Abstract
Background and objective
Congenital diaphragmatic hernia (CDH) is an anomaly of the cardiopulmonary system maturation process that results from both a global embryopathy and concomitant mechanical compression of the cardiopulmonary system from the abdominal contents during fetal maturation. This results in pulmonary hypertension, pulmonary hypoplasia, and cardiac dysfunction, requiring intense critical care management. The patients with highest risk CDH are the most challenging, resource-intensive, and bear most of the mortality. Advances at the basic, translational, and clinical research levels are leading to novel therapies and management strategies for complex, high-risk CDH. Our objective is to review novel approaches in thinking and management for the most complex and high-risk CDH patients. These include patients with prenatal and postnatal indicators of high-risk defects, those receiving extracorporeal life support (ECLS), and those with concomitant anomalies such as complex cardiac and/or chromosomal abnormalities.Methods
PubMed was searched in late 2022 and early 2023 to identify relevant evidence. Search terms included congenital diaphragmatic hernia (CDH)", "extracorporeal life support (ECLS)", "pulmonary hypertension", "dual-hit hypothesis", "risk reduction", "cardiac/chromosomal anomalies", and "novel therapies". We included trials, multicenter studies (prospective and retrospective), single-center reports, and review articles/expert opinion.Key content and findings
CDH is a congenital anomaly of the cardiopulmonary and diaphragmatic systems that represents a spectrum of disease. High-risk or complex patients are defined by prenatal/postnatal risk stratification, receipt of ECLS, and/or having concomitant anomalies, representing the severe end of that spectrum. Overall survival of high-risk CDH is about 50% and comprises the vast majority of mortality, mandating special emphasis. The development of risk-stratification processes, best practices or guidelines of management, and novel therapies is critical to optimize the care of these infants.Conclusions
CDH patients with high-risk disease remain a challenging subset of CDH patients. Increasing opportunities for survival are being realized with novel, investigational approaches.Free full text

Recent advances in the treatment of complex congenital diaphragmatic hernia—a narrative review
Abstract
Background and Objective
Congenital diaphragmatic hernia (CDH) is an anomaly of the cardiopulmonary system maturation process that results from both a global embryopathy and concomitant mechanical compression of the cardiopulmonary system from the abdominal contents during fetal maturation. This results in pulmonary hypertension, pulmonary hypoplasia, and cardiac dysfunction, requiring intense critical care management. The patients with highest risk CDH are the most challenging, resource-intensive, and bear most of the mortality. Advances at the basic, translational, and clinical research levels are leading to novel therapies and management strategies for complex, high-risk CDH. Our objective is to review novel approaches in thinking and management for the most complex and high-risk CDH patients. These include patients with prenatal and postnatal indicators of high-risk defects, those receiving extracorporeal life support (ECLS), and those with concomitant anomalies such as complex cardiac and/or chromosomal abnormalities.
Methods
PubMed was searched in late 2022 and early 2023 to identify relevant evidence. Search terms included congenital diaphragmatic hernia (CDH)”, “extracorporeal life support (ECLS)”, “pulmonary hypertension”, “dual-hit hypothesis”, “risk reduction”, “cardiac/chromosomal anomalies”, and “novel therapies”. We included trials, multicenter studies (prospective and retrospective), single-center reports, and review articles/expert opinion.
Key Content and Findings
CDH is a congenital anomaly of the cardiopulmonary and diaphragmatic systems that represents a spectrum of disease. High-risk or complex patients are defined by prenatal/postnatal risk stratification, receipt of ECLS, and/or having concomitant anomalies, representing the severe end of that spectrum. Overall survival of high-risk CDH is about 50% and comprises the vast majority of mortality, mandating special emphasis. The development of risk-stratification processes, best practices or guidelines of management, and novel therapies is critical to optimize the care of these infants.
Conclusions
CDH patients with high-risk disease remain a challenging subset of CDH patients. Increasing opportunities for survival are being realized with novel, investigational approaches.
Introduction
Congenital diaphragmatic hernia (CDH) is an anomaly where a global embryopathy and concomitant mechanical compression, both associated with a diaphragmatic muscle defect, result in a pathophysiologic trifecta of pulmonary hypertension, pulmonary hypoplasia, and cardiac dysfunction (1,2). These clinical challenges require intense critical care management the instant they reach postnatal life. The management of infants with CDH has improved because of specific advances including pressure-limited ventilation, extracorporeal life support (ECLS), and neonatal critical care expertise. Moreover, breakthroughs in the basic understanding of the pathophysiologic trifecta, along with innovative interventional approaches, are exposing novel therapeutic opportunities. While the overall survival rate among patients with CDH remains approximately 65–80%, depending upon numerous factors, select groups have suggested that further improvements in survival may be achievable through specific, evidence-based, protocol-driven adoption of specific management principles (3-5). Despite this contention, CDH represents a spectrum of disease, and the most challenging, high-risk patients still have a survival around 50% (6,7). This fact underscores the importance of this review’s focus on addressing the needs of this patient population.
