Abstract
Free full text

Antibiotic Use Prior to COVID-19 Vaccine Is Associated with Higher Risk of COVID-19 and Adverse Outcomes: A Propensity-Scored Matched Territory-Wide Cohort
Abstract
Background: Antibiotics may increase the risk of COVID-19 among non-vaccinated subjects via probable gut dysbiosis. We aimed to investigate whether antibiotics also affect the clinical outcomes of COVID-19 vaccine recipients. Methods: This was a territory-wide cohort study of 3,821,302 COVID-19 vaccine recipients (aged ≥ 18 years) with ≥2 doses of either BNT162b2 or CoronaVac. Exclusion criteria included prior COVID-19, prior gastrointestinal surgery, and immunocompromised status. The primary outcome was COVID-19 infection and secondary outcomes included COVID-19-related hospitalization and severe infection (composite of intensive care unit admission, ventilatory support, and/or death). Exposure was pre-vaccination antibiotic use (within 180 days of first vaccine dose). Covariates included age, sex, Charlson Comorbidity Index, and concomitant medication use. Subjects were followed from the index date (first dose vaccination) until outcome occurrence, death, an additional dose of vaccination, or 15 November 2022. Propensity score (PS) matching and a Poisson regression model were used to estimate the adjusted incidence rate ratio (aIRR) of outcomes with antibiotic use. Results: Among 342,338 PS matched three-dose vaccine recipients (mean age: 57.4 years; male: 45.1%) with a median follow-up of 13.6 months (IQR: 9.2–16.3), antibiotics were associated with a higher risk of COVID-19 infection (aIRR: 1.16;95% CI: 1.14–1.19), hospitalization (aIRR: 1.75;95% CI: 1.65–1.86), and severe infection (aIRR: 1.60; 95% CI: 1.21–2.11). Notably, antibiotic use was associated with a higher risk of severe infection and death among CoronaVac recipients (aIRR: 1.62 95% CI: 1.18–2.22 and aIRR: 2.70, 95% CI: 1.54–4.73 for the two secondary outcomes, respectively), but not BNT162b2 recipients. Conclusions: Pre-vaccination use of antibiotics was associated with a higher risk of COVID-19 infection, hospitalization, and severe disease outcomes.
1. Introduction
According to the World Health Organization, COVID-19 has led to nearly 7 million deaths globally as of May 2023. Vaccination is one of the most important measures in preventing severe symptoms and death [1]. There is increasing evidence of the role of the gut microbiome on the impact of COVID-19 disease as well as vaccine immunogenicity [2,3]. Gut microbiota perturbation induced by antibiotics could impair antibody production and affinity among those with low pre-existing antibody titers against influenza virus [4]. Similarly, the early vaccine immunogenicity of BNT162b2 may be impaired by antibiotic usage [5]. The mechanisms underlying these observations include the expression of viral entry receptor angiotensin-converting enzyme 2 (ACE2) [6], the effects of microbiota-derived short chain fatty acids [7], the modulation of B and T cell immune responses [3], and the mediation of the “gut-lung axis”. Gut microbiota alteration by COVID-19 has been associated with inflammatory bowel disease and COVID-19 severity. Intuitively, it has been postulated that gut dysbiosis induced by antibiotics leads to a higher viral load in the gastrointestinal (GI) tract, resulting in a cytokine storm [8] and the impairment of leukocyte function [9]—which is similar to the effects of proton-pump inhibitors (PPIs) in increasing the risk of COVID-19 and severe clinical outcomes in observational studies [10,11].
Worldwide antibiotic use increased by 65% between 2000 and 2015 [12]. Antibiotic misuse was exemplified in a recent study evaluating antibiotic use among 11,047 COVID-19 cases in Hong Kong, carried out between January 2018 to March 2021, that revealed that nearly 30% of cases were given antibiotics—but only less than 2% had confirmed bacterial co-infections [13]. It has been shown that recent usage of antibiotics affects COVID-19 vaccine immunogenicity [5]. However, there is a lack of population-based studies investigating whether antibiotics could increase the risk of COVID-19 and serious clinical outcomes after vaccination. A differential effect may also exist depending on the number of vaccine doses received and the vaccine platform (messenger RNA (mRNA) vs. inactivated virus).
Here, we conducted a territory-wide cohort study on COVID-19 vaccine recipients to determine the effects of antibiotics on the development of COVID-19 and adverse clinical outcomes, with stratification.
2. Methods
2.1. Data Source
A retrospective cohort study was conducted using the electronic medical record database from the Hospital Authority (HA; including all individuals who ever used HA services), linked with territory-wide vaccination records and COVID-19 confirmed case records from the Department of Health using anonymous unique patient identifiers. Medical history and comorbidities were identified by International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, looking back to 2018 at the earliest. Medication use was identified by prescription records using British National Formulary (BNF) codes.
2.2. Setting and Study Population
The Hong Kong government provides the public with COVID-19 vaccines (BNT162b2 and CoronaVac) free of charge. Local residents can choose either vaccine according to their own preference. They can receive vaccination according to the recommended vaccination schedule in designated clinics and community vaccination centers.
