Abstract
Free full text

Critical care and pandemic preparedness and response
Summary
Critical care was established partially in response to a polio epidemic in the 1950s. In the intervening 70 yr, several epidemics and pandemics have placed critical care and allied services under extreme pressure. Pandemics cause wholesale changes to accepted standards of practice, require reallocation and retargeting of resources and goals of care. In addition to clinical acumen, mounting an effective critical care response to a pandemic requires local, national, and international coordination in a diverse array of fields from research collaboration and governance to organisation of critical care networks and applied biomedical ethics in the eventuality of triage situations. This review provides an introduction to an array of topics that pertain to different states of pandemic acuity: interpandemic preparedness, alert, surge activity, recovery and relapse through the literature and experience of recent pandemics including COVID-19, H1N1, Ebola, and SARS.
Pandemics, epidemics, and their relationships to critical care are pertinent topics to reflect on during the 100th year of the British Journal of Anaesthesia. They are the ultimate disruptor, acting as a stress test for healthcare systems with little in the way of a pressure release valve. They expose challenges to infrastructure, organisations, procurement, ethical standards, research collaboration, social justice, and clinical practice, while exacting a physical and mental toll on those who work through them, the toll of patients and relatives notwithstanding.
Definitions
An epidemic is ‘the occurrence in a community or region of cases of an illness, specific health-related behaviour, or other health-related events clearly in excess of normal expectancy’.1 A pandemic can be defined most simply as ‘an epidemic occurring worldwide or over a very wide area, crossing international boundaries and usually affecting a large number of people’.1 Whilst the presence of a pathogen is not incumbent within the definition this is the most common understanding.2 Similarly, disruption to the normal functioning of services and increased mortality or morbidity is expected especially as it pertains to critical care services. For the purposes of this review, we shall use the term pandemic to include some notable epidemics given the similarities in management in critical care at a national/regional level.
History
The origins of critical care are intertwined with the history of the pandemics of the 20th century (Fig. 1). In 1952, an epidemic of poliomyelitis in Copenhagen overwhelmed the limited number of iron lungs available. In the face of widespread death and resource limitation an anaesthetist at Blegdamshospitalet, Bjorn Ibsen, proposed a radical solution; tracheal intubation via tracheostomy with positive pressure ventilation to help clear carbon dioxide and protect the airway from secretions. The scale of the illness necessitated the redeployment of external staff (in this case volunteer nurses, medical students, and retired staff) to ad hoc units in order to manually ventilate the children in 6-h shifts.3, 4 Mortality was greatly reduced (from 87% to 15%) and the next year the first permanent ‘intensive therapy units’ opened in Copenhagen's Kommunehospital, building on the experience of the previous year's polio epidemic.5 From the germ of an epidemic response, critical care as a specialty grew.
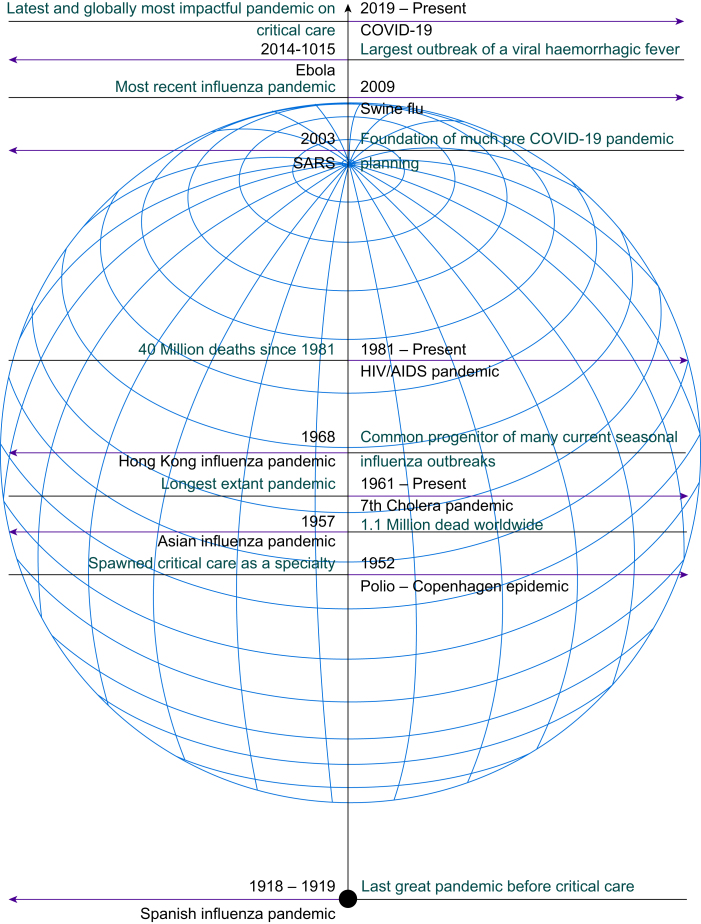
Timeline of some notable pandemics and epidemics since 1918. AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Before the epoch of critical care, the 1918–9 H1N1 Spanish influenza outbreak lasted just more than a year. A trimodal mortality curve demonstrated high disease burden amongst the very young, young adults, and the very old despite many now familiar interventions (school closures, the banning of large gatherings) applied at a public health level.6,7 Comparing the period 1918–9 with the initial 18 months of the COVID-19 pandemic in 103 wealthier countries, there was a 10-fold higher (1% vs ~0.08%) per capita excess mortality in 1918.8 Although not the only difference (the intrinsic lethality of the virus, better organised healthcare and public health systems, freely available antibiotics, widespread therapeutic oxygen, synthetic steroids and latterly vaccines have ameliorated the mortality rate) the mean age of death (27 years in 1918 vs 70 years in 2020)8,9 is perhaps reflective of a demographic likely to have benefitted from critical care today.
