Abstract
Background
The United States Food and Drug Administration issued a black box warning on the mental health adverse effects of montelukast in 2020. Age-related effects on the risk of developing specific neuropsychiatric events in montelukast users remain largely unknown.Objective
To describe the risk of neuropsychiatric events associated with montelukast in adults and children with asthma.Methods
A systematic search of all studies investigating neuropsychiatric events in montelukast users was performed in PubMed, the Cochrane Library and Embase from inception to 7 September 2022. Animal studies and conference abstracts were excluded.Results
59 studies (21 pharmacovigilance studies, four reviews from 172 randomised controlled trials, 20 observational studies, 10 case reports and four case series) evaluating neuropsychiatric events in patients with asthma on montelukast were reviewed. No significant association was shown between montelukast and suicide-related events in six of the observational studies. No association was found for depression as defined by the International Classification of Diseases 10th revision codes in three observational studies and a review of randomised clinical trials. However, findings from four studies using antidepressant prescriptions as the outcome identified significant associations. Consistent with nine pharmacovigilance studies, two large-scale observational studies revealed possible associations of montelukast with anxiety and sleeping disorders in adult patients with asthma, respectively. However, the results were not replicated in two observational studies on children.Conclusion
Montelukast is not associated with suicide- and depression-related events in asthma patients. Older adults may be particularly susceptible to anxiety and sleeping disorders.Free full text

Neuropsychiatric events associated with montelukast in patients with asthma: a systematic review
Abstract
Background:
The United States Food and Drug Administration issued a black box warning on the mental health adverse effects of montelukast in 2020. Age-related effects on the risk of developing specific neuropsychiatric events in montelukast users remain largely unknown.
Objective:
To describe the risk of neuropsychiatric events associated with montelukast in adults and children with asthma.
Methods:
A systematic search of all studies investigating neuropsychiatric events in montelukast users was performed in PubMed, the Cochrane Library and Embase from inception to 7 September 2022. Animal studies and conference abstracts were excluded.
Results:
59 studies (21 pharmacovigilance studies, four reviews from 172 randomised controlled trials, 20 observational studies, 10 case reports and four case series) evaluating neuropsychiatric events in patients with asthma on montelukast were reviewed. No significant association was shown between montelukast and suicide-related events in six of the observational studies. No association was found for depression as defined by the International Classification of Diseases 10th revision codes in three observational studies and a review of randomised clinical trials. However, findings from four studies using antidepressant prescriptions as the outcome identified significant associations. Consistent with nine pharmacovigilance studies, two large-scale observational studies revealed possible associations of montelukast with anxiety and sleeping disorders in adult patients with asthma, respectively. However, the results were not replicated in two observational studies on children.
Conclusion:
Montelukast is not associated with suicide- and depression-related events in asthma patients. Older adults may be particularly susceptible to anxiety and sleeping disorders.
Tweetable abstract
Montelukast was not associated with suicide-related events in asthma patients. Age may be a possible effect modifier on the association between montelukast and anxiety and sleeping disorders, with a higher risk of adverse events in the elderly. https://bit.ly/3DjylmF
Introduction
Montelukast is the most commonly prescribed leukotriene receptor antagonist (LTRA) to reduce airway inflammation in asthma and prevent exercise-induced bronchoconstriction. Montelukast has its own merits in being orally administered as once-daily dosing, and targets alternative inflammatory pathways to those targeted by inhaled corticosteroids (ICS), which may offer therapeutic benefits to specific groups of patients [1]. Therefore, the Global Initiative for Asthma guideline recommends LTRAs as an alternative or add-on controller to ICS in the management of persistent asthma, or as an alternative controller in initial asthma management in children aged ≤5 years [2].
The risk of neuropsychiatric events in montelukast users has long been an alarming issue for regulatory authorities. The issue first came under scrutiny in 2009, after the US Food and Drug Administration (FDA) issued a change in manufacturer labelling over the neuropsychiatric safety of montelukast [3], and contrasted with recommendations from the joint statement issued by the American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma & Immunology [4]. In March 2020, the FDA further issued a black box warning about the risk of mental health side-effects in montelukast users after reviewing case reports submitted to the FDA Adverse Event Reporting System [5]. The FDA also announced the initiation of an observational study using data under the Sentinel Initiative for the surveillance of medical products [6].
Recent work using data from electronic health record databases worldwide has reported possible relationships between montelukast and neuropsychiatric events, such as sleeping disorders [7], depression [6], dementia [8] and attention deficit/hyperactivity disorder (ADHD) [9]. The largest observational study to date initiated by the FDA Sentinel Initiative revealed a reduced risk of outpatient depressive disorders [6], yet paediatric Canadian studies identified increased risk of neuropsychiatric events [10, 11]. Two observational studies from Japan [8] and Norway [12] have also demonstrated possible paradoxical protective effects of montelukast in neurodegenerative diseases like dementia. However, the results are inconclusive to date owing to heterogeneity in study populations and outcomes. Therefore, we conducted this systematic review to describe the risk of neuropsychiatric events stratified by different psychiatric diagnoses in patients with asthma on montelukast. This review not only serves as an update on our 2018 systematic review [13], but also adds new perspectives on the overall safety or the possible therapeutic potential of montelukast in certain neuropsychiatric diseases in selected patient populations.
Methods
Search strategy
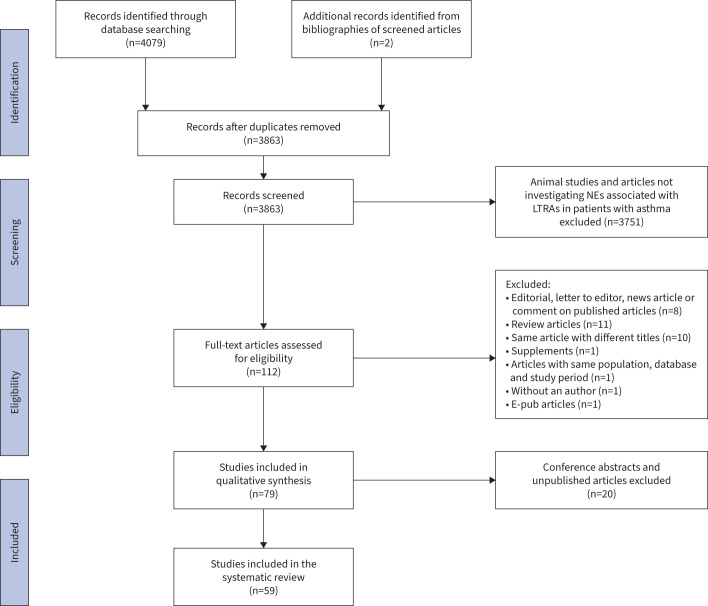
This systematic literature review was carried out from database inception to 7 September 2022. All studies were identified from databases, including PubMed, Cochrane Library and Embase, using Medical Subject Heading (MeSH) terms and keywords relevant to LTRAs and various neuropsychiatric events (figure 1). The list of keywords is included in supplementary material 1. Additional studies were screened from the bibliographies of the identified articles. Studies were screened and identified independently by authors CWHL and SP.
Inclusion and exclusion criteria of studies
All published studies (observational studies, case reports and randomised controlled trials (RCTs)) investigating the associations between montelukast and neuropsychiatric events in patients with asthma were eligible for inclusion, irrespective of age, language and study period. To ensure sufficient sensitivity, studies with all sample sizes of montelukast users were eligible for inclusion as long as the majority (>50%) of the study population consisted of patients with asthma. Articles and studies not in English were translated. Animal studies, commentaries and conference extracts were excluded. Details of results from clinical trials that reported neuropsychiatric events in montelukast users have been summarised in previous systematic reviews of RCTs.
Data extraction
Data from studies were extracted using the PICO (population, intervention, comparison and outcomes) tool independently by CWHL and SP. Discrepancies in the inclusion of studies were discussed and resolved by the two authors. Study results were extracted by CWHL, and SP reviewed and provided additional details whenever necessary. The PICO tool is described in supplementary material 2. No discrepancies were found in the quality assessment process or described otherwise. This paper defined the collation of adverse drug event reports from pharmacovigilance databases worldwide as “pharmacovigilance studies”. Study characteristics of pharmacovigilance studies, case reports and case series are summarised in supplementary material 3–5. Information from case reports, including age, gender, the onset of adverse drug reactions, concomitant medications and prognosis, was extracted. The inclusion and exclusion criteria, exposure groups, study outcome(s), key results and the study periods for observational studies are reported and summarised in tables 1–4.
