Abstract
Purpose of the review
Nonhuman primates (NHPs) are seen as the closest animal model to humans in terms of anatomy and immune system makeup. Here, we review how preclinical studies in this model system are teaching the field of HIV vaccinology the basic immunology that is needed to induce broadly neutralizing antibodies (bnAbs) with vaccination and elicit protective T cell responses. These lessons are being translated into clinical trials to advance towards protective active vaccination against HIV-1 infection.Recent findings
Preclinical vaccination studies in NHPs have shown that highly engineered HIV-1 immunogens can initiate bnAb precursors providing proof of concept for Phase I clinical trials. Additionally, NHP models of HIV-1 infection are elucidating the pathways for bnAb development while serving as systems to evaluate vaccine protection. Innovative immunization strategies have increased affinity maturation of HIV-1 antibodies in long-lived germinal centers. Preclinical studies in macaques have defined the protective level of neutralizing antibodies and have shown that T cell responses can synergize with antibody-mediated immunity to provide protection in the presence of lower neutralizing antibody titers.Summary
The NHP model provides vaccine regimens and desired antibody and T cell responses that serve as benchmarks for clinical trials, accelerating HIV vaccine design.Free full text

Guiding HIV-1 vaccine development with preclinical nonhuman primate research
Abstract
Purpose of the review
Here, we review how preclinical studies in this model system are teaching the field of HIV vaccinology the basic immunology that is needed to induce broadly neutralizing antibodies (bnAbs) with vaccination and elicit protective T cell responses. Nonhuman primates (NHPs) are seen as the closest animal model to humans in terms of anatomy and immune system makeup. These lessons are being translated into clinical trials to advance towards protective active vaccination against HIV-1 infection.
Recent findings
Preclinical vaccination studies in NHPs have shown that highly engineered HIV-1 immunogens can initiate bnAb precursors providing proof of concept for Phase I clinical trials. Additionally, NHP models of HIV-1 infection are elucidating the pathways for bnAb development while serving as systems to evaluate vaccine protection. Innovative immunization strategies have increased affinity maturation of HIV-1 antibodies in long-lived germinal centers. Preclinical studies in macaques have defined the protective level of neutralizing antibodies and have shown that T cell responses can synergize with antibody-mediated immunity to provide protection at lower neutralizing antibody titers.
Summary
The NHP model provides vaccine regimens and desired antibody and T cell responses that serve as benchmarks for clinical trials, accelerating HIV vaccine design.
Introduction
Animal models play a critical role in the design and evaluation of medical interventions. NHPs are an optimal model for predicting human outcomes given the similarities in anatomy and immune systems (1–3). Rhesus and cynomolgus macaques are common NHPs used for research purposes. With recent efforts to better understand the macaque antibody locus, we now have an improved understanding of orthologous antibody gene segments that enable the evaluation of B cell lineage design and germline targeting vaccine strategies (Figure 1A; 4, 5–7). These strategies rely on targeting specific naïve B cell receptors composed of certain immunoglobulin gene segments (Figure 1B; 7, 8, 9). Key virus challenge stocks bearing transmitted/founder HIV-1 envelopes have been developed recently (10, 11), enabling the assessment of the protective capacity of vaccines, elucidating pathways of virus escape from neutralizing antibodies, and tracing bnAb development in NHPs (Figure 1C; 12).