High-risk CDH can be defined in a number of ways: through the Congenital Diaphragmatic Hernia Study Group (CDHSG) diaphragm defect grading system (typically C and D defects, Figure 1) (8), multiple published risk stratification equations (9), lung-to-head ratio (LHR; typically <1.0), precent predictive lung volumes (PPLV), observed-to-expected (O/E) LHR (typically <25%), O/E total fetal lung volumes (TFLV; typically <25%), and/or liver herniation [typically >20% on magnetic resonance imaging (MRI)] (10-12). It is known that “high risk” patients often require interventions such as ECLS, and simply receiving ECLS renders a patient high risk (13). Patients who have a concomitant anomaly (or non-isolated CDH), most commonly cardiac or chromosomal, are also considered to be in a unique risk category, depending on the exact anomaly. Despite these clear risk stratifications, there is unfortunately little evidence to guide complex decision-making in this population, which can be highly controversial given the myriad of factors involved. Alongside multidisciplinary commitment to improving the care of this subset of CDH patients, conflicting data exist (14), and innumerable elements, both known and unknown, influence these outcomes (15,16). By establishing the pre- and postnatal estimated risk associated with infants with CDH, families and medical professionals can educate the family and develop a strategy according to risk and best practices. This includes postnatal interventions like ECLS, but more recently, the potential use for antenatal interventions, like fetoscopic endoluminal tracheal occlusion (FETO), and the use of novel pharmacologic interventions. Our objective is to identify emerging insights, evidence, clinical management approaches, and investigational strategies to consider when facing a neonate with high-risk CDH. We present this article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-240/rc).
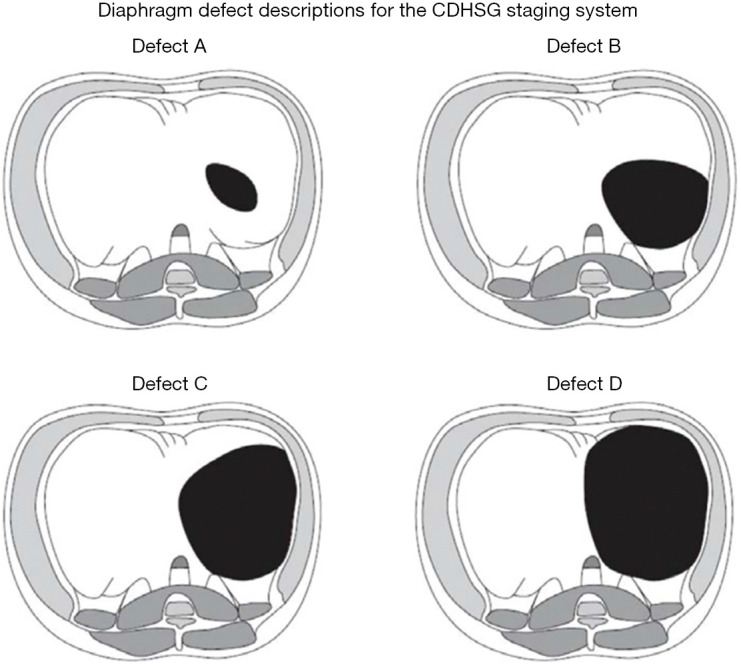
CDHSG diaphragm defect staging system. Defect A is the smallest defect that consists of a mostly intact hemidiaphragm and is completely intramuscular and does not extend to the chest wall. Defect B is a defect that involves 50–75% of the hemidiaphragm and less than half of chest wall is affected. Defect C is a defect where approximately 25% of the hemidiaphragm is present but more than half of the chest wall is involved in the defect. Defect D is the largest defect and has minimal or no diaphragm present and affects most of the chest wall. This condition is often referred to as agenesis. Permissions granted (Lally KP, Lasky RE, Lally PA, et al. Standardized reporting for congenital diaphragmatic hernia--an international consensus. J Pediatr Surg 2013;48:2408-15.). CDHSG, the Congenital Diaphragmatic Hernia Study Group.
Methods
PubMed was searched in the winter of 2022 in order to identify relevant evidence (Table 1). Search terms included “congenital diaphragmatic hernia (CDH)”, “extracorporeal life support (ECLS)”, “pulmonary hypertension”, “dual-hit hypothesis”, “risk reduction”, “cardiac/chromosomal anomalies”, and “novel therapies”. We included trials, multicenter studies (prospective and retrospective), single-center reports, and review articles/expert opinion.
Table 1
| Items | Specification |
|---|---|
| Date of search | 12/2022–1/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “Congenital diaphragmatic hernia (CDH)”, “Extracorporeal Life Support (ECLS)”, “Pulmonary hypertension”, “Dual-Hit hypothesis”, “Risk reduction”, “Cardiac/Chromosomal anomalies”, “Novel therapies” |
| Timeframe | 1988–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: free full text, clinical trial, meta-analysis, randomized controlled trial, review, systemic review, and English language |
| Exclusion criteria: none | |
| Selection process | Identification of articles by the first author, with independent confirmation by the senior author |
Discussion
Novel and emerging understanding of CDH
CDH is a congenital, fetal, neonatal, pediatric, and adolescent anomaly that results from an early embryologic aberration that alters the development of the diaphragm and ultimately the development of the pulmonary vasculature and pulmonary parenchyma. Initially, pulmonary hypoplasia was thought to be solely from compression of the pulmonary tissue, but innovative studies, have shown that mechanical compression, along with early, direct cardiopulmonary embryologic insults, lead to the spectrum of cardiopulmonary pathology, which has been coined the “dual-hit” hypothesis (2). The “dual-hit” hypothesis suggests that initial pulmonary development is hindered by chemical changes in the local environment and then separately, by a defect in diaphragm development, further stifling pulmonary development through mechanical compressive forces (Figure 2) (2). This “dual-hit” hypothesis has been shown with the well-known and established nitrofen model, however there still remains a great deal of exploration in order to fully understand the mechanisms behind CDH pathophysiology. While there are minimal consequences for these infants in utero, after birth, these pathologic hearts and lungs are not able to meet the demands of postnatal life/circulation. Pulmonary hypoplasia results in lower functional lung volumes and increased oxygen and ventilatory requirements, ultimately exacerbating pulmonary vascular resistance and causing progressive respiratory failure (17). Among neonates with CDH, pulmonary hypoplasia is directly associated with risk and survival. For high-risk patients, their measured lung volumes were found to be 39% of the controls, and those who survived had much higher volumes compared to those who did not (18). With the decrease in lung volume, including profound attenuation and responsiveness of the pulmonary vasculature, comes a compromised ability to meet oxygenation and ventilatory requirements, resulting in the inability to wean infants off invasive respiratory support, increased need for tracheostomy, increased incidence of long term pulmonary challenges, and increased mortality (18). Long term, these infants are at high risk to grow into children who struggle with chronic pulmonary infections and asthma (18).
