Abstract
Free full text

Regulation of secondary hair follicle cycle in cashmere goats by miR-877-3p targeting IGFBP5 gene
Abstract
Cashmere, a highly valuable animal product derived from cashmere goats, holds significant economic importance. MiRNAs serve as crucial regulators in the developmental processes of mammalian hair follicles. Understanding the regulation of miRNAs during the hair follicle cycle is essential for enhancing cashmere quality. In this investigation, we employed high-throughput sequencing technology to analyze the expression profiles of miRNAs in the secondary hair follicles of Jiangnan cashmere goats at different stages. Through bioinformatics analysis, we identified differentially expressed miRNAs (DE miRNAs). The regulatory relationships between miRNAs and their target genes were verified using multiple techniques, including RT-qPCR, western blot, Dual-Luciferase Reporter, and CKK-8 assays. Our findings revealed the presence of 193 DE miRNAs during various stages of the hair follicle cycle in Jiangnan cashmere goats. Based on the previously obtained mRNA data, the target genes of DE miRNA were predicted, and 1,472 negative regulatory relationships between DE miRNAs and target genes were obtained. Notably, the expression of chi-miR-877-3p was down-regulated during the telogen (Tn) phase compared to the anagen (An) and catagen (Cn) phases, while the IGFBP5 gene exhibited up-regulation. Further validation experiments confirmed that overexpression of chi-miR-877-3p in dermal papilla cells suppressed IGFBP5 gene expression and facilitated cell proliferation. The results of this study provide novel insights for analyzing the hair follicle cycle.
Introduction
Cashmere is an unpilled, small-diameter fiber that grows in the secondary hair follicles of the goat’s skin. Secondary hair follicles undergo a cycle of growth, degeneration, and reconstruction over the course of a year. Influenced by the activity cycle of secondary hair follicles, the growth of cashmere has obvious seasonality (Krause and Foitzik, 2006; Bernard, 2016). Obviously, the longer the secondary follicle activity period, the longer the growing period of cashmere, thus increasing the production of cashmere. Therefore, it is particularly important to determine the factors that regulate the secondary hair follicle cycle.
MiRNAs, a class of small non-coding RNAs, play a pivotal role in the modulation of gene expression by binding to target mRNAs (Ambros, 2004; Bartel, 2004). Increasing evidence supports their significance in the growth and development of hair follicles. For instance, miR-24 has been found to influence mouse epidermal homeostasis by inhibiting Tcf3 expression, which is crucial for the activation of epidermal stem cells, a key process in hair follicle morphogenesis and regeneration (Amelio et al., 2013). Similarly, miR-148a acts as a critical regulator of gene targets in skin and hair follicles, maintaining the function of stem/progenitor cells during normal tissue homeostasis and regeneration by regulating the expression of Rock1 and Elf5 both in vitro and in vivo (Pickup et al., 2023). MiR-22 directly suppresses the expression of Dlx3, Foxn1, and Hoxc13, thereby affecting the differentiation of keratinocyte progenitor cells (Yuan et al., 2015). Furthermore, miR-205 (Teta et al., 2012), miR-203 (Yi et al., 2008), and miR-31 (Mardaryev et al., 2010) play regulatory roles in epidermal keratinocyte differentiation. MiR-125b inhibits the differentiation of hair follicle stem cells, emphasizing the significance of communication between hair follicle stem cells and dermal papilla cells for periodic hair follicle regeneration (Zhang et al., 2011).
Several miRNAs have been reported to participate in the regulation of cashmere or wool growth. For example, Ba Xiang et al. demonstrated that miRNA-21 can modulate the expression levels of its target genes FGF18 and SMAD7 through Solexa sequencing, quantitative real-time PCR (RT-qPCR), western blot, and dual luciferase reporter gene assays in different periods of skin tissue from cashmere goat secondary hair follicles (Xiang et al., 2023). Guishan Zhang et al. discovered that miRNA-1-3p may regulate hair follicle development in Liaoning cashmere goats by targeting FGF14 (Zhang et al., 2022). Similarly, Tao Ma et al. revealed that miRNA-203 may regulate hair follicle development in cashmere goats by targeting DDOST and NAE1 (Ma et al., 2021). MiRNAs also play a crucial role in embryonic hair follicle development. Shang et al. identified numerous key miRNA-mRNA pairs and elucidated the target relationship between chi-miR-30e-5p and DLL4 in their study of skin tissues from cashmere goat fetuses at different stages (Shang et al., 2022).
Despite the considerable progress in understanding the regulation of hair growth and regeneration through extensive research on miRNAs, challenges persist in expanding the miRNA database and effectively applying miRNAs in hair follicle regeneration. In this study, we constructed miRNA expression profiles in skin tissues from Jiangnan cashmere goats at various stages. We validated the functions of selected key miRNAs and their target genes, with the hope that our findings will contribute new insights into the mechanisms of hair follicle development.
Material and Methods
Animals and sampling
All animal experiments were conducted in strict accordance with the guidelines of the Animal Care and Use Committee of Xinjiang Academy of Animal Science (Approval No. 2020008). In this investigation, three healthy 24-month-old female Jiangnan cashmere goats were chosen as the subjects. A total of nine skin tissue samples with the size of 1 × 1 cm were collected from the left shoulder of goats in September (An, n = 3), January (Cn, n = 3) and March (Tn, n = 3), respectively. The collected skin tissue was thoroughly rinsed with PBS and immediately frozen in liquid nitrogen for transcriptome sequencing. During the sampling in September, the skin tissues of the selected goats were individually collected and washed with PBS containing 3% penicillin-streptomycin (P/S). Subsequently, they were stored in DMEM/F12 medium supplemented with 3% P/S for the isolation of DPCs. Throughout the study, the experimental animals were housed at the Aksu Baihutai Cashmere Goat Breeding Center in Xinjiang, and consistent feeding conditions were maintained both before and after the sampling process.
RNA extraction and sequencing
Nucleic acids were extracted from the nine collected samples using the miRNeasy Mini Kit (Qiagen, 217004). The concentration of the extracted nucleic acids was determined using Nanodrop2000, while RNA integrity was assessed using Agilent 2100. Small RNA libraries were constructed for samples meeting the following criteria: total RNA amount ≥ 1.5 µg, concentration ≥ 200 ng/µL, OD260/280 ratio between 1.7 and 2.5, and OD260/230 ratio between 0.5 and 2.5. The constructed libraries were evaluated using the Qsep-400 method. Single-ended sequencing of the nine Small RNA libraries was conducted on Illumina NovaSeq 6000 sequencers.