Critical care and affiliated services (e.g. anaesthesia, respiratory, geriatrics, and internal medicine) were integral to the secondary care COVID-19 pandemic response with mass diversion of financial and human resources to bolster critical care surge capacity. Maintenance of a functioning critical care network was emphasised by the choice of critical care occupancy as a key endpoint to titrate non-pharmacological societal interventions.10
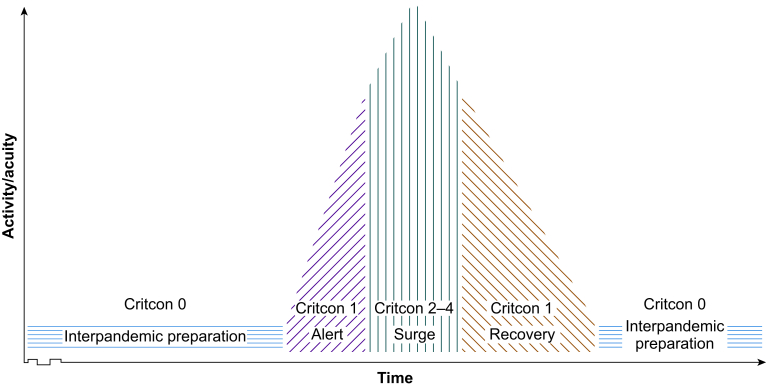
This narrative review is intended as a brisk primer on many subjects that caused issues during the COVID-19 pandemic. Given the magnitude of the subject matter, it is not intended to be, nor could it be, exhaustive but should sign post further reading for interested parties. Because of the relative magnitude and proximity we will make much use of examples from COVID-19. We will also make reference to pertinent lessons drawn from severe acute respiratory syndrome (SARS), pandemic influenzae, Ebola, and polio. We shall examine the different stages of critical care pandemic response: interpandemic preparation, alert, surge, and recovery and relapse (Fig. 2). Literature was gathered by searching for widely cited publications, pertinent news, and editorial content, aiming to gain a range of reports across different geographical areas, including evidence across different medical disciplines and allied health professionals. The content is also reflective of the authors' clinical experience of COVID-19 in the UK at a variety of high acuity London tertiary centres and district general hospitals.
Interpandemic preparation
‘There have been as many plagues as wars in history; yet always plagues and wars take people equally by surprise.’
- Albert Camus, The Plague
Various standards and guidance exist internationally for the provision of critical care services (i.e. Guidelines for the Provision of Intensive Care Services [GPICS] in the UK,11 College of Intensive Care Medicine [CICM] minimum standards in Australia and New Zealand,12 Critical Care Society of Southern Africa (CCSA) Guidance in South Africa13). These standards govern multiple aspects of critical care provision from floor space per patient, specifications for electrical wiring, access to offices, and staffing ratios.