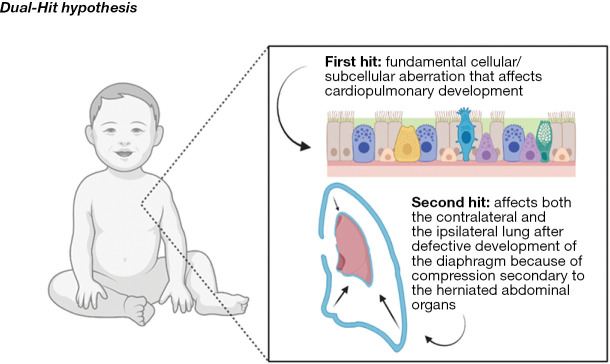
The dual-hit hypothesis. The dual-hit hypothesis is another theory that attributes lung hypoplasia as the primary cause of CDH development with herniation of the abdominal contents as a secondary hit, exacerbating the growth impairment of an already underdeveloped lung. Images created with BioRender. CDH, congenital diaphragmatic hernia.
Pulmonary hypertension is the primary driver of pathophysiology in CDH and is defined as sustained, supernormal pulmonary arterial pressures. It results in compounding disfunction within the pulmonary circulation, suboptimal gas exchange, and exacerbates cardiac dysfunction (19). In the setting of CDH, pulmonary hypertension is caused by aberrant prenatal pulmonary vasculature development, significant for hypertrophic smooth muscle cells and vessel thickening with decreased angiogenesis (19). This causes elevated right-heart pressures, circulatory shunting, poor ventilation and decreased oxygenation in the postnatal setting (19). Pulmonary hypertension is a cornerstone in the pathophysiology of CDHs and is the most important driver of disease and outcome, particularly among high-risk patients. It has been shown that 70% of infants born with CDH will have pulmonary hypertension, and of these patients, 38% of them ultimately receive ECLS support, signifying that pulmonary hypertension is a high-risk feature and that it plays a critical role in the outcome and management of CDH (19). It is determined by postnatal echocardiography through evaluation of the velocity of the tricuspid regurgitation jet, which determines the right ventricular systolic pressure that directly reflects the pulmonary arterial pressure, and then comparing that to the systemic systolic blood pressure (SBP) (19). The ratio of the right ventricular systolic pressure to the SBP is an objective measurement used to stratify pulmonary hypertension. The tricuspid jet or patent ductus arteriosus (PDA) shunt can be identified, and the modified Bernoulli equation used to calculate a pressure (19). This is compared to the systemic, SBP, generating a ratio. A detectable jet but low ratio <0.5 is mild, 0.5–0.67 is considered moderate, a ratio >0.67 is moderate-severe, and >1.0 (supersystemic) is severe, this ratio can be helpful in identifying high-risk patients (19). While, these values are in constant flux, one study showed that 60% of patients had their pressures normalize in the first few days to weeks of life, but those that did not normalize at the end of 3 weeks had a 100% mortality. When measured at one month old, it was shown that those with a right ventricular systolic pressure (RVSP)/SBP less than 0.5 had a 98.6% survival, however those with a ratio greater than 0.67 had only a 43.9% survival (19). In these high-risk neonates, pulmonary hypertension is nearly universally present. With further understanding of the pathophysiology, new management strategies are based on reducing pre-capillary pulmonary vascular resistance and optimizing lung recruitment while avoiding overdistention and de-recruitment (20). Additionally, the use of pulmonary vasodilator therapies like Sildenafil, Bosentan, Epoprostenol, prostaglandin E1 (PGE1), and milrinone have aided in the reduction of the right ventricular (RV) afterload while maintaining systemic blood flow (20).
