Abstract
Introduction
Atopic dermatitis (AD) is a chronic disease that occurs mainly in children. Topical corticosteroids are the main treatment for mild to moderate AD, although they can induce side effects. The efficacy and tolerability of xyloglucan and pea protein (XG-PP) was compared with hydrocortisone in pediatric patients with AD as a steroid-sparing solution.Methods
A prospective, multicenter, comparative study enrolled 42 patients (age 0.5-12 years) with mild-to-moderate AD, assigned 1:1 to XG-PP or hydrocortisone ointment. Treatments were applied twice daily for 14 consecutive days and assessed at baseline, day 8, and day 15. Efficacy endpoints were AD Severity Index (ADSI) score, Scoring Atopic Dermatitis (SCORAD) index, and Patient-Oriented Eczema Measure (POEM). Tolerability was assessed by the occurrence of adverse events (AEs).Results
Both treatments significantly improved ADSI mean score from baseline to day 15; in the XG-PP arm, ADSI score decreased from 10.55 to 4.15 (p = 0.00001), and in the hydrocortisone arm, from 10.65 to 4.30 (p = 0.0001). In the XG-PP arm, the mean SCORAD score decreased from 65.86 to 30.26 (p = 0.00001) and in the hydrocortisone arm from 68.84 to 31.19 (p = 0.0001) at day 15. An overall decrease from moderate to mild AD for both arms (p = 0.0001) was observed with POEM. For all the three indexes evaluated, no statistical significant differences between the study arms evolution from baseline to day 8 or to day 15 were found. No AEs were reported.Conclusion
XG-PP provided a comparable efficacy to hydrocortisone ointment in managing AD, thus representing a safe and effective steroid-sparing alternative in pediatric patients with AD.Trial registration
Retrospectively registered on 24 November 2021 in the ISRCTN registry: 11118799.Free full text

Efficacy and Tolerability of a Novel Topical Treatment Containing Pea Protein and Xyloglucan in the Management of Atopic Dermatitis in Children: A Prospective, Multicenter Clinical Study
Associated Data
Abstract
Introduction
Atopic dermatitis (AD) is a chronic disease that occurs mainly in children. Topical corticosteroids are the main treatment for mild to moderate AD, although they can induce side effects. The efficacy and tolerability of xyloglucan and pea protein (XG-PP) was compared with hydrocortisone in pediatric patients with AD as a steroid-sparing solution.
Methods
A prospective, multicenter, comparative study enrolled 42 patients (age 0.5–12 years) with mild-to-moderate AD, assigned 1:1 to XG-PP or hydrocortisone ointment. Treatments were applied twice daily for 14 consecutive days and assessed at baseline, day 8, and day 15. Efficacy endpoints were AD Severity Index (ADSI) score, Scoring Atopic Dermatitis (SCORAD) index, and Patient-Oriented Eczema Measure (POEM). Tolerability was assessed by the occurrence of adverse events (AEs).
Results
Both treatments significantly improved ADSI mean score from baseline to day 15; in the XG-PP arm, ADSI score decreased from 10.55 to 4.15 (p =
= 0.00001), and in the hydrocortisone arm, from 10.65 to 4.30 (p
0.00001), and in the hydrocortisone arm, from 10.65 to 4.30 (p =
= 0.0001). In the XG-PP arm, the mean SCORAD score decreased from 65.86 to 30.26 (p
0.0001). In the XG-PP arm, the mean SCORAD score decreased from 65.86 to 30.26 (p =
= 0.00001) and in the hydrocortisone arm from 68.84 to 31.19 (p
0.00001) and in the hydrocortisone arm from 68.84 to 31.19 (p =
= 0.0001) at day 15. An overall decrease from moderate to mild AD for both arms (p
0.0001) at day 15. An overall decrease from moderate to mild AD for both arms (p =
= 0.0001) was observed with POEM. For all the three indexes evaluated, no statistical significant differences between the study arms evolution from baseline to day 8 or to day 15 were found. No AEs were reported.