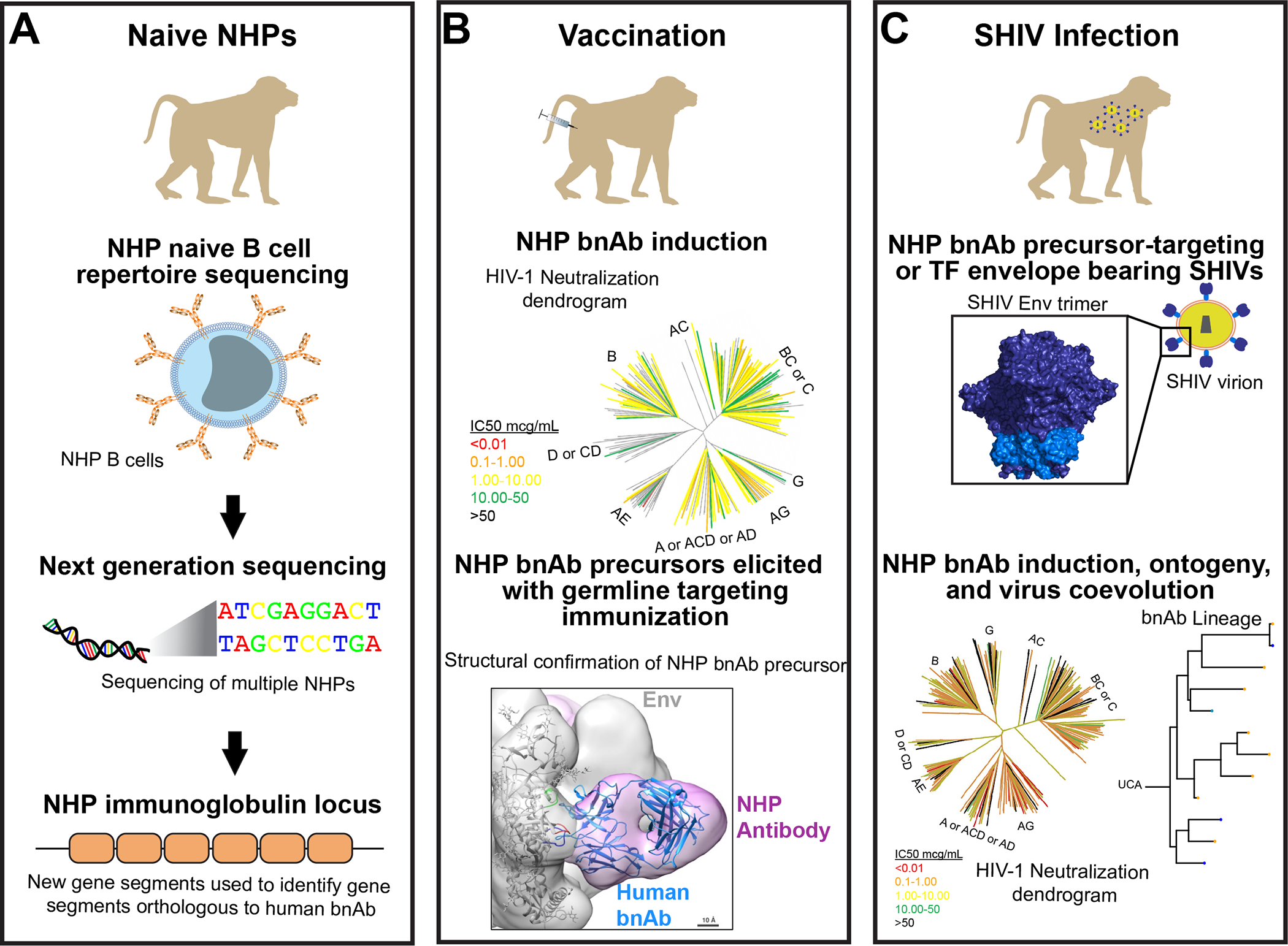
A. The NHP antibody repertoire has been sequenced for tens of macaques allowing a better understanding of the potential antibody responses that macaques can make in response to infection or vaccination. New programs such as IgDiscover have been generated to identify novel variable gene segment alleles. The improved understanding of NHP immunogenetics has facilitated comparisons with known human broadly neutralizing antibodies (bnAbs). B. Vaccination of NHPs has elicited fusion peptide bnAbs using engineered minimal immunogens. The broad neutralization by vaccine-elicited macaque fusion peptide antibody DFPH-a.01 is shown as a color-coded HIV phylogenetic tree. In other studies, the field has found structural evidence that vaccine-induced antibodies recapitulate the binding of human bnAbs. A negative stain electron microscopy 3D reconstruction of a macaque CD4bs antibody binding (pink) to Env (gray) is shown with the ribbon structure of a human CD4bs bnAb (blue) fit inside it. The perfect fit between the two structures shows accurate recapitulation of the human bnAb binding by the macaque antibody. C. Advances in the simian-human immunodeficiency virus (SHIV) infection model has led to multiple uses of this system. New SHIVs have been engineered recently to express relevant challenge viruses with transmitted/founder HIV-1 envelopes that mimic the hard-to-neutralize HIV-1 isolates that are currently circulating. Additionally, SHIVs are being designed to express bnAb precursor-targeting HIV-1 envelopes to elicit specific types of bnAbs in NHPs. The macaque bnAbs are assessed on large virus panels as shown here by the HIV-1 neutralization dendrogram. The induction of bnAbs in the SHIVs enables precise tracking of the bnAb lineage before and after infection, and the identification of virus sequence changes that select for specific bnAb lineage members.
