with the WT group of the same drug concentration group is denoted by ***P < 0.001, **P < 0.01, or *P < 0.01, according to one-way ANOVA test with Tukey’s multiple comparisons.
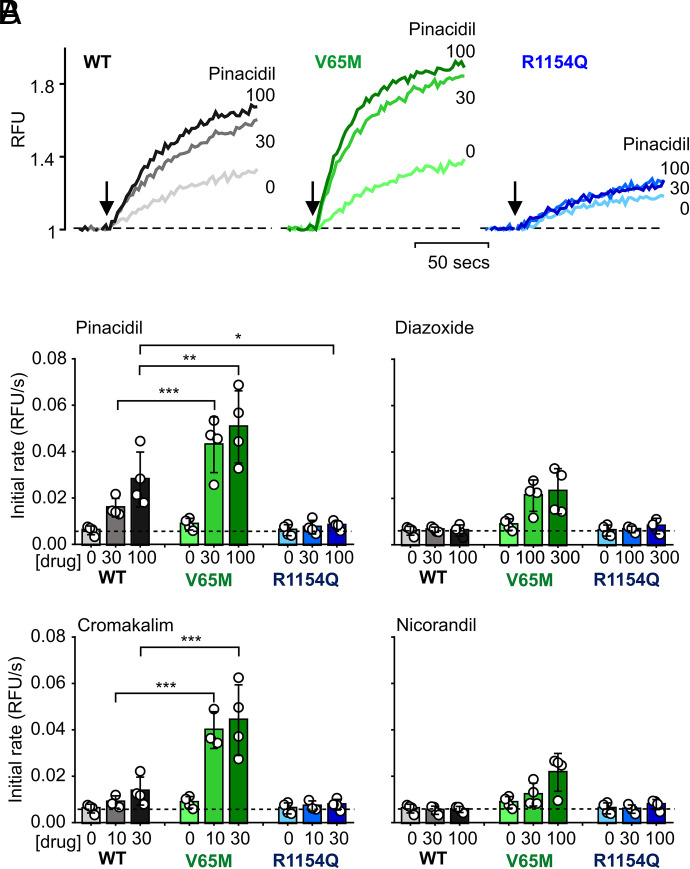
We carried out whole-cell patch-clamp experiments on Kir6.1/SUR2B channels and the apparently pinacidil-insensitive SUR2B[R1154Q] mutant channels (Fig. 5). Nonstationary noise analysis (Stevens, 1972) was used to measure channel density and open probability (Fig. 5B). Although mutant channel density is slightly reduced (to ~60% that of WT) (Fig. 5C), this is not sufficient to explain the lack of pinacidil response in mutant Tl fluxes. These data show that first, although the basal open probability of WT channels is extremely low (~0.02), it is much higher for the SUR2[R1154Q] mutant channels (Fig. 5D). They further show that, although WT current is increased ~150-fold by pinacidil, it is only increased ~10-fold for R1154Q mutant channels (Fig. 5E), and they additionally confirm a markedly reduced glibenclamide sensitivity of R1154Q mutant channels (Fig. 5F), as seen in previous experiments with recombinant expression (McClenaghan et al., 2018).
Loss of pinacidil sensitivity in Kir6.1/SUR2B[R1154Q Cantú mutant channels. (A) Representative whole-cell patch-clamp recordings from stably transfected WT and R1154Q mutant channels exposed to nonconducting (high Na), basal (high K), pinacidil-activated (Pin), and glibenclamide-inhibited (Glib) at concentrations indicated. (B) Nonstationary noise analysis (see Materials and Methods) for the two traces in (A), during exposure to basal, Pin, or Pin+Glib conditions as indicated by colored circles. (C) Number of channels per cell from experiments as in (A) and (B). (D) Open probability from experiments as in (A) and (B) during the final ~30 seconds in each condition. (E) Ratio of the current in pinacidil to the basal current (IPin/IBasal), and (F) ratio of the current in pinacidil+glibenclamide to the current in pinacidil (IPin+Glib/IPin) from experiments as in (A) and (B). In each of graphs in (C)–(F), data from individual experiments are plotted together with mean ± S.D. Statistical significance compared with the WT under each condition is denoted by ***P < 0.001 or *P < 0.05, according to one-way ANOVA test with Tukey’s multiple comparisons.
Dose-dependence of the response of Kir6.1/SUR2B mutants to pinacidil activation was measured to further quantitatively assess drug sensitivities. WT channels only started to show measurable activation of Tl+ influx at 10 μM pinacidil and reached maximum at 100 μM pinacidil. Human Kir6.1 mutations A46V, V65M, E189K, and D338E all showed higher sensitivity to pinacidil, with markedly lower EC50, and C176S additionally showed a much higher maximum channel activity (Fig. 6, A and B). Unexpectedly, at very high concentration of pinacidil (300 μM), the activity of V65M channels was slightly decreased.
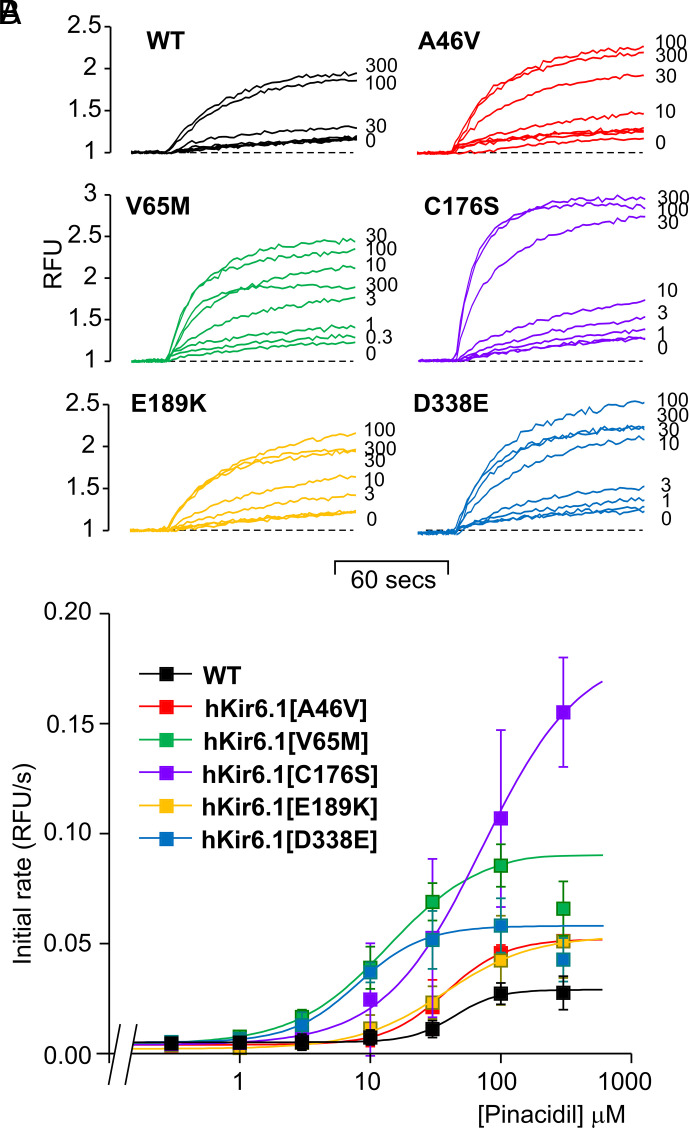
Dose-dependent activation of WT and Cantú Kir6.1 mutant Kir6.1 channels by pinacidil. (A) Representative thallium influx traces of WT and Kir6.1 mutant cells treated with 0, 0.3, 1, 3, 10, 30, 100, and 300 μM pinacidil. (B) Summary of the mean initial rate values of data as in (A). Each data point represents mean ± S.D. of three individual experiments. The mean of average initial rate values were fitted with eq. 3. EC50 and Hill coefficient values are WT: 44.0 ± 9.5 μM, 2.6 ± 1.0; Kir6.1[A46V]: 38.1 ± 3.0 μM, 1.9 ± 0.2; Kir6.1[V65M]: 13.7 ± 0.9 μM, 1.2 ± 0.0; Kir6.1[C176S]: 71.9 ± 18.0 μM, 1.2 ± 0.1; Kir6.1[E189K]: 37.3 ± 17.9 μM, 1.3 ± 0.4; Kir6.1[D338E]: 8.0 ± 0.3 μM, 1.7 ± 0.1.