0.0001) was observed with POEM. For all the three indexes evaluated, no statistical significant differences between the study arms evolution from baseline to day 8 or to day 15 were found. No AEs were reported.
Conclusion
XG-PP provided a comparable efficacy to hydrocortisone ointment in managing AD, thus representing a safe and effective steroid-sparing alternative in pediatric patients with AD.
Trial Registration
Retrospectively registered on 24 November 2021 in the ISRCTN registry: 11118799.
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis (AD) affects between 10% and 20% of the pediatric population worldwide and has increased in developed and low-income countries in recent decades. Topical corticosteroids (TCS) are the mainstay to control skin inflammation and flare-ups in pediatric populations in the short term. |
| Although TCS have a good safety profile, adverse effects are possible, and the skepticism of patients and parents toward TCS often leads to non-compliance to treatment, which may result in unsatisfactory outcomes. |
| On the basis of the efficacy and tolerability of xyloglucan (XG) and pea protein (PP) in adult patients with AD, we expect to observe high tolerability and amelioration of signs and symptoms of AD in infants and children following a 14-day topical treatment with XG and PP. |
| What was learned from the study? |
| Topical treatment with XG and PP provides rapid symptom improvement in pediatric patients affected by mild and moderate AD with an efficacy similar to topical hydrocortisone. |
| This study confirms that the topical combination of XG and PP also evokes benefits in pediatric patients with mild to moderate AD. |
| The study shows that the novel natural-based cream containing XG and PP represents a possible alternative therapy for the treatment of AD in children. |
Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease that usually affects the face, scalp, trunk, flexural surfaces, and hands. The severity of the disease can range from mild to severe disease that is difficult to control [1, 2]. AD is characterized by dryness and lichenification and is often accompanied by a combination of pruritic erythema, excoriations, and serous exudate [3–5]. AD affects between 10% and 20% [6] of the pediatric population worldwide and has increased in developed countries in recent decades. Fifty to 60% of patients diagnosed with AD experience symptoms before the age of 1 year [3, 6, 7]. Numerous studies have shown that skin barrier dysfunction and immune system dysregulation play key roles in AD development. Skin barrier dysfunction has been linked to several gene mutations among which the most notable is the filaggrin (FLG) gene, which encodes the filaggrin protein, a key player in stratum corneum development and structure [8, 9].
Since AD is a chronic condition, the main goal of treatment is to reduce the number of exacerbations of the disease and to limit their severity and duration [10]. Basic therapy focuses on restoring skin barrier function by hydrating the skin with emollients or moisturizers. Topical treatments containing naturally derived non-pharmacological substances have gained much attention in the last few years. These topical solutions act through several synergistic mechanisms of action to preserve the lipid content of the skin barrier, preventing transepidermal water loss (TEWL), and thus moisturizing the skin. These solutions reduce inflammation and itch to improve atopic lesions. These types of moisturizers could be effective not only as maintenance therapy for AD but also when used synergistically with anti-inflammatory pharmacological therapies to prevent flare-ups [11, 12]. Topical corticosteroids (TCS) are the mainstay to control skin inflammation and flare-ups in pediatric populations in the short term. Topical treatments based on calcineurin inhibitors are also used for long-term management of disease flare-ups.