Critical care cannot be given if there are not acceptable numbers of trained staff, appropriate technical equipment, sufficient consumables, key medications, and physical space to provide it in. In prior influenza outbreaks, despite robust pandemic planning, the capacity to deliver critical care has been quickly met or exceeded.14,15
Infrastructure
Traditionally a high level of infrastructure is needed for critical care services. Flexing these standards is possible to the extent of delivery in field hospitals as demonstrated during the 2013–6 Ebola outbreak.16 During COVID-19 many hospitals around the world developed expansion capacity in collaboration with internal medicine, respiratory and theatre teams.17 Ad hoc ICUs, whether they be in theatre space, converted general and acute beds, in new purpose-built facilities, or converted non-healthcare environments, if not supplied with the requisite utilities and familiar equipment, may lead to clinical incidents.18
Given that acuity of patient load and unit strain and proportion of created ICU beds may be related to worsening 30-day mortality,19,20 collaboration between critical care units is crucial when coordinating the pooling of shared resources. District general hospitals with smaller critical care capacities can become overrun with rapid, staccato pulses of patients. A critical care network can allow decompression to larger hubs with greater flex capacity as evidenced by the North West London Experience in early 2020.21,22 A (mostly) successfully coordinated strategy of active decompression was actively used in France with patients transferred intra/inter-regionally and internationally by road, rail, and air to less pressured centres.23,24 Many patients will also require services not available locally and will need referring to networked specialty centres (i.e. a severe acute respiratory failure service).25 As a legacy of COVID-19, there has been a recognition of the need for more established adult critical care transfer services within England with multiple services now in operation (or commissioning process).26
Provision of critical care requires specialist equipment and consumables. In the initial months of COVID-19 there was a possibility that entire healthcare networks could run out of ventilators.27 Rapid innovation and regulatory cooperation allowed the design and production of a mechanical CPAP device to act as an adjunct for invasive ventilation.28 It could be argued that in hindsight, in this case this represented a distraction from the true issues at hand—not the need for more ventilators but many UK hospital's capacity to distribute oxygen. The only UK hospital to reach a near triage event was an oxygen failure at Watford.29 Likewise, industrial oxygen production and supply presented a rate-limiting step in India's response to COVID-19 with traumatic clinical and ethical outcomes.30
Staffing
Guidance specifies that intubated and ventilated patients require 1:111,12 or 2:3 nursing ratios.31 Given that local capacity may increase three-fold in pandemic scenarios,21 consideration needs to be given to achievable ratios and skill mix. It is established that inferior staffing ratios and reduced ITU experience result in poorer patient outcomes (mortality, nosocomial infections, and family satisfaction).32, 33,34 Given the impracticality of rapidly replicating rosters of trained critical care teams, attention has had to turn to both softening acceptable staffing ratios (down as far as 1:4) and sourcing additional staff. Optimal skill mix and staffing ratios may not be attainable; with this, it is almost inevitable that standards of care may have to be reappraised.35 ‘Just-in-time’ training pending the need to expand capacity has been used to improve pathogen-specific knowledge.34,36 Planning should also be in place for rostering of non-patient facing clinical specialties (e.g. microbiology, radiology) and non-clinical support services (e.g. administrative and ancillary services, estates management).37 Likewise staffing may have to be found in unusual places. Much like in 1952 the COVID-19 effort saw retired doctors, surgical specialties, allied health professionals, medical students, and even military doctors and nurses pressed into action in critical care.38,39 Military and charitable services also contribute assistance to many epidemics in the developing world—the Ebola outbreak saw large-scale international logistical and clinical support to augment the overstretched services of Sierra Leone and Liberia.
Alert
‘Some victims were neglected and died; others died despite a great deal of care. There was not a single remedy, you might say, which ought to be applied to give relief, for what helped one sufferer harmed another. No kind of constitution, whether strong or weak, proved sufficient against the plague, but it killed off all, whatever regime was used to care for them.’
- Thucydides' Peloponnesian War
Research
Before COVID-19, SARS 2002–3, H1N1 2009, and the Ebola 2014–6 epidemics highlighted flaws in research infrastructure pertaining to pandemic preparedness. Disjointed coordination, imperfect understanding of disease mechanisms, patient heterogeneity, and sparse multicentre collaboration led to a challenging research space.40,41
For a novel pathogen, having robust descriptive epidemiological data and establishing the natural history of critically ill patients at the outset of a pandemic are the first steps. Critical care services are crucial in defining this process because of their proximity to the most severe cases, and expertise in managing highly significant pathogens.42, 43, 44 Having identified a pathogen as having epidemic/pandemic potential, we are of the opinion that integrating research into the critical care response has a commanding moral and pragmatic imperative. The core processes driving the pathophysiology of a pandemic pathogen need to be investigated to best focus critical care research, as demonstrated, for example, by work into microthrombi formation.45 It has been argued that the search for treatments and the incomplete focus on basic science and pathophysiology hindered the initial response to COVID-19.46
In the UK, the coronial service not only has mandate to investigate the circumstances in which patients have died, but also to utilise inquests to formulate recommendations in order to prevent further deaths.47 In the parts of the UK, digital autopsy (the use of postmortem CT scanning) is the first-line investigation as opposed to a traditional coronial postmortem.48 This may mean key disease insights could be missed early on from lack of tissue examination (although this is the matter of debate).