Quality control and annotation
To ensure the reliability of the miRNA sequencing data, the Clean Reads of miRNAs were aligned against multiple databases using the Bowtie software (Langmead et al., 2009). Specifically, alignments were performed against the Silva database, GtRNAdb database, Rfam database, Repbase database, and the reference genome (ARS1.Capra_hircus.ARS1.genome.fa). Reads matched to the reference genome were compared with the mature sequences of known miRNAs in the miRBase (v22) database and their upstream 2 nt and downstream 5 nt ranges, allowing at most one mismatch. Thus, the identified reads were considered to be the identified known miRNAs. We used the miRDeep2 software package (Friedländer et al., 2012) to obtain possible precursor sequences by comparing reads with genomic location information. Based on the distribution information of reads on precursor sequences and the structural energy information of precursors, a Bayesian model was used to score and finally predict new miRNAs.
Analysis of miRNAs expression levels
The expression levels of miRNAs were quantified and normalized using the Transcripts Per Million (TPM) algorithm (Li et al., 2010). To identify miRNAs with statistically significant expression in anagen, catagen, and telogen among all groups, the R package edgeR (Robinson et al., 2010) was employed with a fold change (FC) threshold of ≥ 1.5 and a P-value threshold of ≤ 0.05.
Prediction of DE miRNAs targeting genes
Prediction of target genes for the DE miRNAs was performed using miRanda-3.3a (Betel et al., 2008) and TargetScan 7.1 (Lewis et al, 2003). The predicted target gene sequences were compared against various databases including NR (Yangyang et al., 2006), Swiss-Prot (Apweiler et al., 2004), GO (Ashburner et al., 2000), COG (Tatusov et al., 2000), KEGG (Kanehisa et al., 2004), KOG (Koonin et al., 2004), and Pfam (Eddy, 1998) using the BLAST software to obtain annotation information for the target genes. Functional annotation of the DE miRNAs’ target genes included gene ontology (GO) functional annotation and enrichment analysis, as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation and enrichment analysis. The Enrichment degree of GO or KEGG was analyzed using Enrichment Factor, and the enrichment significance was calculated using Fisher precision test. Bubble maps visualizing the enriched GO entries and the top 15 pathways in biological processes (BP), cellular components (CC), and molecular functions (MF) were generated using the ggplot2 package.
Verify of transcriptome sequencing results
To validate the reliability of the sequencing data, eight DE miRNAs were randomly selected, and their expression levels were verified using RT-qPCR with U6 as the internal reference gene. Primers for the DE miRNAs were designed using Primer 5.0 (refer to Supplementary Table S1). Following completion of the transcriptome sequencing, the RNA provided by the sequencing company served as the template for the RT-qPCR experiments. The RT-qPCR procedure was performed on the Roche LightCycler 480 II fluorescent quantitative PCR apparatus following established experimental protocols (Wu et al., 2021). The relative expression levels of the miRNAs were analyzed using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Isolation and culture of DPCs
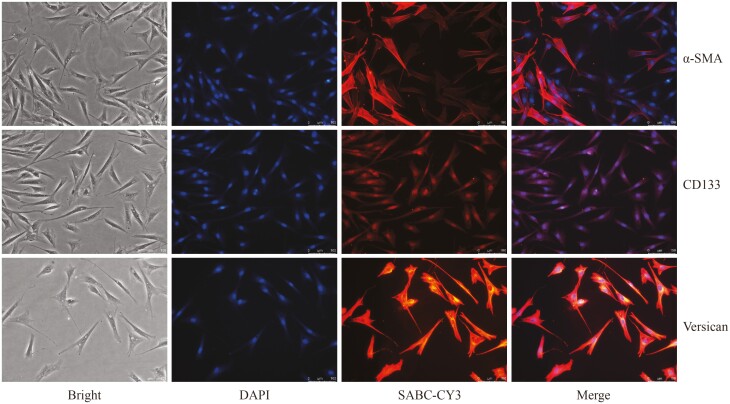
Skin tissues stored in DMEM/F12 medium containing 3% P/S were sequentially washed in six 35 mm Petri dishes. The first Petri dish was filled with 75% alcohol and washed for 10 s, followed by washing with 3% P/S PBS for 30 s in the second Petri dish. Subsequently, the third, fourth, fifth, and sixth Petri dishes were washed with 2% P/S PBS, 1% P/S PBS, 1% P/S PBS, and 1% P/S PBS, respectively, each for 30 s. The rinsed skin was cut into 1 mm strips along the longitudinal section and the epidermal tissue was separated from the dermis along the transverse section, discarding the epidermal tissue. The remaining tissue was finely minced and secondary hair follicle clusters were isolated using tweezers, transferring them to a new 35 mm dish containing 1% P/S PBS. The isolated DPCs were mechanically separated and then cultured in a 12-well plate containing DMEM/F12 medium supplemented with 10% FBS and 1% P/S. Adhesion of DPCs was observed after 6 days, and subculturing was performed after 12 days. The culture medium for the subsequent passages consisted of DMEM/F12 supplemented with 10% FBS. The fourth-generation DPCs were identified using the concentrated SABC three-step kit (Boster, SA1074/SA1041) with the following antibodies: 1:2,000 anti-CD133 antibody (bioss, BS-4770R), 1:2,000 anti-alpha-SMA antibody (bioss, bs-0189R), and 1:2,000 anti-Versican antibody (bioss, bs-2533R).
Dual-luciferase reporter system experiment
The mimics and negative control (NC) of chi-miR-877-3p were synthesized by Suzhou GenePharma Co., Ltd., with sequence information provided in Supplementary Table S1. The wild-type (Wt-IGFBP5) and mutant-type (Mut-IGFBP5) base sequences of the target gene were obtained from NCBI, based on the binding site of chi-miR-877-3p and the 3ʹ UTR region of IGFBP5 (Supplementary Figure S4). GP-miRGLO was enzymatically digested using SacI and XhoI, and the target fragment was cloned into the linearized GP-miRGLO vector using the ClonExpress Entry One Step Cloning Kit (Vazyme, C112-01). The ligated product was then transformed into bacterial receptor cells. The cloned clones were initially enzyme-digested and identified to confirm the successful insertion of the target gene into the target vector. Subsequently, the positive clones were sequenced, analyzed, and compared, and the correct clone was selected as the successfully constructed target gene expression plasmid vector. The constructed plasmid vector was extracted using a plasmid small extraction kit (Tiangen, DP104-02).