Although TCS have a good safety profile, adverse effects are possible, such as cutaneous atrophy, striae, purpura, telangiectasia, acne-like eruption, and systemic absorption with resulting adrenal suppression [13, 14]. The skepticism of patients and parents toward TCS often leads to non-compliance to treatment, which may result in unsatisfactory outcomes [15–17]. Plant-based products have been studied for a long time as an alternative and complementary strategy for the treatment of skin diseases, including AD [18]. Xyloglucan (XG) and pea protein (PP), in addition to emollients and skin conditioners, are the main components of a product manufactured by Devintec SAGL for the treatment of AD in children. XG and PP are substances of natural origin: XG is a non-ionic, neutral, branched polysaccharide consisting of a cellulose-like backbone derived from tamarind seeds [19]. XG is a US Food and Drug Administration (FDA)-approved food additive, a stabilizing agent, and a pharmaceutical excipient. Known for its wound-healing properties, XG can be used as a mucosae and tissue protectant, with restorative functions [20]. PPs are hypoallergenic proteins derived from peas [21], they are particularly rich in glutamine, aspartic acid, arginine, and lysine [22], and have been studied for their ability to help attenuate inflammatory symptoms and microbial colonization [23]. By synergistically creating a protective barrier over the skin, XG and PP have the ability to protect the tissue from external insults, by avoiding the adhesion and proliferation of bacteria, while promoting the natural recovery of the damaged skin barrier. Preclinical AD models demonstrated the ability of XG and PP to preserve epidermal integrity, reduce mast cell degranulation and type 2 interleukins, and support FLG recovery with comparable efficacy to hydrocortisone, suggesting that XG-PP-based products could be a promising approach for the treatment of this condition [24, 25]. Moreover, a double-blind, parallel, randomized, placebo-controlled clinical trial showed that a XG-PP-based formulation significantly improves AD severity even in an adult population, after only 8 days of treatment, representing an alternative solution for mild to moderate AD in adult patients [26].
The aim of the study was to evaluate, in infants and children with AD, the efficacy and tolerability of XG-PP-based topical treatment compared to hydrocortisone, commonly prescribed in the pediatric population.
Methods
Study Design
The present study is a prospective, multicenter, comparative clinical trial sponsored by Novintethical Pharma SA. The study was carried out between August 2019 and April 2020 in five medical centers in Romania, where a hydrocortisone ointment (10 mg/g) is the standard of care for the treatment of AD. Topical corticosteroids are recommended for AD-affected patients who have failed to respond to good skin care and regular use of emollients. The study was performed following the Declaration of Helsinki in accordance with the guideline on Good Clinical Practice (GCP) of the European Community. The study protocol (DERCBS18-06) received full regulatory approval from Romanian National Committee of Bioethics for Medicine and Medical Devices and has been registered in the ISRCTN registry (TN11118799).
Inclusion and Exclusion Criteria
Pediatric patients between 6 months and 12 years old with confirmed diagnosis of AD were considered eligible for the study. Patients were excluded from the study if they presented illness within 4 days before study enrollment or any medical condition that may affect study participation, if they had diagnosis or history of other dermatological conditions, if they had previous allergic reactions or known sensitivity to one or more of the study ingredients, or if they used medications that could affect the study or products that could have an effect similar to the product studied (e.g., corticosteroids, anti-inflammatory drugs). Parents or legal guardians signed a written informed consent prior to treatment.
Treatments
Patients were enrolled in the treatment study in a 1:1 ratio of XG-PP-based cream or hydrocortisone (10 mg/g ointment) with a regimen of two applications/day for 14 consecutive days. It was decided to use hydrocortisone—a low potency topical steroid normally used as standard of care in this category of patients and approved by the ethical committee—for the safety of the children in the study (i.e., to avoid potential complications of high potency topical steroids).
Patients performed three clinical visits: at day 0 (baseline), at day 8 (after 7 days of treatment), at day 15 (end of treatment).
Study Objectives and Endpoints
The primary objective of this study was the evaluation of the efficacy of the product containing XG-PP in alleviating the signs of AD. The primary endpoints were the change from baseline in AD Severity Index (ADSI) [27], a 4-point scale from none (0) to severe (3) for erythema, pruritus, exudation, excoriation, crusts, erosions, and lichenification; the change from baseline in the Scoring Atopic Dermatitis (SCORAD) index [28], calculated according to signs scored by the investigators; and the change from baseline in the Patient-Oriented Eczema Measure (POEM) 5-point scale [29]. All endpoints were evaluated at day 0, day 8, and day 15. ADSI score results can range between minimum 0 and maximum 15; the latter was considered the highest severity in AD evaluation. The SCORAD index assesses the extent (0–100), intensity (0–18), and subjective symptoms (0–20) in patients with AD. The intensity consists of six items: erythema, edema and papules, excoriations, lichenification, vesicles and crusts, and dryness. The subjective items included daily pruritus and sleeplessness. Both subjective items were scored on a 100-mm visual analogue scale where zero corresponds to not at all and 100 to the worst condition. The POEM assesses AD severity according to the following categories: POEM score 0–2, clear or almost clear; score 3–7, mild eczema; score 8–16, moderate eczema; score 17–24, severe eczema; score 25–28, very severe eczema.