While it is well established that pulmonary hypoplasia and pulmonary hypertension are central features of CDH, more recent evidence identified the importance of cardiac dysfunction as an equally important marker for disease severity, along with its association with adverse outcomes in patients with CDH (21). Recent studies have suggested that cardiac dysfunction is associated with the need for ECLS, increased mortality risk, and overall worse outcomes for CDH infants (22). A study by Patel et al. showed that those with early ventricular dysfunction had an increased mortality and need for ECLS, and that their overall survival to discharge was significantly affected by cardiac function (22). They focused on cardiac function, identified via early, postnatal echocardiography, with the goal of characterizing the role of right and left ventricular function in CDH patients (22). They found that left ventricular (LV) dysfunction is associated with more severe consequences, as compared to RV dysfunction, and those with biventricular dysfunction had the highest risk of mortality and need for ECLS (22). They also found that ventricular dysfunction was highest in those with large diaphragmatic defects (types C and D), however, early dysfunction was also present in those with small defects (types A and B) potentially contributing to their unexplained or unexpected adverse outcomes. This concept of early ventricular dysfunction in “low risk” CDH infants, or those with type A and B defects, was further confirmed by Dao et al. (21). While echocardiography remains the mainstay diagnostic tool for clinical cardiac dysfunction at this time, the molecular underpinnings of CDH-associated ventricular dysfunction are slowly being identified. Some studies have suggested that pro brain natriuretic peptide (pro-BNP) can act as a marker for “high-risk” defects, ventricular dysfunction, and mortality, while others have started to investigate the use of myocardial energy metabolism pathways (23,24). Currently, the nitrofen rat model of CDH has shown an association between cardiac hypoplasia and reduced expression on insulin-like growth factor-1, epidermal growth factor, basic fibroblast growth factor, and platelet derived growth factor (25). Other studies have shown abnormal cardiomyocyte structure with reduced expression of mitochondrial and fatty acid biogenesis genes (25). Additionally, cardiac dysfunction continues to be propagated in the postnatal circulation with modifiable characteristics like hypoxia and acidosis. The LV dysfunction contributes to adverse clinical outcomes through reduced LV output and systemic blood flow resulting in impaired tissue oxygenation and more worsening hypoxia and acidosis (25). These studies provide early evidence that ventricular function is a key determinant of disease severity and highlight that it is emerging as an important predictor of outcome but leave the field open to further investigation.
Pulmonary hypoplasia, pulmonary hypertension, and cardiac dysfunction intersect in the high-risk neonate with CDH. It is how these three pillars of CDH interact, along with concomitant cardiac and/or chromosomal anomalies, in the setting of gestational age and birthweight, that creates various unique phenotypes, or presentations, of CDH. Focusing on cardiopulmonary pathophysiology, the first phenotype described is a left to right shunting at both the ductal and the atrial level with no or mild pulmonary hypertension and no cardiac dysfunction, clearly a low-risk situation (20). The next phenotype is seen with right to left atrial or PDA shunting (20). This phenotype presents with pulmonary hypertension but no cardiac dysfunction (20). The third and final phenotype presents with pulmonary hypertension and primary LV dysfunction and is a left to right atrial shunting with right to left shunting via the PDA (20). Understanding how they interplay is critical for identification of novel therapeutic strategies and for successful management of these patients. For example, in phenotype 1, where there is preserved ventricular function, hypoxia may be associated with parenchymal disease and respond to new management strategies in ventilator management (20). In phenotype 2, pre-ductal hypoxemia worsens pulmonary vasoconstriction and vascular resistance (PVR) so management strategies revolve around reducing pre-capillary PVR by optimizing sedation, vasodilator therapies, and lung recruitment (20). Finally, phenotype three is driven by post capillary pulmonary venous hypertension and primary LV dysfunction, and is best managed by supporting LV function with inotropic therapies (Table 2) (20).
Table 2
| Pathophysiology | 1st phenotype | 2nd phenotype | 3rd phenotype |
|---|---|---|---|
| PDA shunt direction | LàR | RàL | RàL |
| Atrial shunt direction | LàR | RàL | LàR |
| Pulmonary hypertension | No or mild | Present | Present |
| Cardiac dysfunction | No | No | Present (primary LV dysfunction) |
| Interventions and therapies | Changes in ventilator settings | Reducing pre-capillary PVR by optimizing sedation, vasodilation, and lung recruitment | Supporting LV function with inotropic therapies |
PDA, patent ductus arteriosus; L, left; R, right; LV, left ventricular; PVR, pulmonary vasoconstriction and vascular resistance.
Early management
When an infant with CDH is delivered there are a few critical steps that must be taken to initially stabilize the child and confirm the disease. Then in these high-risk, high acuity patients with high morbidity and mortality, following an establishing a strict protocol at delivery ensures the best outcome possible. This protocol is designed for early recognition, a risk-stratified approach, lung protective ventilation, and early consideration of ECLS (if the patient is deemed an appropriate candidate) (4). Studies have shown that scheduling a planned delivery after 39 weeks’ gestation at a high-volume tertiary center is best, either via cesarean section or vaginal delivery, as there is no difference in outcome by delivery strategy (5). Once the infant is born, initial stabilization includes measurements of heart rate, pre and post-ductal saturations (5). In high risk infants, it is recommended that these infants are intubated immediately after birth, however, exceptions may rarely be made for infants who have good predicted lung development and excellent postnatal respiratory effort (5). Ventilation goals should be to avoid high airway pressures and establish adequate perfusion and oxygenation, with low peak pressures (<25 cmH2O), avoiding volutrauma and barotrauma (5). An orogastric tube to intermittent suction should be placed to prevent bowel distention. An arterial line should be placed for accurate blood pressure monitoring to ensure adequate perfusion of the peripheral tissues. In the setting of hypotension with poor tissue perfusion, a fluid bolus of 10–20 cc/kg of normal saline (NS) can be given but no more than twice (mitigating fluid overload), and if tissue perfusion and blood pressures do not improve, inotropic and/or vasopressors can be added accordingly (5). Studies have shown there is no role for surfactant therapy. After the infant is stabilized, they should be moved quickly to the neonatal intensive care unit (NICU). In the NICU, CDH infants are allowed permissive hypercapnia (arterial CO2 levels between 50–70 mmHg) with preductal saturations between 80–95% and post ductal saturations above ~70%. Pressure-controlled ventilation should aim for peak inspiratory pressure (PIP) <25 cmH2O and an initial positive end-expiratory pressure (PEEP) of 3–5 cmH2O with a ventilator rate of 40–60/breaths per minute. Once stabilized, the FiO2 can be gradually reduced to maintain preductal saturations between 85–95% (5). Infants should be sedated accordingly but neuromuscular blocking agents should be avoided if possible. Finally, the use of ECLS should be considered early (without isolated physiologic status limits) and conversations with families about realistic outcomes should be discussed so that palliative/comfort care are options for families and patients in the setting of severe, concomitant genetic abnormalities, profound prematurity, low birthweight, and/or complex cardiac disease unable to be stabilized (16,26).