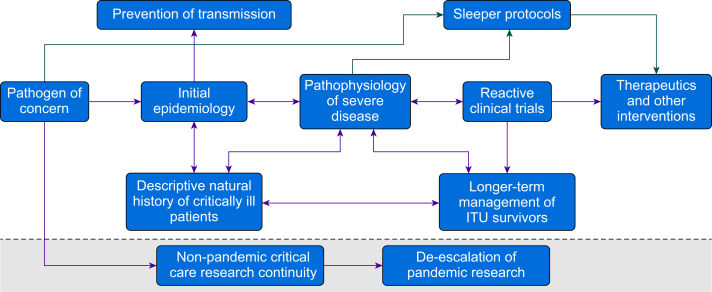
Efforts were made before COVID-19 to reduce, at points, cumbersome governance and funding structures by introducing ‘sleeping’ trials (Fig. 3), dormant adaptable RCTs ready to recruit in the eventuality of a pandemic, in order to rapidly develop high-quality evidence in the early days of an outbreak culminating in the success of REMAP CAP.49 Combined with the development and integration of multi-arm adaptive platform trials allowed the rapid testing and verification of promising treatments, and minimised risk to patients. The aforementioned REMAP CAP (tocilizumab) along with RECOVERY (dexamethasone, baricitinib)50 and the yet to report HEAL COVID-1951 trials generated rapid and efficacious RCT evidence by quicky excluding ineffective treatments. Combined with the application of new messenger RNA technology, these trial methodologies generated many of the treatments that are allowing COVID-19 to transition from pandemic to endemic.52
The rapidity of knowledge sharing, and global connectivity, allowed the COVID-19 pandemic to be investigated and published in the scientific record at an unprecedented rate. That is not to say it has been an unalloyed success. By the end of May 2020 there were >1870 critical care COVID-19 articles already published.53 Such was the ‘maelstrom’ of global research, the problem became that there was too much, often poor-quality evidence.53 An interplay developed between established and social media, and the demand for promising COVID-19 research leading to the prominence of fringe or inefficacious therapies such as hydroxychloroquine and ivermectin.54,55 Access to preprints caused issues through the promulgation and amplification of weak evidence: non-scientific media accessed not yet published studies lending an aura of authenticity to fringe treatments.56 Likewise even major journals were noted to retract published articles because of flaws in their peer review and data verification processes.57,58
Inter- and post-pandemic research capacity has been affected by COVID-19. This is multifactorial secondary to elective surgical pauses, saturated research and funding capacity, workforce pressures, and the need to complete outstanding COVID-19 research.59 Plans should be put in place for non-pandemic research continuity during a pandemic, and realignment and de-escalation during the aftermath.60
Infection control
The identification of new pathogens may occur after the most extreme presentations. Critical care is often at the face of this process.42,61 An established infection control strategy (which is adequately resourced) is a vital part of pandemic preparedness. Most modern pandemics have been driven by respiratory pathogens spread via droplets and aerosols, other routes include direct contact (Ebola), faeco-oral (cholera), and blood borne (human immunodeficiency virus). There remains a possibility of transmission of pathogens via animal vectors (Yersinia pestis, Zika virus) although this is of less relevance for hospital infection control strategies.
Pragmatically, primacy should be given to clinician safety when handling ultra-high-risk pathogens such as viral haemorrhagic fevers (VHFs), or ultra-virulent ones such as measles. This may generate complexities when providing critical care to patients: cumbersome personal protective equipment (PPE), isolation units, and altered technical processes may hamper the delivery of optimal care, although they do not preclude it.62, 63, 64 Staff delivering critical care to patients are at increased risk over some other healthcare staff because of the nature of procedures (instrumentation of airways, jugular line insertions), close contact with aerosolised bodily secretions, and potential for patient agitation.62,65 During COVID-19, staff faced threats on two fronts; a lack of PPE as a result of global supply issues and rampant demand; and uncertainty about what defined an exposure—most pertinently aerosol generating procedures.66
If adequate PPE and training are provided then pathogenic risks to staff can be ameliorated (as evidenced during the SARS outbreak).67 Conversely, poor infection prevention and control planning, scarce or poor-quality PPE, or poor concordance with PPE lead to clinical and nonclinical attrition of healthcare staff through infection and anxiety.68,69, 70, 71
Surge activity
‘No one knows anything about them up here, a number around each one’s neck in turn, no coffin, no shroud, nothing but a covering of quicklime. And so on to the next one.’