Variable Sensitivity of Kir6.1 Mutants to Glibenclamide Inhibition
WT and hKir6.1 mutant channels were next evaluated for their response to glibenclamide, in the presence of 100 μM pinacidil (Fig. 7, A and B). All channels were inhibited to a similar maximal extent, but all GOF mutations showed reduced sensitivity to glibenclamide (Fig. 7B), with a similar rank order to that seen for enhancement of pinacidil sensitivity (Fig. 6B). Plotting the IC50 values for glibenclamide inhibition against EC50 values for pinacidil activation (Fig. 7C) reveals a close fit of the data to the dashed line that represents the hypothesis that the action of glibenclamide is purely on decreasing the binding affinity for pinacidil (i.e., that they are reciprocally related).
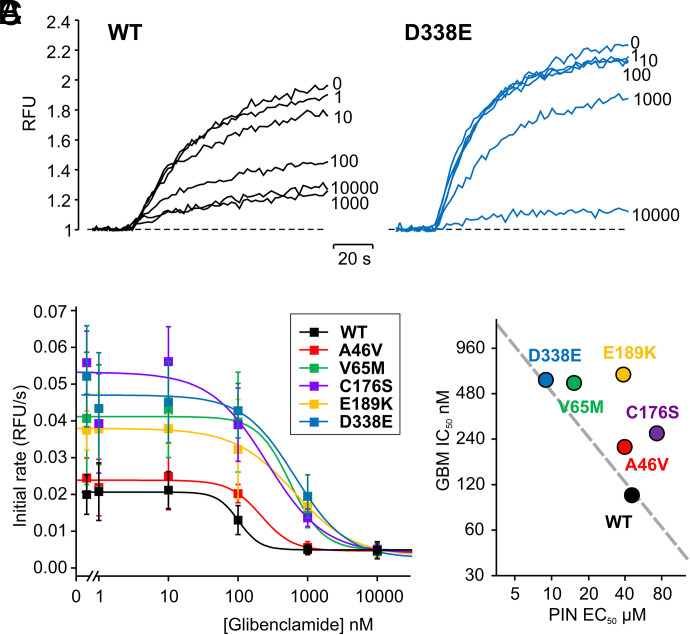
Dose-dependent inhibition of pinacidil activated WT and Cantú mutant Kir6.1/SUR2B channel current by glibenclamide. (A) Representative thallium influx traces of WT and Kir6.1[D338E] cells treated with 100 μM pinacidil with 0, 1 nM, 10 nM, 100 nM, 1 μM, and 10 μM glibenclamide. (B) Summary of the mean initial rate values of data as in (A). Each data point represents mean ± S.D. of four to seven experiments. The data were fitted with eq. 4. IC50 and Hill coefficient values are WT: 102.0 ± 6.7 nM, 2.9 ± 6.0; Kir6.1[A46V]: 213.5 ± 31.9 nM, 1.9 ± 0.3; Kir6.1[V65M]: 565.1 ± 132.4 nM, 1.8 ± 0.8; Kir6.1[C176S]: 261.8 ± 139.0 nM, 1.1 ± 0.5; Kir6.1[E189K]: 642.0 ± 32.6 nM, 1.0 ± 0.2; Kir6.1[D338E]: 591.3 ± 172.4 nM, 1.5 ± 0.4. (C) IC50 for glibenclamide inhibition versus EC50 for pinacidil activation. The dashed line models the hypothesis that glibenclamide acts solely by displacing pinacidil, with the equation: EC50(WT) × IC50(WT) = EC50(mut) × IC50(mut).
Measurements of KATP Channel Activities by Voltage Sensing Dye DiBAC4(3)
The sensitivity of the thallium flux assay being such that we can only distinguish channel-specific signals in the presence of KCOs is a major limitation to assessing physiologic activities. We therefore developed a complementary method, using DiBAC4(3) to detect channel activity by effects on the membrane potential that allows us to characterize mutational effects under conditions without KCOs (Fig. 8A). Intracellular DiBAC4(3) fluorescence is reported to be linearly positively correlated with cell membrane potential (Yamada et al., 2001). To confirm this, pilot experiments were carried out in which untransfected Landing pad cells, or WT, hKir6.1[A46V], and hKir6.1[V65M] transfected cells, loaded with 3 μM DiBAC4(3) dissolved in buffer, were incubated in extracellular K+ concentrations between 1–140 mM (Fig. 8B). Consistent with a relatively high K+ conductance that clamped the membrane potential close to EK under basal conditions, the fluorescence from V65M cells was linear with log[K]o, to below 3 mM (Fig. 8B). Both WT and Landing pad-only cells showed a much shallower dependence on [K]o, consistent with much lower overall K+ conductances, and essentially no KATP-specific conductance, whereas A46V behavior was intermediate (Fig. 8B).
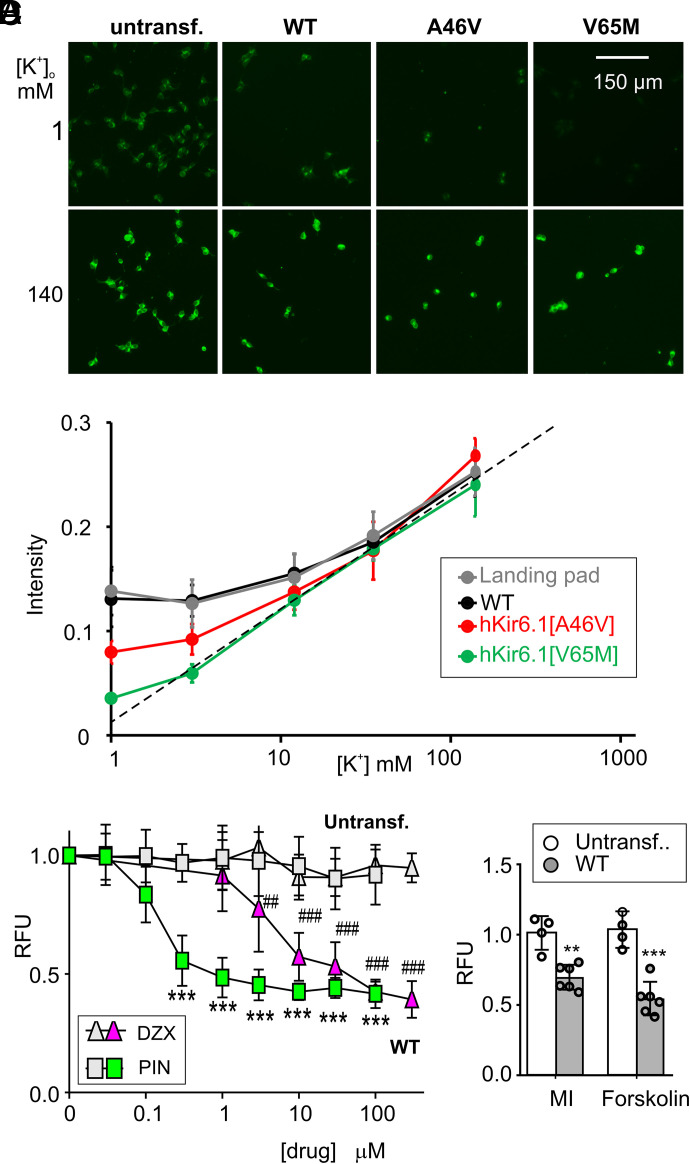
Characterization of Kir6.1/SUR2B channel activities with DiBAC4(3) assay. (A) Representative images of untransfected Landing pad, WT, Kir6.1[A46V], and Kir6.1[V65M] cells, 30 minutes after incubation with 3 μM DiBAC4(3) in [K+]o 1 mM or 140 mM. (B) Fluorescence intensities of DiBAC4(3)-treated untransfected Landing pad, WT, Kir6.1[A46V], and Kir6.1[V65M] cells as a function of [K+]o (1–140 mM). The data are expressed as mean ± S.D. (n = 3 experiments in each case). (C) DiBAC4(3) fluorescence of untransfected Landing Pad and WT cells to different concentrations of pinacidil (PIN) and diazoxide (DZX), normalized to untreated cells in each group. The data are expressed as mean ± S.D. (n = 5–6 for PIN, n = 4 for DZX). ***P < 0.001 for pinacidil group, ##P < 0.01, and ###P < 0.001 for diazoxide group compared untreated control in each group. One-way ANOVA with Dunnett’s multiple comparisons test. (D) DiBAC4(3) fluorescence (relative to untreated group) for untransfected Landing pad and WT cells in response to MI (oligomycin, 2.5 mM) and 2-deoxy-D-glucose, 1 mM) or forskolin (10 μM). Data are expressed as mean ± S.D. (n = 4–6). **P < 0.01, ***P < 0.001 compared with data points of control cells. One-way ANOVA with Dunnett’s multiple comparisons test.