Envelope protein (Env), present at a low frequency as a trimer of heterodimers, is the only surface-exposed protein on HIV virions (13), making it the sole target for nAbs (14). Additionally, there are several regions of hypervariability on the protein surface and extensive, variable glycan masking which present a cryptic target for humoral immunity (15–17). Despite these obstacles, the investigation of natural infections identified numerous surface epitopes of conserved vulnerability, for which potent, bnAbs have arisen (18). Consequently, a longstanding HIV vaccinology goal is the recapitulation of these humoral responses in HIV-naïve individuals (18). Advancing this goal requires preclinical animal models (i.e. NHPs), with relevance to human immunological responses that can guide the design process and demonstrate comparable humoral responses. This review will highlight the recent advances in NHP research that has bolstered our ability to test novel vaccine strategies in NHPs and thereby determine the key immune responses that Phase I trials should measure.
Advances in macaque immunogenomics.
To compare the antibody response in NHPs and humans, a better understanding of the antibody repertoire in macaques was needed. To this end, major efforts have gone towards sequencing the macaque immunoglobulin locus (Figure 1A) (4, 6, 7, 19). The early attempts to sequence the macaque immunoglobulin variable region generated databases managed by King’s College or IMGT (20). Among these gene segments variable region gene segments were identified that had greater than 93% sequence homology with orthologous gene segments in humans (21, 22). However, the combination of next generation sequencing and Sanger sequencing revealed that the macaque immunoglobulin locus was more complex and diverse than previously thought (6, 7). For example, after sequencing 10 macaques, only 75 of the 761 alleles observed in the study were present in the IMGT database (4). Additionally, macaques not only differed in single polymorphisms, but also one macaque may lack a particular allele that is present in another macaque (4). Thus, the genomic structure differed among macaques. Online databases of immunoglobulin sequences, such as KIMDB continue to aid interpretation of vaccine-specific antibody responses (6). With this new knowledge, investigators can then determine whether a vaccine is expanding the types of antibodies for which the vaccine was designed.
Immunization strategies targeting bnAb epitopes.
Inducing bnAbs to the CD4 binding site (CD4bs) is a major focus for vaccine design (23–25). Several highly potent human broadly-neutralizing antibodies against the CD4bs have been characterized in the past 13 years (e.g. VRC01(26), CH235.12(27), 8ANC131(28), N6(29)), derived from conserved variable gene segments, which suggest universal genetic targets, and shared evolutionary pathways to bnAb maturation. In particular, the VH1–46 gene segment-restricted bnAb lineage CH235, has been longitudinally characterized, yielding a highly probable unmutated common ancestor, for which an immunogen CH505.M5.G458Y was designed to engage similar precursors in HIV naïve individuals (27, 30). This immunogen elicited a potent CD4bs-directed immune response in outbred rhesus macaques (23). Further macaque trials with soluble Env trimers identified CD4-mimicking bnAb “precursors,” utilizing signature residues in the HCDR2 (e.g. Y54) like CD4 and human bnAbs. The structure of the vaccine induced antibody paratope possessed the antiparallel beta sheets like CD4 and the arginine at position 71 (31) that creates a salt bridge with Env in all known human bnAbs against this site (Figure 2B; 32). Clinical trials will determine whether this vaccine elicits CD4-mimicking antibodies in humans (the HVTN 309 study).