- Letters from a Mourning City, Axel Munthe
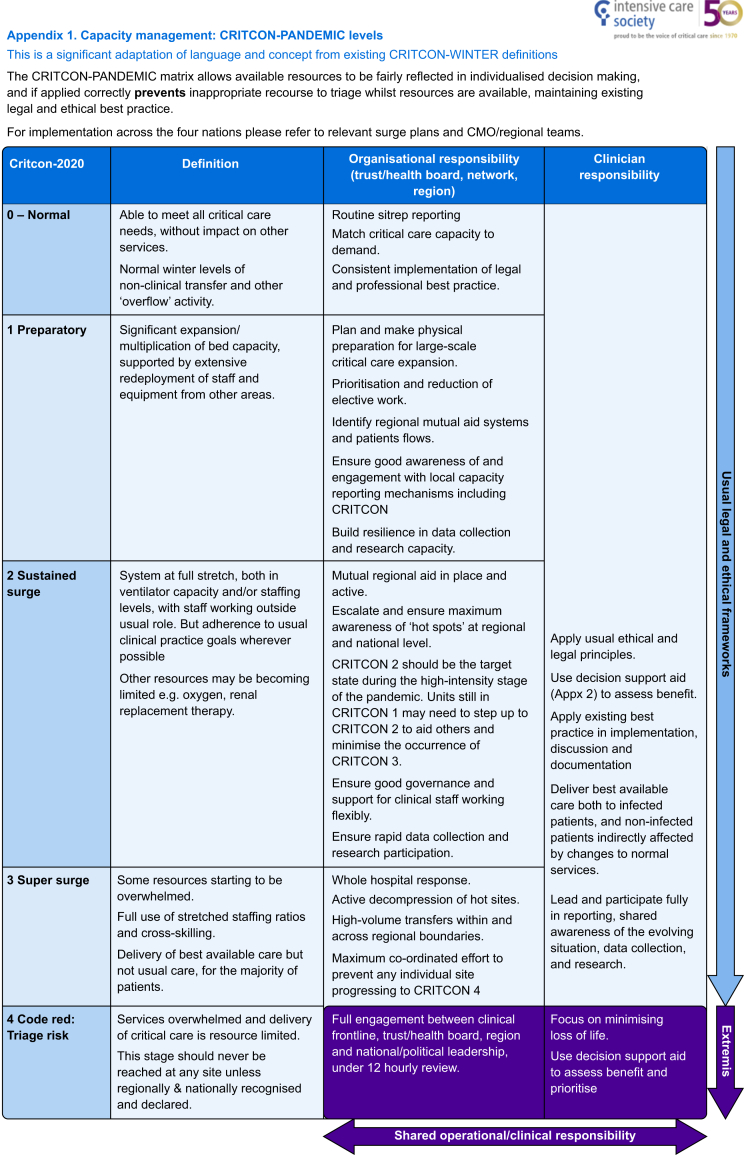
The UK critical care networks have an established system of categorising system strain, known as CRITCON, stemming from the 2009 H1N1 pandemic,72 initially giving levels 0–4 from 0 ‘business as usual’, through 1 ‘bad winter’ peaking at 4 ‘triage’. This was adapted in 2020 at the outset of COVID-19 to cater for the anticipated influx of critically ill patients that the pandemic would bring73 (Fig. 4). The targeted level for the pandemic was that of CRITCON 2 or ‘sustained surge’—a system in which was working markedly above normal capacity, with the cancellation of routine business, and relying on staff redeployed from normal roles—but crucially adhering to usual practice goals. Several hospitals reached CRITCON 3, at peak stretch, however, none officially crossed the threshold into CRITCON 4, triage risk.74
Triage
Pandemics and epidemics may pose direct challenges to accepted socio-cultural, ethical, moral, and legal precepts. Critical care is skill and resource intensive by definition; day-to-day provision therefore is limited by reasonable skill limitations and cost implications. In the worst-case scenario, when a system fails or is unable to increment capacity to a sufficient extent, the demand for a given service may vastly outstrip supply.10 There need not be a mass catastrophe, or entire saturation of critical care capacity to provoke ethical dilemmas. Even at CRITCON 1 there is an anticipation of at least partial reallocation of staff and equipment and reprioritisation of elective work. Many of these decisions are pragmatic and equitable, however if incorrectly applied then may lead to ‘drawbridge ethics’ (where operative capacity is removed long before a pandemic arrives, in effect pulling up the drawbridge) or moral panics.75
At the most extreme, triage risks may occur. At this stage services are resource limited and interventions may even need to be removed from existing patients. For many years there have been efforts to create pandemic triage guidelines in the anticipation that critical care services may be overwhelmed. The aim of these is to provide ethical, or scientific ethical hybrid models to delineate those most appropriate to use the finite resource available.
At the simplest, some have advocated a ‘first come, first served’ approach as the most egalitatrian.76,77 Others identify those with the best chance of benefitting from critical care by excluding those with minimal chance of surviving because of pronounced derangements in physiology or advanced comorbidities and those without need for critical care input at time of review. Various strategies can then be used to prioritise the remainder.78, 79, 80, 81, 82 Care should be paid after the admission to continually reassess whether the patient is benefitting or being harmed through invasive management, as changes in information about the patient's ability to benefit from critical care come to light or the level of acuity affecting the system change.83 So called ‘reverse triage’, removal of established support from one patient to another more likely to benefit, can be ethically justifiable but is also likely to be morally challenging for clinicians.84 Classical biomedical ethical frameworks, justice, autonomy, beneficence, and non-maleficence are a basis of many triages of resource protocols.74 84 No one ethical instrument can be utilised to triage, but a multi-faceted model should be adopted to operationalise complementary values.75,85,86 Ethical values may also not be compatible with the laws of countries in which they are applied; early engagement with legal services and regulatory bodies may be necessary.87,88 There should be transparency in the institution of resource triage.79,89 The National Institute for Health and Care Excellence (NICE), the Faculty of Intensive Care Medicine (FICM), and the Royal College of Physicians provide numerous recognised decision-making frameworks around treatment escalation and end-of-life decisions which provide structure.90,91
The activation of altered triage protocols, which deny some patients critical care treatments deemed necessary by the treating clinicians, must only occur once all possible national and feasible international calls for resource have been exhausted and there is still an unmanageable demand on local resources. All decisions should be made by two experienced clinicians disconnected from the patient's care.61,72,84
Health inequalities
Historical examples including Māori populations in 1918–9 and 1950s New Zealand, and more recently Canadian First Nations and Indigenous Australians in 2009, demonstrate that morbidity and mortality fall heavily on historically discriminated against indigenous communities.14,92,93 COVID-19 has further highlighted longstanding health inequalities across underserved population groups with greater rates of disease acquisition, severity, hospital admission, organ failure, long-term morbidity, and mortality.43,94, 95, 96 Despite making up <14% of the UK population, Black, Asian, and minority ethnic groups accounted for 19% of deaths in hospital and 35% of critical care admissions following COVID-19.97 In East London, Black and Asian patients hospitalised as a result of COVID-19 were younger and more likely to die.98, 99 These disparities are intrinsically linked to wider social determinants of health and reflect underlying inequalities with higher levels of pre-existing comorbidities, higher levels of socioeconomic deprivations, and structural determinants of healthcare access and provision.100, 101, 102 Despite improvement in overall outcomes, better treatments, and processes of care as the pandemic progressed, Asian and Black ethnic groups continued to have increased hospital admissions and death in hospital associated with COVID-19.99 This pattern was repeated in the United States where arguably high levels of inequality, lack of centralised healthcare, and polarised political views led to large disparities in clinical outcomes.103 There remains the crucial need to better identify, understand, and mitigate the driving factors to prevent continued health inequalities in the future.