Individuals aged ≥18 who had received 3 doses of either BNT162b2 or CoronaVac vaccine during the study period (23 February 2021 to 15 November 2022) were included (n = 3,299,819). Figure S1 shows the study subject selection process. Subsequently, patients with a history of GI surgery (n = 17,372), who were immunocompromised (cancer; transplant receipient; primary immunodeficiency; immune-related inflammatory disorders including rheumatoid arthritis, inflammatory bowel disease, and others; splenectomy; end-stage renal disease/dialysis; n = 129,984), who had a history of COVID-19 before the first dose of the vaccination (n = 18,017), or who used any antibiotic between the first and third dose of vaccination (n = 140,060) were excluded. The cohort size of our study was 2,994,386 after exclusion.
Patients were followed from the index date (date of first-dose vaccination) until the earliest of the following: outcome occurrence, death, date of vaccination of an additional dose, or end of data availability (15 November 2022).
2.3. Exposures of Interest
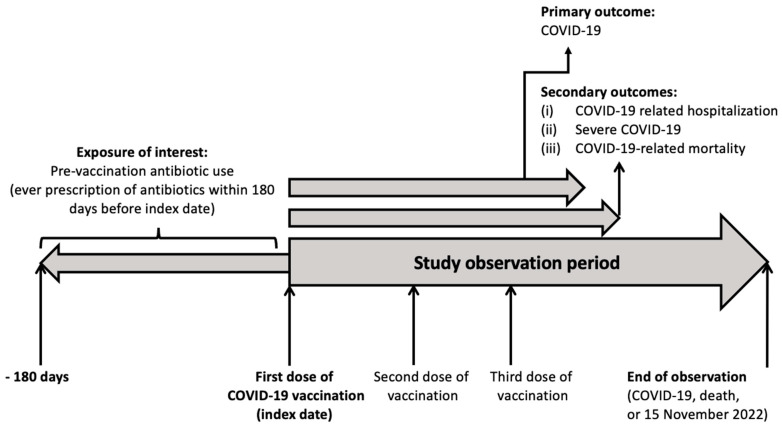
The primary exposure of interest is the pre-vaccination use of antibiotics. Table S2 shows the list of antibiotics included and their classification in terms of their anti-aerobic/anti-anaerobic effects and anti-bacterial spectrum. Pre-vaccination antibiotic users were those who received any prescription of any antibiotics within 180 days before their first-dose vaccination [5]. Antibiotic non-users were those who did not receive any prescription of antibiotics within 180 days from the first dose of vaccination. Figure 1 shows the study timeframe.
2.4. Outcomes of Interest
Outcomes of interest included: (i) COVID-19, defined by a positive polymerase chain reaction (PCR) test (voluntary reporting of rapid antigen test (RAT) positive test was not included) as confirmed by the Department of Health of Hong Kong Special Administrative Region Government; (ii) COVID-19-related hospitalization, defined as hospital admission within 28 days following a positive PCR test; (iii) COVID-19-related mortality, defined as all-cause mortality within 28 days following a positive PCR test; (iv) severe COVID-19, defined as a composite of COVID-19-related mortality or intensive care unit (ICU) admission or ventilatory support (ICD-9 procedure codes: 39.65, 89.18, 93.90, 93.95, 93.96, 96.7, 96.04) within 28 days following a positive PCR test.
2.4.1. Statistical Analyses
R software (version 3.2.3) was used for all statistical analyses. Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR). Discrete variables were expressed as counts with percentage.
Covariates included age, sex, Charlson Comorbidity Index (CCI) score [14], index date, presence of comorbidities (hypertension [15], diabetes mellitus [16], dyslipidemia [17], cardiovascular disease [18], respiratory disease, obesity diagnosis [19], smoking [20], alcohol use disorders [21], ulcers, moderate-to-severe liver disease [22,23,24], chronic renal failure [25]), and medication use within the past 90 days (angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) [26], metformin, lipid-modifying drugs, antiplatelets, nonsteroidal anti-inflammatory drugs (NSAIDs), oral anticoagulants, oral corticosteroids, antidepressants, antiviral drugs, PPIs [10,11], or H2 receptor antagonists (H2RAs) [27].
Propensity-score matching was conducted to minimize potential confounding between pre-vaccination antibiotic users versus non-users at a 1:1 ratio using the nearest-neighbor algorithm with a caliper of 0.2, where the propensity score was estimated using logistic regression for the covariates listed above. A standardized mean difference (SMD) of 0.2 or less was considered negligible.
Poisson regression adjusted for covariates was used to estimate the incidence rate ratio (IRR) of outcomes among pre-vaccination antibiotic users versus non-users in the propensity-score (PS)-matched cohort. Stratified analyses by age (<60 and ≥60), sex, Charlson Comorbidity Index categories (0 and ≥1), and vaccine platform were conducted. Effects of the nature of various antibiotics (anti-aerobic vs. anti-anaerobic, narrow- vs. broad-spectrum, and intravenous vs. oral) and different antibiotic classes used pre-vaccination on vaccine effectiveness were also estimated.
To increase the robustness of the study results by demonstrating a biological gradient, the association between pre-vaccination cumulative antibiotic exposure and outcomes were estimated using Poisson regression adjusted for the covariates listed above. Cumulative antibiotic exposure in the past 180 days before first dose vaccination were categorized by their cumulative duration (0 (non-users), 1–7, and ≥8 days). Incidence rate ratios (IRRs) and 95% confidence intervals were reported.
A two-sided p-value of less than 0.05 was considered statistically significant.