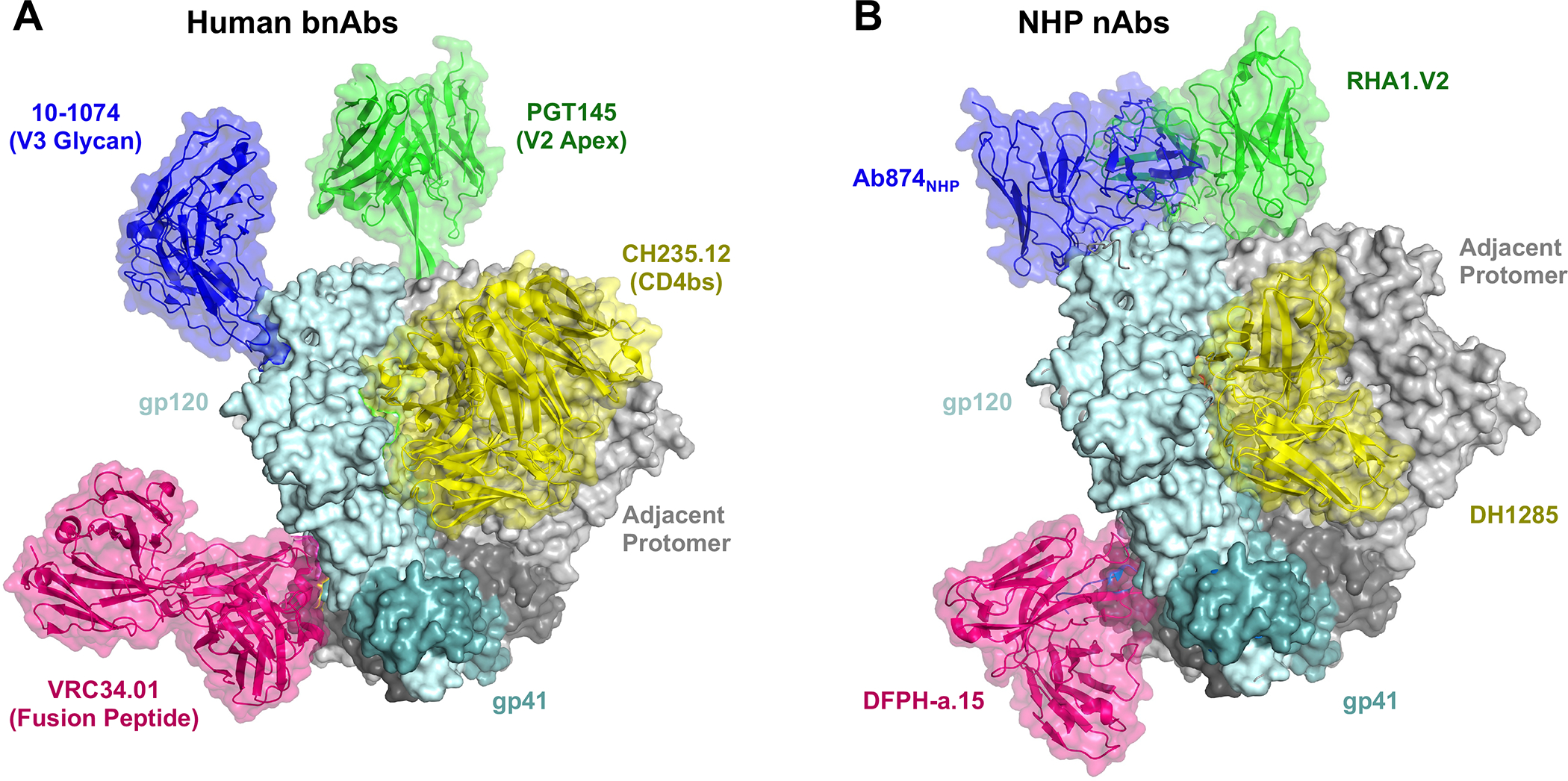
A. Human bnAbs and their respective epitopes of vulnerability aligned using Pymol to a BG505:PGT145 model using gp120 or gp41 structure (PDB: 5V8L). Other bnAb models with Env masking: 10–1074 (8CZZ), VRC34.01 (5I8H), CH235.12 (5F96). B. SHIV and vaccine-induced nAbs from rhesus macaques aligned via gp120/gp41 to BG505 (5V8L): Ab874NHP (6ORO), DFPH-a.15 (6N1W), RHA1.V2 (6XRT), DH1285 (EMD-27621 and EMD-27622). Induction of the rhesus nAbs validates the use of macaques as a model system for evaluating vaccine immunogens and studying antibody responses to virus infection.