Engagement with families
Increasing patients' families' engagement in their loved ones' care and their presence on the intensive care unit improves patient outcomes, and the psychological impact on their relatives.104, 105, 106, 107, 108, 109 This drive was obstructed at the outbreak of COVID-19 with more restrictive visitation policies in the interests of public health, security, and scarcity of human resource.110,111 In the initial waves of COVID-19 these policies prevented some families from being able to stay by their relatives' sides at their deaths.105 Whilst there was understandable public health concern, it could be arguable that this is not only not in the interests of dying patients but also deleterious to the staff tasked with caring for them.112 In order to circumvent the worst sequalae of this, many critical care units introduced daily phone updates, video calling, and specific family liaison teams staffed by non-critical care clinicians.113 It is foreseeable that in the case of a different pathology (i.e. Ebola or another viral haemorrhagic fever virus), such is the significance of the pathogen that these issues would likely be compounded because of the perceived near impossibility of family visiting on public health grounds and need to provide care in isolator tents making video calls, etc., more challenging.
Training opportunities
Critical care and anaesthesia share an interwoven history in many countries. The significant overlap in patient care skillsets mean that both anaesthesia services and critical care will be profoundly affected directly and indirectly by significant pandemics.114 This is especially true when it comes to the training of the next generation of anaesthetists and critical care physicians. On a direct level occupation of theatre capacity, the redeployment and reallocation of theatre teams comes at an opportunity cost of reduced elective caseload and subspecialty training for anaesthetists.115,116 Experientially, airway guidance advocated the most experienced intubator as first operator, potentially limiting the opportunities for trainees, at the beginning of their careers, learning core techniques (direct laryngoscopy, bag mask ventilation) over concerns surrounding droplet spread and aerosolization.115,117 Training issues were further compounded by concerns surrounding curriculum adherence, recognition of ‘time served’, delay and format changes of mandatory examinations, reduction in overseas career development opportunities, and changes in specialty recruitment processes.115
Novel strategies needed to be adopted to continue academic and clinical teaching programmes. Zoom and Teams teaching sessions became ubiquitous and are still present in many departments today.118 Likewise, many learned societies developed online training platforms to prepare staff for the pandemic and to disseminate information and guidelines as they developed.36,119
That is not to say pandemics are not without benefit for the training of anaesthetists and intensivists. Exposure to critical care is a cornerstone of traditional anaesthetic training, and this was without doubt abundant during the last pandemic. Complimentary and associated specialties redeployed to ICU took away a greater insight of the critically ill patient and have benefitted their own specialties through their experience, developing better communication skills, resilience, and knowledge of patient care outside of their specialty interest.120 Impressionable foundation doctors (those in rotational training during their first 2 years out of medical school) were redeployed to critical care units around the UK—this has anecdotally contributed to the large increase in demands for anaesthesia training programmes in the UK and awareness of critical care more generally.121,122
Maintaining high-risk surgery
Even in normal demand ‘CRITCON 0’ periods, perioperative critical care is a limited resource for high-risk patients. Outside of pandemics high-risk operations are frequently cancelled because of a lack of critical care capacity. Given that at the first escalation of systemic stress CRITCON 1—cancellation and delay of non-urgent elective work is one of the first levers available—anaesthetic and surgical practice will be disrupted by severe epidemics and pandemics. Likewise, concerns surrounding operating on patients with occult or recent infection can reduce flow.123
The scale of surgical backlogs after the COVID-19 pandemics is one sequalae of the intensity of the ongoing COVID-19 pandemic (routine, non-urgent surgical volumes were reduced by up to 97% in the UK during the first wave).124 The consequences of the choke on high-risk surgical capacity led to indirect mortality from the pandemic, including previously curatively resectable cancers, surgically operable cardiovascular disease, or solid organ transplantations.125, 126, 127
As successive waves of COVID-19 have arrived, so has the response to ensuring high-risk surgery can continue as independently as possible from general critical care capacity. In the authors' experience the adoption of surgical hub and spoke models, external surgical ‘green sites’, private sector facilities, and ring-fenced surgical monitored beds in contextually appropriate ways can allow surgical activity to trend back towards normal levels. The continued development of enhanced perioperative ‘Level 1.5’ care facilities represents an ongoing pathway to develop pandemic (and later endemic) surgical continuity in the absence of available critical care beds.128,129 In addition to ring-fencing beds, the benefits of dedicated enhanced perioperative care units (amongst other terminology),130 the use of enhanced recovery after surgery (ERAS) and ERAS-type pathways reduces length of stay allowing higher turnover; ergo in theory, more patient flow through limited bed stock.131 Given that elective surgery is a core pillar of healthcare not only in the developed world, but also in low- and middle-income countries, work has begun for the assessment of surgical preparedness across a range of socioeconomic realities in preparation for the next external shock inclusive of ring-fencing of electrical and oxygen supplies, theatre space, and critical care beds.132