In addition to these established guidelines, there are new emerging practices and therapies that show promise for improved outcomes, particularly for patients with high-risk CDH. The first is the timing for umbilical cord clamping. The current practice of umbilical cord clamping before lung aeration and perinatal stabilization may contribute to worsening pulmonary hypertension. A study using the ovine model for diaphragmatic hernia shows that cord clamping after lung aeration, known as physiologic-base cord clamping, avoids initial high pressures in the lung vasculature while maintaining adequate blood flow (27). The use of PGE1 has also altered the management of pulmonary hypertension in CDH infants. PGE1 is typically used to maintain ductal patency in patients with congenital cardiac disease, this facilitates pulmonary and systemic blood flow. This can be directly applied to infants with CDH who also experience supra-systemic right ventricular pressure (28). PGE1 maintains ductal patency as a “pressure relief valve” to reduce the effect of afterload on the right heart (Figure 3) (28). Finally, for the most severe cases, new more aggressive approaches are being tried. One example is seen in those with lethal pulmonary hypoplasia, the SPHERE (severe pulmonary hypoplasia and evaluation for resuscitative efforts) protocol was developed (26). These patients were started on a trial ventilation and if they met physiologic criteria were offered ECLS therapy, and those who did not, were directed to comfort care (26). Notably, there was no difference in mortality, showing that “predetermined” physiologic limits predicting mortality were inaccurate. This is consistent with previous evidence that an aggressive approach, giving every isolated CDH an opportunity for ECLS support and attempt at diaphragm repair, optimized survival (16). This evidence changed prenatal counseling and has warranted additional studies to be considered by the larger CDH community (26). These changes in early resuscitation, leveraging an aggressive approach and an opportunity for survival, offer many new avenues for change and open the door for further exploration. As we start to make advances in genomics and functional studies, the underlying causes and pathophysiologic challenges in CDH are becoming more apparent. By understanding the molecular mechanisms and pathways that are most frequently disrupted, medical teams are able to tailor each infants’ care based on their needs, using a “personalized medicine” approach, outcomes will continue to improve. Despite advances, discovering each mutation and the phenotypic variability associated with the myriad of variables remains a challenge and one that requires further exploration (29).
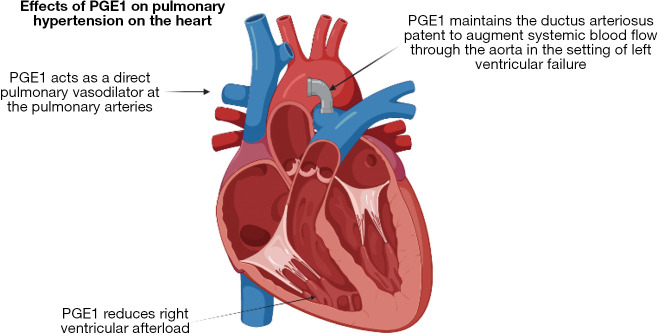
Effects of PGE1 on pulmonary hypertension on the heart. PGE1 is administered to maintain ductal patency in congenital heart disease allowing improved pulmonary and systemic blood flow. This approach can also be beneficial in infants with CDH who experience supra-systemic right ventricular pressures. A patent PDA can reduce the afterload on the right heart by serving as a “pressure relief valve”. Images created with BioRender. PGE1, prostaglandin E1; CDH, congenital diaphragmatic hernia; PDA, patent ductus arteriosus.
Defining and identifying current survival in high-risk CDH
Currently, the overall mortality in CDH remains between 65% and 80%, largely depending on the institution, region, timing of identification of defect (early prenatal, late prenatal, liveborn, etc.), and length/completeness of follow-up (1). Classification of high risk CDH is now being based on prenatally identified factors, including ultrasound and MRI derived measurements, and/or postnatal factors including birth weight, Apgar score, severity of pulmonary hypertension, arterial blood gas values, oxygen saturation, and/or vital signs. The presence of major cardiac or chromosomal anomalies, irrespective of timing of identification, also may alter risk in CDH (30). High risk infants are those that are more likely to end up on ECLS therapies or those with a greater than 50% chance of mortality (30). The survival of these infant’s hinges on early detection and appropriate risk stratification for appropriate deployment of critical therapeutic interventions, family counseling, resource allocation, and establishment of care targets, including appropriate transitions in care (9,30). In many cases, prenatal imaging detects and appropriately risk-stratifies the patient’s prior to birth, but risk is not static in these patients and variables like gestational age of birth, the transition from fetal circulation, and iatrogenic factors after birth all affect the patient’s outcomes and alter their evolving “risk profile” (9).