To determine the target relationship between miRNAs and mRNAs, 293T cells were used as experimental vectors to validate the binding of miRNAs to the predicted target gene’s 3ʹ UTR region using the Dual-Luciferase Reporter Assay System (Promega, E1910). The experimental groups consisted of: (1) mimics + Wt-IGFBP5, (2) NC + Wt-IGFBP5, (3) mimics + Mut-IGFBP5, and (4) NC + Mut-IGFBP5.
Function verification of chi-miR-877-3p
To verify the regulatory effect of chi-miR-877-3p on target genes in dermal papilla cells, fourth-generation dermal papilla cells in good condition were seeded in 6-well Petri dishes at a density of 1 × 105 cells per well. When the cell confluence reached approximately 70%, the culture medium was removed, and the cells were washed three times with PBS. Mimics and NC were transfected into the dermal papilla cells using Lipofectamine 3000 (Invitrogen, L3000001), with each group repeated three times. After transfection for 6 h, fresh medium was added to continue the culture. After 48 h, total RNA was extracted from the cells using TRIzol. The expression of miRNAs, target genes, and related genes was detected by RT-qPCR, with primer information provided in Supplementary Table S1. After 72 h, total protein was extracted using RIPA lysis buffer (Biyuntian, P0013B), and the protein concentration was determined using a BCA protein concentration assay kit (Biyuntian, P0010S). The protein expression of IGFBP5 was examined by western blotting, using the following antibodies: 1:2,000 IGFBP5 Polyclonal Antibody (bioss, BS-0406R) and 1:10,000 β-Tubulin Monoclonal Antibody (Immunoway, YM3030).
To verify the effect of chi-miR-877-3p on the proliferation of DPCs, DPCs were seeded in 96-well plates at a density of 5 × 103 cells per well and transfected with chi-miR-877-3p mimics and NC, respectively, with 6 replicates per group. After 12 h, 10 μL of CCK8 reagent was added to each well and incubated for 4 h. The optical density (OD) value was measured at 450 nm using an enzyme-labeled instrument.
Results
Statistics of sequencing data
A total of 144.86 million clean reads were obtained from small RNA sequencing of nine samples. After quality control, the clean data for each sample exceeded 12.80 million reads. The Q30 score, indicating base call accuracy, was ≥ 95.73% for each sample (Supplementary Table S2), and the mapping rate was ≥ 76.80% (Supplementary Table S3). In total, 1,359 miRNAs were detected across the nine samples, comprising 422 known miRNAs and 937 novel predicted miRNAs. The mature miRNAs generated had a length primarily ranging from 20 to 24 nucleotides. The length distribution of identified known and novel miRNAs is depicted in Supplementary Figure S1. Both known and novel miRNAs predominantly exhibited a length of 22 nucleotides, consistent with the characteristics of animal miRNAs. Family analysis based on sequence similarity revealed that 345 novel miRNAs belonged to the mir-2284 family, 24 miRNAs belonged to the mir-1154 family, 19 miRNAs belonged to the let-7 family, and 16 miRNAs belonged to the mir-10 family.
Analysis of miRNAs expression
The expression levels of miRNAs in each sample were analyzed. The top three miRNAs with the highest average expression across all samples were chi-miR-148a-3p, chi-miR-143-3p, and chi-miR-26a-5p. Supplementary Figure S2 shows that the overall expression patterns of miRNAs were similar across the nine samples.
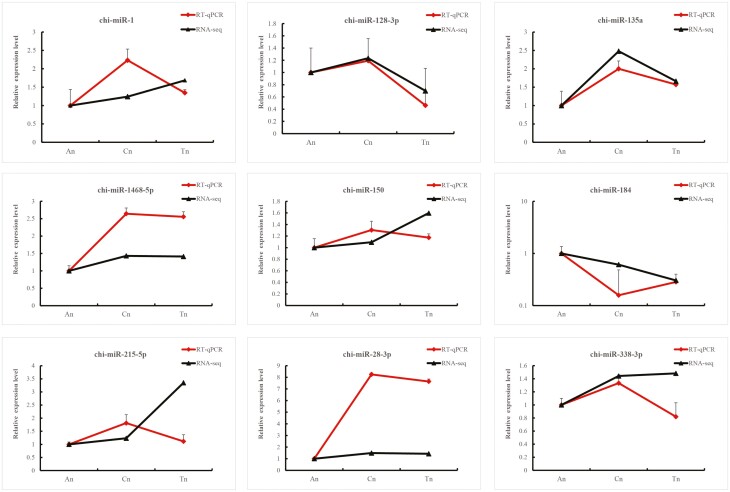
Table 1 (Supplementary Figure S3) presents the statistics of DE miRNAs between samples. In the catagen vs. anagen (Cn vs. An) comparison, 80 DE miRNAs were identified, with 59 up-regulated and 21 down-regulated. In the catagen vs. telogen (Cn vs. Tn) comparison, 60 DE miRNAs were detected, comprising 21 up-regulated and 39 down-regulated. The anagen vs. telogen (Tn vs. An) comparison revealed 121 DE miRNAs, of which 80 were up-regulated and 41 were down-regulated. To validate the results of the miRNA transcriptome sequencing, we selected nine DE miRNAs (chi-miR-1, chi-miR-128-3p, chi-miR-135a, chi-miR-1468-5p, chi-miR-150, chi-miR-184, chi-miR-215-5p, chi-miR-28-3p, and chi-miR-338-3p) for verification using RT-qPCR. The expression trends observed in the transcriptome sequencing data were consistent with those obtained from RT-qPCR analysis (Figure 1).
Table 1.
Statistical table of the number of DE mRNA
| DE mRNA set | DE mRNA number | Up-regulated | Down-regulated |
|---|---|---|---|
| Cn vs. An | 80 | 59 | 21 |
| Cn vs. Tn | 60 | 21 | 39 |
| Tn vs. An | 121 | 80 | 41 |
Prediction and functional annotation of miRNAs target genes
All predicted miRNAs targeted a total of 27,043 genes, with 21,086 genes obtaining annotation information. In the catagen and anagen phases, DE mRNAs were associated with 10,578 target genes, which were annotated with 9,278 GO terms and 8,938 pathways. Similarly, during the Cn vs. An comparison, DE mRNAs were linked to 10,578 target genes, which annotated 9,278 GO terms and 8,938 pathways. In the Cn vs. Tn comparison, DE mRNAs predicted 7,600 target genes, which were annotated with 6,815 GO terms and 6,604 pathways. Furthermore, DE miRNAs predicted 11,122 target genes in the Tn vs. An comparison and annotated them with 9,754 GO terms and 9,434 pathways.