At 1 mM [K+]o, fluorescence signals of the four cell lines were well detected and well separated (hKir6.1[V65M] < hKir6.1[A46V] < WT ≈ untransfected cells) (Fig. 8B), and we thus used this extracellular [K+] for the following measurements. We first conducted DiBAC4(3) assays on WT hKir6.1/hSUR2B channels under the same experimental conditions used in the thallium influx assay. Although pinacidil and diazoxide had apparently qualitatively different effects in the thallium flux assay (i.e., no effect of 100 μM diazoxide) (Fig. 4B), both drugs dose-dependently hyperpolarized WT cells (Fig. 8C), with pinacidil being more potent than diazoxide. Notably, diazoxide generated clear KATP-dependent hyperpolarization signals in the DiBAC4(3) assay at all concentrations above 1 μM (Fig. 8C). Similarly, although MI and 10 μM forskolin failed to generate measurable Tl+ influx signals, both caused significant hyperpolarization of WT cells (Fig. 8D), confirming that intracellular ATP depletion and phosphorylation can cause channel activation.
Overlapping the dose response to pinacidil in the two assays, we can more clearly compare the sensitivity of the two methods. With any given concentration of pinacidil, the potassium channel activity in the two assays should theoretically be the same. However, the pinacidil-sensitivity of signal amplitudes was very different between the two assays. Although thallium assay signal changes are only detected above 10 μM, DiBAC4(3) signal changes are almost saturated at this concentration (Fig. 9A). We used a simple model of the system (see Materials and Methods), assuming that the membrane potential is controlled only by K conductance and Na conductance to predict the K conductance reported by the DiBAC4(3) signal for WT channels (Fig. 9B). In the range of pinacidil from ~0.3–10 μM, the model provides reasonable agreement with the thallium flux-reported conductance, suggesting that relative conductance could be directly inferred with the DiBAC4(3) method, but also that ~80% of the DiBAC4(3) signal change (that occurs at <3 μM pinacidil) results from activation of <20% of the maximal available WT conductance. With this recognition that very low levels of WT channel activity are detected by the DiBAC4(3) assay, we measured the DiBAC4(3) fluorescence of each of the mutant cell lines under basal conditions (Fig. 9C). WT cells showed similar fluorescence amplitude to untransfected cells, but all of the mutant cells exhibited markedly lower fluorescence. Notably, CS hSUR2B mutations, all of which showed no activation by pinacidil in the thallium flux assay, clearly showed comparably significant cell hyperpolarization effects (Fig. 9C), reflecting some level of basal activation. For the hKir6.1 mutations, we plotted the calculated relative conductance from these DiBAC4(3) measurements versus the activity index assessed from Tl+ flux assays (see Materials and Methods). Consistent with both methods assessing the same underlying behaviors, there is a reasonable correlation between the two (Fig. 9D, see Discussion).
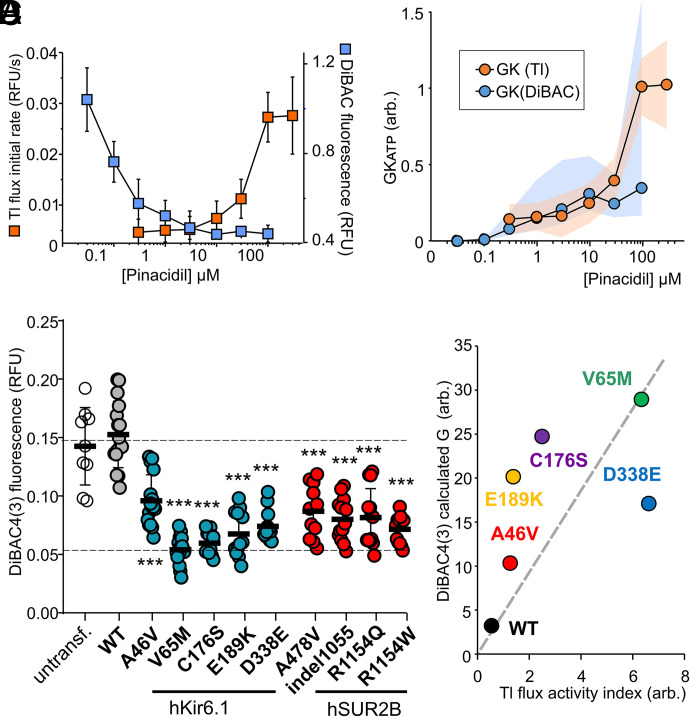
Sensitivity of DiBAC4(3) assays in response to Kir6.1/SUR2B activities and cellular diagnosis of CS mutations. (A) Dose–response of pinacidil-induced signal changes in thallium influx (orange, from Fig. 5B) and DiBAC4(3) (blue, n = 4–5, [K+]o 1 mM) assays. Data are expressed as mean ± S.D. in each case. (B) Calculated GKATP from Tl flux and DiBAC4(3) assays. Data shown for mean values. For Tl flux assay, the conductance is directly proportional to initial rate and scaled to 1. For DiBAC4(3) assay, eq. 6, with values of background gNa = 0.001, gK = 0.003. (C) Basal DiBAC4(3) fluorescence from untransfected Landing pad, WT, and indicated Kir6.1 or SUR2B mutant cells, measured without drug treatment in 1 mM [K+]o. Individual values, mean, and S.D. are plotted (n = 9–16). One-way ANOVA with Dunnett’s multiple comparisons test. ***P< 0 .001. (D) Calculated conductance in the DiBAC4(3) assay plotted versus activity index in the Tl+ flux assay for Kir6.1 mutants.