TABLE 1
Study characteristics of research studies on suicide-related outcomes
| Study (publication year) | Study design | Study/inclusion period | Inclusion criteria | Exclusion criteria | Subjects (n) | End-points | Key findings |
| Paljarvi et al. [7] (2022) | Retrospective cohort study | 2015–2019 | For asthma group: 1. Asthma-related healthcare contact 2. Aged 15–64 years at index prescription of MTK 3. Alive for 12 months after index prescription | 1. Dx of COPD 2. Dx of obstructive sleep apnoea 3. Dx of neoplasm 4. Pregnancy during study period | MTK users (36 245) vs non-users (36 245) vs non-users (36 245) 245) | 1. 12-month incident Dx for NEs, including bipolar disorders, depression, anxiety disorders, OCD and sleeping disorders 2. 12-month incident dispensed prescriptions for psychiatric medications | OR (95% CI) (propensity score-matched) for: 1. any non-fatal self-harm in 1 year: 1.06 (0.72–1.55) |
| Sansing-Foster et al. [6] (2020) | Retrospective cohort study | 2000–2015 | 1. Aged >6 years with both medical and drug coverage for at least 183 days before medication initiation 2. Asthma Dx in any care setting 3. No Dx of COPD during 183 days prior to index date | 1. Experienced outcome on cohort entry day 2. >45-day gap between consecutive enrolment periods for medical and drug coverage 3. Recipients of LTRA, LAMA and ICS 183 days prior to index date 4. Same-day dispensing for both MTK and ICS on index date | MTK (457 377) vs ICS (457 377) vs ICS (457 377) 377) | 1. Hospitalisation for depressive disorders defined as inpatient claims 2. Treated outpatient depressive disorders 3. Hospitalisation for self-harm 4. Modified self-harm (by E-codes) | HR (95% CI) for 1. self-harm: 0.92 (0.69–1.21) 2. modified self-harm: 0.81 (0.63–1.05) |
| Lu et al. [14] (2015) | ITS | 2005–2010 | 1. Aged 5–64 years 2. Continuous health enrolment plan for past 12 months 3. Enrolled for health plan for current month 4. At least one outpatient or inpatient visit in the past year due to asthma diagnosis | History of: 1. COPD, cystic fibrosis, bronchiectasis 2. Pulmonary hypertension or embolism 3. Bronchopulmonary dysplasia 4. CHF | 140 000 (rolling cohort with asthma) 000 (rolling cohort with asthma) | Monthly percentage of patients 1. dispensed an LTRA and non-LTRA 2. on mental health visits Quarterly percentage of patients 3. medically treated for suicide attempts | 1 year after the FDA label change in 2009, suicide attempts: 1. did not change in adolescents 2. increased by 0.03 (95% CI 0.01–0.05) percentage points in young adults (aged 18–29 years) 3. increased by 0.01 (95% CI 0.00–0.01) percentage points in adults (aged 30–64 years) |
| Chen et al. [15] (2014) | Retrospective cohort study | 2000–2008 | 1. Aged >10 years 2. Inpatient asthma Dx or ≥2 recorded outpatient asthma Dx 3. 1 year duration of asthma | 1. Received asthma Dx from 1997–1999 2. Completed suicide (deaths within 2 weeks of self-harm) | cDDD 0–90: 165 960 960cDDD ≥90: 726 | 1. Self-harm (ICD-9 E950–E959, E980–E989) | 1. Adjusted HR (95% CI) for asthma diagnosis: 1.70 (1.35–2.14) 2. Adjusted HR (95% CI) for MTK cDDD ≥90: 0.92 (0.29–2.91) |
| Schumock et al. [16] (2012) | Case–control | 1997–2006 | 1. Aged 5–24 years 2. ≥1 prescription for asthma controller medication (ICS, LTRA, LABA, methylxanthines, immunomodulators, mast cell stabilisers, inhaled anticholinergics) | 1. Received asthma controller medication >30 days before date of asthma diagnosis 2. Last enrolment date on or before index date 3. Not continuously enrolled for ≥6 months 4. Not continuously enrolled for ≥2 months and 20% or more of total months | Case: 344 Control: 3438 | 1. Suicide attempt (ICD-9 E950–E959) | Adjusted OR (95% CI) for 1. ever users of LTRA: 0.74 (0.46–1.20) 2. current users of LTRA: 0.70 (0.36–1.39) |
| Schumock et al. [17] (2011) | Ecological study | 1999–2006 | Poisson regression analysis of association between LTRA and suicide deaths at country level | – | 249 872 suicide deaths 872 suicide deaths | 1. Suicide deaths | 1. Association between rate of MTK prescriptions dispensed and suicide rate: −0.0003, p=0.0217 2. Estimate rate multiplier for MTK: 0.9997 (95% CI 0.9994–0.9999) |
| Jick et al. [18] (2009) | Cohort | 1998–2007 | 1. All patients receiving ≥1 MTK prescriptions | – | MTK (23 500) 500) | 1. Rate of suicide completed 2. Person-year(s) at risk of suicide | 1. Rate (95% CI) of suicide with asthma Dx: 1.02 (0.6–1.5) per 100 000 person-years 000 person-years2. At risk for suicide: 21  050 person-years 050 person-years |
| Philip et al. [19] (2009) | Reviews of RCTs (116 trials#) | Up to 11 March 2008 | 1. All age ranges 2. Regardless of approval status for any indication completed by 11 March 2008 3. Early (Phase 1 and Phase 2a trials)/Late (Phase 2b/Phase 3) | 1. Subjects who had clinically significant psychiatric disorders | MTK (20 131) vs Placebo (9287) vs Active control (8346) 131) vs Placebo (9287) vs Active control (8346) | 1. ADRs¶ | 1. No completed suicides reported in any study 2. 1 patient each in active-controlled and open-label studies |
ADR: adverse drug reaction; cDDD: cumulative defined daily dose; CHF: congestive heart failure; Dx: diagnosis; FDA: US Food and Drug Administration; HR: hazard ratio; ICD-9: International Classification of Diseases, ninth revision; ICS: inhaled corticosteroid; ITS: interrupted time series; LAMA: long-acting muscarinic antagonist; LTRA: leukotriene receptor antagonist; MTK: montelukast; NE: neuropsychiatric event; OCD: obsessive compulsive disorder; OR: odds ratio; RCT: randomised controlled trial; vs: versus. #: controls include placebo and active control while trials included randomised, double-blind, placebo-controlled parallel-group trials; randomised, double-blind, placebo-controlled crossover trials; and open-label studies; ¶: ADRs include completed suicide, suicide attempt, suicidal ideation and other terms suggesting self-injurious behaviour.