To gain further insights into the role of DE miRNAs in hair follicles, we conducted an intersection analysis between the target genes of DE miRNAs and the mRNAs differentially expressed in the secondary hair follicles of Jiangnan cashmere goats at different time periods (Wu et al., 2022). This analysis revealed that 133 DE miRNAs targeted 1,147 differentially expressed genes. GO analysis indicated that these genes were primarily associated with functions such as keratin filament (GO:0045095), animal organ development (GO:0048513), and the external side of the plasma membrane (GO:0009897; Figure 2A). KEGG analysis revealed that these genes were mainly involved in pathways such as Staphylococcus aureus infection (ko05150), the Estrogen signaling pathway (ko04915), and the PI3K-Akt signaling pathway (ko04151; Figure 2B).

Functional enrichment analysis of target genes with differential expression of DE miRNAs. (A) GO enrichment analysis. (B) KEGG enrichment analysis. The X-axis represents the Fold Enrichment. The Y-axis represents the GO/KEGG. The size of the bubble represents the number of genes annotated to a GO/KEGG, the color represents the enrichment P value, where the darker the color, the smaller the P value is. The bubble graph shows the top 15 GO/KEGG terms with the smallest P values.
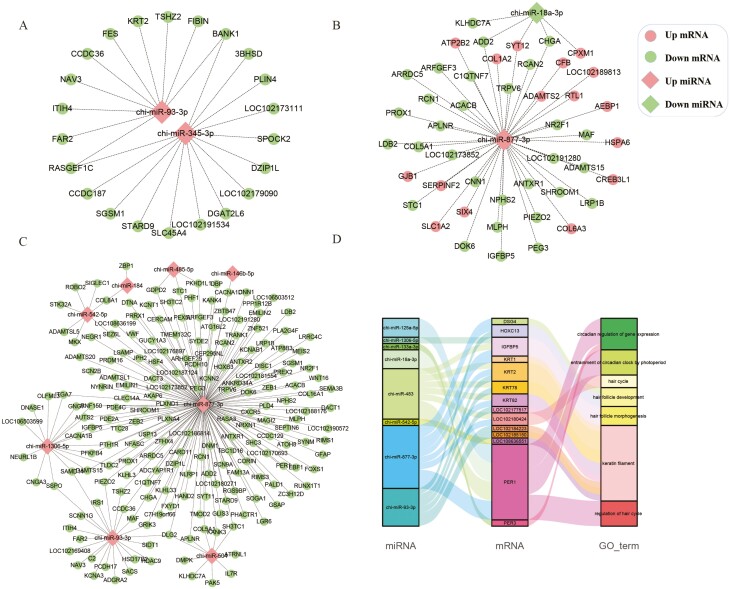
To elucidate the regulatory mechanisms of miRNAs, we focused on negatively regulated miRNA-mRNA pairs in each control group and retained networks with more than 10 connection points. In the Cn vs. An comparison, we identified 352 target relationships, among which seven differentially expressed miRNAs were found to negatively regulate 39 known target genes (Figure 3A). Similarly, in the Cn vs. Tn comparison, we obtained 133 target relationships, and 5 known DE miRNAs were found to negatively associate with 50 target genes (Figure 3B). Furthermore, in the Tn vs. An comparison, we identified 989 target relationships, involving 17 known DE miRNAs that negatively regulated 211 target genes. Figure 3C illustrates a complex network of targets, comprising eight DE miRNAs and 193 target genes. Notably, chi-miR-877-3p exhibited differential expression in both the Cn vs. Tn and Tn vs. An groups and displayed the highest number of target genes. Moreover, when the DE target genes were subjected to GO annotation analysis, we found that 14 target genes among the eight DE miRNAs were associated with hair follicle development, hair cycle, and hair shaft formation (Figure 3D).
Verification of chi-miR-877-3p
Figure 4 depicts the immunofluorescence identification of fourth-generation DPCs. The results demonstrated positive expression of α-SMA, CD133, and Versican molecular markers in each cell, indicating the successful isolation of relatively pure DPCs.
The transcriptome sequencing analysis revealed that the expression of chi-miR-877-3p was up-regulated in An compared to Tn, while the predicted target gene IGFBP5 was down-regulated (Figure 5A). To verify the regulatory effect of chi-miR-877-3p on IGFBP5 expression, mimics and NC were separately transfected into DPCs. The RT-qPCR results demonstrated a significant increase in the expression level of chi-miR-877-3p in the mimics group compared to the NC group (P < 0.001). Conversely, the expression level of IGFBP5 was significantly reduced in the mimics group compared to the NC group (P < 0.001; Figure 5B). The western blot and RT-qPCR results exhibited a consistent trend (Figure 5C). Furthermore, the dual luciferase reporter assay indicated a significant decrease in the ratio of firefly luciferase substrate fluorescence to Renilla luciferase substrate fluorescence in the mimics and IGFBP5-WT group (P < 0.05), while no significant change in enzyme activity ratio was observed between the NC group and IGFBP5-WT group (P > 0.05) (Figure 5D). Additionally, to gain further insights into the effect of chi-miR-877-3p on DPCs, the CCK-8 assay revealed a significant increase in cell viability of dermal papilla cells after transfection with chi-miR-877-3p mimics compared to the NC group (P < 0.01; Figure 5E).
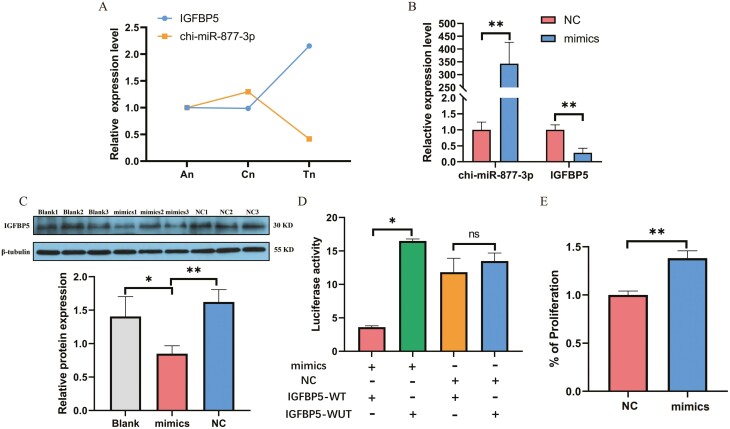
Functional verification of chi-miR-877-3p and target gene IGFBP5. (A) Expression trend analysis of chi-miR-877-3p and IGFBP5 in secondary hair follicles at different periods. (B) Verification of the regulation of chi-miR-877-3p on IGFBP5 expression level in dermal papilla cells. (C) Western blot analysis results. (D) Dual luciferase reporter assay. E. The effect of chi-miR-877-3p on cell proliferation was detected by CCK8. Data are mean ± SEM, and statistical analysis was performed using t-test. * Represents a significant difference, P < 0.05, **represents a very significant difference, P < 0.01.