Effect of KATP Inhibitors on Cantú Mutations Measured by Voltage Sensing Dye DiBAC4(3)
With measurable basal activities for both hSUR2B and hKir6.1 mutations in the DiBAC4(3) assay, we were able to characterize the effects of channel blockers, glibenclamide, repaglinide, and PNU37883A on basal channel activity without KCO activation (Fig. 10). Again, very variable drug sensitivities were apparent. Hyperpolarization caused by the hKir6.1 mutations, particularly the three most active (V65M, C176S, and E189K), were very poorly sensitive to reversal by all three inhibitors. Hyperpolarization caused by hSUR2B[R1154W] and by hKir6.1[G338E] were uniquely insensitive to glibenclamide and PNU37883A, respectively, whereas hyperpolarization caused by hKir6.1[A46V] and the other hSUR2B mutations (A478V, R1154Q, indel1055) were readily reversed by each of the drugs.
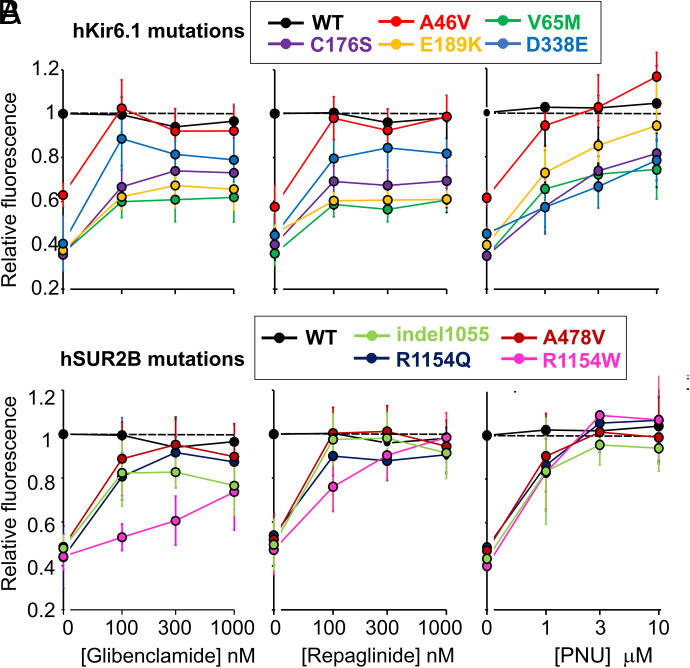
Effect of Cantú mutations on sensitivity to distinct channel inhibitors DiBAC4(3) fluorescence (relative to untreated WT group) of indicated Kir6.1 (A) or SUR2B (B) mutant channels in response to glibenclamide, repaglinide, and PNU37883A at the indicated concentrations. The data are expressed as mean ± S.D. (n = 4–5).
Discussion
All CS patients to date are heterozygous for mutations in ABCC9 (SUR2) (Harakalova et al., 2012; van Bon et al., 2012; Grange et al., 2019) or KCNJ8 (Kir6.1) (Brownstein et al., 2013; Cooper et al., 2014; Chihara et al., 2020; Apuril Velgara et al., 2022), and knock-in mouse models show that key CS features result from Kir6.1/SUR2B channel GOF (Huang et al., 2018; McClenaghan et al., 2020a; York et al., 2020). Determining molecular consequences of any given mutation is important: first, because accurate CS diagnosis requires confirmation of a molecular GOF (Gao et al., 2023) and second, to develop a molecular phenotype-human phenotype correlation, ultimately important for personalized stratification of treatment. Third, variable sensitivity of mutations to different potential inhibitors (Cooper et al., 2015; McClenaghan et al., 2018; Houtman et al., 2019), makes it essential to assay potential therapies for each mutation.
Recombinant patch-clamp experiments have shown all analyzed CS mutations to form overactive KATP channels when expressed in, or with, SUR2A and Kir6.2 subunits (Harakalova et al., 2012; Cooper et al., 2014, 2015; McClenaghan et al., 2018; Houtman et al., 2019), but CS mutations have not been electrophysiologically assayed in, or with, the relevant SUR2B or Kir6.1 subunits. Kir6.1 and Kir6.2, as well as SUR2A and SUR2B, generate distinct channel properties and pharmacology: Kir6.1 requires activating effects of MgADP or MgATP on the SUR subunit (Yamada et al., 1997; Reimann et al., 2000; Sim et al., 2002; Li et al., 2013), and SUR2B is more sensitive than SUR2A to activation by MgATP (Reimann et al., 2000) or MI (Li et al., 2016) and KCO (Shindo et al., 1998; Matsuoka et al., 2000; Reimann et al., 2000). There can also be clear species-dependent effects on channel pharmacology (Paajanen and Vornanen, 2002; Singareddy et al., 2022), and hence it is important to assay Kir6.1/SUR2B channels and human channels specifically.
High Throughput Approaches to Ion Channel Mutation Screening
We have therefore adapted the Landing pad system (Matreyek et al., 2020) to provide a rapid approach to generating stable cell lines expressing hKir6.1/hSUR2B channels and variants at similar expression levels. Second, we have developed thallium flux (Raphemot et al., 2014) and DiBAC3(4) assays (Gopalakrishnan et al., 1999; Yamada et al., 2001; Baczko et al., 2004) for high throughput and semi-quantitative assessment of channel activity. In both assays, cells are intact with normal cellular composition—critical for measuring physiologically relevant KATP channel activities. One potential shortcoming of our diagnosis pipeline is that mutations are expressed homomerically, whereas all patients to date are heterozygous. Heterozygous expression might be achieved using an additional IRES to drive a normal gene copy, but this will increase the complexity of the attB plasmid and require further optimization. Otherwise, the present assays should provide accurate reflection of CS mutational effects in relevant physiologic conditions. They can now be used for comprehensive mutational analysis and may be further scaled up for random mutagenic library-based approaches and combined with fluorescence-activated cell sorting and next-generation sequencing for large-scale high throughput (Coyote-Maestas et al., 2022) and generation of clinical “look up” tables.
In considering the thallium flux versus DiBAC4(3) assays, it is clear that the dynamic range of K conductance assayed by the two is quite distinct and overlaps over only a small range. The thallium flux data indicate that basal KATP conductance is very low in CS Kir6.1 mutations but is activated by pinacidil with higher sensitivity and to a greater extent than in WT. However, thallium flux data also suggest the erroneous conclusion that none of the SUR2B mutations result in any meaningful GOF. That this is not correct is made clear by the DiBAC4(3) data, which reveal a hyperpolarizing effect of all SUR2B mutations and provide an estimate of the relative effect of different mutations. In the thallium flux assay, there was also no observable effect of MI or forskolin on WT Kir6.1/SUR2B channels, but again there was significant decrease of DiBAC4(3) fluorescence, confirming the activating effects of both on Kir6.1/SUR2B channels (Shi et al., 2007; Yang et al., 2008; Li et al., 2016).
Our data (Fig. 9A) show that the DiBAC4(3) assay is extremely sensitive to even very small changes of conductance, such that only 1%–10% of the maximal pinacidil-activated conductance measured in the thallium flux being sufficient to maximally hyperpolarizes the cells. The simplified analysis in Fig. 9B suggests that both assays do indeed assess the same conductances but over a very different absolute range. Although neither assay is as precise as patch-clamp, both are relatively high throughput and technically easy and do not require the advanced operator skills of electrophysiology. By predicting basal conductance of Kir6.1 mutants from DiBAC4(3) measurements, and the predicted basal activity given the pinacidil sensitivity in the Tl flux assay, we see that there is reasonable agreement between the two methods in the rank effect of mutations (Fig. 9D). The much higher sensitivity of the DiBAC4(3) assay, avoiding the need for pinacidil activation, may make it particularly advantageous over the thallium assay for clinical diagnosis.