The secondary objective was to assess the tolerability of the product studied by monitoring the occurrence of adverse events (AEs), defined as any unwarranted medical occurrence in a patient (any adverse event whether device related or not). The following parameters were evaluated: percentage of participants who experienced AEs, number of patients who discontinued the study as a result of AEs, and AD progression.
Statistical Analysis
Assuming a mean difference of 10 in the change in SCORAD index from baseline to day 15 based on previous studies and expert agreement, a standard deviation of 11, a significance level (α) of 0.05, a power of 80%, and a dropout rate of 10%, we calculated a sample size of 21 patients in each group [30]. Continuous data were expressed in terms of mean, standard deviation (SD), and number of observations. Categorical data were summarized in terms of the number of patients and percentages. To evaluate statistically significant differences in the demographic characteristics at baseline between the two study groups, t test or chi-square test was used. To examine the primary outcome, the t test and Wilcoxon signed-rank test were applied to compare the mean scores of ASDI scale, SCORAD index, and POEM scale in the two treatment groups. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 20.
Results
Population Characteristics
A total of 42 patients were enrolled in the study, 21 patients in each treatment group. There were 11 and 8 male patients in the XG-PP-based product and hydrocortisone groups, respectively (p =
= 0.53). The mean age of patients was 4 years (range 1–11) in the study product group and 7 years (range 1–12) in the hydrocortisone group (p
0.53). The mean age of patients was 4 years (range 1–11) in the study product group and 7 years (range 1–12) in the hydrocortisone group (p =
= 0.18). The study groups showed similar results and no statistical differences at baseline were observed (Table 1).
0.18). The study groups showed similar results and no statistical differences at baseline were observed (Table 1).
Table 1
Clinical characteristics of the comparative treatment groups
| Topical treatment containing XG and PP | Hydrocortisone | p value | |
|---|---|---|---|
| Age (years) | 4.90 (± 3.19) 3.19) | 6.30 (± 3.15) 3.15) | 0.18 |
| Gender (n) | 0.53 | ||
| Male | 10 | 13 | |
| Female | 11 | 8 | |
| Weight (kg) | 20.04 (± 9.81) 9.81) | 22.39 (± 8.48) 8.48) | 0.43 |
| Height (cm) | 107.20 (± 22.85) 22.85) | 114.85 (± 21.28) 21.28) | 0.29 |
| Body mass index | 16.57 (± 1.51) 1.51) | 16.39 (± 1.68) 1.68) | 0.72 |
| Systolic blood pressure (mmHg) | 105.00 (± 6.71) 6.71) | 106.25 (± 7.89) 7.89) | 0.60 |
| Diastolic blood pressure (mmHg) | 58.9 (± 6.48) 6.48) | 58.15 (± 4.53) 4.53) | 0.68 |
| Pulse (beats/min) | 88.65 (± 5.93) 5.93) | 87.05 (± 4.53) 4.53) | 0.41 |
| Body temperature (°C) | 36.30 (± 0.11) 0.11) | 36.05 (± 0.22) 0.22) | 0.32 |
| Breathing rate (breath/min) | 30.60 (± 6.48) 6.48) | 31.85 (± 6.31) 6.31) | 0.71 |
Data are mean ±
± SD unless otherwise stated
SD unless otherwise stated
XG xyloglucan, PP pea protein, SD standard deviation
Primary Endpoints
The primary endpoints of the study were the change in mean ADSI score, SCORAD index, and POEM score in both treatment arms from baseline to days 8 and 15. Both arms showed clinically significant improvement in ADSI score at day 8 and day 15. In the XG-PP treatment group, ADSI score decreased from a mean of 10.55 at baseline to 6.25 at day 8 (p =
= 0.00001), and 4.15 at day 15 (p
0.00001), and 4.15 at day 15 (p =
= 0.00001). In the hydrocortisone group, the ADSI mean score decreased from 10.65 at baseline to 6.85 at day 8 (p
0.00001). In the hydrocortisone group, the ADSI mean score decreased from 10.65 at baseline to 6.85 at day 8 (p =
= 0.00001), and to 4.30 at day 15 (p
0.00001), and to 4.30 at day 15 (p =
= 0.00001). No statistically significant differences were found between the study arms when comparing the decrease from baseline to day 8 (p
0.00001). No statistically significant differences were found between the study arms when comparing the decrease from baseline to day 8 (p =
= 0.91) or to day 15 (p
0.91) or to day 15 (p =
= 0.92) (Fig. 1a).