TABLE 4
Study characteristics of research studies on neurodegenerative disease-related outcomes
| Study (publication year) | Study design | Study period | Inclusion criteria | Exclusion criteria | Subjects (n) | End-points | Key findings |
| Ishikura et al. [8] (2021) | Retrospective cohort study | 2006–2015 | 1. New diagnosis of bronchial asthma during study period (defined as ≥2 outpatient claims or ≥1 inpatient claims) 2. Aged ≥50 years at the time of diagnosis 3. ≥2 prescriptions of LTRA during study period | 1. Zafirlukast users 2. Diagnosis of dementia before bronchial asthma diagnosis, before the first prescription or between the first and second prescription of LTRA 3. Enrolment period of <6 months 4. Diagnosis based on “Suspected” diagnostic codes | LTRA users (10 471) vs non-users (10 471) vs non-users (10 471) 471) | 1. Onset of dementia of all types identified by ICD-10 codes 2. Subtypes of dementia (Alzheimer's dementia and vascular dementia) | 1. Adjusted HR (95% CI) for dementia: 0.42 (0.20–0.87) 2. Incidence rates of dementia in LTRA users: 0.52 cases per 1000 person-years |
| Xiong et al. [30] (2021) | Longitudinal observational study | 2005–2021 | 1. Cognitively normal: normal cognition and did not use any medications for dementia 2. MCI: MCI diagnosis 3. AD: all-cause dementia diagnosis with AD as primary or contributing cause of cognitive impairment defined by NINCDS-ADRDA or NIA-AA criteria | 1. Patients who did not complete the medication form 2. Patients who did not have baseline data to identify the variables used in propensity score matching | Number (Cognitively normal/MCI/AD): LTRA users (350/200/151) vs non-users (1050/600/453) | 1. Immediate logical memory 2. Delayed logical memory 3. Psychomotor processing speed 4. Language test scores | Adjusted relative risk (95% CI) (cognitively normal/MCI/AD) for 1. Immediate memory: 0.992 (0.985–0.998)#/1.015 (0.991–1.039)/1.048 (0.972–1.130) 2. Delayed memory: 0.995 (0.987–1.002)/1.032 (0.993–1.074)/1.065 (0.932–1.217) Unstandardised coefficient (95% CI) (AD) for 1. Digit Symbol Substitution Test: 1.466 (0.253–2.678)# 2. Boston Naming Test: 0.529 (0.215–0.866)# 3. animal naming: 0.541 (0.215–0.866)# 4. vegetable naming: 0.309 (0.056–0.561)# |
| Grinde and Endahgl [12] (2017) | Retrospective cohort study | 2004–2015 | 1. ≥2 prescriptions of MTKs or inhalation-type asthma medication 2. aged ≥60 years in 2014 | – | MTK (23 636) vs non-users (179 636) vs non-users (179 837) 837) | 1. Use of dementia medicine¶,+ 2. Admission to nursing home 3. Death 4. Parkinson's medicine+ 5. Diabetes medicine+ | Adjusted HR (95% CI) for 1. dementia medicine: 0.89 (0.81–0.98) 2. Parkinson's medicine: 1.06 (0.98–1.15) 3. diabetes medicine: 0.85 (0.80–0.90) |
AD: Alzheimer’s disease; HR: hazard ratio; ICD-10: International Classification of Diseases, tenth revision; LTRA: leukotriene receptor antagonist; MCI: mild cognitive impairment; MTK: montelukast; NIA-AA: National Institute on Aging–Alzheimer's Association; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association; vs: versus. #: significant after adjusting for a false discovery rate of 10%; ¶: includes memantine, donepezil, rivastigmine or galantamine; +: use of drugs defined under the Anatomical Therapeutic Chemical classification system.
TABLE 2
Study characteristics of research studies on depression-related outcomes
| Study (publication year) | Study design | Study/inclusion period | Inclusion criteria | Exclusion criteria | Subjects (n) | End-points | Key findings |
| Paljarvi et al. [7] (2022) | Retrospective cohort study | 2015–2019 | For asthma group: 1. Asthma-related healthcare contact 2. Aged 15–64 years at index prescription of MTK 3. Alive for 12 months after index prescription | 1. Dx of COPD 2. Dx of obstructive sleep apnoea 3. Dx of neoplasm 4. Pregnancy during study period | MTK users (36 245) vs non-users (36 245) vs non-users (36 245) 245) | 1. 12-month incident Dx for NEs, including bipolar disorders, depression, anxiety disorders, OCD and sleeping disorders 2. 12-month incident dispensed prescriptions for psychiatric medications | OR (95% CI) (propensity score-matched) for: 1. single episode of major depression in 1 year: 1.05 (0.96–1.15) 2. receipt of antidepressant: 1.16 (1.07–1.26) |
| Sansing-Foster et al. [6] (2020) | Retrospective cohort study | 2000–2015 | 1. Aged >6 years with both medical and drug coverage for at least 183 days before medication initiation 2. Asthma Dx in any care setting 3. No Dx of COPD during 183 days prior to index date | 1. Experienced outcome on cohort entry day 2. >45-day gap between consecutive enrolment periods for medical and drug coverage 3. Recipients of LTRA, LAMA and ICS 183 days prior to index date 4. Same-day dispensing for both MTK and ICS on index date | MTK (457 377) vs ICS (457 377) vs ICS (457 377) 377) | 1. Hospitalisation for depressive disorders defined as inpatient claims 2. Treated outpatient depressive disorders 3. Hospitalisation for self-harm 4. Modified self-harm (by E-codes) | HR (95% CI) for 1. hospitalisation for depressive disorders: 1.06 (0.90–1.24) 2. hospitalisation for depressive disorders in patients without a psychiatric history: 0.63 (0.37–1.07) 3. treated outpatient depressive disorders: 0.91 (0.89–0.93) |
| Ali et al. [20] (2015) | Case–control | 1998–2009 | 1. Aged 1–17 years 2. Primary Dx of asthma 3. Health plan enrolment in the 12 months before and after asthma claim | 1. Developmental disorders 2. Receiving long-term care 3. Pre-existing NE in 365 days before asthma claims | Case: 1920 Control: 5760 | As post hoc analyses: 1. Anxiety disorders 2. Depressive disorders 3. Aggressive disorders | Adjusted OR (95% CI) for patients exposed to MTK in the past 365 days for: 1. depressive disorders: 1.01 (0.66–1.54) |
| Zhou et al. [21] (2013) | ITS | 2003–2007 | 1. Aged ≤45 years 2. ≥2 asthma controller prescriptions in the study period 3. ≥1 medical and pharmacy claim 12 months before and after index date (1st prescription) | 1. Aged ≥46 years (higher likelihood of COPD) 2. Other asthma controllers during 12 months before and up to 3 months after index date | MTK (232 159) vs fluticasone (264 159) vs fluticasone (264 704) vs LABA/ICS (89 704) vs LABA/ICS (89 635) 635) | 1. Antidepressant dispensing rate (only medications as monotherapy are included) | Immediate change (95% CI) for antidepressant dispensing rates after index date: 1. Patient aged 1–11 years: MTK (0.14% (0.06–0.22%))# vs fluticasone (0.17% (−0.01–0.35%)) vs LABA/ICS (0.16% (−0.06–0.37%)) 2. Patient aged 12–17 years: MTK (0.80% (0.31–1.29%))# vs fluticasone (0.83% (−0.08–1.73%)) vs LABA/ICS (0.76% (0.22–1.30%))# 3. Patient aged 18–24 years: MTK (1.93% (1.55–2.32%))# vs fluticasone (1.72% (1.30–2.15%))# vs LABA/ICS (2.76% (2.35–3.17%))# 4. Patient aged 25–45 years: MTK (3.62% (3.15–4.09%))# vs fluticasone (2.38% (1.54–3.21%))# vs LABA/ICS (4.03% (3.34–4.73%))# |
| Holbrook and Harik-Khan [22] (2008) | Reviews of RCTs (3 trials¶) | + | Randomised, double-masked, controlled trials conducted by the ALA-ACRC including MTK as treatment arm | - | MTK (569) vs placebo/active control (900) | 1. Emotional well-being§ | 1. No statistically significant changes in quality-of-life scores from all three trials for MTK arm |
ALA-ACRC: American Lung Association Asthma Clinical Research Centers; Dx: diagnosis; HR: hazard ratio; ICS: inhaled corticosteroid; ITS: interrupted time series; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; LTRA: leukotriene receptor antagonist; MTK: montelukast; NE: neuropsychiatric event; OCD: obsessive compulsive disorder; OR: odds ratio; RCT: randomised controlled trial; vs: versus. #: p<0.05; ¶: controls included placebo and active control, plus randomised, double-blind, placebo-controlled parallel-group trials; +: trials included were conducted by the ALA-ACRC; §: emotional well-being evaluated by the Juniper Mini Asthma Quality of Life emotional dimension scores.