2.4.2. Statements of Ethics
The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of the University of Hong Kong and the Hong Kong West Cluster of Hospital Authority.
3. Results
3.1. Patient Characteristics
In total, 342,338 three-dose vaccine recipients (171,169 antibiotic users and 171,169 antibiotic non-users) were PS matched. The mean age was 57.2 years (SD: 18.2) in antibiotic non-users and 57.6 (SD: 19.1) in antibiotic users. The median follow-up was 13.6 months (IQR: 9.2–16.3), and the median duration of pre-vaccination antibiotic use was 7.0 days (IQR: 6.0–9.0). The difference in baseline characteristics between antibiotic users and non-users before PS matching and after PS matching (all with SMD < 0.2) is shown in Table S1 and Table 1, respectively.
Table 1
Baseline characteristics of pre-vaccination antibiotic users and non-users after propensity score matching.
| Pre-Vaccination Antibiotic Non-Users (n = 171,169) | Pre-Vaccination Antibiotic Users (n = 171,169) | SMD | |
|---|---|---|---|
| Age, years (mean (SD)) | 57.21 (18.2) | 57.60 (19.1) | 0.021 |
| Sex, male (%) | 76,808 (44.9) | 77,638 (45.4) | 0.010 |
| Charlson Comorbidity Index (mean (SD)) | 0.40 (0.8) | 0.41 (0.8) | 0.019 |
| Vaccine platform—CoronaVac (%) | 91,576 (53.5) | 93,168 (54.4) | 0.019 |
| Received 3rd dose (%) | 1.00 (0.0) | 1.00 (0.0) | <0.001 |
| Comorbidities—no. (%) | |||
| Hypertension | 51,436 (30.0) | 51,294 (30.0) | 0.002 |
| Diabetes mellitus | 26,124 (15.3) | 26,414 (15.4) | 0.005 |
| Dyslipidemia | 29,851 (17.4) | 29,351 (17.1) | 0.008 |
| Cardiovascular diseases | 57,263 (33.5) | 57,375 (33.5) | 0.001 |
| Respiratory diseases | 8634 (5.0) | 9169 (5.4) | 0.014 |
| Obesity diagnosis | 8377 (4.9) | 8626 (5.0) | 0.007 |
| Smoking | 2100 (1.2) | 2279 (1.3) | 0.009 |
| Alcohol use disorders | 1047 (0.6) | 1066 (0.6) | 0.001 |
| Ulcers | 3569 (2.1) | 3921 (2.3) | 0.014 |
| Moderate-severe liver disease | 334 (0.2) | 372 (0.2) | 0.005 |
| Chronic renal failure | 3358 (2.0) | 3459 (2.0) | 0.004 |
| Medication use in past 6 months—no. (%) | |||
| ACEIs | 15,317 (8.9) | 15,693 (9.2) | 0.008 |
| ARBs | 18,010 (10.5) | 18,074 (10.6) | 0.001 |
| Metformin | 21,302 (12.4) | 21,316 (12.5) | <0.001 |
| Lipid lowering agents | 46,982 (27.4) | 46,076 (26.9) | 0.012 |
| Antiplatelets | 23,053 (13.5) | 23,134 (13.5) | 0.001 |
| NSAIDs | 28,861 (16.9) | 26,385 (15.4) | 0.039 |
| Oral anticoagulants | 4739 (2.8) | 4897 (2.9) | 0.006 |
| Steroids | 3478 (2.0) | 4104 (2.4) | 0.025 |
| Antidepressants | 12,873 (7.5) | 12,939 (7.6) | 0.001 |
| Antiviral drugs | 3214 (1.9) | 3231 (1.9) | 0.001 |
| PPIs | 36,374 (21.3) | 36,571 (21.4) | 0.003 |
| H2RAs | 41,112 (24.0) | 38,394 (22.4) | 0.038 |
SMD: standardized mean difference, SD: standard deviation, ACEIs: angiotensin-converting enzyme inhibitors, ARBs: angiotensin receptor blockers, NSAIDs: non-steroidal anti-inflammatory drugs, PPIs: proton pump inhibitors, H2RAs: H2 receptor antagonists.
3.2. Association between Pre-Vaccination Antibiotic Use and COVID-19 Outcomes
Among the PS-matched vaccine recipients, 45,173 (13.2%) developed COVID-19. The median time from the index date to development of infection was 7.9 months (IQR: 5.7–10.8), respectively. Pre-vaccination antibiotic use was associated with a higher risk of COVID-19 (aIRR: 1.16; 95% CI: 1.14–1.18), COVID-19-related hospitalization (aIRR: 1.75; 95% CI: 1.65–1.86), and severe disease outcomes (composite of ICU admission/ventilatory support/death—aIRR: 1.60; 95% CI: 1.21–2.11) and death (aIRR: 2.56; 95% CI: 1.56–4.20) (Table 2).