Discussion
The hair follicle, a highly conserved organ, serves various functions such as immune response against pathogens, thermoregulation, sebum production, angiogenesis, neurogenesis, and wound healing. Recent studies have highlighted the crucial role of miRNAs in maintaining normal skin and hair follicle development, homeostasis, and circulation (Ji et al., 2021). In Jiangnan cashmere goats, a total of 1,359 miRNAs were detected in the skin tissue, with chi-miR-143-3p exhibiting high expression abundance. However, most miRNA studies related to cashmere have focused on goat skin, leaving the question of highly expressed miRNAs in secondary hair follicles and their impact on cashmere growth unanswered. To address this, Minglin Wang et al. investigated the preferential expression of miR-143-3p in the secondary hair follicles of cashmere goats compared to the epidermis and primary hair follicles. They demonstrated that miR-143-3p could inhibit the expression of ITGA6 and regulate the hair follicle cycle (Wang et al., 2023). Additionally, differential expression of miR-143 was observed in different waveform furs of lambs of Hu sheep, suggesting its significance in hair follicle development (Hu et al., 2021).
In our study, we compared the expression profiles of miRNAs at different stages of secondary hair follicles and identified a total of 193 DE miRNAs. The largest number of differentially expressed genes was observed between telogen and anagen, followed by catagen and anagen, while catagen and telogen showed the least differential gene expression. To validate the accuracy of transcriptome data, nine DE miRNAs were selected for RT-qPCR analysis. Chi-miR-184 exhibited high expression during anagen. Previous research on mice has indicated that miR-184 induces the activation and epidermal differentiation of the Notch signaling pathway by targeting KRT15 and FIH1 (Nagosa et al., 2017). In catagen, both chi-miR-1 and chi-miR-1468-5p demonstrated high expression. Yan et al. (2019, 2022) discovered that miR-1 was highly expressed in the exosomes of dermal papilla cells and regulated the proliferation and differentiation of cashmere hair follicle stem cells by suppressing IGF1R and LEF1 gene expression. Zhou et al. (2018) also reported higher expression levels of chi-miR-1468-5p in catagen secondary hair follicles of Shanbei white cashmere goats, consistent with our findings. Moreover, chi-miR-150 exhibited high expression during telogen. Zhao et al. (2021) integrated whole transcriptome data from different periods of sheep fetuses and found that the target genes of miR-150 were enriched in epithelial development and the Notch signaling pathway, influencing hair follicle development in sheep fetuses. Fu (2021) conducted a combined analysis of whole transcriptome and protein and discovered that chi-miR-105a negatively regulated and predicted the expression of the target gene ETV6, thus affecting cashmere fineness.
To gain further insight into the role of DE miRNAs in cashmere goat hair follicles, GO and KEGG analyses were conducted. The results revealed that a substantial number of DE miRNAs targeted genes were associated with GO terms and signaling pathways related to hair follicle development. For instance, FOXN1, a target gene of chi-miR-1306-5p, is implicated in hair follicle morphogenesis (GO:0031069). In hair follicle development, Foxe1 serves as a downstream target of the Shh/Gli pathway, playing a crucial role in the correct positioning of hair follicles within the dermis and subdermis (Brancaccio et al., 2004). The involvement of the Shh signaling pathway in regulating the hair follicle cycle and epithelial cell growth is well-established (Huntzicker et al., 2006; Woo et al., 2012). Multiple novel DE miRNAs (novel_miR_463, novel_miR_589, and novel_miR_790) share FOXN1 as a common target gene, positively regulating hair follicle development (GO:0051798) and hair follicle morphogenesis (GO:0031069). Several studies have highlighted the unique role of FOXN1 in hair follicle differentiation (Hu et al., 2010; Potter et al., 2011). Furthermore, the predicted target gene of chi-miR-483, INHBA, is associated with negative regulation of hair follicle development (GO:0051799), hair follicle development (GO:0001942), and hair follicle morphogenesis (GO:0031069). Ruijun Shi’s transcriptome sequencing findings suggested that INHBA may influence wool growth and density in Hetian sheep (Shi et al., 2022).
Moreover, a significant number of DE miRNA target gene annotations were found in the Wnt signaling pathway (ko04310) and Notch signaling pathway (ko04330). For example, the target genes of chi-miR-345-3p include LOC102178253, THSD7B, and RNF43. Chi-miR-93-3p targets TLE3, TBL1X, and VANGL1, while chi-miR-18a-3p targets DTX4 and DAAM2. Wnt, Shh, Notch, and BMP signaling pathways are crucial in hair follicle morphogenesis (Rishikaysh et al., 2014) and play particularly significant roles in the initial stages of hair follicle development as necessary initiating factors for substrate formation (Choi, 2020). The Notch signaling pathway promotes the differentiation of hair follicle stem cells into hair follicle cells, inhibits their differentiation into epidermal cells, and ensures normal differentiation of hair shafts, inner root sheaths, and the periodic growth of hair follicles during hair follicle development (Madaan et al., 2018; Wang et al., 2022).
The study of miRNAs primarily relies on complex regulatory patterns that govern the expression of target genes. By combining the target genes of DE miRNAs with previous mRNA transcriptome data, numerous DE miRNA-DE mRNA relationship pairs were identified. Extensive research by numerous scholars has demonstrated that the relationship between miRNAs and mRNAs is not one-to-one, but rather miRNAs can regulate the expression of multiple genes, sometimes even hundreds (Stark et al., 2003). Notably, among the many target gene relationship pairs, chi-miR-877-3p and its target gene IGFBP5 stood out.