TABLE 3
Study characteristics of research studies on other neuropsychiatric outcomes
| Study (publication year) | Study design | Study period | Inclusion criteria | Exclusion criteria | Subjects (n) | End-points | Key findings |
| Park et al. [23] (2022) | Self-controlled case series | 2005–2007 and 2016–2018 | 1. Aged 3–30 years at the beginning of each study period 2. Dx of NE 3. Record of LTRA use (MTK or pranlukast) 4. Dx of asthma or allergic rhinitis | 1. Patients who died during the study period 2. Patients who received LTRA or had a Dx of NE within 1 year before the observation period | 17 001# 001# | 1. Newly diagnosed NE during Obs period, categorised into psychotic, mood, anxiety, sleep, cognitive, movement and personality disorders | Incidence rate ratio (95% CI) (total/Obs period 1/Obs period 2) of NEs for: 1. all patients: 1.05/0.88/1.11¶ 2. patients started LTRA for 1–3 days: 0.68+/0.65¶/0.69+ 3. patients started LTRA for 4–7 days: 2.10+/1.35/2.36+ 4. patients started LTRA for 8–14 days: 1.60+/1.11/1.78+ Incidence rate ratio (95% CI) in patients with: 1. asthma: 1.00 (0.90–1.10) 2. allergic rhinitis only: 1.19 (1.01–1.39)¶ |
| Paljarvi et al. [7] (2022) | Retrospective cohort study | 2015–2019 | For asthma group: 1. Asthma-related healthcare contact 2. Aged 15–64 years at index prescription of MTK 3. Alive for 12 months after index prescription | 1. Dx of COPD 2. Dx of obstructive sleep apnoea 3. Dx of neoplasm 4. Pregnancy during study period | MTK users (36 245) vs non-users (36 245) vs non-users (36 245) 245) | 1. 12-month incident Dx for NEs, including bipolar disorders, depression, anxiety disorders, OCD and sleeping disorders 2. 12-month incident dispensed prescriptions for psychiatric medications | OR (95% CI) propensity score-matched) for: 1. any incident NE in 1 year: 1.11 (1.04–1.19) 2. any sleep problem: 1.13 (1.02–1.25) 3. insomnia: 1.13 (1.01–1.27) 4. receipt of sleep medication: 1.11 (0.99–1.25) 5. hypersomnia: 1.06 (0.78–1.44) 6. circadian rhythm disorder: 1.00 (0.67–1.48) 7. parasomnia: 1.04 (0.70–1.53) 8. anxiety or related disorder: 1.21 (1.05–1.20) 9. phobic anxiety: 1.13 (0.86–1.48) 10. generalised anxiety: 1.18 (1.05–1.33) 11. other anxiety: 1.11 (1.02–1.21) |
| Özata et al. [24] (2021) | Prospective cohort study | Nov 2017–Jun 2018 | 1. Age 1.5–5 years 2. Asthma diagnosed by an independent specialist physician based on the Global Initiative for Asthma guideline | 1. Chronic diseases other than asthma 2. Psychiatric disease and a history of regular use of medication 3. Illiterate parents or non-Turkish speakers 4. Failure to attend follow-ups or not fully completing the CBCLs 5. ADHD and other psychiatric disorders | MTK users (50) vs ICS users (45) | 1. CBCL scores for aged 1.5–5 years | Scores: ICS vs MTK in: 1. total CBCL score: 40 (IQR 27–52.5) vs 42.5 (IQR 29–61.5), p=0.34 2. internalisation score: 13 (IQR 8–18) vs 12.5 (IQR 9.75–21.5), p=0.41 3. externalisation score: 13 (IQR 7–17.5) vs 12.5 (IQR 7–20.25), p=0.40 |
| Kang et al. [25] (2021) | Case–control | 2003–2013 | 1. Newly diagnosed asthma during study period 2. Prescribed asthma disease controller 3. Aged >60 years | 1. Case group: patients diagnosed with NEs before asthma diagnosis | Case: 31 922 922Control: 31  922 922 | 1. NEs including mood disorder, sleep disorder, anxiety disorder, personality disorder, substance-related disorder, agitation, schizophrenia and self-harm | Adjusted OR (95% CI) for: 1. overall NEs: 1.67 (1.58–1.78) 2. sleep disorder: 1.54 (1.42–1.68) 3. mood disorder: 1.65 (1.51–1.81) 4. anxiety disorder: 1.63 (1.50–1.77) |
| Shim et al. [26] (2021) | Retrospective cohort study | 2002–2015 | 1. Received health screening examinations between 2009 and 2010 2. Asthma diagnosis before the date of health screening 3. Aged 40–79 years | 1. Use of LTRA prior to the date of health screening 2. NEs before the date of health screening | LTRA users (12 168) vs non-users (49 168) vs non-users (49 403) 403) | 1. NEs identified using ICD-10 codes§ | Adjusted HR (95% CI) for: 1. LTRA use: 1.01 (0.83–1.23) Adjusted HR (95% CI) for LTRA use of: 2. <6 months: 1.01 (0.83–1.24) 3. 6–11 months: 0.81 (0.36–1.84) 4. 12–23 months: 1.37 (0.66–2.86) 5. ≥24 months: 0.71 (0.26–1.98) |
| Bayer et al. [27] (2020) | Prospective cohort study | Sep 2013–Mar 2014 | 1. Aged 3–18 years 2. Taking MTK for the first time due to asthma during study period | 1. Experienced asthma attack when started MTK 2. Uncontrolled asthma symptoms 3. Existing chronic disease (diabetes, CKD) 4. Existing neuropsychiatric disease (epilepsy/ADHD) | 125 | 1. Neuropsychiatric ADRs occurred 2. Effects of neuropsychiatric ADRs on patients’ QoL | 1. NE in patients (62.4%) 2. Statistically significant elevations (p<0.001) of NEs post-MTK treatment in temperamental behaviour, nightmare and sleep disorders Child-reported QoL OR in: 3. preschool age: 2.66, p=0.048 4. school age: 5.95, p=0.027 |
| Huang et al. [9] (2020) | Retrospective cohort study | 1 Jan 1997–31 Dec 2013 | 1. Aged ≤12 years 2. ≥1 claim of inpatient admission or ≥3 claims of ambulatory visit 3. New-onset asthma diagnosed during study period | 1. ADHD diagnosis before asthma diagnosis or MTK treatment 2. Patients with follow-up periods <6 months | MTK (12 806) vs non-MTK users (12 806) vs non-MTK users (12 806) 806) | 1. Occurrence of ADHD using ICD-9 codes (314.X) | 1. Adjusted HR (95% CI) for MTK on association with ADHD: 1.04 (0.93–1.17) 2. Prolonged use of MTK (>90 days) did not increase the risk of ADHD when compared with ≤90 days (1.08, 95% CI 0.95–1.23) and >90 days (1.00, 95% CI 0.86–1.15) |
| Glockler-Lauf et al. [10] (2019) | Case–control | 2004–2015 | 1. Aged 5–18 years with physician-diagnosed asthma in study period 2. Asthma maintenance medication prescribed | 1. No valid health card number/residence code 2. Existing surgery, psychiatric disorders or non-pharmacologically treated asthma (<1 record of asthma maintenance drug) | Case: 898 Control: 3497 | 1. First NEƒ following physician-diagnosed asthma | 1. Adjusted OR (95% CI) for MTK use: 1.91 (1.15–3.18) 2. 42.4% of NE in 90 days 3. Most prevalent presenting complaint for first NE: anxiety (436 out of 898, 48.6%); sleep disturbance (234 out of 898, 26.1%) |
| Benard et al. [11] (2017) | Retrospective cohort | 2011–2016 | 1. Aged 1–17 years 2. Physician-confirmed asthma 3. A clinic visit during MTK initiation as monotherapy or adjunct therapy to ICS/ICS+LABA | Without MTK prescription or maintenance asthma medications, or denied taking medications of interest | MTK (84) vs ICS monotherapy (84) | 1. Incidence of parent-reported neuropsychiatric ADR leading to drug cessation 2. Characteristics of parent-reported NE ADRs | 1. Relative risk (95% CI) for ADR reported by parents: 12.0 (1.6–90.2) 2. Incidence (95% CI) of MTK cessation: 12% (7–21%) |
| Ali et al. [20] (2015) | Case–control | 1998–2009 | 1. Aged 1–17 years 2. Primary Dx of asthma 3. Health plan enrolment in the 12 months before and after asthma claim | 1. Developmental disorders 2. Receiving long-term care 3. Pre-existing NE in 365 days before asthma claims | Case: 1920 Control: 5760 | 1. ND (composite end-point) 2. Psychiatric disorder diagnosis 3. NE diagnosis 4. Psychotropic medication receipt As post hoc analyses: 1. Anxiety disorders 2. Depressive disorders 3. Aggressive disorders | Adjusted OR (95% CI) for 1. ND with MTK exposure in the year prior to index date: 1.01 (0.88–1.14) 2. ND with high chronic cumulative dose of MTK: 0.64 (0.50–0.82) Psychiatric disorder diagnosis with high chronic cumulative dose of MTK: 0.64 (0.49–0.83) 3. Anxiety disorders for patients exposed to MTK in the year prior to index date: 0.95 (0.66–1.37) 4. Aggressive disorders for patients exposed to MTK in the year prior to index date: 0.92 (0.58–1.45) |
| Lu et al. [14] (2015) | ITS | 2005–2010 | 1. Aged 5–64 years 2. Continuous health enrolment plan for past 12 months 3. Enrolled for health plan for current month 4. At least one outpatient or inpatient visit in the past year due to asthma diagnosis | History of: 1. COPD, cystic fibrosis, bronchiectasis 2. pulmonary hypertension or embolism 3. bronchopulmonary dysplasia 4. CHF | 140 000 (rolling cohort with asthma) 000 (rolling cohort with asthma) | Monthly percentage of patients 1. dispensed an LTRA and non-LTRA 2. on mental health visits 3. medically treated for suicide attempts (quarterly percentage) | 1 year after the FDA label change in 2009, mental health visits 1. increased by 0.25 (95% CI 0.01–0.49) percentage points in adolescents 2. increased by 1.00 (95% CI 1.00–1.00) percentage points among young adults (aged 18–29 years) 3. increased by 0.61 (95% CI 0.31–0.91) percentage points in adults (aged 30–64 years) |