CS: Mechanistic Implications
One potential explanation for the remarkable, unexpected, loss of pinacidil sensitivity for each SUR2B mutation is that these mutations dramatically decrease trafficking of the channel complex to the surface, with a small number of channels that do make it to the surface still being sufficient to elevate basal KATP activities to cause measurable hyperpolarization. Whole-cell patch-clamp experiments confirm dramatic loss of pinacidil activation for SUR2B[R1154Q], but with only a slight reduction in channel density that cannot explain lack of conductance in Tl flux experiments. A more probable explanation is that these mutations decrease KCO binding to the SUR2 subunit or decrease the coupling between KCO binding and channel activation. MgADP decreases the binding of the related KCO P1075 to SUR2B (Hambrock et al., 1999), and it is possible that the SUR2B mutations favor this MgADP induced dissociation of KCOs.
Additionally, hKir6.1 CS mutations all caused variable increases in maximal conductance in saturating [pinacidil] and leftward shifts of the [pinacidil]-dependence. Assuming that pinacidil binding affinities are unaffected, the left-shifted dose–response curves reflect increased coupling of binding to channel activation. In general, analogous mutations in Kir6.2 increase the stability of the open channel, and hence the maximum open probability (Trapp et al., 1998; Loussouarn et al., 2000; Enkvetchakul et al., 2001; Cooper et al., 2017), which may give rise to the higher plateau fluxes at saturating pinacidil concentrations.
Implications for Pharmacotherapy Development
Multiple sulphonylurea and glinide drugs are Food and Drug Administration-approved for the treatment of type 2 and neonatal diabetes (Pearson et al., 2006; Tonini et al., 2006), and could potentially be repurposed as CS therapies (Ma et al., 2019; Nichols, 2023). However, KATP mutations that increase channel open state stability also typically decrease sulfonylurea sensitivity (Koster et al., 2005; Takagi et al., 2013), and hence decrease therapeutic efficacy. Moreover, sulfonylurea sensitivity of SUR2-based channels is generally lower than SUR1-based channels (Dorschner et al., 1999). Thus, use of KATP inhibitors to treat CS patients might be limited by inefficacy and high dose requirements, such that hypoglycemia, due to undesired inhibition of SUR1-based KATP channels in the pancreas, becomes a problem. We show that basal activity of most SUR2B CS mutations is effectively blocked by 100–300 nM glibenclamide, and more effectively by the glinide repaglinide, which is also reportedly more effective than glibenclamide at stimulating insulin secretion from islet cells (Fuhlendorff et al., 1998). Both drugs interact with high and low affinity sites on the channel complex (Gribble et al., 1997; Koster et al., 1999), with the high affinity site having a higher affinity for repaglinide (Fuhlendorff et al., 1998). It is possible that the resistance of R1154W to glibenclamide, substantiated by Kir6.2/SUR2A patch-clamp analyses (McClenaghan et al., 2018), is due to a reduction in binding affinity, given the close localization of R1154 to the glibenclamide binding site (Martin et al., 2017a).
Glibenclamide can dissociate KCOs from KATP channels (Quast et al., 1993; Löffler-Walz and Quast, 1998), providing a reasonable explanation for the apparent inverse relationship between activatory pinacidil sensitivity and inhibitory glibenclamide sensitivity for Kir6.1 mutants (Fig. 6). C176S and E189K are outliers, indicating additional attenuating effects on glibenclamide sensitivity. Kir6.2 residue C166 (equivalent to Kir6.1[C176]) is reported to have complex actions involving palmitoylation (Yang et al., 2020) that may be relevant. In addition, Kir6.2[E179K] (corresponding to Kir6.1[E189K]) decreased channel sensitivity to neomycin and increased the binding of phosphatidylinositol 4,5-bisphosphate (Pipatpolkai et al., 2020), thereby reducing high affinity sulfonylurea-induced channel inhibition (Koster et al., 1999). Reduced coupling to the high affinity SUR1 site (Koster et al., 1999) may explain why sulfonylureas do not effectively reverse hyperpolarization caused by open state stabilizing Kir6.1 CS mutations (e.g., V65M and C176S) (Cooper et al., 2017) and suggests that these drugs will not be effective therapies for such mutations.
Although PNU37883A, glibenclamide, and repaglinide have similar rank orders for mutation-dependence of their inhibition, we see quite different responses of specific mutations to each drug class. PNU37883A inhibits KATP channels via the Kir6.1 subunit (Kovalev et al., 2004). Kir6.1[D338E] specifically decreased the effect of PNU37883A in the DiBAC4(3) assay, but the mechanism is unclear. However, at saturating doses, PNU37883A was more effective than glibenclamide or repaglinide at inhibiting basal Kir6.1 mutation activities. Fatal effects of PNU37883A on cardiac function (Humphrey et al., 1996) mean this drug is not currently approved, but the present results suggest it might be a promising lead compound for developing a specific Kir6.1 inhibitor for CS.
Finally, we note that automated patch-clamp methods may rival fluorescence approaches in terms of throughput, but the latter remain technically easier for potential drug screening initiatives. Our data highlight distinct sensitivity ranges of the Tl flux versus DiBAC4(3) assays and will be important to consider when developing screening assays for any nonvoltage-dependent K channels. In the specific case of KATP channels, our data indicate that screening using pinacidil preactivated channels may not work for SUR2B mutant channels, and basal, or at least physiologically activated, channel activity may be important to assess.
Acknowledgments
The synthesized human SUR2A cDNA was a kind gift of Rod MacKinnon (Rockefeller University). The authors declare that all the data supporting the findings of this study are available within the paper.
Data Availability
All original data will be made available to interested parties on reasonable request.
Abbreviations
| CS | Cantú syndrome |
| DiBAC4(3) | bis-(1,3-dibutylbarbituric acid)trimethine oxonol |
| GOF | gain-of-function |
| Grel | relative conductance |
| Gsat | conductance in saturating [drug] |
| G0 | conductance in zero drug |
| HBSS | Hanks’ balanced salt solution |
| IRES | internal ribosome entry sites |
| KATP | ATP-sensitive potassium |
| KCO | potassium channel opener |
| Kir6 | inward rectifier sub-family 6 |
| KOH | potassium hydroxide |
| LOF | loss-of-function |
| NBD | nucleotide binding domain |
| PKA | protein kinase A |
| RFU | relative fluorescence units |
| SUR | sulphonylurea receptor |
| WT | wild type |
Authorship Contributions
Participated in research design: Gao, McClenaghan, Nichols.
Conducted experiments: Gao.
Contributed new reagents or analytic tools: Matreyek, Grange.
Performed data analysis: Gao, Nichols.
Wrote or contributed to the writing of the manuscript: Gao, McClenaghan, Matreyek, Grange, Nichols.
Footnotes
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute [Grant R35 HL140024] (to C.G.N.), [Grant K99 HL150277] (to C.M.C.), and Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R21 HD103347] (to D.K.G. and C.G.N.).
1Current affiliation: Center for Advanced Biotechnology and Medicine, Departments of Pharmacology and Medicine, Rutgers University, Piscataway, New Jersey.