Recovery and relapse
‘If we are victorious in one more battle, we shall be utterly ruined.’
- Life of Pyrrhus, Plutarch
Burnout and moral injury
On the ascent to peak activity, we have seen that multiple hazards are faced by critical care teams. Whilst the initial surges may pass by on adrenaline and morale, the long tail of weaning from organ support, an aggregation of moral injury from ceaseless mortality, a limitation of resource and crisis standards of care, a pervasive paranoia of novel pathogens, and over capacity work schedules can lead to attrition in workforce. In pandemics from SARS through Ebola through to COVID-19, healthcare workers report high levels of burnout, post-traumatic stress disorder (PTSD), concerns about pathogenic transmission to family members, and sleeping difficulties.133 Risks to team members can be divided into clinical (i.e. direct risks from the pathogen) and nonclinical risks (i.e. threats of violence, professional criticism or litigation, burnout, and other associated mental health disorders).35
Burnout is a syndrome comprising emotional exhaustion, depersonalisation, and low personal accomplishment caused by chronic exposure to the accretion of physical and mental stressors involved in providing healthcare.134 The weight of the pandemic stretched many staff beyond their elastic limit to the point of burnout and even breakage, leading to the loss of many experienced critical care nurses in the post-pandemic period.135 Burnout presents a risk to patient safety with worse outcomes from overstretched and overburdened teams.136 This can be ameliorated by providing certainty of legal safe standing and extraordinary circumstances, sharing acceptable crisis standards of care and adequate rest periods and facilities, equitable pay and conditions, appropriate protective equipment, applicable training, and supervision.35
Long-term outcomes
In addition to bearing the mental and physical scars of general critical illness complications, such as PTSD, digital injuries from pressors, ICU acquired weakness, patients can have disease specific complications from pandemic illnesses. Whilst they may not bear the visible stigmata of post-polio neuropathies or smallpox scars, the survivors of critical COVID-19 continue to suffer with persistent organ dysfunction well after the initial insult. In one large-scale study of a large United States Veterans Administration data set of the patients admitted with to critical care units with COVID-19 compared with patients admitted to hospital without a positive test, there was a 6-month hazard ratio of 13.26 for developing end stage kidney disease—this represents a truly life-changing event.137
One peculiarity of critical COVID-19 was its propensity to require attritional periods of tracheal intubation with high pressures from poorly compliant lungs. In addition, tracheostomies for profound neuromuscular weakness and parenchymal damage aggregated to lead to a remarkable incidence of tracheal lesions, including tracheo-oesophageal fistulae, subglottic stenosis, and tracheomalacia.138,139 It is our experience that many of these complications will need outpatient surgical management, and some might lead to the need for long-term artificial airways.
Patients also bear the mental scars of their illness, for example Ebola survivors are haunted by traumatic memories of their illness, and brush with mortality. This is especially challenging in environments such as Liberia, with one psychiatrist and one mental health hospital in the country as of 2015 at the end of the outbreak.140 GPICS and NICE guidance both advocate the follow-up of critical care survivors inclusive of psychological sequalae.11
Management of critically ill pandemic survivors through their acute stay can become a Pyrrhic victory if their longer-term health needs are not met upon discharge. A structured and multidisciplinary approach is fundamental to tackling the multiple sequalae of post-pandemic critical care. The post-intensive care syndrome—a constellation of: skeletal muscle wasting and neuromuscular dysfunction, cognitive impairment, mood disorders, and speech problems can persist for many years, greatly affecting functional status and quality of life.141 This is equally applicable to post-pandemic survivors as a general ITU population.142 The Post ICU Presentation Screen Tool (PICUPS) was developed during the pandemic and is the first multiprofessional tool to facilitate selection of patients for closer follow-up and generation of an appropriate rehabilitation prescription.142,143
As we pass the third anniversary of the initial surges of COVID-19 activity, literature is becoming available on functional outcomes of critical care survivors. Patients demonstrate persistent neuromuscular dysfunction, ongoing dyspnoea, and mental health issues in keeping with non-pandemic acute respiratory distress syndrome (ARDS) and post-intensive care syndrome (PICS).144 However, the scale is different because of the volume and incidence of COVID-19 representing a significant demand on health services. In addition to having a scalable acute response, we suggest it may be prudent to have plans made for selective scalable rehabilitation capacity and long-term critical care specific follow-up.