0.92) (Fig. 1a).
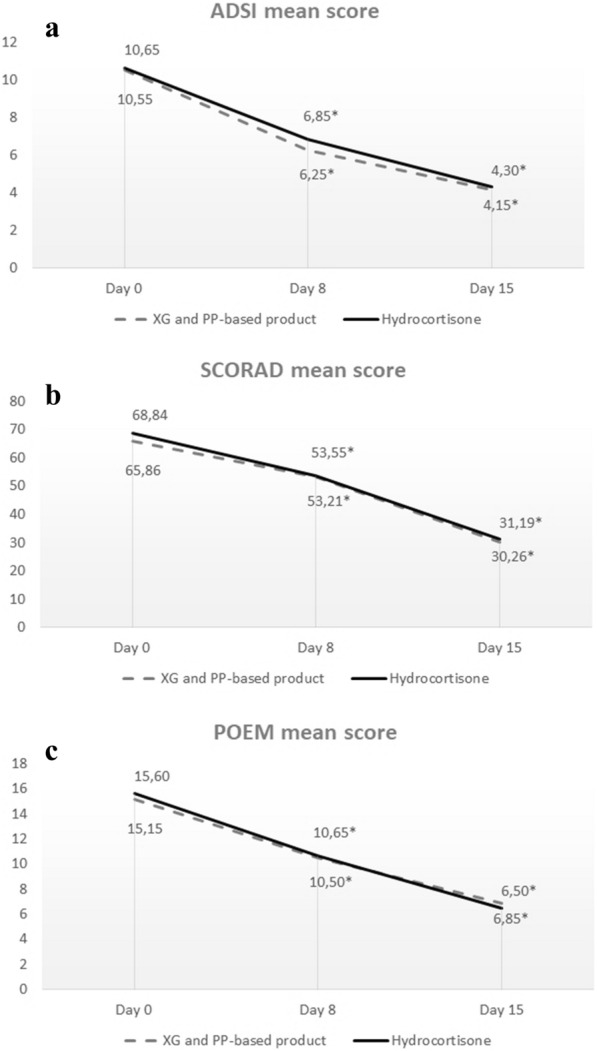
a ADSI mean score results at the time-point evaluations. No statistically significant differences were found between the study arms when comparing the decrease from baseline to day 8 (p =
= 0.91) or to day 15 (p
0.91) or to day 15 (p =
= 0.92). Statistically significant differences were found in the study arm compared to day 0 with p
0.92). Statistically significant differences were found in the study arm compared to day 0 with p =
= 0.00001 at day 8 and at day 15 in both the XG-PP and hydrocortisone treatment groups (*). b SCORAD mean score results at the time-point evaluations. No statistically significant differences were observed between study arms when comparing the decrease in SCORAD index from baseline to day 8 (p
0.00001 at day 8 and at day 15 in both the XG-PP and hydrocortisone treatment groups (*). b SCORAD mean score results at the time-point evaluations. No statistically significant differences were observed between study arms when comparing the decrease in SCORAD index from baseline to day 8 (p =
= 0.88) or to day 15 (p
0.88) or to day 15 (p =
= 0.93). Statistically significant differences were found in the study arm compared to day 0: p
0.93). Statistically significant differences were found in the study arm compared to day 0: p =
= 0.00001 at day 8 and at day 15 in the XG-PP treatment arm; p
0.00001 at day 8 and at day 15 in the XG-PP treatment arm; p =
= 0.0002 at day 8 and p
0.0002 at day 8 and p =
= 0.00001 at day 15 in the hydrocortisone treatment group (*). c Score results at the time-point evaluations. No statistically significant differences were found between study arms when comparing score decrease from baseline to day 8 (p
0.00001 at day 15 in the hydrocortisone treatment group (*). c Score results at the time-point evaluations. No statistically significant differences were found between study arms when comparing score decrease from baseline to day 8 (p =
= 0.89) or from baseline to day 15 (p
0.89) or from baseline to day 15 (p =
= 0.75). Statistically significant differences were found in the study arm compared to day 0 with a value of p
0.75). Statistically significant differences were found in the study arm compared to day 0 with a value of p =
= 0.00001 at day 8 and at day 15 in both the XG-PP and hydrocortisone treatment groups (*)
0.00001 at day 8 and at day 15 in both the XG-PP and hydrocortisone treatment groups (*)
Analysis of the SCORAD index showed a significant improvement in mean scores for both arms. In the XG-PP arm, the mean SCORAD score decreased significantly from baseline (65.86) to day 8 (53.21; p =