Prenatally, image derived measurements best predict high risk CDH. The most frequently obtained and investigated imaging findings include LHR, O/E LHR, O/E TFLV, absolute fetal lung volume (FLV), PPLV, percentage of liver herniation, and stomach herniation. O/E-LHR is the most commonly studied metric (6) and is better than other parameters in predicting survival of fetuses with CDH, with a major benefit in its ability to control for gestational age (31). An O/E LHR value that is less than 25% is specific for neonatal mortality, while an O/E-TFLV that was less than 35% had a significantly lower survival rate compared to those with greater than 35%. Those with an LHR >1.4 had the highest survival rates while those with a LHR < one had the lowest, and those with a lower degree of liver herniation, below 20% had a significantly higher survival compared to those with a higher degree (>20%) of herniation (10,32). Liver herniation implies a large defect that is associated with a greater degree of lung hypoplasia and need for patch repair postnatally (9). ECLS is used more frequently in those with an O/E-TFLV <35% and, similarly, in those with a more than 20% liver herniation (30). PPLV is a newer measure that is based off lung volumes and fetal size that was found to be a much more intuitive measurement with studies showing that values less than 15 were associated lower survival rates, longer lengths of stay, and prolonged ECLS courses (33).
Equally as important to the prenatal risk predictors are the postnatal predicters. Risk is not static, and many elements of delivery, birth weight, and neonatal care in the first 24 hours can alter the course of infants with CDH. Apgar score and birth weight are useful in generating survival probability (34). A study completed by Terui et al. looked at this postnatal 24-hour window of care to establish a risk stratification system and showed that Apgar scores at one minute was capable of predicting mortality (35). Additionally, using the arterial or capillary blood gas, gives information about gas exchange and ventilation and gives a dynamic reflection of both the physiology and the respiratory care provided (9). Poor gas exchange with the failure to improve markers of oxygenation and ventilation is a poor prognostic sign seen in high risk CDH infants and has been shown to accurately predict outcomes and serve as a marker for transition in care (9). The inability to clear CO2 is a measure of overall cardiopulmonary function in the CDH patient and can be used to predict the use of ECLS (9).
Use of ECLS
ECLS is an artificial support of the lung and/or heart function, which allows recovery from a reversible respiratory problem and is used in ~30% of infants with CDH (17). Those that receive ECLS support are classified as “high risk” infants and parameters like: karyotype abnormalities, syndromic features, the presence of CDH, left and right ventricular function/proportions, clinical status and trajectory, liver herniation, O/E LHR, and PPLV all go into consideration when making a decision regarding ECLS candidacy (17).
Jancelewicz et al. looked at the survival benefit of neonates with CDH who were supported with ECLS comparted to those who weren’t (36). This was a retrospective study that used in-hospital mortality as the primary outcome (36). Of the ECLS-eligible CDH patients, only 29% of them were placed on ECLS (36). They then divided the patients into low, intermediate and high risk with “high risk” patients being defined as those with the lowest achievable first-day arterial partial pressure of CO2 ≥60 mmHg (36). They found that the use of ECLS is associated with mortality for the low and intermediate groups and associated with a significant survival advantage in the high risk group, but that the degree of this advantage was highly dependent on the centers volume of CDH and experience with ECLS (36). In additional to the diverse nature in the management and experience of ECLS, many vary decision for repair timing while on ECLS. After stabilization, patients who do not receive ECLS typically undergo CDH repair surgery after 24 hours of life but within the first seven days of life. The optimal timing and surgical technique used for repair will vary depending on the severity of CDH and the presence of any additional anomalies, which exceeds the scope of this review. Dao et al. examined this in a 2-aim, retrospective cohort study. Aim one was to compare and contrast repair on vs. off ECLS while aim two was to compare and contrast early vs. late repair while on ECLS (37). Results for aim one showed that there is a lower mortality rate to repairing while on ECLS vs. off; and aim two showed that early on repair had lower mortality rates compared to the late on group (37). These studies show that high-quality ECLS benefits high-risk CDH patients and that an early on ECLS diaphragmatic repair strategy confers the best chance for survival, particularly among high-risk patients.
It is noted that roughly 50% of the infants placed on ECLS for CDH do not survive and that those who do survive are at increased risk for long term morbidity like neurodevelopmental abnormalities and chronic pulmonary hypertension (13,17). The patients who receive ECLS interventions are of the most high risk in the CDH population. Prenatally, imaging like O/E LHR, liver herniation, and O/E TFLV are used. Values for an O/E LHR less than 25%, if more than 20% of the liver is found in the chest, and O/E TFLV less than 25% all predict the severity of lung hypoplasia need for ECLS (13). These values have been chosen as they are associated with postnatal survival, however, there is heterogeneity with these measurements and each ECLS center has the ability to make their own cut-offs for which infants they deem will benefit from ECLS support (17). For example, those with structural and/or genetic anomalies impact the decision to initial ECLS as well as the overall outcome of the fetus, but there are no clear regulations or guidelines in this infant population (17).
CDH plus congenital cardiac disease, chromosomal anomalies and other syndromes
Infants with CDH can be born with other syndromic presentation, abnormal genetic testing, and other significant anomalies like structural cardiac disease, all influence the decision to procedure with ECLS and alter their overall survival risk but there is limited data on this (13).