Conclusions
‘Who taught you all this, doctor? The reply came promptly: ‘Suffering.’
- Albert Camus, The Plague
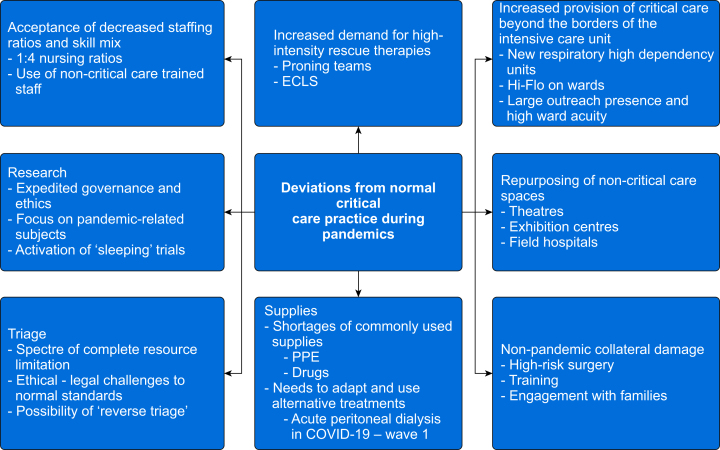
Pandemics are an inevitable product of living in organised societies. This is as true now as it was in 1918 (or during the Athenian Plague of 430 BC for that fact). Critical care represents the last redoubt of treatment for the infected and therefore society at large. Nearly every infected patient who was admitted to an ICU during the first salvos of the COVID-19 pandemic would have died when compared with their forebears from just 100 yr before (as would many of those not even admitted but ‘merely’ on supplemental oxygen). This highlights the enormous marginal benefit of having a functional critical care system, within and without the unit. There however remains room for reflection and reiteration of plans. Pandemics will continue to force critical care units to act outside their normal processes (Fig. 5). Areas of excellence in COVID-19, such as flexibility and scalability of research capacity, and the use of sleeping trials came from the recognition of the failings of prior pandemics. Areas where there was perceived failure this time, such as availability of PPE, oxygen supplies, and other consumables, stemmed from relative complacency (on a global level) regarding known risks. There has been monumental progress in the 105 yr since 1918. Pandemic outcomes have overall greatly improved, not just in part because of the founding of critical care as a specialty in 1952. Various pandemics globally since then have honed the armamentarium of the intensivist, but it should be noted with every great leap forward in pandemic-inspired technology a price has been paid in human suffering that should always be acknowledged.
Authors’ contributions
Original concept: all authors
Drafting of original article: WT, YW
Critical revisions of the manuscript for important intellectual content: all authors
Final approval: all authors
Declaration of interest
The authors declare that they have no conflicts of interest.
Notes
Handling Editor: Jonathan Hardman
References
Articles from BJA: British Journal of Anaesthesia are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/154504551
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.bja.2023.07.026
Article citations
Monocyte derived large extracellular vesicles in polytrauma.
J Extracell Biol, 3(9):e70005, 02 Sep 2024
Cited by: 0 articles | PMID: 39224236 | PMCID: PMC11367151
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Expanding the pandemic influenza preparedness framework to the epidemic of COVID-19].
Zhonghua Yu Fang Yi Xue Za Zhi, 54(6):597-601, 01 Jun 2020
Cited by: 3 articles | PMID: 32842276
The Swine Flu Triage (SwiFT) study: development and ongoing refinement of a triage tool to provide regular information to guide immediate policy and practice for the use of critical care services during the H1N1 swine influenza pandemic.
Health Technol Assess, 14(55):335-492, 01 Dec 2010
Cited by: 18 articles | PMID: 21208551
Review
Addressing challenges for clinical research responses to emerging epidemics and pandemics: a scoping review.
BMC Med, 18(1):190, 25 Jun 2020
Cited by: 24 articles | PMID: 32586391 | PMCID: PMC7315698
Review Free full text in Europe PMC
Pandemic planning and response in academic pediatric emergency departments during the 2009 H1N1 influenza pandemic.
Acad Emerg Med, 20(1):54-62, 01 Jan 2013
Cited by: 10 articles | PMID: 23570479