The authors declare no conflicts of interest.
dx.doi.org/10.1124/jpet.123.001659.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aguilar-BryanLClementJP4thGonzalezGKunjilwarKBabenkoABryanJ (1998) Toward understanding the assembly and structure of KATP channels. Physiol Rev 78:227–245. [Abstract] [Google Scholar]
- Apuril VelgaraESMarianiMTorellaAMusacchiaFNigroVSelicorniA; Telethon Undiagnosed Diseases Program Consortium (2022) Cantù syndrome: report of a patient with a novel variant in KCNJ8 and revision of literature. Am J Med Genet A 188:1661–1666. [Abstract] [Google Scholar]
- BabenkoAPPolakMCavéHBusiahKCzernichowPScharfmannRBryanJAguilar-BryanLVaxillaireMFroguelP (2006) Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 355:456–466. [Abstract] [Google Scholar]
- BaczkóIGilesWRLightPE (2004) Pharmacological activation of plasma-membrane KATP channels reduces reoxygenation-induced Ca(2+) overload in cardiac myocytes via modulation of the diastolic membrane potential. Br J Pharmacol 141:1059–1067. [Europe PMC free article] [Abstract] [Google Scholar]
- BrownsteinCATowneMCLuquetteLJHarrisDJMarinakisNSMeineckePKutscheKCampeauPMYuTWMarguliesDMet al. (2013) Mutation of KCNJ8 in a patient with Cantú syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this condition. Eur J Med Genet 56:678–682. [Europe PMC free article] [Abstract] [Google Scholar]
- ChiharaMAsahinaAItohM (2020) A novel mutation in the KCNJ8 gene encoding the Kir6.1 subunit of an ATP-sensitive potassium channel in a Japanese patient with Cantú syndrome. J Eur Acad Dermatol Venereol 34:e476–e478. [Abstract] [Google Scholar]
- ChutkowWASimonMCLe BeauMMBurantCF (1996) Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes 45:1439–1445. [Abstract] [Google Scholar]
- CooperPEMcClenaghanCChenXStary-WeinzingerANicholsCG (2017) Conserved functional consequences of disease-associated mutations in the slide helix of Kir6.1 and Kir6.2 subunits of the ATP-sensitive potassium channel. J Biol Chem 292:17387–17398. [Europe PMC free article] [Abstract] [Google Scholar]
- CooperPEReutterHWoelfleJEngelsHGrangeDKvan HaaftenGvan BonBWHoischenANicholsCG (2014) Cantú syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat 35:809–813. [Europe PMC free article] [Abstract] [Google Scholar]
- CooperPESala-RabanalMLeeSJNicholsCG (2015) Differential mechanisms of Cantú syndrome-associated gain of function mutations in the ABCC9 (SUR2) subunit of the KATP channel. J Gen Physiol 146:527–540. [Europe PMC free article] [Abstract] [Google Scholar]
- Coyote-MaestasWNedrudDHeYSchmidtD (2022) Determinants of trafficking, conduction, and disease within a K+ channel revealed through multiparametric deep mutational scanning. eLife 11:e76903. [Europe PMC free article] [Abstract] [Google Scholar]
- DavisMJKimHJZawiejaSDCastorena-GonzalezJAGuiPLiMSaundersBTZinselmeyerBHRandolphGJRemediMSet al. (2020) Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function. J Physiol 598:3107–3127. [Europe PMC free article] [Abstract] [Google Scholar]
- DörschnerHBrekardinEUhdeISchwanstecherCSchwanstecherM (1999) Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol Pharmacol 55:1060–1066. [Abstract] [Google Scholar]
- EnkvetchakulDLoussouarnGMakhinaENicholsCG (2001) ATP interaction with the open state of the K(ATP) channel. Biophys J 80:719–728. [Europe PMC free article] [Abstract] [Google Scholar]
- FlaggTPEnkvetchakulDKosterJCNicholsCG (2010) Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev 90:799–829. [Europe PMC free article] [Abstract] [Google Scholar]
- FosterMNCoetzeeWA (2016) KATP channels in the cardiovascular system. Physiol Rev 96:177–252. [Europe PMC free article] [Abstract] [Google Scholar]
- FuhlendorffJRorsmanPKofodHBrandCLRolinBMacKayPShymkoRCarrRD (1998) Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes 47:345–351. [Abstract] [Google Scholar]
- GaoJMcClenaghanCChristiaansIAldersMvan DuinenKvan HaelstMMvan HaaftenGNicholsCG (2023) Lymphedema as first clinical presentation of Cantu Syndrome: reversed phenotyping after identification of gain-of-function variant in ABCC9. Eur J Hum Genet 31:188–194. [Europe PMC free article] [Abstract] [Google Scholar]
- GloynALPearsonERAntcliffJFProksPBruiningGJSlingerlandASHowardNSrinivasanSSilvaJMMolnesJet al. (2004) Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350:1838–1849. [Abstract] [Google Scholar]
- GopalakrishnanMWhiteakerKLMolinariEJDavis-TaberRScottVEShiehCCBucknerSAMilicicICainJCPostlSet al. (1999) Characterization of the ATP-sensitive potassium channels (KATP) expressed in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther 289:551–558. [Abstract] [Google Scholar]
- GrangeDKRoesslerHIMcClenaghanCDuranKShieldsKRemediMSKnoersNVAMLeeJMKirkEPScurrIet al. (2019) Cantú syndrome: findings from 74 patients in the International Cantú Syndrome Registry. Am J Med Genet C Semin Med Genet 181:658–681. [Europe PMC free article] [Abstract] [Google Scholar]
- GribbleFMTuckerSJAshcroftFM (1997) The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol 504:35–45. [Abstract] [Google Scholar]
- HambrockALöffler-WalzCKloorDDelabarUHorioYKurachiYQuastU (1999) ATP-sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol Pharmacol 55:832–840. [Abstract] [Google Scholar]
- HarakalovaMvan HarsselJJTerhalPAvan LieshoutSDuranKRenkensIAmorDJWilsonLCKirkEPTurnerCLet al. (2012) Dominant missense mutations in ABCC9 cause Cantú syndrome. Nat Genet 44:793–796. [Abstract] [Google Scholar]
- HaynesJMCookAL (2006) Protein kinase G-induced activation of K(ATP) channels reduces contractility of human prostate tissue. Prostate 66:377–385. [Abstract] [Google Scholar]
- HoutmanMJCChenXQileMDuranKvan HaaftenGStary-WeinzingerAvan der HeydenMAG (2019) Glibenclamide and HMR1098 normalize Cantú syndrome-associated gain-of-function currents. J Cell Mol Med 23:4962–4969. [Europe PMC free article] [Abstract] [Google Scholar]
- HuangYMcClenaghanCHarterTMHinmanKHalabiCMMatkovichSJZhangHBrownGSMechamRPEnglandSKet al. (2018) Cardiovascular consequences of KATP overactivity in Cantu syndrome. JCI Insight 3:e121153. [Europe PMC free article] [Abstract] [Google Scholar]
- HumphreySJSmithMPCiminiMGBuchananLVGibsonJKKhanSAMeisheriKD (1996) Cardiovascular effects of the K-ATP channel blocker U-37883A and structurally related morpholinoguanidines. Methods Find Exp Clin Pharmacol 18:247–260. [Abstract] [Google Scholar]
- HuopioHShyngSLOtonkoskiTNicholsCG (2002) K(ATP) channels and insulin secretion disorders. Am J Physiol Endocrinol Metab 283:E207–E216. [Abstract] [Google Scholar]
- InagakiNGonoiTClementJP4thNambaNInazawaJGonzalezGAguilar-BryanLSeinoSBryanJ (1995) Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270:1166–1170. [Abstract] [Google Scholar]