| Bisgaard et al. [28] (2009) | Reviews of RCTs (9 trials##) | 1995–2004 | 1. Aged 6 months to 14 years 2. Asthmatic patients without significant comorbidities | – | MTK (1999) vs placebo (873) vs active control (379)¶¶ | 1. All ADRs | 1. One case of NE in open-label extension studies, in which the patient had background attention deficit disorder and depression, later withdrawn due to asthma exacerbation 2. One case of epilepsy in long-term double-blind studies, leading to discontinuation |
| Philip et al. [29] (2009) | Reviews of RCTs (46 trials++) | Up to 25 April 2008§§ | 1. Randomised, double-blind, placebo-controlled trials 2. Parallel-group or crossover trials 3. Multiple dose administration 4. Inclusive of ≥20 patients per treatment group of all ages 5. Patients aged ≥3 months 6. Completed by 25 April 2008 | Patients with clinically significant psychiatric illnesses at baseline (in trials) | MTK (11 673) vs Placebo (8827) vs Active control (4724) 673) vs Placebo (8827) vs Active control (4724) | 1. BRAEs | OR (95% CI) for 1. patients with BRAEs: 1.12 (0.93–1.36) 2. BRAE leading to discontinuation: 0.52 (0.17–1.51) |
ADHD: attention deficit hyperactivity disorder; ADR: adverse drug reaction; BRAE: behaviour-related adverse experience; CBCL: Child Behavior Checklist; CHF: congestive heart failure; CKD: chronic kidney disease; Dx: diagnosis; FDA: US Food and Drug Administration; HR: hazard ratio; ICD-9/10: International Classification of Diseases, ninth/tenth revision; ICS: inhaled corticosteroids; IQR: interquartile range; ITS: interrupted time series; LABA: long-acting β-agonist; LTRA: leukotriene receptor antagonist; Obs: observation period; OCD: obsessive compulsive disorder; MTK: montelukast; ND: neuropsychiatric disturbance; NE: neuropsychiatric event; OR: odds ratio; QoL: quality of life; RCT: randomised controlled trial; vs: versus. #: including 4300 patients enrolled in observation period 1 (1 January 2005–31 December 2007) and 12 701 patients enrolled in observation period 2 (1 January 2016–31 December 2018); study also included 6484 (38.14%) patients with only diagnoses for allergic rhinitis; ¶: p<0.05; +: p<0.005; §: NEs include F00–F09 organic, including symptomatic mental disorders; F10–F19 mental and behavioural disorders due to psychoactive substance use; F20–F29 mood disorders; F40–F49 neurotic, stress-related and somatoform disorders; F50–F59 behavioural syndromes associated with physiological disturbances and physical factors; F99 unspecified mental disorder; X60–X84 suicide attempt; and R45.8 suicide ideation; ƒ: NEs include substance-related disorders, schizophrenia, anxiety, sleep disturbance, mood and personality disorders, and agitation; NEs with outpatient visits were excluded; ##: controls included placebo and active control, plus randomised, double-blind, placebo-controlled parallel-group trials and open-label studies; ¶¶: only including participants in trials enrolling asthma patients; ++: controls included placebo and active control, plus randomised, double-blind, placebo-controlled parallel-group trials and open-label studies and randomised, double-blind, placebo-controlled crossover trials; §§: including 30 trials on asthma, 12 on seasonal allergic rhinitis, two on perennial allergic rhinitis, one on respiratory syncytial virus-induced bronchiolitis and one on migraine headaches.
701 patients enrolled in observation period 2 (1 January 2016–31 December 2018); study also included 6484 (38.14%) patients with only diagnoses for allergic rhinitis; ¶: p<0.05; +: p<0.005; §: NEs include F00–F09 organic, including symptomatic mental disorders; F10–F19 mental and behavioural disorders due to psychoactive substance use; F20–F29 mood disorders; F40–F49 neurotic, stress-related and somatoform disorders; F50–F59 behavioural syndromes associated with physiological disturbances and physical factors; F99 unspecified mental disorder; X60–X84 suicide attempt; and R45.8 suicide ideation; ƒ: NEs include substance-related disorders, schizophrenia, anxiety, sleep disturbance, mood and personality disorders, and agitation; NEs with outpatient visits were excluded; ##: controls included placebo and active control, plus randomised, double-blind, placebo-controlled parallel-group trials and open-label studies; ¶¶: only including participants in trials enrolling asthma patients; ++: controls included placebo and active control, plus randomised, double-blind, placebo-controlled parallel-group trials and open-label studies and randomised, double-blind, placebo-controlled crossover trials; §§: including 30 trials on asthma, 12 on seasonal allergic rhinitis, two on perennial allergic rhinitis, one on respiratory syncytial virus-induced bronchiolitis and one on migraine headaches.
Quality assessment
Quality assessment of articles included in this review was performed independently by CWHL and SP. The Newcastle–Ottawa Scale was used to assess the methodological quality of observational studies, including cohort studies and case–control studies. For observational studies adopting an interrupted time series design, longitudinal observational studies, pharmacovigilance studies, case series and case reports, the findings are described narratively owing to a lack of assessment tools for these studies.
Outcomes
A wide range of neuropsychiatric outcomes, including neuropsychiatric events that were not listed in the montelukast product label, was assessed to ensure the overall psychiatric safety of montelukast. For depression-related outcomes, suicide-related outcomes, anxiety-related outcomes, sleeping disorders and neurodegenerative disease-related outcomes, the outcomes themselves and the proxy measures of the particular outcome were grouped together and reported as the primary outcomes of interest for this review. Secondary outcomes included other neuropsychiatric outcomes, such as agitation, disorientation, hallucinations, irritability, restlessness, tremor, drowsiness, seizures, ADHDs, bipolar disorders, psychosis, confusion, abnormal behaviour, speech disorders, impulse control disorders and schizophrenia. Terminologies defined as primary and secondary outcomes are listed in supplementary material 1.
Results
Study characteristics
This systematic search yielded a total of 4081 articles. After removing 218 duplicates and 3751 articles not investigating neuropsychiatric events associated with montelukast in patients with asthma, 112 articles were assessed for full-text screening. After completion of full-text screening, 59 studies were eligible for inclusion, including 21 pharmacovigilance studies, four reviews of 172 RCTs, 20 observational studies, 10 case reports and four case series (figure 1). Observational studies were curated from population-wide electronic health record databases in eight countries in the USA, Europe and Asia. Databases in the USA were mostly representative of commercially or publicly insured populations based on insurance claims on prescriptions, while national databases in Korea and Taiwan were representative of the whole population. Among all included observational studies and reviews of RCTs, eight studies included children and adolescents aged <24 years old and three studies exclusively enrolled patients aged >50 years old.
Quality assessment of observational studies
The Newcastle–Ottawa scale scores of observational studies are summarised and reported in supplementary material 6 and 7. The methodological quality of case–control studies included in this review was high in terms of participant selection, adjustment for covariates and exposure [10, 16, 20, 25] compared to that of cohort studies. The representativeness of study cohorts was not adequate in eight cohort studies [7–9, 11, 12, 24, 27, 30] because the associations were investigated only in specific subgroups of patients. Two studies scored low in quality assessment because no comparison cohort was present [18] and covariates were not adjusted in statistical analyses [24]. Five cohort studies did not report follow-up period specifically [6, 12, 18, 24, 27]. Three cohort studies obtained high quality assessment scores, because independent assessments of study outcomes and follow-up periods were sufficient, for over 1 year without significant loss to follow-up (>20%) [9, 15, 26].