IGF1 signaling profoundly influences hair follicle proliferation, tissue remodeling, hair growth cycle, and hair follicle differentiation. The regulation of the IGF1 gene is associated with androgens. Overexpression of IGF1 in hair follicles leads to increased size in certain follicles and changes in hair morphology. Conversely, forced expression of IGFBP3 and IGFBP5 reduces IGF1 signaling, resulting in thinner and slightly shorter hair (Weger and Schlake, 2005; Trüeb, 2018). Elevated expression of IGFBP5 in mutant dermal papilla cells may cause reduced hair growth, as observed in the mutant. Mouse studies have shown that transgenic IGFBP5 expression in hair follicles leads to abnormal bending and thinning of hair shafts, making IGFBP5 a molecular marker for distinguishing hair shaft curvature (Ji et al., 2021). The Wnt/β-catenin signaling pathway coordinates interactions between follicles to guide the signal cycle of hair morphogenesis. Evidence suggests that IGF is involved in regulating β-catenin activity in dermal papilla cells. Deficiency in β-catenin in these cells leads to a four-fold increase in IGFBP5 expression. Beta-catenin deficiency can cause hair loss and disrupt the hair cycle (Enshell-Seijffers et al., 2010). Furthermore, the expression patterns of IGF1 and IGF-binding proteins differ in hair follicles. Previous studies have reported IGF1 expression only in the dermis and dermal papilla’s mesenchymal cells. In humans, IGFBP3 and IGFBP5 are primarily expressed in the dermal papilla of the hair follicle, while IGFBP4 exhibits strong expression at the junction between the dermal papilla and the hair matrix. Additionally, IGFBP5 is highly expressed during the resting and rest periods of hair follicles in mice, consistent with our findings (Figure 5A). Collectively, these findings indicate that IGFBP5 is a critical regulator in hair follicle biology. Through transcriptome sequencing and target prediction, we discovered that IGFBP5 may be a target gene of chi-miR-877-3p. RNA22 software predicted multiple binding sites between chi-miR-877-3p and the 3ʹ UTR region of IGFBP5. This result was subsequently validated through dual luciferase reporter gene assays. Moreover, overexpression of chi-miR-877-3p in dermal papilla cells of cashmere goats down-regulated IGFBP5 expression and promoted dermal papilla cell proliferation. These findings indicate that IGFBP5 is indeed a target gene of chi-miR-877-3p, and the Chi-miR-877-3p- IGFBP5 pathway likely plays a role in regulating the cycle of secondary hair follicles in cashmere goats.
Conclusion
Transcriptome sequencing revealed significant miRNA expression in the skin tissue of cashmere goats, with distinct expression patterns observed in secondary hair follicles at different stages. Several target genes predicted for chi-miR-877-3p are closely related to hair growth and the development of hair follicles. Our study also demonstrated that chi-miR-877-3p plays a regulatory role in controlling the expression of IGFBP5 in dermal papilla cells. In summary, chi-miR-877-3p represents a promising novel target for modulating the development of the hair follicle cycle.
Supplementary Material
skad314_suppl_Supplementary_Tables_S1-S3_Figures_S1-S4
Glossary
Abbreviations:
| An | anagen |
| BP | Biological Processes |
| CC | Cellular Components |
| Cn | catagen |
| DE miRNAs | differentially expressed miRNAs |
| DPCs | dermal papilla cells |
| GO | Gene Ontology |
| IGFBP5 | Insulin like growth factor binding protein 5 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MF | Molecular Functions |
| MiRNAs | microRNAs |
| NC | negative control |
| P/S | penicillin-streptomycin |
| RT-qPCR | quantitative real-time PCR |
| Tn | telogen |
Contributor Information
Cuiling Wu, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Liang Yuan, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Wenzhi Cao, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Xiaofang Ye, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Xiaolin Ma, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Chongkai Qin, Xinjiang Aksu Prefecture Animal Husbandry Technology Extension Center, Aksu, China.
Bin Li, Xinjiang Aksu Prefecture Animal Husbandry Technology Extension Center, Aksu, China.
Fei Yu, Key Laboratory of Special Environments Biodiversity Application and Regulation in Xinjiang, School of Life Sciences, Xinjiang Normal University, Xinjiang, Urumqi, China.
Xuefeng Fu, Key Laboratory of Genetics Breeding and Reproduction of Xinjiang Wool-sheep Cashmere-goat (XJYS1105), Institute of Animal Science, Xinjiang Academy of Animal Sciences, Xinjiang Urumqi, China.
Funding
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01B107), Xinjiang Uygur Autonomous Region “Tianchi Talents”, National Key Research and Development Program of China (2021YFD1200902), National Natural Science Foundation of China (31360814) and Xinjiang Autonomous Region Innovation Environment Construction Special Project (2021D04008)
Literature Cited
- Ambros, V. 2004. The functions of animal microRNAs. Nature 431:350–355. 10.1038/nature02871 [Abstract] [CrossRef] [Google Scholar]
- Amelio I., Lena A. M., Bonanno E., Melino G., and Candi E.. 2013. miR-24 affects hair follicle morphogenesis targeting Tcf-3. Cell Death Dis. 4:e922. 10.1038/cddis.2013.426 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Apweiler R., Bairoch A., Wu C. H., Barker W. C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., et al.. 2004. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 32:D115–D119. 10.1093/nar/gkh131 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., et al.. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25:25–29. 10.1038/75556 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. 10.1016/s0092-8674(04)00045-5 [Abstract] [CrossRef] [Google Scholar]
- Bernard, B. A. 2016. Advances in understanding hair growth. F1000Res 5:F1000 Faculty Rev–F1000 Faculty 147. 10.12688/f1000research.7520.1 [CrossRef] [Google Scholar]
- Betel D., Wilson M., Gabow A., Marks D. S., and Sander C.. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res. 36:D149–D153. 10.1093/nar/gkm995 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brancaccio A., Minichiello A., Grachtchouk M., Antonini D., Sheng H., Parlato R., Dathan N., Dlugosz A. A., and Missero C.. 2004. Requirement of the forkhead gene Foxe1, a target of sonic hedgehog signaling, in hair follicle morphogenesis. Hum. Mol. Genet. 13:2595–2606. 10.1093/hmg/ddh292 [Abstract] [CrossRef] [Google Scholar]