Table 2
Association between pre-vaccination antibiotic use and COVID-19 outcomes after three doses of BNT162b2/CoronaVac.
| Events | Person-Days | No. of Persons | Incidence Rate (per 100,000 Person-Days) | Adjusted IRR (95% CI) | |
|---|---|---|---|---|---|
| COVID-19 | |||||
| Never users | 21,110 | 66,365,213 | 171,169 | 31.80883 | - |
| Pre-vaccination antibiotics users | 24,063 | 65,191,587 | 171,169 | 36.9112 | 1.16 (1.14–1.18) |
| COVID-19-related hospitalization | |||||
| Never users | 1550 | 70,426,545 | 171,169 | 2.200875 | - |
| Pre-vaccination antibiotics users | 2889 | 69,621,220 | 171,169 | 4.149597 | 1.75 (1.65–1.86) |
| Severe COVID-19 (ICU admission/ventilatory support/death) | |||||
| Never users | 78 | 70,655,136 | 171,169 | 0.110395 | - |
| Pre-vaccination antibiotics users | 138 | 70,067,227 | 171,169 | 0.196954 | 1.60 (1.21–2.11) |
| COVID-19-related death | |||||
| Never users | 21 | 70,665,654 | 171,169 | 0.029717 | - |
| Pre-vaccination antibiotics users | 66 | 70,080,467 | 171,169 | 0.094177 | 2.56 (1.56–4.20) |
IRR: incidence rate ratio; CI: confidence interval.
Antibiotic use was associated with a similarly higher risk of infection regardless of the duration of use (Table 3). A biological gradient existed for COVID-19 hospitalization and severe COVID-19. Compared with antibiotic non-use, the use of antibiotics for 7 days or less was associated with an approximately 1.5-fold higher risk of these outcomes (hospitalization aIRR: 1.57 (95% CI: 1.47–1.69); severe outcomes aIRR: 1.49 (95% CI: 1.08–2.04), respectively); while use of antibiotics for ≥8 days was associated with a 1.8- to 2.0-fold higher risk of these outcomes (hospitalization aIRR: 2.07 (95% CI: 1.92–2.23); severe outcomes aIRR: 1.77 (95% CI: 1.26–2.50), respectively; Table 3). However, a longer duration of antibiotic use did not pose a higher risk of COVID-19 mortality compared with shorter durations.
Table 3
Association between cumulative duration of pre-vaccination antibiotic exposure and COVID-19, hospitalization and severe COVID-19.
| Cumulative Duration (within 180 Days Pre-Vaccination) | Events | Person-Days | Persons | Incidence Rate (per 100,000 Person-Days) | Adjusted IRR (95% CI) |
|---|---|---|---|---|---|
| COVID-19 infection | |||||
| 0 days (non-users) | 21,110 | 66,365,213 | 171,169 | 31.80883 | - |
| 1–7 days | 16,911 | 47,596,958 | 122,112 | 35.52958 | 1.140 (1.117–1.164) |
| ≥8 days | 7152 | 17,594,629 | 49,057 | 40.64877 | 1.212 (1.180–1.246) |
| COVID-19 hospitalisation | |||||
| 0 days (non-users) | 1550 | 70,426,545 | 171,169 | 2.200875 | - |
| 1–7 days | 1641 | 50,791,737 | 122,112 | 3.23084 | 1.572 (1.466–1.685) |
| ≥8 days | 1248 | 18,829,483 | 49,057 | 6.627904 | 2.070 (1.919–2.232) |
| Severe COVID-19 | |||||
| 0 days (non-users) | 78 | 70,655,136 | 171,169 | 0.110395 | - |
| 1–7 days | 78 | 51,044,140 | 122,112 | 0.152809 | 1.485 (1.083–2.035) |
| ≥8 days | 60 | 19,023,087 | 49,057 | 0.315406 | 1.772 (1.258–2.496) |
| COVID-19 mortality | |||||
| 0 days (non-users) | 21 | 70,665,654 | 171,169 | 0.029717 | - |
| 1–7 days | 40 | 51,051,180 | 122,112 | 0.078353 | 2.626 (1.543–4.469) |
| ≥8 days | 26 | 19,029,287 | 49,057 | 0.136631 | 2.459 (1.373–4.403) |
IRR: incidence rate ratio; CI: confidence interval.
3.3. Stratified Analysis
3.3.1. Stratified by Vaccine Platform, Age, Sex, and Charlson Comorbidity Index
The magnitude of the higher infection risk and hospitalization associated with antibiotic use was similar regardless of vaccine platform, age, sex, or CCI (Table 4).
Table 4
Association between pre-vaccination antibiotic use and COVID-19 outcomes, stratified by vaccine platform, age, sex, and Charlson Comorbidity Index.
| Pre-Vaccination Antibiotic Use | Adjusted Incidence Rate Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| BNT162b2 | CoronaVac | Age < 60 | Age ≥ 60 | Male | Female | CCI 0 | CCI ≥ 1 | |
| COVID-19 | ||||||||
| 1.17 (1.13–1.20) | 1.15 (1.13–1.18) | 1.15 (1.12–1.18) | 1.18 (1.15–1.21) | 1.15 (1.12–1.19) | 1.17 (1.14–1.20) | 1.14 (1.12–1.16) | 1.22 (1.17–1.26) | |
| COVID-19-related hospitalization | ||||||||
| 1.69 (1.48–1.92) | 1.76 (1.64–1.89) | 1.63 (1.40–1.89) | 1.76 (1.64–1.88) | 1.74 (1.60–1.90) | 1.76 (1.61–1.93) | 1.73 (1.57–1.90) | 1.75 (1.61–1.89) | |
| Severe COVID-19 | ||||||||
| 1.50 (0.83–2.72) | 1.62 (1.18–2.22) | 1.80 (0.71–4.54) | 1.57 (1.17–2.11) | 1.65 (1.14–2.38) | 1.56 (1.01–2.40) | 0.98 (0.59–1.62) | 1.94 (1.38–2.74) | |
| COVID-19-related mortality | ||||||||
| 2.02 (0.70–5.85) | 2.70 (1.54–4.73) | - | 2.53 (1.54–4.15) | 2.71 (1.41–5.22) | 2.30 (1.07–4.90) | 2.18 (0.84–5.64) | 2.66 (1.49–4.76) | |
CI: confidence interval; CCI: Charlson Comorbidity Index.