Some CD4bs bnAbs require low probability immunogenetic events such as insertions or deletions (33), which creates a potential precursor elicitation bottleneck; and almost all require extensive somatic hypermutation (over 40% of amino acid sequence divergence from germline for some antibodies) (34), which may require long and arduously designed vaccine regimens. In contrast, a more recently identified class of bnAb, known as IOMA, requires much less somatic hypermutation and no apparent indels, with moderately reduced breadth and potency (35). Recently designed IOMA germline-targeting immunogens IGT1 and IGT2 showed promise in multiple animal models, including outbred macaques, where vaccination elicited some CD4bs-directed neutralization (36).
Another epitope, termed the V3-glycan site has also garnered interest for its ability to elicit bnAbs (e.g. BG18, PGT121, 10–1074) which can interact with the highly conserved GDIR motif and N332 glycan (Figure 2A; 37). Escolano et al. designed the RC1–4fill immunogen as a virus-like particle and immunized mice, rabbits, and rhesus macaques, yielding V3-glycan-specific antibodies with characteristically long HCDR3 (Figure 2B; 38). Macaques immunized with this germline-targeting Env virus-like particle were boosted with several heterologous envelopes, sequentially, resulting in modest heterologous pseudovirus neutralization and no protection against chimeric simian-human immunodeficiency virus (SHIV) infection (39). Neutralizing serum titers may have been adversely affected by the persistence of off-target responses to the CD4bs of boosting immunogens (40).
Perhaps the most effective recapitulation of bnAb responses to date in NHPs used immunogens targeting the fusion peptide domain of gp41. Initial studies of FP bnAb responses identified the human bnAb VRC34.01, which targeted the fusion peptide at the N terminus of gp41 (Figure 2A; 41). This discovery led to the development of a small peptide immunogen capable of initiating FP-directed antibody responses in NHPs (42). Further NHP studies have demonstrated that these small FP peptides focus potent autologous neutralizing antibody responses against the FP region. These FP antibodies can then be affinity matured with boosting using Env trimers to exhibit broad neutralization (Figure 1B; 43, 44). This immunogen has yielded the most potent anti-HIV NHP bnAbs, DFPH-a.01, capable of neutralizing 59% of a 208-virus global panel with a geometric mean IC50 of 3.12 μg/mL (Figures 1B and and2B;2B; 44).
Optimization of immunization strategies.
To-date, the best attempts to elicit bnAbs and the prevailing wisdom in the field suggests that HIV-1 vaccine designs will require regimens with multiple immunogens and prolonged germinal center responses to mirror the maturation pathway in vivo (9, 18, 45, 46). To that end, nucleoside-modified mRNA has been explored using macaque preclinical models given the ease of rapid manufacturing of multiple immunogens. Early studies provided evidence that similarly designed nucleoside-modified mRNA encapsulated in lipid nanoparticles (mRNA-LNP) can perform as well as protein-based vaccine platforms for eliciting robust autologous neutralizing antibody responses (47, 48). Further, there is evidence that mRNA-based vaccines can stimulate Env-specific T follicular helper cells, suggesting their ability to drive germinal center reactions (48, 49). While the COVID-19 mRNA vaccines have raised questions about whether mRNA can elicit durable neutralizing antibody responses, we have observed durable neutralizing antibody responses against HIV-1 can be elicited with mRNA-LNP vaccines in macaques (47, 48). Recent experiments have used mRNA-LNP vaccines to express virus-like particles that protected macaques in a model of HIV-1 infection (48). Neutralizing antibody responses, as well as non-neutralizing antibody effector functions, likely account for this efficacy against SHIV challenge. Similar antibody induction will be examined in an upcoming Phase I trial called HVTN 310 (48).
In addition to novel vaccine platforms like mRNA-LNPs, novel strategies for immunization route have been developed in NHPs. In a SHIV challenge study, Felber et al. delivered DNA vectored HIV-1 envelope and recombinant gp120 vaccines simultaneously in each leg or delivered the DNA in one leg and the protein in the other leg (50). Macaques who received DNA and protein together in each leg were significantly protected from repetitive low-dose SHIV CH505 TF challenge, whereas separating the DNA and protein administration into independent legs provided no protection (50). This study suggested that activation of T cells and B cells simultaneously in the same draining lymph node may provide a benefit for inducing protective immunity.