= 0.00001) and to day 15 (30.26; p
0.00001) and to day 15 (30.26; p =
= 0.00001). In the hydrocortisone arm, a statistically significant decrease in the mean SCORAD score was observed between baseline (68.84) and day 8 (53.55; p
0.00001). In the hydrocortisone arm, a statistically significant decrease in the mean SCORAD score was observed between baseline (68.84) and day 8 (53.55; p =
= 0.0002) and baseline and day 15 (31.19; p
0.0002) and baseline and day 15 (31.19; p =
= 0.00001). No statistically significant differences were observed between study arms when comparing the decrease in SCORAD index from baseline to day 8 (p
0.00001). No statistically significant differences were observed between study arms when comparing the decrease in SCORAD index from baseline to day 8 (p =
= 0.88) or to day 15 (p
0.88) or to day 15 (p =
= 0.93) (Fig. 1b). Assessment of the POEM score showed improvement in the group of patients treated with the XG-PP-based product. The mean score of 15.15 at baseline, indicating moderate eczema, decreased to 10.50 (p
0.93) (Fig. 1b). Assessment of the POEM score showed improvement in the group of patients treated with the XG-PP-based product. The mean score of 15.15 at baseline, indicating moderate eczema, decreased to 10.50 (p =
= 0.00001) at day 8 and to 6.85 at day 15 (p
0.00001) at day 8 and to 6.85 at day 15 (p =
= 0.00001), indicating mild eczema. In the hydrocortisone group, the POEM mean score at baseline was 15.60, indicating moderate eczema, which improved to 10.65 and to 6.50 on day 15, indicating mild eczema; comparison between baseline and day 8 or day 15 showed a statistically significant difference in patient evolution (p
0.00001), indicating mild eczema. In the hydrocortisone group, the POEM mean score at baseline was 15.60, indicating moderate eczema, which improved to 10.65 and to 6.50 on day 15, indicating mild eczema; comparison between baseline and day 8 or day 15 showed a statistically significant difference in patient evolution (p =
= 0.00001). No statistically significant differences were found between study arms when comparing score decrease from baseline to day 8 (p
0.00001). No statistically significant differences were found between study arms when comparing score decrease from baseline to day 8 (p =
= 0.89) or from baseline to day 15 (p
0.89) or from baseline to day 15 (p =
= 0.75) (Fig. 1c).
0.75) (Fig. 1c).
Secondary Endpoints
Evaluation of the secondary endpoints showed that there was no AE or AD progression on day 8, 15, and 28 of treatment with XG-PP.