Montelukast and suicide-related outcomes
The evidence investigating the associations between montelukast and suicide-related outcomes has been reported in a previous review [13], including reviews of RCTs [19, 28] and analysis from VigiBase, a pharmacovigilance database maintained by the World Health Organization (WHO) [31]. VigiBase appeared to detect disproportionality between the actual and the expected reporting rates in developing suicidal behaviour in montelukast users [31], which were not captured by RCTs.
Six observational studies consistently indicated that the risk of suicide-related outcomes attributable to montelukast was statistically insignificant [6, 7, 15–18]. Currently available evidence has shown a lack of associations between LTRA use and suicide attempts [16] and self-harm, the strongest predictor of suicide, in three other cohort studies [6, 7, 15]. These studies included a national retrospective cohort study in Taiwan (adjusted HR 0.92, 95% CI 0.29–2.91) [15], the FDA Sentinel database (HR 0.92, 95% CI 0.69–1.21) [6] and the TriNetX Analytics Network, a patient repository database from pharmaceuticals and healthcare organisations between 2015 and 2019 (HR 1.06, 95% CI 0.72–1.55) [7].
Montelukast and depression-related outcomes
Original research publications did not identify associations between montelukast and depression in a review of RCTs [22] and observational studies [6, 7, 20]. The findings were consistent in recent propensity score-matched cohort studies [6, 7], with no significant associations identified between montelukast and 1-year incident depression (OR 1.05, 95% CI 0.96–1.15) in the TriNetX patient repository [7] and hospitalisation for depression (HR 1.06, 95% CI 0.90–1.24) in the FDA Sentinel database [6]. Montelukast use was, however, associated with a reduced risk of outpatient depressive disorders in the same FDA study (HR 0.91, 95% CI 0.89–0.93) [6].
To date, the results have been inconclusive when incident antidepressant prescriptions are used as a proxy for depression. Evidence from a previous asymmetry analysis in Denmark [32] and a study adopting an interrupted time series design [21] demonstrated associations between montelukast and subsequent prescription or initiation of antidepressants, yet comparable trends were also found for other treatment arms such as corticosteroids [21]. While not associated with depression, montelukast was found to be associated with 1-year incident prescription of antidepressants in the same US cohort study (OR 1.16, 95% CI 1.07–1.26) [7]. The results were not replicated in US veterans however (sequence symmetry ratio 0.75, 95% CI 0.68–0.83) [33].
Montelukast and anxiety-related outcomes
Case reports of anxiety, irritability and aggressive behaviours were identified in montelukast users [34–40], consistent with signals from pharmacovigilance databases that collected case reports of anxiety disorders [41–44], aggressiveness [41–45], irritability [43] and restlessness [44].
Montelukast was associated with 1-year incident anxiety or related disorders (OR 1.21, 95% CI 1.05–1.20), generalised anxiety (OR 1.18, 95% CI 1.05–1.33) and other anxiety (OR 1.11, 95% CI 1.02–1.21) in the US study utilising TriNetX patient repositories [7]. Similar findings were obtained in a case–control study in Korean patients aged >60 years old (OR 1.63, 95% CI 1.50–1.77) [25]. In contrast, a US case–control study found montelukast was not associated with a higher risk of anxiety disorders (OR 0.95, 95% CI 0.66–1.37) and aggressive disorders (OR 0.92, 95% CI 0.58–1.45) in children aged 1–17 years old [20].
Montelukast and sleeping disorders
Incidents of sleeping disorders after montelukast use were described in case reports [35–40, 46] and case series [47–50]. The symptoms reported included insomnia [36, 39], vivid dreams or night terrors [35, 38, 48], disturbed sleep or difficulty sleeping [35, 37, 38] and parasomnia [37, 38, 46]. Sleep disturbances were also reported in studies involving four pharmacovigilance databases [42, 45, 51], with adverse drug reaction reports from VigiBase showing disproportionately elevated reporting rates for insomnia (reporting OR 5.08, 95% CI 4.77–5.41) and nightmare (reporting OR 22.48, 95% CI 20.87–24.21) [42] in montelukast users.
Montelukast was associated with a higher risk of 1-year incident sleeping problems of any kind (HR 1.13, 95% CI 1.02–1.25) and insomnia (HR 1.01, 95% CI 1.01–1.27) [7]. This was also echoed by a case–control study in Korea that enrolled patients aged >60 years old (OR 1.54, 95% CI 1.42–1.68) [25]. A self-controlled case series in adolescents and young adults did not reveal such associations (incidence rate ratio 0.92, 95% CI 0.80–1.06), yet 38.14% of the patients enrolled were only diagnosed with allergic rhinitis rather than asthma [23].
Although an observational study enrolling 276 413 patients with asthma with the TriNetX Analytics Network showed an elevated risk of sleeping disorders, the harmful effects of montelukast were less evident in specific subtypes of sleeping disorders [7]. While associated with sleeping problems of any kind, montelukast was not associated with an elevated risk of hypersomnia (OR 1.06, 95% CI 0.78–1.44), circadian rhythm disorder (OR 1.00, 95% CI 0.67–1.48) or parasomnia (OR 1.04, 95% CI 0.70–1.53) in the same propensity score-matched cohort [7]. The 1-year incidence of these outcomes in asthma cohorts ranged from one to two per 1000 individuals compared to 25 and 22 in montelukast users and non-users, respectively, for any sleep problems [7]. Montelukast initiation was not associated with incident prescription of sleep medications in the following year either (OR 1.11, 95% CI 0.99–1.25) [7].
413 patients with asthma with the TriNetX Analytics Network showed an elevated risk of sleeping disorders, the harmful effects of montelukast were less evident in specific subtypes of sleeping disorders [7]. While associated with sleeping problems of any kind, montelukast was not associated with an elevated risk of hypersomnia (OR 1.06, 95% CI 0.78–1.44), circadian rhythm disorder (OR 1.00, 95% CI 0.67–1.48) or parasomnia (OR 1.04, 95% CI 0.70–1.53) in the same propensity score-matched cohort [7]. The 1-year incidence of these outcomes in asthma cohorts ranged from one to two per 1000 individuals compared to 25 and 22 in montelukast users and non-users, respectively, for any sleep problems [7]. Montelukast initiation was not associated with incident prescription of sleep medications in the following year either (OR 1.11, 95% CI 0.99–1.25) [7].
Montelukast and other neuropsychiatric events in adults and elderly
Montelukast may be associated with a reduced risk of developing pre-existing and new-onset dementia in observational studies. In a US study, montelukast was associated with a slower decline in cognitive test scores in patients with pre-existing Alzheimer's disease dementia [30]. Similar results were also identified in montelukast users in a Norwegian prescription study for the use of dementia medicine (adjusted HR 0.89, 95% CI 0.81–0.98) [12] and new-onset dementia (adjusted HR 0.42, 95% CI 0.20–0.87, p=0.019) in over 20 000 newly diagnosed asthma patients aged ≥50 years in Japan [8]. However, important differences existed between LTRA users and non-users at baseline, including comorbidities and the use of benzodiazepines (14.3% versus 15.5%, p=0.012), which were independently associated with dementia [8].
000 newly diagnosed asthma patients aged ≥50 years in Japan [8]. However, important differences existed between LTRA users and non-users at baseline, including comorbidities and the use of benzodiazepines (14.3% versus 15.5%, p=0.012), which were independently associated with dementia [8].
In Korea, LTRAs were associated with an increased risk of developing neuropsychiatric events (adjusted OR 1.67, 95% CI 1.58–1.78), mood disorders (adjusted OR 1.65, 95% CI 1.51–1.81), anxiety disorders (adjusted OR 1.63, 95% CI 1.51–1.81) and sleep disorders (adjusted OR 1.54, 95% CI 1.42–1.68) in 63 844 asthma patients aged >60 years [25]. This contrasted with the findings from another Korean cohort study using the same population-wide National Health Insurance Service database [26]. Analyses of 61
844 asthma patients aged >60 years [25]. This contrasted with the findings from another Korean cohort study using the same population-wide National Health Insurance Service database [26]. Analyses of 61 571 Korean patients aged 40–79 years did not reveal any statistically significant relationships upon LTRA initiation (HR 1.01, 95% CI 0.83–1.23). A prolonged duration of LTRA use of over 24 months was not associated with an increased risk of neuropsychiatric events (HR 0.71, 95% CI 0.26–1.98) [26].