However, antibiotic use was associated with a higher risk of severe disease outcomes and death with CoronaVac only (aIRR: 1.62 95% CI: 1.18–2.22; aIRR: 2.70, 95% CI: 1.54–4.73 for the two secondary outcomes, respectively), aged ≥60 years (aIRR: 1.57 95% CI: 1.17–2.11; aIRR: 2.53, 95% CI: 1.54–4.15 for the two secondary outcomes, respectively), and those with CCI > 1 (aIRR: 1.94 95% CI: 1.38–2.74; aIRR: 2.66, 95% CI: 1.49–4.76 for the two secondary outcomes, respectively). On the other hand, the magnitude of risk for the secondary outcomes was similar for males and females.
3.3.2. Stratified by Nature and Class of Antibiotics
Compared with antibiotic non-use, anti-anaerobic, broad-spectrum, and oral antibiotics were associated with a higher infection risk (aIRR: 1.16, 95% CI: 1.14–1.19; aIRR: 1.16, 95% CI: 1.14–1.18; aIRR: 1.16, 95% CI: 1.14–1.18, respectively), hospitalization (aIRR: 1.71, 95% CI: 1.60–1.83; aIRR: 1.77, 95% CI: 1.67–1.89; aIRR: 1.66, 95% CI: 1.60–1.77, respectively) and severe infection outcomes (aIRR: 1.70, 95% CI: 1.26–2.29; aIRR: 1.68, 95% CI: 1.26–2.22; aIRR: 1.53, 95% CI: 1.14–2.04, respectively; Table 5). Broad-spectrum antibiotics were associated with a higher infection risk (aIRR: 1.16; 95% CI: 1.14–1.18) and severe infection outcomes (aIRR: 1.67; 95% CI: 1.26–2.22). Anti-aerobic, narrow-spectrum, and intravenous antibiotics were associated with a higher infection risk (aIRR: 1.11, 95% CI: 1.07–1.15; aIRR: 1.14, 95% CI: 1.09–1.20; aIRR: 1.15, 95% CI: 1.02–1.29, respectively), but not severe infection outcomes (aIRR: 1.49, 95% CI: 0.88–2.53; aIRR: 0.79, 95% CI: 0.25–2.51; aIRR: 1.92, 95% CI: 0.77–4.76, respectively).
Table 5
Association between nature of the antibiotics and COVID-19 outcomes after three doses of BNT162b2/CoronaVac.
| Events | Person-Days | No. of Persons | Incidence Rate (per 100,000 Person-Days) | Adjusted IRR (95% CI) | |
|---|---|---|---|---|---|
| COVID-19 infection | |||||
| Anti-aerobic vs. anti-anaerobic | |||||
| Never users | 21,110 | 66,365,213 | 171,169 | 31.80883 | - |
| Anti-aerobic | 3527 | 10,273,829 | 26,363 | 34.32995 | 1.11 (1.07–1.15) |
| Anti-anaerobic | 16,879 | 45,822,622 | 119,732 | 36.83552 | 1.16 (1.14–1.19) |
| Both | 3657 | 9,095,136 | 25,074 | 40.2083 | 1.21 (1.16–1.25) |
| Narrow- vs. broad-spectrum | |||||
| Never users | 21,110 | 66,365,213 | 171,169 | 31.80883 | - |
| Narrow-spectrum | 1588 | 4,630,583 | 11,613 | 34.29374 | 1.14 (1.09–1.20) |
| Broad-spectrum | 20,276 | 54,929,426 | 144,534 | 36.91282 | 1.16 (1.14–1.18) |
| Both | 2199 | 5,631,578 | 15,022 | 39.04767 | 1.19 (1.14–1.24) |
| Intravenous vs. oral | |||||
| Never users | 21,110 | 66,365,213 | 171,169 | 31.80883 | - |
| Oral | 22,447 | 61,588,197 | 159,487 | 36.44692 | 1.16 (1.14–1.18) |
| Intravenous | 302 | 704,960 | 2359 | 42.83931 | 1.15 (1.02–1.29) |
| Both | 1314 | 2,898,430 | 9323 | 45.33489 | 1.24 (1.17–1.31) |
| COVID-19-related hospitalization | |||||
| Anti-aerobic vs. anti-anaerobic | |||||
| Never users | 1550 | 70,426,545 | 171,169 | 2.200875 | - |
| Anti-aerobic | 368 | 10,926,345 | 26,363 | 3.368006 | 1.65 (1.47–1.85) |
| Anti-anaerobic | 1932 | 48,955,027 | 119,732 | 3.946479 | 1.71 (1.60–1.83) |
| Both | 589 | 9,739,848 | 25,074 | 6.047322 | 1.98 (1.80–2.18) |
| Narrow- vs. broad-spectrum | |||||
| Never users | 1550 | 70,426,545 | 171,169 | 2.200875 | - |