Discussion
This study confirms the beneficial effects of the novel topical treatment containing XG-PP in relieving symptoms associated with AD in pediatric patients, with a comparable efficacy to that of hydrocortisone, confirming the initial assumption that XG and PP are effective to protect the tissue from external insults by synergistically creating a protective barrier over the skin [31]. Improvements assessed by the primary endpoints demonstrated a rapid and continuous effect of the product. Indeed, ADSI, SCORAD, and POEM mean scores showed a statistically significant decrease from baseline to day 8 of the assessment and another significant decrease on day 15. In addition, patients enrolled had moderate AD at baseline, as indicated by the POEM score, and 2 weeks of treatment with XG and PP reduced the mean AD grade of the study cohorts to “mild,” indicating that pediatric patients with moderate AD are the potential target population for XG and PP cream. In particular, POEM is well recognized to be a feasible tool also for clinical practice [1, 7, 32]. Tolerability analysis confirmed the good safety profile of the study product as no AEs nor dropouts were reported.
Several clinical trials comparing the efficacy of TCS with emollients are available, but they are mostly characterized by inconsistent study design [33, 34]. A recently published meta-analysis of AD pediatric studies found that treatments with TCS tended to be more effective than vehicles or moisturizers, but generalization is not possible because of the wide heterogeneity of products with different moisturizing properties [33]. Interestingly, several studies have shown that the co-administration of TCS and emollients or moisturizers improves symptoms associated with AD [35, 36]. In this regard, a new generation of emollients containing active non-pharmacological ingredients has gained attention for their corticosteroid-sparing effect when used as an alternative to TCS [11]. A multicenter, open-label trial using an oat-based emollient in the maintenance therapy of 108 children (ages 6 months–6 years) with moderate AD demonstrated that the number of flares and use of TCS significantly decreased during 3 months of treatment [37]. Additionally, in subjects above 3 years of age with mild and stable AD, the treatment containing vitamin E, tocopherol, glycerine, and enriched with Aqua posae filiformis and microresyl significantly reduces AD clinical signs and symptoms and increases skin hydration after 28 days, thus improving the patients’ quality of life [38].
The results of this study show that the novel natural-based cream containing XG and PP represents a possible substitute therapy for the treatment of AD in children taking TCS. Notably, XG and PP can significantly reduce AD-associated symptoms within a week of treatment which may avoid short- and long-term AEs caused by TCS and improve treatment adherence [39–42].
Importantly, this is the first clinical study to verify the efficacy and safety of a topical formulation containing XG and PP in children, confirming the results observed in preclinical studies [25]. Additionally, oral utilization of XG and PP in adults demonstrated no systemic adverse events during 6 months of treatment [43, 44]. To date, the literature revealed that phytotherapeutic treatments for AD and products of natural origin are often considered adjunctive therapies for the treatment of moderate to severe AD [18, 45]. Clinical trials assessing the efficacy and safety of topical phytotherapy for AD treatment are limited. In one randomized controlled study, 55% of subjects treated with aloe vera reported drying up of the skin on test areas while no serious adverse effects were recorded [46]. More recent data showed that aloe vera [47] or Cardiospermum halicacabum [48] based creams exhibit a good efficacy profile. On the other hand, other studies indicated that a prolonged use of topical aloe vera-based products could lead to unwanted side effects such as urticaria [49], contact dermatitis [50–52], and widespread dermatitis [53].
The reported results are significant because the treatment studied was shown to be as effective and safe as the standard short-term steroid-sparing therapy for pediatric patients with mild to moderate AD. The reported results of XG and PP also suggest that this novel therapeutic could act as an effective and safe alternative maintenance treatment after the patient has undergone reactive therapy with topical corticosteroids.
The importance of a fast-acting product with the ability to decrease symptom severity within 1 week greatly impacts the daily lives of patients and their families which leads to an improved AD management [54]. Controlled clinical trials are advisable to explore the possibility that such treatment may reduce the severity of AD over time, thereby reducing the burden of disease on the patients and caregivers. In addition, the inclusion of a quality of life tool in the study endpoints would be useful to assess the impact of the treatment on everyday life; the same applies to objective measures such as self-report questionnaires that can provide information about patients’ feelings regarding product tolerability.