571 Korean patients aged 40–79 years did not reveal any statistically significant relationships upon LTRA initiation (HR 1.01, 95% CI 0.83–1.23). A prolonged duration of LTRA use of over 24 months was not associated with an increased risk of neuropsychiatric events (HR 0.71, 95% CI 0.26–1.98) [26].
Montelukast and neuropsychiatric events in children
Neuropsychiatric events were commonly reported in children taking montelukast in case reports [34, 36–38, 46] and pharmacovigilance studies [31, 42]. In a recently published prospective cohort study, neuropsychiatric events were reported in 62.4% of 125 patients with asthma aged 3–18 years [27].
Observational studies showed conflicting evidence on the relationship between montelukast and neuropsychiatric events when paediatric montelukast users were enrolled exclusively [9–11, 20]. Ali et al. [20] did not reveal significant associations between montelukast and the composite outcome of psychiatric diagnosis or psychotropic medication receipt in a case–control study of 1920 patients with asthma aged 1–17 years old with montelukast exposure a year prior to the outcome occurring (OR 1.01, 95% CI 0.88–1.14). This contrasted with the results from a case–control study in Ontario involving 4395 paediatric patients with asthma, which showed a higher risk of new-onset neuropsychiatric events for montelukast users (adjusted OR 1.91, 95% CI 1.15–3.18) [10]. Similar findings were observed in another retrospective cohort study in 2011–2016 in Quebec, with a higher relative risk for parent-reported neuropsychiatric events leading to drug discontinuation in montelukast users (relative risk 12.0, 95% CI 1.60–90.2) [11].
The relationships between montelukast and neuropsychiatric events were also reported for specific neuropsychiatric diagnoses. A Taiwanese cohort study of 12 086 patients with asthma aged ≤12 years old did not find an elevated risk for ADHD diagnosis with montelukast use after a 6-month follow-up (HR 1.04, 95% CI 0.93–1.17) [9]. While there were reservations over the validity of the International Classification of Diseases ninth revision (ICD-9) codes in diagnosing ADHD, the codes were validated with high sensitivity and specificity in electronic health databases elsewhere [52]. Montelukast was not significantly associated with changes in the Child Behavior Checklist scores, an externally validated tool to assess behavioural changes, in Turkish children with asthma aged 1.5–5 years [24].
086 patients with asthma aged ≤12 years old did not find an elevated risk for ADHD diagnosis with montelukast use after a 6-month follow-up (HR 1.04, 95% CI 0.93–1.17) [9]. While there were reservations over the validity of the International Classification of Diseases ninth revision (ICD-9) codes in diagnosing ADHD, the codes were validated with high sensitivity and specificity in electronic health databases elsewhere [52]. Montelukast was not significantly associated with changes in the Child Behavior Checklist scores, an externally validated tool to assess behavioural changes, in Turkish children with asthma aged 1.5–5 years [24].
Discussion
This systematic review did not reveal significant associations between montelukast use and suicide-related events. While the evidence on depression remains conflicting, older adults may be particularly susceptible to specific neuropsychiatric events such as anxiety and sleeping disorders. Future studies are required to confirm the associations between montelukast and neurodegenerative diseases.
Montelukast was not found to be harmful in suicide-related events in reviews of RCTs and observational studies using ICD codes to define study outcomes [6, 15, 16]. In clinical trials, suicidal events might be too rare to be detected given a rate of 1.02 cases per 100 000 person-years [18], resulting in nonsignificant associations observed in subsequent reviews [19, 28]. Besides, the sensitivity of ICD codes in capturing suicide could be somewhat compromised in observational studies owing to a conservative approach adopted by clinicians when classifying deaths as suicides [53]. The findings may also be susceptible to residual confounding because most studies investigated the associations of montelukast and suicidal events with administrative data, which may not capture nonclinical characteristics, such as socioeconomic factors, in the study cohorts. Despite these methodological limitations, the consistency of findings in research studies suggests that montelukast poses no additional risk for suicide-related events at a population-wide level.
000 person-years [18], resulting in nonsignificant associations observed in subsequent reviews [19, 28]. Besides, the sensitivity of ICD codes in capturing suicide could be somewhat compromised in observational studies owing to a conservative approach adopted by clinicians when classifying deaths as suicides [53]. The findings may also be susceptible to residual confounding because most studies investigated the associations of montelukast and suicidal events with administrative data, which may not capture nonclinical characteristics, such as socioeconomic factors, in the study cohorts. Despite these methodological limitations, the consistency of findings in research studies suggests that montelukast poses no additional risk for suicide-related events at a population-wide level.
However, the possibility of montelukast inducing suicide-related events in certain individuals should not be excluded, because current methodologies struggle to address differences in baseline characteristics at an individual level. Asthma diagnosis itself might be a contributor to suicidal events, as revealed by a Taiwanese cohort study showing self-harm to be associated with asthma (adjusted HR 1.70, 95% CI 1.35–2.14) [15]. Other clinical diagnoses may also contribute to the risk of suicide, the magnitude of which remains largely unknown. Statistical adjustments for these diagnoses in observational studies would be useful in accounting for the differences in baseline characteristics. The findings, however, imply associations of montelukast with suicide-related events in the whole study population, rather than the safety of the drug in every single individual involved. Montelukast may still be harmful in individuals with specific combinations of baseline comorbidities associated with suicide whom the current study designs failed to capture. Developing risk-scoring tools for suicide risk and matching patients based on the risk scores may be useful in reflecting individual differences on the baseline risk of suicide as we aim to further unmask the relationship between montelukast and the risk of suicidal events on an individual level.
Despite the identification of adverse drug reactions on depression by pharmacovigilance databases, these associations were not found in RCTs and observational studies. Asthma may be associated with depression [54, 55], and results from observational studies did not show an increased risk of depression after adjusting for asthma severity [6, 16]. Besides, comparable increases in antidepressant prescription rates observed in comparison groups [21, 32] may suggest that the reports of depression identified from pharmacovigilance databases could be attributed to both poor asthma control and the use of LTRAs, rather than either factor alone. While montelukast was associated with incident antidepressant prescriptions [21, 32], the outcome definition may be prone to classification bias because antidepressants may be indicated for other disorders such as neuropathic pain [7]. In addition, the follow-up periods in previous RCTs of up to 24 weeks [22, 29] may be too short to detect the associations between montelukast and depression on long-term use. Future research studies should look to address these methodological limitations in terms of adjustments for baseline asthma severity, outcome definitions and duration of follow-up.
Interestingly, the risk for depression was reduced in montelukast users in observational studies [6, 7], which could be attributed to the immunomodulatory effects of montelukast. M1 macrophage-mediated inflammation was associated with depression, and many antidepressants were shown to possess immunomodulatory effects by reducing plasma interleukin-1β and interleukin-6 levels [56]. By lowering serum proinflammatory tumour necrosis factor-α and interleukin-6, montelukast may offer benefits in depression [57]. LTRAs have also shown antidepressant activities by boosting the proliferation of neural progenitors and neurogenesis in mice [58]. However, corticosteroids, a drug class known to be associated with depression, were often used as a comparison arm in observational studies [6]. Therefore, the findings cannot exclude the possibility that montelukast induces depression, but to a lesser extent than ICS. Besides, the sole inclusion of psychiatric outcomes resulting in healthcare claims may fail to capture mild cases of neuropsychiatric events related to montelukast or ICS use [6]. The protective effects reported in a recent cohort study, but only before propensity score matching [7], may suggest undiscovered confounding.