- IsomotoSKondoCYamadaMMatsumotoSHigashiguchiOHorioYMatsuzawaYKurachiY (1996) A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem 271:24321–24324. [Abstract] [Google Scholar]
- KharadeSVNicholsCDentonJS (2016) The shifting landscape of KATP channelopathies and the need for ‘sharper’ therapeutics. Future Med Chem 8:789–802. [Europe PMC free article] [Abstract] [Google Scholar]
- KosterJCRemediMSDaoCNicholsCG (2005) ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 54:2645–2654. [Abstract] [Google Scholar]
- KosterJCMarshallBAEnsorNCorbettJANicholsCG (2000) Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 100:645–654. [Abstract] [Google Scholar]
- KosterJCShaQNicholsCG (1999) Sulfonylurea and K(+)-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir6.2 and SUR1 subunits. J Gen Physiol 114:203–213. [Europe PMC free article] [Abstract] [Google Scholar]
- KovalevHQuayleJMKamishimaTLodwickD (2004) Molecular analysis of the subtype-selective inhibition of cloned KATP channels by PNU-37883A. Br J Pharmacol 141:867–873. [Europe PMC free article] [Abstract] [Google Scholar]
- LiAKnutsenRHZhangHOsei-OwusuPMoreno-DominguezAHarterTMUchidaKRemediMSDietrichHHBernal-MizrachiCet al. (2013) Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc 2:e000365. [Europe PMC free article] [Abstract] [Google Scholar]
- LiCGCuiWYWangH (2016) Sensitivity of KATP channels to cellular metabolic disorders and the underlying structural basis. Acta Pharmacol Sin 37:134–142. [Europe PMC free article] [Abstract] [Google Scholar]
- LiNWuJ-XDingDChengJGaoNChenL (2017) Structure of a pancreatic ATP-sensitive potassium channel. Cell 168:101–110.e10. [Abstract] [Google Scholar]
- Löffler-WalzCQuastU (1998) Binding of K(ATP) channel modulators in rat cardiac membranes. Br J Pharmacol 123:1395–1402. [Europe PMC free article] [Abstract] [Google Scholar]
- LoussouarnGMakhinaENRoseTNicholsCG (2000) Structure and dynamics of the pore of inwardly rectifying K(ATP) channels. J Biol Chem 275:1137–1144. [Abstract] [Google Scholar]
- MaAGurnasinghaniSKirkEPMcClenaghanCSinghGKGrangeDKPanditCZhuYRoscioliTElakisGet al. (2019) Glibenclamide treatment in a Cantú syndrome patient with a pathogenic ABCC9 gain-of-function variant: initial experience. Am J Med Genet A 179:1585–1590. [Europe PMC free article] [Abstract] [Google Scholar]
- MartinGMKandasamyBDiMaioFYoshiokaCShyngSL (2017a) Anti-diabetic drug binding site in a mammalian KATP channel revealed by cryo-EM. eLife 6:e31054. [Europe PMC free article] [Abstract] [Google Scholar]
- MartinGMYoshiokaCRexEAFayJFXieQWhortonMRChenJZShyngS-L (2017b) Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. eLife 6:e24149. [Europe PMC free article] [Abstract] [Google Scholar]
- MatreyekKAStephanyJJChiassonMAHasleNFowlerDM (2020) An improved platform for functional assessment of large protein libraries in mammalian cells. Nucleic Acids Res 48:e1. [Europe PMC free article] [Abstract] [Google Scholar]
- MatreyekKAStephanyJJFowlerDM (2017) A platform for functional assessment of large variant libraries in mammalian cells. Nucleic Acids Res 45:e102. [Europe PMC free article] [Abstract] [Google Scholar]
- MatsuokaTMatsushitaKKatayamaYFujitaAInagedaKTanemotoMInanobeAYamashitaSMatsuzawaYKurachiY (2000) C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ Res 87:873–880. [Abstract] [Google Scholar]
- McClenaghanCHansonASala-RabanalMRoesslerHIJosifovaDGrangeDKvan HaaftenGNicholsCG (2018) Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms. J Biol Chem 293:2041–2052. [Europe PMC free article] [Abstract] [Google Scholar]
- McClenaghanCHuangYMatkovichSJKovacsAWeinheimerCJPerezRBroekelmannTJHarterTMLeeJMRemediMS, et al. (2020a) The mechanism of high-output cardiac hypertrophy arising from potassium channel gain-of-function in Cantu syndrome. Function (Oxf) 1:zqaa004. [Europe PMC free article] [Abstract] [Google Scholar]
- McClenaghanCHuangYYanZHarterTMHalabiCMChalkRKovacsAvan HaaftenGRemediMSNicholsCG (2020b) Glibenclamide reverses cardiovascular abnormalities of Cantu syndrome driven by KATP channel overactivity. J Clin Invest 130:1116–1121. [Europe PMC free article] [Abstract] [Google Scholar]
- MeyerMChudziakFSchwanstecherCSchwanstecherMPantenU (1999) Structural requirements of sulphonylureas and analogues for interaction with sulphonylurea receptor subtypes. Br J Pharmacol 128:27–34. [Europe PMC free article] [Abstract] [Google Scholar]
- NicholsCG (2006) KATP channels as molecular sensors of cellular metabolism. Nature 440:470–476. [Abstract] [Google Scholar]
- NicholsCG (2023) Personalized therapeutics for KATP-dependent pathologies. Annu Rev Pharmacol Toxicol 63:541–563. [Europe PMC free article] [Abstract] [Google Scholar]
- NicholsCGShyngSLNestorowiczAGlaserBClementJP4thGonzalezGAguilar-BryanLPermuttMABryanJ (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272:1785–1787. [Abstract] [Google Scholar]
- PaajanenVVornanenM (2002) The induction of an ATP-sensitive K(+) current in cardiac myocytes of air- and water-breathing vertebrates. Pflugers Arch 444:760–770. [Abstract] [Google Scholar]
- PearsonERFlechtnerINjølstadPRMaleckiMTFlanaganSELarkinBAshcroftFMKlimesICodnerEIotovaVet al. ; Neonatal Diabetes International Collaborative Group (2006) Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355:467–477. [Abstract] [Google Scholar]
- PipatpolkaiTCoreyRAProksPAshcroftFMStansfeldPJ (2020) Evaluating inositol phospholipid interactions with inward rectifier potassium channels and characterising their role in disease. Commun Chem 3:147. [Europe PMC free article] [Abstract] [Google Scholar]
- QuastUBrayKMAndresHManleyPWBaumlinYDosogneJ (1993) Binding of the K+ channel opener [3H]P1075 in rat isolated aorta: relationship to functional effects of openers and blockers. Mol Pharmacol 43:474–481. [Abstract] [Google Scholar]
- RaphemotRSwaleDRDadiPKJacobsonDACooperPWojtovichAPBanerjeeSNicholsCGDentonJS (2014) Direct activation of β-cell KATP channels with a novel xanthine derivative. Mol Pharmacol 85:858–865. [Europe PMC free article] [Abstract] [Google Scholar]
- RaphemotRWeaverCDDentonJS (2013) High-throughput screening for small-molecule modulators of inward rectifier potassium channels. J Vis Exp (71):4209. [Europe PMC free article] [Abstract] [Google Scholar]
- ReimannFGribbleFMAshcroftFM (2000) Differential response of K(ATP) channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol Pharmacol 58:1318–1325. [Abstract] [Google Scholar]
- ScalaRMaqoudFZizzoNMeleACamerinoGMZitoFARanieriGMcClenaghanCHarterTMNicholsCGet al. (2020) Pathophysiological consequences of KATP channel overactivity and pharmacological response to glibenclamide in skeletal muscle of a murine model of Cantù syndrome. Front Pharmacol 11:604885. [Europe PMC free article] [Abstract] [Google Scholar]