Slow delivery immunization is another immunization approach being developed in NHPs (19, 51, 52). For this strategy called escalating dose immunization, macaques were immunized every 2 days for 12 days with an increasing amount of vaccine (19). This approach elicited higher HIV-1 autologous tier 2 neutralizing antibodies in rhesus macaques than conventional immunization. Sampling the lymph nodes over time using fine needle aspiration showed the Env-reactive germinal center B cell frequency and Env-specific T follicular helper cell frequency were increased by escalating dose immunization compared to single bolus immunization (19). The epitopes recognized by antibodies elicited with the escalating dose regimen were different than the epitopes targeted by single bolus vaccination (19). Similar results could also be obtained by delivering the antigen via an implantable osmotic pump (19, 52). Overall, the results in macaques suggested slow persistent delivery of vaccine immunogen generated a better-quality antibody response. A recent study from Lee et al. extended further these observations, showing that the germinal center response to HIV-1 vaccination lasted 30 weeks or more in NHPs (53). During this time the clones evolved higher affinity and some neutralization of heterologous viruses was observed (53). Given the high level of somatic mutation required for bnAb development (54, 55), the protracted affinity maturation after an escalating dose immunization gives hope that requisite high levels of affinity maturation can be achieved if immunogens are antigenic for bnAb lineage members. A modified escalating dose regimen—aiming to mimic the slow, constant rise in antigen during acute infection—is now being tested in a Phase I trial called HVTN 301 (NCT05471076) and HVTN 144 (NCT# still to be assigned).
Preclinical studies in macaques identify the next wave of vaccine adjuvants.
Vaccine adjuvants are a critical component of HIV-1 vaccines (56, 57). Recently, vaccine adjuvants have targeted toll-like receptors as a means for stimulating adaptive and innate immune cells (57–59). Monophosphoryl lipid A (MPLA), AS01B, ALFQ, GLA-SE, and 3M-052 are principal examples of TLR agonist adjuvants. NHPs have been critical in evaluating these adjuvants since TLR expression, sequence homology, and cellular distribution can differ in rodents versus primates (60). Hence, the TLR4 agonist GLA-SE is a potent adjuvant in mice (61), however in macaques it did not activate the immune systems as determined by serum cytokine analyses (59, 62). In contrast, the TLR7/8 agonist 3M052-SE potently activated the immune system and led to upregulation of expression of more than half of the cytokines analyzed in vaccinated macaques (62). Serum neutralization was higher in the 3M-052-SE group than the GLA-SE group (62). 3M-052 is being investigated in upcoming or ongoing HIV-1 Phase I trials (NCT04177355; NCT04915768; NCT05471076; NCT05903339; NCT05828095).
Saponins including QS21 have been combined with toll-like receptor agonists such as MPLA to generate potent adjuvants (63). This approach is widely used across vaccinology producing approved vaccines for malaria or the Novavax COVID-19 vaccine (64, 65). Recently, a particular formulation of saponin was combined with TLR4 agonist MPLA to form a nanoparticle adjuvant called Saponin/MPLA nanoparticles (SMNP) (66). Immunizations of macaques with soluble Env trimer immunogens adjuvanted with SMNP induced superior neutralizing antibodies, antibody effector functions, and germinal center B cell responses compared to immune-stimulating complexes (ISCOMs) or TLR agonist adjuvants (66). Given its success in macaque preclinical studies, this potent adjuvant is being evaluated in HVTN 144.
New SHIV Models Furthering Vaccine Research Efforts.
A long-standing need in HIV research is the development of NHP models of HIV-1 that faithfully recapitulate the pathogenesis and viral evolutionary dynamics of human infection. However, the generation of SHIVs utilizing primary or transmitted/founder (TF) Envs with higher barriers to neutralization has long been hampered by the lack of NHP CD4 binding affinity for HIV-1 Env(67). Recently, the recognition of sequence divergence between humans and NHPs at critical residues in CD4, led to the hypothesis that bulky aromatic residues at Env position 375 might facilitate rhesus CD4 binding(10, 11).
These designs have been gainfully employed in the demonstration of sustained infectivity with initial replication spikes and setpoint viremias reminiscent of natural infection in humans and even capable of progressing to AIDS in rhesus macaques(10, 12). Further, the platform has shown multiple Envs with demonstrated bnAb elicitation in humans (CH505, CH848, and CAP256), can elicit neutralizing antibodies against critical Env epitopes observed in humans (Figure 1C; 12). This system also provides a means to intimately study the co-evolution of such antibodies alongside virus escape mutants to better elucidate the pathways required to mature bnAbs in vivo (Figure 1C). For instance, the V2 apex antibody lineage RHA.1 is found concurrent with viruses who have mutations in the C strand of the second variable region where RHA.1 binds (12). The ability to sample virus and antibody sequences before infection and during acute infection provides sequence information for potential immunogens to start and affinity mature bnAb lineages.