There are a few limitations to the study. First, the number of participants included into the study is limited. Further studies should be based on larger groups of patients. Moreover, patients were screened and enrolled by dermatologists at their individual offices and/or medical centers. The doctors had to confirm a diagnosis of AD through a clinical evaluation based on their experience in order to enroll a patient. However, a more stringent selection process would be beneficial. Secondly, it was not possible to collect some information such as the date of eczema onset, comorbidities, and the percentage of participants previously treated with systemic agents and/or topical corticosteroids. Lastly, the follow-up period was 2 weeks. Given the promising safety and efficacy results obtained, longer follow-up studies, including other scores such as EASI, VAS pruritus, and IGA, are needed to demonstrate the efficacy in preventing flare-ups and the establishment of long-term disease control.
Conclusion
Topical treatment with XG and PP provides rapid and durable benefits in pediatric patients affected by mild and moderate AD. The efficacy of this treatment is comparable to mild-potency topical corticosteroid therapy. Use of XG-PP can therefore be considered as a steroid-sparing strategy. XG-PP is also characterized by an excellent tolerability, embodying a potential maintenance treatment. Future research in children should extend studies on the fast action of the treatment to improve quality of life and the ability to reduce flare-ups for long-term AD management.
Acknowledgements
We thank the participants of the study.
Medical Writing and Editorial Assistance
The authors didn’t receive any medical writing or editorial assistance for this article.
Author Contributions
Roni P. Dodiuk-Gad contributed to results, discussion, reading and approval of the manuscript. Stefano Veraldi contributed to study conception and design, discussion of results, manuscript reading and approval (but did not participate in data collection and analysis). All other authors contributed to the study conception, design, data collection and analysis. All authors discussed the results, contributed to the final manuscript, read and approved the final manuscript.
Funding
Sponsorship for this study was funded by Novintethical Pharma SA, and Rapid Service Fee was funded by Devintec SAGL.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
The authors have no conflict of interests to declare.
The study was performed following the Declaration of Helsinki in accordance with the guideline on Good Clinical Practice (GCP) of the European Community. The study protocol (DERCBS18-06) received full regulatory approval from Romanian National Committee of Bioethics for Medicine and Medical Devices and has been registered in the ISRCTN registry (TN11118799).
References
Articles from Dermatology and Therapy are provided here courtesy of Springer
Full text links
Read article at publisher's site: https://doi.org/10.1007/s13555-023-01035-6
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s13555-023-01035-6.pdf
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/155328923
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Topical Application of a Formulation Containing Pea Proteins and Xyloglucan in Adult Patients with Atopic Dermatitis: A Double-blind, Parallel, Randomized, Placebo-controlled, Multicenter Study.
J Clin Aesthet Dermatol, 16(7):35-41, 01 Jul 2023
Cited by: 2 articles | PMID: 37560506 | PMCID: PMC10409507
Once-Daily Crisaborole Ointment, 2%, as a Long-Term Maintenance Treatment in Patients Aged ≥ 3 Months with Mild-to-Moderate Atopic Dermatitis: A 52-Week Clinical Study.
Am J Clin Dermatol, 24(4):623-635, 15 May 2023
Cited by: 4 articles | PMID: 37184828 | PMCID: PMC10184626
Crisaborole Topical Ointment, 2% in Adults With Atopic Dermatitis: A Phase 2a, Vehicle-Controlled, Proof-of-Concept Study.
J Drugs Dermatol, 14(10):1108-1112, 01 Oct 2015
Cited by: 31 articles | PMID: 26461821
Omalizumab for severe atopic dermatitis in 4- to 19-year-olds: the ADAPT RCT
National Institute for Health and Care Research, Southampton (UK), 10 Jun 2022
Cited by: 0 articles | PMID: 35679442
ReviewBooks & documents Free full text in Europe PMC
Funding
Funders who supported this work.

 1
1 