- Choi, B. Y. 2020. Targeting Wnt/β-catenin pathway for developing therapies for hair loss. Int. J. Mol. Sci. 21:4915. 10.3390/ijms21144915 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Eddy, S. R. 1998. Profile hidden Markov models. Bioinformatics 14:755–763. 10.1093/bioinformatics/14.9.755 [Abstract] [CrossRef] [Google Scholar]
- Enshell-Seijffers D., Lindon C., Kashiwagi M., and Morgan B. A.. 2010. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 18:633–642. 10.1016/j.devcel.2010.01.016 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Friedländer M. R., Mackowiak S. D., Li N., Chen W., and Rajewsky N.. 2012. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40:37–52. 10.1093/nar/gkr688 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fu, X. 2021. Selection of regulatory factors related to cashmere fiber diameter trait from transcriptome and proteome profiles in Tibetan cashmere goats. Gansu Agricultural University. 10.27025/d.cnki.ggsnu.2021.000030. [CrossRef] [Google Scholar]
- Hu B., Lefort K., Qiu W., Nguyen B. C., Rajaram R. D., Castillo E., He F., Chen Y., Angel P., Brisken C., et al.. 2010. Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes Dev. 24:1519–1532. 10.1101/gad.1886910 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hu T., Huang S., Lv X., Wang S., Getachew T., Mwacharo J. M., Haile A., and Sun W.. 2021. miR-143 targeting CUX1 to regulate proliferation of dermal papilla cells in Hu sheep. Genes (Basel) 12:2017. 10.3390/genes12122017 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Huntzicker E. G., Estay I. S., Zhen H., Lokteva L. A., Jackson P. K., and Oro A. E.. 2006. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 20:276–281. 10.1101/gad.1380906 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ji S., Zhu Z., Sun X., and Fu X.. 2021. Functional hair follicle regeneration: an updated review. Signal Transduct Target Ther. 6:66. 10.1038/s41392-020-00441-y [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kanehisa M., Goto S., Kawashima S., Okuno Y., and Hattori M.. 2004. The KEGG resource for deciphering the genome. Nucleic Acids Res. 32:D277–D280. 10.1093/nar/gkh063 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Koonin E. V., Fedorova N. D., Jackson J. D., Jacobs A., Krylov D. M., Makarova K. S., Mazumder R., Mekhedov S. L., Nikolskaya A. N., et al.. 2004. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 5:R7–R7. 10.1186/gb-2004-5-2-r7 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Krause K., and Foitzik K.. 2006. Biology of the hair follicle: the basics. Semin. Cutan. Med. Surg. 25:2–10. 10.1016/j.sder.2006.01.002 [Abstract] [CrossRef] [Google Scholar]
- Langmead B., Trapnell C., Pop M., and Salzberg S. L.. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10:R25. 10.1186/gb-2009-10-3-r25 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., and Burge C. B.. 2003. Prediction of mammalian microRNA targets. Cell 115:787–798. 10.1016/s0092-8674(03)01018-3 [Abstract] [CrossRef] [Google Scholar]
- Li B., Ruotti V., Stewart R. M., Thomson J. A., and Dewey C. N.. 2010. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26:493–500. 10.1093/bioinformatics/btp692 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [Abstract] [CrossRef] [Google Scholar]
- Ma T., Li J., Li J., Wu S., Xiangba, Jiang H., Zhang Q. 2021. Expression of miRNA-203 and its target gene in hair follicle cycle development of Cashmere goat. Cell Cycle 20:204–210. 10.1080/15384101.2020.1867789 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Madaan A., Verma R., Singh A. T., and Jaggi M.. 2018. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 40:429–450. 10.1111/ics.12489 [Abstract] [CrossRef] [Google Scholar]
- Mardaryev A. N., Ahmed M. I., Vlahov N. V., Fessing M. Y., Gill J. H., Sharov A. A., and Botchkareva N. V.. 2010. Micro-RNA-31 controls hair cycle-associated changes in gene expression programs of the skin and hair follicle. FASEB J. 24:3869–3881. 10.1096/fj.10-160663 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Nagosa S., Leesch F., Putin D., Bhattacharya S., Altshuler A., Serror L., Amitai-Lange A., Nasser W., Aberdam E., Rouleau M., et al.. 2017. microRNA-184 induces a commitment switch to epidermal differentiation. Stem Cell Rep. 9:1991–2004. 10.1016/j.stemcr.2017.10.030 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pickup M. E., Hu A., Patel H. J., and Ahmed M. I.. 2023. MicroRNA-148a controls epidermal and hair follicle stem/progenitor cells by modulating the activities of ROCK1 and ELF5. J. Invest. Dermatol. 143:480–491.e5. 10.1016/j.jid.2022.06.028 [Abstract] [CrossRef] [Google Scholar]
- Potter C. S., Pruett N. D., Kern M. J., Baybo M. A., Godwin A. R., Potter K. A., Peterson R. L., Sundberg J. P., and Awgulewitsch A.. 2011. The nude mutant gene Foxn1 is a HOXC13 regulatory target during hair follicle and nail differentiation. J. Invest. Dermatol. 131:828–837. 10.1038/jid.2010.391 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rishikaysh P., Dev K., Diaz D., Qureshi W. M., Filip S., and Mokry J.. 2014. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 15:1647–1670. 10.3390/ijms15011647 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Robinson M. D., McCarthy D. J., and Smyth G. K.. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. 10.1093/bioinformatics/btp616 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shang F., Wang Y., Ma R., Rong Y., Wang M., Wu Z., Hai E., Pan J., Liang L., Wang Z., et al.. 2022. Screening of microRNA and mRNA related to secondary hair follicle morphogenesis and development and functional analysis in cashmere goats. Funct Integr Genomics 22:835–848. 10.1007/s10142-022-00842-y [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shi R., Li S., Liu P., Zhang S., Wu Z., Wu T., Gong S., and Wan Y.. 2022. Identification of key genes and signaling pathways related to Hetian sheep wool density by RNA-seq technology. PLoS One 17:e0265989. 10.1371/journal.pone.0265989 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Stark A., Brennecke J., Russell R. B., and Cohen S. M.. 2003. Identification of Drosophila MicroRNA targets. PLoS Biol. 1:E60. 10.1371/journal.pbio.0000060 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tatusov R. L., Galperin M. Y., Natale D. A., and Koonin E. V.. 2000. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28:33–36. 10.1093/nar/28.1.33 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Teta M., Choi Y. S., Okegbe T., Wong G., Tam O. H., Chong M. M., Seykora J. T., Nagy A., Littman D. R., Andl T., et al.. 2012. Inducible deletion of epidermal Dicer and Drosha reveals multiple functions for miRNAs in postnatal skin. Development 139:1405–1416. 