Defining mechanisms of protection from infection.
Due to the difficulty with inducing bnAbs by active vaccination, non-neutralizing antibody effector functions such as antibody-dependent cellular phagocytosis or cytotoxicity have been foci of previous vaccine efficacy trials (68). However, with the inefficacy seen in the recent Phase IIb or III trials (HVTN 702, 705, and 706), which aimed to elicit non-neutralizing antibodies (69), the field has refocused on antibody neutralization. A recent meta-analysis of 18 passive transfer studies indicated that neutralizing antibody titer correlated with protection and depending on the antibody specificity and virus, a nAb 50% inhibitory dilution (ID50) titer of 685 (95% CI:319, 1471) was needed to protect macaques form SHIV infection 90% of the time (70). Similar protective titers have been calculated in macaques vaccinated with Env trimers and challenged with SHIV. Recently, a panel of rhesus macaques immunized with a variety of BG505 immunogen technologies and regimens yielded a cohort of animals with varying degrees of tier 2 autologous neutralization (52). Repetitive SHIV challenges of the animals in high and low nAb titer groups demonstrated that nAb titer levels were highly correlative of protection against SHIVBG505 challenge; further, regular tracking of BG505 pseudovirus serum neutralization identified an ID50 was equal to 476 (CI: 272–991) above which the probability of macaque being protected was 90%(71). Similar studies that stratified macaques based on tier 2 autologous nAb responses to CH505 TF demonstrated a similar pattern with low responding macaques showing delayed but inevitable onset of viremia during repeat low-dose intrarectal challenge with SHIV, while high nAb titer animals achieved prolonged protection against repeated challenge. The 90% protective ID50 was equal to 1954 (CI: 417–8913) against the SHIVCH505 TF virus produced in 293T cells (62).
While bnAb induction with vaccination is the principal vaccine goal currently, T cell responses have emerged as a complementary immune response that vaccines should target (72). The focus on T cells was reinvigorated because of a study where the Pulendran group immunized one group of macaques with Env trimer formulated with 3M-052 adjuvant alone and another group with Env trimer formulated with 3M-052 adjuvant plus a trivalent gag T cell vaccine (73). Both vaccines afforded significant protection against homologous virus challenge. Macaques with ID50 neutralizing antibody titers above 300 were protected regardless of vaccine administered. However, when neutralizing antibodies were below an ID50 titer of 300, macaques given the trivalent gag T cell vaccine and adjuvanted Env trimers were protected significantly better than macaques that received only adjuvanted Env trimers (73). This preclinical study suggests that when neutralizing antibodies are at a low level, tissue resident CD8+ T cells can confer protection. Thus, highly engineered Env trimer vaccines that trigger bnAbs are being combined with CD8+ T cell vaccines to provide the best protection.
Challenges in the macaque model to consider.
NHP research is still hindered by the limited access to NHPs, which ultimately increases the cost to perform NHP studies. Additionally, the differences in immunogenicity in macaques and humans may complicate the ability of NHP preclinical study outcomes to predict human outcomes. The Phase IIb clinical trial HVTN 705 was ineffective at preventing infection in humans but showed efficacy in macaque-SHIV preclinical studies (74). Underlying the different vaccine outcomes may be differences in immunoglobulin allelic diversity and certain antibody subclasses such as IgG3 having very different structures and functionality, or the transmissibility and resistant nature of circulating HIV-1 isolates. Thus, care must still be taken to not over interpret results in NHP preclinical trials.
Conclusions.
The successful elicitation of bnAbs against the fusion peptide of HIV-1 envelope and induction of bnAb precursors in macaques has generated enthusiasm for upcoming clinical trials aiming to initiate the precursor antibodies that can develop into bnAbs. Combining these antibody-targeting vaccines with T cells will be the next step to determine whether synergistic, protective B cell and T cell responses can be elicited in humans as achieved in macaques. Clear goals for upcoming Phase I trials have been elucidated based on the types of immune responses elicited by vaccination in protected NHPs.