CDH is associated with a variety of congenital heart defects, but a study by Fraser et al. suggested CDH had the highest associated with tetralogy of Fallot, followed by coarctation, and finally, complete atrioventricular septal defects (21). Rarely, some patients are also found to have isolated aortic arch anomalies such as coarctation of aorta, hypoplastic aortic arch, interrupted aortic arch, and aortic aneurysmal disease. A study by Gupta et al. examined patient with these isolated anomalies and found that while there is an increased mortality, it is likely due to the severe thoracic anatomic derangement, rather than a unique pathophysiology of the combined disease processes (38). These aortic anomalies rarely are intervened on and act as an indicator for high risk CDH. The significance of this association implies that those with the concomitant diagnosis of CDH and congenital heart disease (CHD) have an increase morbidity, mortality, and the need for ECLS. Those with CDH and CHD have a lower survival rate compared to those with just CDH or just an isolated cardiac defect (39). There is little information on this topic and outcomes of newborns with CDH and major heart defects has not improved over the last decade (40). Making this area a needed target for innovation, intervention, and allows a great opportunity to develop collaborative strategies involving pediatric and congenital cardiac surgeons.
Cardiac disease complicates the management of CDH; however, it is not the only thing that can complicate patient care. CDH has been known to be associated with a variety of other chromosomal anomalies and syndromes. One documented association is that of Cornelia de Lange syndrome (CdLS) (41). CdLS is a genetic disorder with a widely varied phenotype but predominately consists of thick or long eyebrows, a small nose, small stature, developmental delay, long or smooth philtrum, thin upper lip, and a downturned mouth. Infants that have both CdLS and CDH have a poor prognosis with the CDH being the cause of death in 5–20% of cases; however, if they undergo repair they can survive to discharge, so CdLS is not necessarily viewed as a contraindication to repair but should be something that prompts early discussion and family counseling so they can make the best informed decision in their child’s care. There are many other chromosomal abnormalities associated with CDH including Pallister-Killian syndrome, Fryns syndrome, Mattew-Wood syndrome, and Donnai-Barrow syndrome, however, there is little information that dictates management in these complicated infants. Perhaps a reasonable approach is a clear prenatal discussion, including palliative care, to inform the family and gather value-specific information (42). If maximal intervention is desired, supporting the patients postnatally, without proceeding to ECLS, in an attempt to stabilize and, ultimately, repair the diaphragmatic defect may be a balanced approach.
Finally, not all anomalies that affect the management of CDH are structural, prematurity and low birth weight are two major anomalies that influence the management of CDH. A study conducted by Gupta et al. compared infants with CDH who were born with low birthweight infants to those born with normal birthweights (43). This study found that infants with low birth weight had more congenital anomalies, larger diaphragmatic defects, a higher mortality, and a decreased utilization of ECLS (43). This study found that just a 1 kg increase in birthweight increased survival by 34% (43).
Novel and emerging therapies
By knowing pre and postnatally, the estimated risk associated with infants with CDH, families and the medical team can start to plan accordingly. This includes postnatal interventions like ECLS, but more recently, the potential use for antenatal interventions and the use of novel pharmacologic interventions. While postnatal interventions have made great improvements in those with less severe disease, the overall survival for those with “severe” CDH still remains stagnate with an estimate 50% survival and acts as the driver to find alternative therapies to conventional therapy (44). Antenatal interventions like FETO have evolved to become a promising adjunct to the treatment of CDH (44). Fetal lung development occurs in overlapping stages and any impedance to this development will hinder the proper pulmonary vascular development resulting in the large ventilation/perfusion mismatch seen with CDH infants (44). The FETO procedure occludes the trachea and prevents the regression of pulmonary fluid forcing pulmonary tissue and pulmonary vasculature into a hyperplastic state, ultimately causing growth and expansion to the alveoli and vasculature (44). A randomized trial completed by Deprest et al. shows that by performing FETO in fetuses between 27–29 weeks gestation there is a significantly higher survival to discharge (40%) compared to those who received expectant care (15%) (45). However, there is a high risk of preterm labor, prelabor rupture of membranes, and preterm birth with this intervention making it a risky intervention that still requires additional investigations prior to becoming a mainstay in therapy (45). Another avenue of therapy that is still developing is that of new pharmacologic interventions that are targeted to key components of CDH pathophysiology (20). The ongoing investigations to better understand the mechanism of pulmonary hypertension in CDH and its close interplay with cardiac dysfunction will allow for the development of novel physiology-based treatments like target mitochondrial treatments, prostaglandins, new classes of anti-pulmonary hypertension drugs, and improved oxygen delivery (20). For example, early prostacyclin (PG12) therapy may decrease the need and/or duration of ECLS and the administration of amniotic fluid stem cell extracellular vesicles (AFSC-EVs) may be a way to rescue pulmonary hypoplasia (46) (Figure 4). One experimental study by Khalaj et al. demonstrates how extracellular vesicles (EVs), are isolated from a c-kit+ amniotic fluid stem cells (AFSCs)-conditioned medium and then administered to explants of human fetal lung tissue and/or in-vivo rat dams (47). The results show these AFSC-EVs rescue pulmonary tissue hypoplasia by restoring autophagy through microRNA cargo transfers (47). A follow up study by Khalaj et al. shows that these AFSC-EVs rescue the expression of alveolar type one and two cell markers at both the canalicular and saccular stages as well as restored markers of lipofibroblasts and PDGFRA+ cells to that of the levels seen in the control group (48). This follow up study also identified the AFSC-EV RNA is carried throughout the mRNA-miRNA network that is responsible for regulating lung development suggesting this EV method could provide the base for future therapies for restoring fetal lung growth and maturation in infants with pulmonary hypoplasia secondary to CDH (48). Another route these EVs have been found another potential use is in the management of pulmonary hypertension. The study by Monroe et al. examined how the extracellular matrix (ECM) in the pulmonary arteries was changed with the use of mesenchymal stem-cell extracellular vesicles (MSC-EVs) (49). In a similar method to Khalaj’s group, MSC-EVs were administered and found that these MSC-EVs have the power to attenuate pathological ECM remodeling by inhibiting lung inflammation and vascular remodeling (49,50). Mesenchymal stem cells have also been able to be used in the transamniotic stem cell therapy (TRASCET) method and have shown to improve pulmonary vascular resistance and impact fetal pulmonary hemodynamics. These studies are just a start to how targeted therapy may improve the outcomes in high-risk CDH and offer a window for translationally relevant therapies to impact clinical interventions (50).