| Narrow-spectrum | 106 | 4,933,949 | 11,613 | 2.148381 | 1.32 (1.08–1.61) |
| Broad-spectrum | 2516 | 58,654,252 | 14,4534 | 4.289544 | 1.77 (1.67–1.89) |
| Both | 267 | 6,033,019 | 15,022 | 4.425645 | 1.77 (1.55–2.02) |
| Intravenous vs. oral | |||||
| Never users | 1550 | 70,426,545 | 171,169 | 2.200875 | - |
| Oral | 2373 | 65,784,698 | 159,487 | 3.607222 | 1.66 (1.56–1.77) |
| Intravenous | 86 | 749,421 | 2359 | 11.47553 | 1.90 (1.53–2.36) |
| Both | 430 | 3,087,101 | 9323 | 13.92893 | 2.49 (2.23–2.78) |
| Severe COVID-19 (ICU admission/ventilatory support/death) | |||||
| Anti-aerobic vs. anti-anaerobic | |||||
| Never users | 78 | 70,655,136 | 171,169 | 0.110395 | - |
| Anti-aerobic | 17 | 10,982,965 | 26,363 | 0.154785 | 1.49 (0.88–2.53) |
| Anti-anaerobic | 100 | 49,255,135 | 119,732 | 0.203025 | 1.70 (1.26–2.29) |
| Both | 21 | 9,829,127 | 25,074 | 0.213651 | 1.28 (0.79–2.08) |
| Narrow- vs. broad-spectrum | |||||
| Never users | 78 | 70,655,136 | 171,169 | 0.110395 | - |
| Narrow-spectrum | 3 | 4,950,897 | 11,613 | 0.060595 | 0.79 (0.25–2.51) |
| Broad-spectrum | 125 | 59,041,017 | 144,534 | 0.211717 | 1.67 (1.26–2.22) |
| Both | 10 | 6,075,313 | 15,022 | 0.164601 | 1.28 (0.66–2.47) |
| Intravenous vs. oral | |||||
| Never users | 78 | 70,655,136 | 171,169 | 0.110395 | - |
| Oral | 112 | 66,147,965 | 159,487 | 0.169317 | 1.53 (1.14–2.04) |
| Intravenous | 5 | 763,064 | 2359 | 0.655253 | 1.92 (0.77–4.76) |
| Both | 21 | 3,156,198 | 9323 | 0.665357 | 2.04 (1.25–3.34) |
| COVID-19-related death | |||||
| Anti-aerobic vs. anti-anaerobic | |||||
| Never users | 21 | 70,665,654 | 171,169 | 0.029717 | - |
| Anti-aerobic | 7 | 10,984,950 | 26,363 | 0.063724 | 2.24 (0.95–5.27) |
| Anti-anaerobic | 51 | 49,263,702 | 119,732 | 0.103524 | 2.89 (1.73–4.83) |
| Both | 8 | 9,831,815 | 25,074 | 0.081368 | 1.59 (0.70–3.61) |
| Narrow- vs. broad-spectrum | |||||
| Never users | 21 | 70,665,654 | 171,169 | 0.029717 | - |
| Narrow-spectrum | 2 | 4,951,046 | 11,613 | 0.040396 | 2.19 (0.51–9.39) |
| Broad-spectrum | 59 | 59,053,118 | 144,534 | 0.09991 | 2.60 (1.57–4.30) |
| Both | 5 | 6,076,303 | 15,022 | 0.082287 | 2.31 (0.87–6.15) |
| Intravenous vs. oral | |||||
| Never users | 21 | 70,665,654 | 171,169 | 0.029717 | - |
| Oral | 53 | 66,158,741 | 159,487 | 0.08011 | 2.51 (1.51–4.17) |
| Intravenous | 3 | 763,471 | 2359 | 0.392942 | 3.23 (0.95–10.93) |
| Both | 10 | 3,158,255 | 9323 | 0.316631 | 2.72 (1.26–5.86) |
IRR: incidence rate ratio; CI: confidence interval.
Regarding antibiotic class (Table S3), compared with antibiotic non-use, penicillins (aIRR: 1.17; 95% CI: 1.15–1.19), cephalosporins (aIRR: 1.12; 95% CI: 1.04–1.21), macrolides (aIRR: 1.12; 95% CI: 1.05–1.19), carbapenems (aIRR: 1.35; 95% CI: 1.16–1.58), quinolones (aIRR: 1.16; 95% CI: 1.11–1.21), tetracyclines (aIRR: 1.20; 95% CI: 1.13–1.28), and aminoglycosides (aIRR: 1.45; 95% CI: 1.11–1.89) were associated with a higher risk of COVID-19—but this was not the case with nitroimidazoles (aIRR: 1.04; 95% CI: 0.98–1.10) or glycopeptides (aIRR: 1.14; 95% CI: 0.92–1.42). All antibiotic classes were associated with a higher risk of COVID-19-related hospitalization. Penicillins and quinolones were associated with a higher risk of severe COVID-19 (aIRR: 1.62, 95% CI: 1.22–2.16 and aIRR: 1.75, 95% CI: 1.06–2.91, respectively).