Emerging evidence from pharmacovigilance databases and observational studies indicated adverse relationships between montelukast and anxiety in older adults, but not in children. First, it is worth noting that cytochrome P450 enzymes [35] and the additive neurotoxicity of eicosapentaenoic acid, a precursor for the biosynthesis of leukotrienes [39], may be alternative culprits, as pointed out by another review of case reports [59]. Whether genetic polymorphisms in cytochrome P450 enzymes may affect the associations remains unknown. While asthma control is associated with anxiety [55], the possible involvement of leukotriene pathways in inducing anxiety-like behaviour was also highlighted [60]. Knockout of the 5-lipooxygenase activating protein, a key protein involved in eicosanoid metabolism, resulted in lower expression of transcription factor cFOS and induced anxiety in mice [60]. More importantly, the decline of cFOS was associated with age-dependent anxiety phenotypes [60], which may explain the adverse associations identified particularly in elderly patients. Using real-world data, two large population-based studies enrolling over 50 000 adults [7, 25] revealed associations of montelukast with the risk of anxiety, and the findings should be robust.
000 adults [7, 25] revealed associations of montelukast with the risk of anxiety, and the findings should be robust.
Montelukast was consistently associated with sleeping disorders in adults and the elderly in studies with different designs. However, these associations were called into question when patients without asthma were enrolled [23], and when subtypes of sleeping disorders were defined as study outcomes. The fact that montelukast was used off-label for patients with obstructive sleep apnoea [61] further complicated the interpretation of findings when various types of sleeping disorders were analysed as a composite outcome. Therefore, stratification of patient populations and outcomes of sleeping disorders are crucial in future studies.
Based on data from Europe [12] and Asia [8, 30], montelukast appears to be associated with a lower risk of developing neurodegenerative diseases in the elderly. Statistically significant associations were found using dementia medications [12] and new-onset dementia [8] as outcome definitions. However, some studies [12, 30] were not designed to evaluate the safety of montelukast in developing neurodegenerative diseases, but rather to explore the therapeutic potential of montelukast in these outcomes. Therefore, the study populations may not be representative of patients with asthma in general. The Japanese cohort study on dementia only included new users of LTRAs to avoid results being distorted by the previous use of medications [8], yet the effect sizes were small using cognitive test scores as the outcome [30]. Montelukast was found to modulate neuroinflammation in animal models when used at high doses [62]. A recent case series also demonstrated the potential of high-dose montelukast in improving memory [63]. Future work shall continue to focus on establishing clinical evidence and the optimal dose-response relationship of montelukast in the treatment of neurodegenerative diseases. The safety of montelukast for neurodegenerative diseases in general asthma patients remains to be established.
Evidence from previous literature on the relationship between montelukast use and neuropsychiatric events in children was inconclusive. To date, case reports on neuropsychiatric events related to montelukast use in paediatric subjects are more common [10, 11, 16, 20, 28, 31, 41, 43–45, 64]. While this could be attributed to the fact that montelukast is more commonly prescribed for children [13], physiological alterations in brain development and the hypothalamic–pituitary–adrenal axis during adolescence may cause dysregulations in neurotransmitters and stress hormones, which could also act as possible contributors to the neuropsychiatric events observed [65].
The three observational studies enrolling paediatric patients [10, 11, 27] revealed statistically significant increases in the risks of developing various neuropsychiatric events. However, small cohort sizes [11], narrow scopes of definitions of neuropsychiatric events [10] and over-representation of paediatric subjects from low-income families [10] limited the generalisability of the results. While molecular models suggest that neurogenesis declines in adulthood and offers protective effects against molecules entering the brain via the blood–brain barrier [66], emerging evidence has suggested otherwise because montelukast was found to be associated with various neuropsychiatric events in the elderly, rather than in children. The heterogeneity in study outcomes across paediatric observational studies also implies further work is required to confirm the neuropsychiatric safety of montelukast in children.
Strengths and limitations
To date, this is the largest systematic review that includes studies investigating the associations between montelukast and a variety of neuropsychiatric events after FDA labelling changes in 2009 and reflects possible changes in associations due to awareness from physicians. In this review, we stratified studies based on different neuropsychiatric events, including suicide-related outcomes, depression, anxiety, sleeping disorders and neurodegenerative diseases. This approach helped to reveal differences in the evidence levels and the direction of associations in terms of different neuropsychiatric events, which were not reported previously. Our systematic review also included studies with various types of study designs and studies enrolling patients with different age groups, revealing that age affects the relationship between neuropsychiatric events and montelukast. However, this review was limited by heterogeneity in study populations, lack of access to data from the original clinical trials and different definitions of neuropsychiatric outcomes across studies. The assessment of methodological quality using the Newcastle–Ottawa Scale may be limited by low agreement on scores between reviewers, and the lack of validation for cross-sectional studies [67] that also applied to certain studies [18] in this review.
Conclusion
Montelukast has not been shown to increase the risk of suicide attempts in patients with asthma in observational studies. The evidence on depression was inconsistent, and possible protective effects are yet to be unmasked. However, current evidence revealed possible associations between montelukast and anxiety, as well as specific subtypes of sleeping disorders. The signals from observational studies were alarming for older patients, although the associations could not be confirmed in children because the study outcomes in this patient group were heterogeneous. The stratification of specific subtypes of psychiatric disorders and age is warranted in future studies to reveal how age and the types of psychiatric diagnoses may affect the relationship.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0079-2023.SUPPLEMENT
Acknowledgements
The authors thank Lisa Y. Lam (Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, the University of Hong Kong, Hong Kong SAR, China) for proofreading the manuscript before being submitted for publication.
Provenance: Submitted article, peer reviewed.
Conflict of interest: C.W.H. Lo received a student internship award by the University of Hong Kong under the Undergraduate Research Fellowship Programme. I.C.K. Wong received grants from the Food and Health Bureau of the Government of Hong Kong Special Administrative Region (HKSAR); funding from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Hong Kong Health and Medical Research Fund in Hong Kong, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia for pharmacoepidemiology to the University of Hong Kong, outside submitted work; consulting fees from IQVIA for offering advisory services on pharmacoepidemiology studies and payment from the Appeal Court in Hong Kong for expert testimony on the effects of cannabis outside submitted work; and salary as an independent nonexecutive director of Jacobson Medical in Hong Kong. E.W.Y. Chan received grants from the Health Bureau of the Government of the HKSAR, Hong Kong Research Grants Council, National Natural Science Fund of China, AstraZeneca, Novartis, RGA Reinsurance Company, Pfizer and Narcotics Division of the Security Bureau of HKSAR; consulting fees from Pfizer, Novartis and AstraZeneca; and travel support from Novartis. E.W.Y. Chan received medical device samples from GlaxoSmithKline Ltd., AstraZeneca, Boehringer Ingelheim and Novartis for teaching purposes. The other authors declare no conflicts of interest.
References
Articles from European Respiratory Review are provided here courtesy of European Respiratory Society
Full text links
Read article at publisher's site: https://doi.org/10.1183/16000617.0079-2023
Read article for free, from open access legal sources, via Unpaywall:
https://err.ersjournals.com/content/errev/32/169/230079.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/154778840
Article citations
Montelukast deprescribing in outpatient specialty clinics: A single center cross-sectional study.
Explor Res Clin Soc Pharm, 16:100509, 17 Sep 2024
Cited by: 0 articles | PMID: 39351122 | PMCID: PMC11439829
A Longitudinal Analysis of Black Box Warnings: Trends and Implications for Drug Safety.
Cureus, 16(4):e57597, 04 Apr 2024
Cited by: 0 articles | PMID: 38706997 | PMCID: PMC11069364
Review Free full text in Europe PMC
Promising Effects of Montelukast for Critically Ill Asthma Patients via a Reduction in Delirium.
Pharmaceuticals (Basel), 17(1):125, 18 Jan 2024
Cited by: 0 articles | PMID: 38256958 | PMCID: PMC10819207
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Assessing the risk of intentional self-harm in montelukast users: an updated Sentinel System analysis using ICD-10 coding.
J Asthma, 61(7):653-662, 14 Dec 2023
Cited by: 1 article | PMID: 38064517
Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case-Control Study.
J Pediatr, 209:176-182.e4, 21 Mar 2019
Cited by: 27 articles | PMID: 30905424
Analysis of Neuropsychiatric Diagnoses After Montelukast Initiation.
JAMA Netw Open, 5(5):e2213643, 02 May 2022
Cited by: 11 articles | PMID: 35608857 | PMCID: PMC9131741
Montelukast: a review of its therapeutic potential in persistent asthma.
Drugs, 59(4):891-928, 01 Apr 2000
Cited by: 47 articles | PMID: 10804041
Review

 1,4
1,4