10.1242/dev.070920 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Trüeb, R. M. 2018. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Skin Appendage Disord. 4:90–95. 10.1159/000479333 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang X., Liu Y., He J., Wang J., Chen X., and Yang R.. 2022. Regulation of signaling pathways in hair follicle stem cells. Burns Trauma 10:tkac022. 10.1093/burnst/tkac022 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang M., Dai H., Sheng S., Liu Y., Zhang S., Bai W., and Xue H.. 2023. Discovery and functional analysis of secondary hair follicle miRNAs during annual cashmere growth. Int. J. Mol. Sci. 24:1063. 10.3390/ijms24021063 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weger N., and Schlake T.. 2005. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J. Invest. Dermatol. 125:873–882. 10.1111/j.0022-202X.2005.23946.x [Abstract] [CrossRef] [Google Scholar]
- Woo W. M., Zhen H. H., and Oro A. E.. 2012. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 26:1235–1246. 10.1101/gad.187401.112 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wu C., Wang C., Zhai B., Zhao Y., Zhao Z., Yuan Z., Zhang M., Tian K., and Fu X.. 2021. Study of microRNA expression profile in different regions of ram epididymis. Reprod. Domest. Anim. 56:1209–1219. 10.1111/rda.13978 [Abstract] [CrossRef] [Google Scholar]
- Wu C., Qin C., Fu X., Huang X., and Tian K.. 2022. Integrated analysis of lncRNAs and mRNAs by RNA-Seq in secondary hair follicle development and cycling (anagen, catagen and telogen) of Jiangnan cashmere goat (Capra hircus). BMC Vet. Res. 18:167. 10.1186/s12917-022-03253-0 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xiang B., Li Y., Li J., Zhang B., Li J., Jiang H., and Zhang Q.. 2023. MiR-21 regulated hair follicle cycle development in Cashmere goats by targeting FGF18 and SMAD7. Anim. Biotechnol 10:1–8. 10.1080/10495398.2023.2186891 [Abstract] [CrossRef] [Google Scholar]
- Yan H., Gao Y., Ding Q., Liu J., Li Y., Jin M., Xu H., Ma S., Wang X., Zeng W., et al.. 2019. Exosomal micro RNAs derived from dermal papilla cells mediate hair follicle stem cell proliferation and differentiation. Int. J. Biol. Sci. 15:1368–1382. 10.7150/ijbs.33233 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yan H., Jin M., Li Y., Gao Y., Ding Q., Wang X., Zeng W., and Chen Y.. 2022. miR-1 regulates differentiation and proliferation of goat hair follicle stem cells by targeting IGF1R and LEF1 genes. DNA Cell Biol. 41:190–201. 10.1089/dna.2021.0288 [Abstract] [CrossRef] [Google Scholar]
- Yangyang D., Jianqi L. I., Songfeng W. U., Yunping Z. H. U., Yaowen C., and Fuchu H. E.. 2006. Integrated nr database in protein annotation system and its localization. Comput. Eng. 32:71–72. 10.1109/INFOCOM.2006.241. [CrossRef] [Google Scholar]
- Yi R., Poy M. N., Stoffel M., and Fuchs E.. 2008. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 452:225–229. 10.1038/nature06642 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yuan S., Li F., Meng Q., Zhao Y., Chen L., Zhang H., Xue L., Zhang X., Lengner C., and Yu Z.. 2015. Post-transcriptional regulation of keratinocyte progenitor cell expansion, differentiation and hair follicle regression by miR-22. PLoS Genet. 11:e1005253. 10.1371/journal.pgen.1005253 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang X., Wan G., Berger F. G., He X., and Lu X.. 2011. The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol. Cell 41:371–383. 10.1016/j.molcel.2011.01.020 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhang G., Xu J., Zhang Y., Yang S., and Jiang H.. 2022. Expression of miRNA-1-3p and its target gene in hair follicle cycle development of Liaoning Cashmere goat. Anim. Biotechnol. 34:1937–1942. 10.1080/10495398.2022.2058519 [Abstract] [CrossRef] [Google Scholar]
- Zhao B., Luo H., He J., Huang X., Chen S., Fu X., Zeng W., Tian Y., Liu S., Li C. J., et al.. 2021. Comprehensive transcriptome and methylome analysis delineates the biological basis of hair follicle development and wool-related traits in Merino sheep. BMC Biol. 19:197. 10.1186/s12915-021-01127-9 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou G., Kang D., Ma S., Wang X., Gao Y., Yang Y., Wang X., and Chen Y.. 2018. Integrative analysis reveals ncRNA-mediated molecular regulatory network driving secondary hair follicle regression in cashmere goats. BMC Genomics 19:222. 10.1186/s12864-018-4603-3 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Articles from Journal of Animal Science are provided here courtesy of Oxford University Press
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/154819721
Article citations
MicroRNA-181a Targets GNAI2 and Affects the Proliferation and Induction Ability of Dermal Papilla Cells: The Potential Involvement of the Wnt/β-Catenin Signaling Pathway.
Int J Mol Sci, 25(14):7950, 20 Jul 2024
Cited by: 0 articles | PMID: 39063192 | PMCID: PMC11277120
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
Quick GO (5)
- (2 citations) GO - GO0031069
- (1 citation) GO - GO0045095
- (1 citation) GO - GO0001942
- (1 citation) GO - GO0009897
- (1 citation) GO - GO0048513
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inner Mongolian Cashmere Goat Secondary Follicle Development Regulation Research Based on mRNA-miRNA Co-analysis.
Sci Rep, 10(1):4519, 11 Mar 2020
Cited by: 21 articles | PMID: 32161290 | PMCID: PMC7066195
Integrative analysis reveals ncRNA-mediated molecular regulatory network driving secondary hair follicle regression in cashmere goats.
BMC Genomics, 19(1):222, 27 Mar 2018
Cited by: 26 articles | PMID: 29587631 | PMCID: PMC5870523
Chi-miR-30b-5p inhibits dermal papilla cells proliferation by targeting CaMKIIδ gene in cashmere goat.
BMC Genomics, 21(1):430, 26 Jun 2020
Cited by: 9 articles | PMID: 32586272 | PMCID: PMC7318507
Comprehensive Transcriptome Analysis of Hair Follicle Morphogenesis Reveals That lncRNA-H19 Promotes Dermal Papilla Cell Proliferation through the Chi-miR-214-3p/β-Catenin Axis in Cashmere Goats.
Int J Mol Sci, 23(17):10006, 02 Sep 2022
Cited by: 3 articles | PMID: 36077403 | PMCID: PMC9456307
Funding
Funders who supported this work.
National Key Research and Development Program of China (1)
Grant ID: 2021YFD1200902
National Natural Science Foundation of China (1)
Grant ID: 31360814
Natural Science Foundation of Xinjiang Province (1)
Grant ID: 2022D01B107