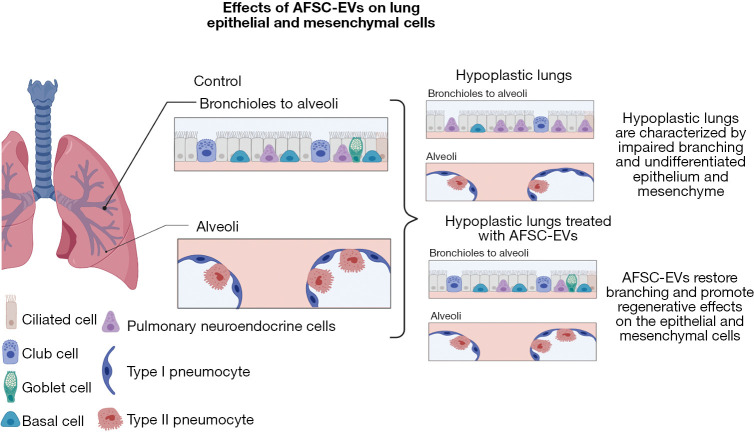
Effects of AFSC-EVs on lung epithelial and mesenchymal cells. The presence of AFSC-EVs RNA in the mRNA regulatory network involved in lung development indicated that this EV-based therapy can promote fetal lung growth and restore the regeneration of the epithelial and mesenchymal cells. Images created with BioRender. AFSC-EVs, amniotic fluid stem cell extracellular vesicles.
Conclusions
In conclusion, neonates with high-risk CDH, including the severe patients on the isolated CDH spectrum, along with those who have a CDH plus an associated anomaly, require special consideration and benefit from nuanced, multidisciplinary management. Detailed and thorough prenatal imaging and risk stratification, followed by prenatal counselling, help families make informed decisions about ongoing management. While many of these children can survive both high-risk isolated CDH, as well as CDH plus situations, special considerations are clearly warranted, morbidity is high, clinical course more difficult to predict, and evolving goals of care important to consider. Additional evidence is necessary to advance our understanding of this uniquely complex cohort of CDH patients, so that we may develop new novel therapies to improve outcomes of CDH and provide evidence-based guidance for these unique patients and their families.
Acknowledgments
We would like to acknowledge the Fore Hadley Foundation and the Ladybug Foundation for their contributions.
Funding: None.
Notes
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Antonio F. Corno) for the series “The Impact of the Progresses of Knowledge and Technologies in Pediatrics” published in Translational Pediatrics. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-240/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-240/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-240/coif). The series “The Impact of the Progresses of Knowledge and Technologies in Pediatrics” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
 h after birth.
J Perinatol
2017;37:805-8. 10.1038/jp.2017.11 [Abstract] [CrossRef] [Google Scholar]
h after birth.
J Perinatol
2017;37:805-8. 10.1038/jp.2017.11 [Abstract] [CrossRef] [Google Scholar]Articles from Translational Pediatrics are provided here courtesy of AME Publications
Full text links
Read article at publisher's site: https://doi.org/10.21037/tp-23-240
Read article for free, from open access legal sources, via Unpaywall:
https://tp.amegroups.org/article/viewFile/115367/pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.21037/tp-23-240
Article citations
Development of a prediction nomogram for 1-month mortality in neonates with congenital diaphragmatic hernia.
BMC Surg, 24(1):198, 27 Jun 2024
Cited by: 0 articles | PMID: 38937726
Hemodynamic management of congenital diaphragmatic hernia: the role of targeted neonatal echocardiography.
World J Pediatr Surg, 7(2):e000790, 08 May 2024
Cited by: 0 articles | PMID: 38737963 | PMCID: PMC11086387
Review Free full text in Europe PMC
Use of Barbed Sutures for Congenital Diaphragmatic Hernia Repair.
Children (Basel), 11(1):35, 28 Dec 2023
Cited by: 0 articles | PMID: 38255349 | PMCID: PMC10814386
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Extracorporeal life support in congenital diaphragmatic hernia.
Semin Pediatr Surg, 32(4):151328, 01 Aug 2023
Cited by: 2 articles | PMID: 37939639
Congenital diaphragmatic hernia-associated pulmonary hypertension.
Semin Pediatr Surg, 33(4):151437, 02 Jul 2024
Cited by: 0 articles | PMID: 39018718
Review
Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization.
J Pediatr Surg, 42(9):1526-1532, 01 Sep 2007
Cited by: 51 articles | PMID: 17848243
Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia.
Pediatrics, 116(3):e356-63, 01 Sep 2005
Cited by: 217 articles | PMID: 16140678

 1
,
2
1
,
2