4. Discussion
This is the first study to demonstrate the potential undesirable effects of pre-vaccination antibiotics on the COVID-19 disease course, with a higher risk of infection, hospitalization, and severe disease outcomes being demonstrated in a territory-wide cohort study involving >300,000 PS-matched three-dose vaccine recipients, with a biological gradient being demonstrated. Notably, severe infection and death associated with pre-vaccination antibiotic use was only observed in CoronaVac recipients, but not in BNT162b2 recipients.
Antibiotics can cause significant changes in gut microbiota that have both short- and long-term health consequences [28]. Antibiotics reduce the overall diversity of gut microbiota species—including the loss of some important taxa—which causes metabolic shifts, increases gut susceptibility to colonization, and stimulates the development of bacterial antibiotic resistance [29]. Given the high efficacy of two doses of BNT162b2 and CoronaVac (protection from symptomatic COVID-19 is 95% [30] and 70% [31], respectively), it is hardly surprising that the effects of antibiotics on increasing infection risk was only slightly elevated (5–7%) in two- or three-dose vaccine recipients in the current study.
Although booster doses can enhance antibody production [32,33] and reduce the severity of infection outcomes [34], we showed that antibiotics were still associated with 1.8-fold and 1.6-fold higher risks of hospitalization and severe clinical outcomes (including ICU admission, ventilatory support, and death), respectively, in the three-dose vaccine recipients. Bacterial co-infection causes life-threatening complications in patients with severe viral infections [35], including COVID-19. Studies show that early antibiotic exposure poses risks for childhood asthma, allergies, and airway illnesses [36]. Indiscriminate administration of broad-spectrum antibiotics may increase nosocomial bloodstream infection rates in immunocompromised cancer patients [37]. While the baseline composition of the gut microbiota is mostly restored within 1.5 months, several common species (e.g., Bifidobacterium adolescentis, Coprococcus eutactus, Coliinsella aerofaciens, Methanobrevibacter smithii, Bididobacterium catenulatum, Bifidobacterium angulatum, and Eubacterium ventriosum) remain undetectable for at least 180 days [38]. With the combination of the immunocompromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, it has been proven that microorganisms from the dysbiotic gut microbiome translocate into the blood of COVID-19 patients, leading to dangerous blood-streamed infection during COVID-19 [39,40].
Subgroup analysis showed that antibiotic use was associated with a higher risk of adverse outcomes for COVID-19 with CoronaVac, but not BNT162b2, among the three-dose recipients. BNT162b2 mounts a higher neutralizing antibody level of nearly 10-fold more than CoronaVac after two doses of vaccine [40]. Owing to the waning effects of serum antibody levels, vaccine effectiveness against infection progressively diminishes [41]. This corroborates the hypothesis that a higher humoral response induced by BNT162b2 may minimize the unfavorable effects of antibiotics on vaccine immunogenicity and adverse clinical outcomes. A similar phenomenon was observed among older individuals and those with comorbidities, but not among younger age groups and those without comorbidities. The presence of comorbidities is linked to decreased immunity, reduced functional status, and increased healthcare utilization [42]. Advanced age and the number of concurrent comorbidities could also have a synergistic adverse effect on the health status of the elderly, explaining the higher COVID-19 mortality observed among elderly comorbid patients [43].
It was observed that broad-spectrum antibiotics were associated with a higher infection risk in the three-dose vaccine recipients, but not narrow-spectrum antibiotics. Furthermore, the intravenous route of the antibiotics had an overall higher risk of adverse infection outcomes than the oral route. Both gram-positive and gram-negative bacteria are disrupted by broad-spectrum antibiotics, as opposed to narrow spectrum antibiotics [44]. Broad-spectrum antibiotics decrease the Firmicutes to Bacteroidetes (F/B) ratio [45], which is associated with maintaining homeostasis, and changes in this ratio are regarded as dysbiosis. Models have shown that broad-spectrum antimicrobials (specifically vancomycin, metronidazole, and lacticin) cause population shifts from Firmicutes to Proteobacteria in the distal colon. In contrast, the narrow-spectrum bacteriocin (thuricin CD) seems to cause negligible changes in gut flora [46]. One study revealed a significant difference in the antibiotic resistance gene pools in mice treated with tetracycline between intravenous injection and oral administration of the drug, suggesting that drug administration routes influence the level of antibiotic resistance in gut microbiota [47]. The exact mechanism between the type, dosage, duration, administration, and pharmacokinetic and pharmacodynamics properties of antibiotics with vaccine immunogenicity and disease outcomes requires further investigation.
This study had several strengths: First, the population-based study with a large sample size allowed us to detect a statistically significant, albeit small in magnitude, increase in risk of infection—which is important for verifying the hypothesis of antibiotic-induced gut microbiota dysbiosis leading to impaired vaccine immunogenicity. Second, the demonstration of a biological gradient for the majority of outcomes increased the robustness of the study results and strengthened our underlying hypothesis. Third, reverse causality (i.e., symptomatic COVID-19 leading to antibiotic use before infection diagnosis) was minimized, as antibiotic use was defined before the first dose of vaccination while infection outcomes were only counted after the second dose of vaccination. With such a study design, the minimal interval between the commencement of antibiotics was at least three and four weeks for BNT162b2 and CoronaVac recipients, respectively. Fourth, since this was a cohort study, there was less recall and interviewer bias in terms of antibiotics as compared with a case–control study. To minimize the effects of confounding factors on the causality, PS matching ensured the similarity of the baseline characteristics between antibiotic users and non-users in order to reach a quasi-experimental design that may not be otherwise have been achieved by a randomized clinical trial (RCT).