- ScalaRMaqoudFZizzoNPassantinoGMeleACamerinoGMMcClenaghanCHarterTMNicholsCGTricaricoD (2021) Consequences of SUR2[A478V] mutation in skeletal muscle of murine model of Cantu syndrome. Cells 10:1791. [Europe PMC free article] [Abstract] [Google Scholar]
- ShiYWuZCuiNShiWYangYZhangXRojasAHaBTJiangC (2007) PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293:R1205–R1214. [Europe PMC free article] [Abstract] [Google Scholar]
- ShindoTYamadaMIsomotoSHorioYKurachiY (1998) SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 124:985–991. [Europe PMC free article] [Abstract] [Google Scholar]
- ShyngSNicholsCG (1997) Octameric stoichiometry of the KATP channel complex. J Gen Physiol 110:655–664. [Europe PMC free article] [Abstract] [Google Scholar]
- SimJHYangDKKimYCParkSJKangTMSoIKimKW (2002) ATP-sensitive K(+) channels composed of Kir6.1 and SUR2B subunits in guinea pig gastric myocytes. Am J Physiol Gastrointest Liver Physiol 282:G137–G144. [Abstract] [Google Scholar]
- SingareddySSRoesslerHIMcClenaghanCIkleJMTryonRCvan HaaftenGNicholsCG (2022) ATP-sensitive potassium channels in zebrafish cardiac and vascular smooth muscle. J Physiol 600:299–312. [Europe PMC free article] [Abstract] [Google Scholar]
- SinghGKMcClenaghanCAggarwalMGuHRemediMSGrangeDKNicholsCG (2022) A unique high-output cardiac hypertrophy phenotype arising from low systemic vascular resistance in Cantu syndrome. J Am Heart Assoc 11:e027363. [Europe PMC free article] [Abstract] [Google Scholar]
- StevensCF (1972) Inferences about membrane properties from electrical noise measurements. Biophys J 12:1028–1047. [Europe PMC free article] [Abstract] [Google Scholar]
- SungMWYangZDriggersCMPattonBLMostofianBRussoJDZuckermanDMShyngS-L (2021) Vascular KATP channel structural dynamics reveal regulatory mechanism by Mg-nucleotides. Proc Natl Acad Sci USA 118:e2109441118. [Europe PMC free article] [Abstract] [Google Scholar]
- TakagiTFurutaHMiyawakiMNagashimaKShimadaTDoiAMatsunoSTanakaDNishiMSasakiHet al. (2013) Clinical and functional characterization of the Pro1198Leu ABCC8 gene mutation associated with permanent neonatal diabetes mellitus. J Diabetes Investig 4:269–273. [Europe PMC free article] [Abstract] [Google Scholar]
- TeramotoNTomodaTYunokiTItoY (2006) Different glibenclamide-sensitivity of ATP-sensitive K+ currents using different patch-clamp recording methods. Eur J Pharmacol 531:34–40. [Abstract] [Google Scholar]
- TinkerAAzizQLiYSpectermanM (2018) ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol 8:1463–1511. [Abstract] [Google Scholar]
- ToniniGBizzarriCBonfantiRVanelliMCeruttiFFaleschiniEMeschiFPriscoFCiaccoECappaMet al. ; Early-Onset Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology (2006) Sulfonylurea treatment outweighs insulin therapy in short-term metabolic control of patients with permanent neonatal diabetes mellitus due to activating mutations of the KCNJ11 (KIR6.2) gene. Diabetologia 49:2210–2213. [Abstract] [Google Scholar]
- TrappSProksPTuckerSJAshcroftFM (1998) Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J Gen Physiol 112:333–349. [Europe PMC free article] [Abstract] [Google Scholar]
- TuckerSJGribbleFMZhaoCTrappSAshcroftFM (1997) Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387:179–183. [Abstract] [Google Scholar]
- van BonBWGilissenCGrangeDKHennekamRCKayseriliHEngelsHReutterHOstergaardJRMoravaETsiakasKet al. (2012) Cantú syndrome is caused by mutations in ABCC9. Am J Hum Genet 90:1094–1101. [Europe PMC free article] [Abstract] [Google Scholar]
- YamadaAGajaNOhyaSMurakiKNaritaHOhwadaTImaizumiY (2001) Usefulness and limitation of DiBAC4(3), a voltage-sensitive fluorescent dye, for the measurement of membrane potentials regulated by recombinant large conductance Ca2+-activated K+ channels in HEK293 cells. Jpn J Pharmacol 86:342–350. [Abstract] [Google Scholar]
- YamadaMIsomotoSMatsumotoSKondoCShindoTHorioYKurachiY (1997) Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol 499:715–720. [Abstract] [Google Scholar]
- YangHQMartinez-OrtizWHwangJFanXCardozoTJCoetzeeWA (2020) Palmitoylation of the KATP channel Kir6.2 subunit promotes channel opening by regulating PIP2 sensitivity. Proc Natl Acad Sci USA 117:10593–10602. [Europe PMC free article] [Abstract] [Google Scholar]
- YangYShiYGuoSZhangSCuiNShiWZhuDJiangC (2008) PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta 1778:88–96. [Europe PMC free article] [Abstract] [Google Scholar]
- York NWParker HXie ZTyus DWaheed MAYan ZGrange DKRemedi MSEngland SKHu H, et al. (2020) Kir6.1- and SUR2-dependent KATP over-activity disrupts intestinal motility in murine models of Cantu syndrome. JCI Insight 5:e141443. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from The Journal of Pharmacology and Experimental Therapeutics are provided here courtesy of American Society for Pharmacology and Experimental Therapeutics
Full text links
Read article at publisher's site: https://doi.org/10.1124/jpet.123.001659
Read article for free, from open access legal sources, via Unpaywall:
https://jpet.aspetjournals.org/content/jpet/386/3/298.full.pdf
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/153250853
Article citations
Non-radioactive Rb+ Efflux Assay for Screening KATP Channel Modulators.
Methods Mol Biol, 2796:191-210, 01 Jan 2024
Cited by: 1 article | PMID: 38856903
Personalized Therapeutics for KATP-Dependent Pathologies.
Annu Rev Pharmacol Toxicol, 63:541-563, 28 Sep 2022
Cited by: 9 articles | PMID: 36170658 | PMCID: PMC9868118
Review Free full text in Europe PMC
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe
-
(1 citation)
PDBe - 7MJOView structure
RefSeq - NCBI Reference Sequence Database (2)
- (1 citation) RefSeq - NM_004982.4
- (1 citation) RefSeq - NM_020297.4
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function.
J Physiol, 598(15):3107-3127, 30 May 2020
Cited by: 32 articles | PMID: 32372450 | PMCID: PMC7641979
Zoledronic Acid Blocks Overactive Kir6.1/SUR2-Dependent KATP Channels in Skeletal Muscle and Osteoblasts in a Murine Model of Cantú Syndrome.
Cells, 12(6):928, 17 Mar 2023
Cited by: 3 articles | PMID: 36980269 | PMCID: PMC10047381
Cantu syndrome-associated SUR2 (ABCC9) mutations in distinct structural domains result in KATP channel gain-of-function by differential mechanisms.
J Biol Chem, 293(6):2041-2052, 22 Dec 2017
Cited by: 29 articles | PMID: 29275331 | PMCID: PMC5808765
Kir6.1 and SUR2B in Cantú syndrome.
Am J Physiol Cell Physiol, 323(3):C920-C935, 25 Jul 2022
Cited by: 13 articles | PMID: 35876283 | PMCID: PMC9467476
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R35 HL140024
Grant ID: K99 HL150277
NICHD NIH HHS (1)
Grant ID: R21 HD103347