Acknowledgments
We thank Robert Edwards for structural biology analyses and Elizabeth Donahue for assistance with the preparation of the manuscript.
Financial support and sponsorship
Division of AIDS in the National Institute of Allergy and Infectious Disease (R01-AI120801, UM1-AI144371, and U19-Al160546)
Footnotes
Conflicts of Interest
JC and KOS each have patents submitted on immunogens discussed in this review.
Contributor Information
James Counts, Duke Human Vaccine Institute, Department of Medicine, Duke University School of Medicine, Durham, NC 27710.
Kevin O. Saunders, Duke Human Vaccine Institute, Departments of Surgery, Immunology, and Molecular Genetics and Microbiology, Duke University School of Medicine, Durham, NC 27710.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
*of special interest
**of outstanding interest
This manuscript describes the macaque immunoglobulin locus database KIMDB. The results added significantly to the number of macaque gene sequences.
This manuscript was the first to identify the substantial allelic diversity in macaque immunoglobulin variable gene seqments.
The first review article to explain the concept of designing immunogens to target specific broadly neutralizing antibodies.
This manuscript traced neutralizing antibody development in macaques infected with a SHIV expressing a transmitted founder envelope.
This study showed the improved immunogenicity with slow delivery vaccination in macaques.
This manuscript was the first evidence that neutralizing antibodies with a neutralization profile like CH235 bnAb precursors could be elicited in macaques with an engineered immunogen.
This study provides the structural and molecular evidence that CD4bs bnAb precursors resembling human VH1–46 bnAb precursors could be elicited with vaccination.
This study reports the induction of V3-glycan bnAb precursors in macaques. The immunogen was designed to elicit PGT121-like precursors and vaccine responses shared common HCDR3 signatures with PGT121.
This study generated the broadest neutralizing antibodies elicited with vaccination in macaques. The antibodies targeted the fusion peptide of HIV-1 envelope.
This study shows that long-lived germinal center responses can be generated with slow delivery immunization strategies. The long-lived germinal center responses led to affinity maturation of HIV antibodies.
Determined the protective concentration of nAbs needed in vaccinated macaques, and epitopes targeted by the nAbs. Importantly, the CD4 binding site was among the nAb specificities elicited in vaccinated macaques.
This manuscript showed that nAbs correlate with protection from SHIV infection and determined the protective concentration of nAbs needed in vaccinated macaques.
This study estabilished that CD8+ T cells could synergize with neutralizing antibodies to reduce the titer of neutralizing antibodies needed for protection. This study has led to the pursuit of combination vaccines targeting neutralizing antibodies and T cells.
Full text links
Read article at publisher's site: https://doi.org/10.1097/coh.0000000000000819
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10810179
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/154233195
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/coh.0000000000000819
Article citations
A platform for the rapid screening of equine immunoglobins F (ab)2 derived from single equine memory B cells able to cross-neutralize to influenza virus.
Emerg Microbes Infect, 13(1):2396864, 27 Sep 2024
Cited by: 0 articles | PMID: 39331815 | PMCID: PMC11441081
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.
J Int AIDS Soc, 24 Suppl 7:e25831, 01 Nov 2021
Cited by: 20 articles | PMID: 34806332 | PMCID: PMC8606870
Review Free full text in Europe PMC
Progress with induction of HIV broadly neutralizing antibodies in the Duke Consortia for HIV/AIDS Vaccine Development.
Curr Opin HIV AIDS, 18(6):300-308, 25 Sep 2023
Cited by: 1 article | PMID: 37751363 | PMCID: PMC10552807
Review Free full text in Europe PMC
Coadministration of CH31 Broadly Neutralizing Antibody Does Not Affect Development of Vaccine-Induced Anti-HIV-1 Envelope Antibody Responses in Infant Rhesus Macaques.
J Virol, 93(5):e01783-18, 19 Feb 2019
Cited by: 8 articles | PMID: 30541851 | PMCID: PMC6384077
Targeting broadly neutralizing antibody precursors: a naïve approach to vaccine design.
Curr Opin HIV AIDS, 14(4):294-301, 01 Jul 2019
Cited by: 11 articles | PMID: 30946041
Review
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: U19 AI160546
Grant ID: UM1 AI144371
Grant ID: R01 AI120801
Grant ID: T32 AI007392