Several limitations of this study should be noted: First, unmeasured confounding may still exist due to the observational study design, although all major confounding factors affecting vaccine immunogenicity and disease outcomes were minimized by the large sample size and PS matching design (Table 1). Second, gut microbiota data was not available; thus, the exact mechanisms of antibiotic-induced gut dysbiosis on vaccine immunogenicity and COVID-19 disease outcomes require further study. Third, asymptomatic infection cannot be ruled out, as this study included only PCR-confirmed positive case, which may overestimate the effects of antibiotics. Fourth, data on further booster doses and bivalent vaccines were not available before the study end date. Fifth, over-the-counter antibiotic usage information was not available, which may bias the study results. For example, self administered antibiotics may be taken incorrectly for too short a time, with wrong doses or timing with food intake. Lastly, COVID-19 vaccination effectiveness may vary in different waves of the epidemic due to the difference in the number of infected cases and prevention protocol/testing criteria from government policies. A prior study showed different frequencies of COVID-19 cases in different waves before the rollout of the vaccination program [48].
5. Conclusions
Antibiotics were associated with a higher COVID-19 risk, hospitalization, and severe infection in three-dose vaccine recipients. Further studies are warranted to investigate the interactions between gut microbiota and antibiotics and their effects on the COVID-19 vaccine’s immunogenicity and clinical outcomes. These results further reinforce the importance of the judicious use of antibiotics to minimize their direct and indirect impact on health.
Abbreviations
Adjusted incidence rate ratio, aIRR; angiotensin-converting enzyme 2, ACE2; angiotensin-converting enzyme inhibitors, ACEIs; angiotensin receptor blockers, ARBs; British National Formulary, BNF; Charlson Comorbidity Index, CCI; gastrointestinal, GI; H2 receptor antagonists, H2RAs; Hospital Authority, HA; incidence rate ratio, IRR; intensive care unit, ICU; International Classification of Diseases, Ninth Revision, ICD-9; interquartile range, IQR; messenger RNA, mRNA; nonsteroidal anti-inflammatory drugs, NSAIDs; polymerase chain reaction, PCR; propensity score, PS; proton-pump inhibitors, PPIs; rapid antigen test, RAT; standard deviation, SD; standardized mean difference, SMD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11081341/s1, Figure S1: Patient selection flow chart; Table S1: Baseline characteristics of pre-vaccination antibiotic users and non-users before propensity score matching; Table S2: Anti-anaerobic/anti-aerobic activity and anti-bacterial spectrum of various classes of antibiotics; Table S3: Association between pre-vaccination use of different classes of antibiotics and COVID-19 outcomes after vaccination with three doses of BNT162b2/CoronaVac.
Funding Statement
This work was funded by a research grant from the the Food and Health Bureau; HMRF Research on COVID-19, The Government of the Hong Kong Special Administrative Region (Principal Investigator (WP2): EWC; Ref: COVID1903011).
Author Contributions
Conceptualization, K.S.C. and W.K.L.; methodology, K.S.C., V.K.C.Y., E.W.C. and W.K.L.; data collection, V.K.C.Y. and E.W.C.; statistical analysis, V.K.C.Y. and X.Y.; drafting of manuscript, K.S.C., V.K.C.Y. and L.K.L.; supervision and revision of manuscript; I.F.N.H., E.W.C. and W.K.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the DH Ethics Committee (LM171/2021).
Informed Consent Statement
Informed consent was not needed as all subjects were anonymized in the electronic healthcare database system.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
Articles from Vaccines are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/vaccines11081341
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/2076-393X/11/8/1341/pdf?version=1691474583
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/156705452
Article citations
Association between Gut Microbiota Composition and Long-Term Vaccine Immunogenicity following Three Doses of CoronaVac.
Vaccines (Basel), 12(4):365, 27 Mar 2024
Cited by: 0 articles | PMID: 38675747 | PMCID: PMC11055114
The Predictive Value of Gut Microbiota Composition for Sustained Immunogenicity following Two Doses of CoronaVac.
Int J Mol Sci, 25(5):2583, 23 Feb 2024
Cited by: 1 article | PMID: 38473829 | PMCID: PMC10931755
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Proton pump inhibitors associated with severe COVID-19 among two-dose but not three-dose vaccine recipients.
J Gastroenterol Hepatol, 39(9):1837-1846, 05 May 2024
Cited by: 0 articles | PMID: 38705849
Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: A self-controlled case series and nested case-control study.
Lancet Reg Health West Pac, 21:100393, 02 Feb 2022
Cited by: 43 articles | PMID: 35128500 | PMCID: PMC8808060
Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 omicron infection and related hospital admission among people with substance use disorder in Hong Kong: a matched case-control study.
Lancet Psychiatry, 10(6):403-413, 01 May 2023
Cited by: 4 articles | PMID: 37141907 | PMCID: PMC10191606
Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study.
Thyroid, 32(5):505-514, 07 Apr 2022
Cited by: 25 articles | PMID: 35216517
Funding
Funders who supported this work.
Food and Health Bureau (1)
Grant ID: COVID1903011